Abstract
The voltage dependence of activation of the HCN hyperpolarization-activated cation channels is shifted in inside-out patches by −40 to −60 mV relative to activation in intact cells, a phenomenon referred to as rundown. Less than 20 mV of this hyperpolarizing shift can be due to the influence of the canonical modulator of HCN channels, cAMP. Here we study the role of phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2) in HCN channel rundown, as hydrolysis of PI(4,5)P2 by lipid phosphatases is thought to underlie rundown of several other channels. We find that bath application of exogenous PI(4,5)P2 reverses the effect of rundown, producing a large depolarizing shift in HCN2 activation. A synthetic short chain analogue of PI(4,5)P2, dioctanoyl phosphatidylinositol 4,5-bisphosphate, shifts the HCN2 activation curve to more positive potentials in a dose-dependent manner. Other dioctanoyl phosphatidylinositides with one or more phosphates on the lipid headgroup also shift activation, although phosphatidylinositol (PI) is ineffective. Several lines of evidence suggest that HCN2 is also regulated by endogenous PI(4,5)P2: (a) blockade of phosphatases slows the hyperpolarizing shift upon patch excision; (b) application of an antibody that binds and depletes membrane PIP2 causes a further hyperpolarizing shift in activation; (c) the shift in activation upon patch excision can be partially reversed by MgATP; and (d) the effect of MgATP is blocked by wortmannin, an inhibitor of PI kinases. Finally, recordings from rabbit sinoatrial cells demonstrate that diC8 PI(4,5)P2 delays the rundown of native HCN currents. Thus, both native and recombinant HCN channels are regulated by PI(4,5)P2.
INTRODUCTION
The HCN channel gene family (HCN1–4) encodes the hyperpolarization-activated cation channels that generate the excitatory pacemaker current (Ih or If), which contributes to the rhythmic activity of both neurons and cardiac myocytes (Robinson and Siegelbaum, 2003; DiFrancesco, 2006). Cyclic AMP has long been known to directly enhance Ih by shifting its activation to more positive potentials (DiFrancesco and Tortora, 1991). More recently, HCN channel function has been found to be regulated by several other mechanisms, including tyrosine phosphorylation (Yu et al., 2004; Zong et al., 2005; Arinsburg et al., 2006); association with MiRP1, a potential HCN channel β subunit (Yu et al., 2001); and association with TRIP8b, a cytoplasmic protein that controls channel trafficking (Santoro et al., 2004). Despite the discovery of these new modes of Ih regulation, several lines of evidence, including the phenomenon of channel rundown, suggest the existence of other regulatory mechanisms that have yet to be identified.
The activation of Ih shifts by as much as −40 to −60 mV relative to that in intact cells during recordings from inside-out patches or during prolonged dialysis associated with whole-cell recordings, for both native HCN channels in cardiac myocytes (DiFrancesco et al., 1986; DiFrancesco and Tortora, 1991; DiFrancesco and Mangoni, 1994; Bois et al., 1997) and recombinant channels in heterologous cells (Chen et al., 2001). In contrast, this rundown phenomenon is not observed in perforated-patch whole-cell recordings, suggesting that rundown may be due to the loss of intracellular constituents that regulate Ih in intact cells (Zhou and Lipsius, 1993). Since cAMP produces, at most, a 20-mV positive shift in the activation of Ih, loss of a modulatory influence of cyclic nucleotides after patch excision can account for no more than half of the 40–60-mV shift seen during rundown (DiFrancesco and Tortora, 1991; Chen et al., 2001), suggesting the presence of other modulatory factors. Neither MiRP1 (Yu et al., 2001) nor TRIP8b (Santoro et al., 2004) are likely candidates for this factor since these proteins do not act to shift HCN activation gating to more positive potentials in intact cells. Although overexpression of Src tyrosine kinase in HEK-293 cells does shift the voltage dependence of HCN channel activation to more positive potentials (Arinsburg et al., 2006), loss of Src activity is also unlikely to underlie rundown because pharmacological inhibition of endogenous Src has relatively little effect on the voltage dependence of HCN channel activation in intact cells (Zong et al., 2005; Arinsburg et al., 2006). Thus, the identity of the regulatory mechanism responsible for the rundown of Ih activation remains unclear.
Interestingly, rundown may be of physiological relevance since a −40-mV hyperpolarizing shift in HCN channel activation is observed in intact ventricular myocytes during postnatal development (Robinson et al., 1997) and this shift does not appear to be due to changes in basal cAMP concentration (Qu et al., 2001). Moreover, during heart failure, HCN channel activation in ventricular myocytes shifts back toward more positive potentials, producing a proarrhythmic increase in pacemaker current (Cerbai et al., 1997).
One promising candidate molecule for underlying rundown is the membrane phospholipid, phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2). Although PI(4,5)P2 was initially discovered as a precursor for two other second messengers, inositol trisphosphate and diacylglycerol, it has more recently been shown to play an active role in cellular physiology, including enzyme activation, membrane trafficking, and, more specifically, the regulation of ion channels (Hilgemann et al., 2001; McLaughlin et al., 2002; Suh and Hille, 2005). Here we examine the hypothesis that the hyperpolarizing shift in HCN channel gating upon patch excision results from the loss of a basal regulatory action of PI(4,5)P2 during rundown due to lipid dephosphorylation by membrane-bound phosphatases. Such a mechanism has been previously found to underlie the functional changes observed upon patch excision for a variety of other ion channels, including inward rectifying K+ channels (Zhang et al., 1999) and P/Q-type voltage-gated Ca2+ channels (Wu et al., 2002; Suh and Hille, 2005).
To address this hypothesis, we examined both recombinant HCN2 channels expressed in Xenopus oocytes and native HCN currents in cardiac sinoatrial cells. We employed several techniques to manipulate membrane levels of PI(4,5)P2 in both excised patches and whole oocytes, while monitoring the consequences on HCN2 gating. The results from these experiments are consistent with the idea that HCN2 and native sinoatrial HCN currents are regulated by both exogenous and endogenous PI(4,5)P2.
MATERIALS AND METHODS
Expression in Xenopus Oocytes
Mouse HCN2 was previously cloned into pGEMHE (Chen et al., 2001) and the HCN2/FPN mutant was previously constructed (Zhou et al., 2004). PIP5K (phosphatidylinositol-4-phosphate 5-kinase) in the pGEMHE vector was the generous gift of the Logothetis laboratory. pGEMHE:HCN2 and pGEMHE:PIP5K were linearized by SphI and HpaI, respectively. cRNA was transcribed from linearized DNA using T7 RNA polymerase (mMessage mMachine; Ambion) and injected into Xenopus oocytes as described previously (Goulding et al., 1992). Oocytes were injected with 5 ng HCN2 cRNA; in coexpression experiments, oocytes were injected with 20 ng HCN2 and 20 ng PIP5K cRNA.
Xenopus Oocyte Electrophysiological Recordings
Cell-free inside-out patches were obtained 5–6 d after cRNA injection, and data were acquired using a patch-clamp amplifier (Axopatch 200B; Axon Instruments, Inc.). Patch pipettes had resistances of 1–3 MΩ and were filled with a pipette solution containing (in mM) 96 KCl, 1 NaCl, 10 HEPES, 1.8 CaCl2, and 1 MgCl2, pH 7.4. The bath solution contained 96 KCl, 1 NaCl, 10 HEPES, and 5 EGTA, pH 7.4. The FV bath solution used to inhibit phosphatases contained 96 KCl, 0.4 NaCl, 10 HEPES, 5 EGTA, 0.1 KF, 0.2 Na3VO4, pH 7.4. An Ag-AgCl ground wire was connected to the bath solution by a 3 M KCl agar bridge electrode, and junction potential was compensated before the formation of each patch. Linear leak current was not subtracted. Data were filtered at 1 kHz with an 8-pole low-pass Bessel filter (Frequency Devices) and sampled at 2 kHz with an ITC-16 interface (Instrutech) and Pulse software (HEKA).
Two microelectrode voltage-clamp recordings were obtained 1 d after cRNA injection using an oocyte clamp amplifier (model OC-725C; Warner Instruments). Data were filtered at 1 kHz and sampled at 2 kHz. The recordings were obtained with the oocytes bathed in an extracellular solution containing (in mM) 96 KCl, 2 NaCl, 10 HEPES, and 2 MgCl2, pH 7.5. Microelectrodes were filled with 3 M KCl and had resistances of 0.5–2 MΩ.
Hyperpolarizing voltages in 10-mV step increments were applied to either inside-out patches or intact oocytes from a holding potential of −30 mV. All recordings were obtained at room temperature (18–22°C).
Data Analysis
HCN currents were analyzed as previously described (Chen et al., 2001). Activation curves were determined from plots of tail current amplitude (measured at −40 mV) as a function of test voltage during 3-s hyperpolarizing steps. Activation curves were fit with a Boltzmann equation to obtain the midpoint voltage of activation (V1/2) and slope factor (s) of the relation (both in units of mV). Dose–response curves plotting the magnitude of the shift in V1/2 as a function of diC8 PI(4,5)P2 concentration were measured and fitted with the Hill equation to obtain the maximal shift in V1/2 at saturating lipid concentration, the concentration of lipid producing a half-maximal shift (K1/2), and the Hill coefficient (h). Analysis was done using PulseFit (HEKA), Excel (Microsoft), and Origin (Microcal). Single comparisons between two experimental conditions were evaluated by a Student's t test. Comparisons involving multiple populations were evaluated by ANOVA with a Scheffe Post-Hoc test.
Experiments on Rabbit Sinoatrial Cells
Animal protocols conformed to the guidelines for the care and use of laboratory animals (National Institutes of Health publication no. 85–23). Rabbit sinoatrial cells were isolated as reported (DiFrancesco et al., 1986). Whole-cell patch-clamp experiments were performed using pipettes with a resistance of 2.5–4 MΩ filled with a solution containing (in mM) 130 aspartic acid, 146 KOH, 10 NaCl, 2 CaCl2, 5 EGTA-KOH, 2 Mg-ATP, and 10 HEPES-KOH, pH 7.2. Cells were superfused with an extracellular solution containing 140 NaCl, 2.3 NaOH, 1 MgCl2, 5.4 KCl, 1.8 CaCl2, 5 HEPES, 10 glucose, 2 MnCl2, and 1 BaCl2, pH 7.4. Currents were recorded and filtered on-line at 1 kHz with an Axopatch 200B amplifier, and acquired using pClamp 9.0 software (Axon Instruments, Inc.). Recordings were performed at 32°C.
Activation curves were measured using a two-step protocol in which voltage steps from −25 to −115 or −145 mV were applied from a holding potential of −35 mV, followed by a step to −70 mV. To allow HCN channels to reach steady-state activation at all voltages, the duration of test steps varied from 10 s at −25 mV to 5 s at −145 mV while the duration of the step to −70 mV increased from 8 to 13.5 s after steps from more hyperpolarized voltages. The normalized plot of tail current versus test voltage was fitted with a Boltzmann function to obtain the V1/2 and slope factor. Values are given as the mean ± SEM and data were compared using Student's t test for single comparisons and two-way ANOVA followed by a Bonferroni Post-Hoc test for multiple comparisons. Values of P < 0.05 were considered significant.
Reagents and Drugs
An aqueous stock solution of 0.5 mM native PI(4,5)P2 (Calbiochem) was sonicated and aliquoted before snap freezing in liquid nitrogen. Aliquots were stored at −80°C until the day of the experiment, when working solutions were sonicated a second time. Dioctanoyl (diC8) forms of PI, PI(4)P, PI(3,4)P2, PI(4,5)P2, and PI(3,4,5)P3 (Echelon) were dissolved in water to make 2.5 mM stock solutions and stored in aliquots at −80°C. Oleoyl CoA (Sigma-Aldrich) was dissolved in water at a concentration of 5 mM. Anti-PIP2 mAb and anti-hGM-CSF Ab (Assay Designs) were stored at −20°C at a concentration of 0.909 μg/μl. Antibodies were diluted 1:30 with bath solution to make working solutions. Heat-inactivated anti-PIP2 mAb was prepared using a 90-min incubation in a 95°C water bath. Wortmannin (Sigma-Aldrich) was dissolved in DMSO at a concentration of 30 mM and stored in aliquots at −20°C. LY294002 (Biomol) was dissolved in DMSO at a concentration of 50 mM and stored in aliquots at −20°C. Poly-d-lysine HBr with a molecular weight of 1–4 kD was applied to patches at a final concentration of 25 μg/ml. Materials were purchased from Sigma-Aldrich unless otherwise noted.
Online Supplemental Material
Additional experimental data (available online at http://www.jgp.org/cgi/content/full/jgp.200609648/DC1) show the effect of rundown on the V1/2 of HCN2 (Fig. S1) and the effect of phosphatidylinositides on maximal tail current amplitude (Fig. S2).
RESULTS
Exogenous Phosphatidylinositol Phosphates Alter the Voltage-dependent Gating of HCN2 Channels
Hyperpolarization-activated currents were elicited by a series of 3-s hyperpolarizing test pulses in membrane patches from Xenopus oocytes expressing HCN2. The midpoint voltage of activation from tail current activation curves (V1/2) was approximately −80 mV when recorded in cell-attached patches before patch excision or from intact oocytes using two-microelectrode voltage clamp (Chen et al., 2001). However, within 1 min of formation of an inside-out patch, the V1/2 had shifted by −40 mV, to approximately −120 mV. The V1/2 was then stable over the next 10 min, indicating that this rundown in the voltage dependence of activation rapidly reached completion (Fig. S1, available at http://www.jgp.org/cgi/content/full/jgp.200609648/DC1).
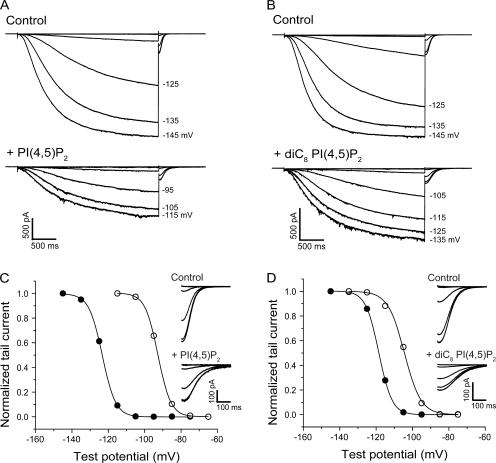
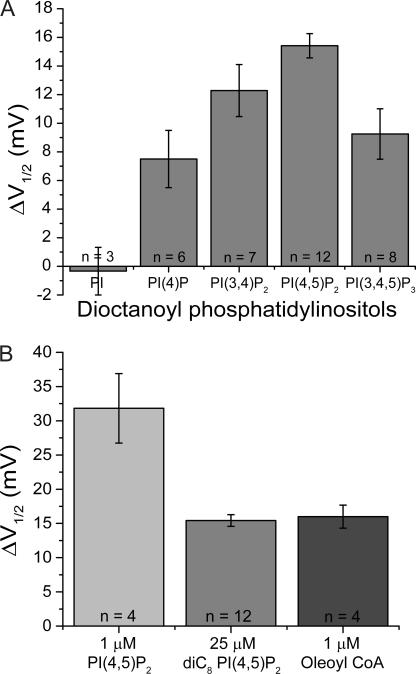
The role of PI(4,5)P2 in HCN channel gating was first studied by application of exogenous PI(4,5)P2 to inside-out patches after rundown was complete. Bath application of 1 μM PI(4,5)P2 isolated from native tissues, which consists predominantly of phosphatidylinositol 4,5-bisphosphate with an arachidonyl acyl chain in position 2 and a stearyl acyl chain at position 3 (AASt) (Rohacs et al., 2002), produced a large, gradual positive shift in the voltage dependence of HCN2 activation (Fig. 1, A and C). The time course of the shift in V1/2 upon application of PI(4,5)P2 was fit by a single exponential function with a t1/2 value (time to produce a half maximal effect) of ∼250 s. The average shift in V1/2 after 28 min of exposure to phospholipid was +31.8 ± 5.1 mV (n = 4; see Fig. 3 B).
Figure 1.
Phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2) and dioctanoyl phosphatidylinositol 4,5-bisphosphate (diC8 PI(4,5)P2) cause depolarizing shifts in the activation of HCN2. Macroscopic HCN2 currents are shown for individual inside-out patches in response to a series of hyperpolarizing voltage steps. (A) Before (top) and after (bottom) a 15′ bath application of 1 μM native PI(4,5)P2. (B) Before (top) and after (bottom) a 10′ bath application of 25 μM diC8 PI(4,5)P2. (C and D) Normalized tail currents from A and B, respectively, plotted as a function of test potential and fit with the Boltzmann equation. Data obtained in absence (filled circles) or presence (open circles) of PI(4,5)P2 and diC8 PI(4,5)P2 application. Insets show expanded records of tail currents from A and B, measured at −40 mV.
Figure 3.
Shift in V1/2 of HCN2 depends on number and location of inositol phosphates and on acyl chain length. (A) V1/2 shifts with 10-min applications of dioctanoyl phosphatidylinositol (PI) (−0.3 ± 1.7 mV, n = 3), dioctanoyl phosphatidylinositol 4-phosphate (PI(4)P) (+7.5 ± 2.0 mV, n = 6), dioctanoyl phosphatidylinositol 3,4-bisphosphate (PI(3,4)P2) (+12.3 ± 1.8 mV, n = 7), dioctanoyl phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2), and dioctanoyl phosphatidylinositol 3,4,5-trisphosphate (PI(3,4,5)P3) (+9.3 ± 1.8 mV, n = 8). Differences between the responses to diC8 PI(4,5)P2 and diC8 PI(3,4,5)P3 and between the responses to diC8 PI(4,5)P2 and diC8 PI(4)P are statistically significant (P < 0.05; ANOVA, Post Hoc). (B) V1/2 shifts after 28-min application of 1 μM of native PI(4,5)P2 (+31.8 ± 5.1 mV, n = 4), 10-min application of 25 μM diC8 PI(4,5)P2 (+15.4 ± 0.8 mV, n = 12), and 2-min application of 1 μM oleoyl coA (+16.0 ± 1.7 mV, n = 4).
Given the slow rate at which native PI(4,5)P2 altered the voltage-dependent activation of HCN2, we next examined the short-acyl chain analogue of PI(4,5)P2, dioctanoyl phosphatidylinositol 4,5-bisphosphate (diC8 PI(4,5)P2), since this analogue has been reported to act more rapidly than long chain lipids (Rohacs et al., 2002). Application of 25 μM diC8 PI(4,5)P2 caused a significant positive shift in the activation curve of HCN2, which occurred more quickly but was smaller in magnitude than the shift caused by 1 μM AASt PI(4,5)P2 (Fig. 1, B and D). The onset of the shift in V1/2 with diC8 PI(4,5)P2 (n = 10) had a t1/2 value of 86 s, three times faster than the effect of full-length PI(4,5)P2 (the increased rate of action of diC8 PI(4,5)P2 may reflect the fact that it needed to be applied at a higher concentration than the more potent full-length AASt PI(4,5)P2). The average shift in V1/2 of HCN2 after 10 min exposure to 25 μM diC8 PI(4,5)P2 was +15.4 ± 0.8 mV (n = 12; see Fig. 3 B), and there was a dose-dependent relation between the shift in V1/2 and the concentration of diC8 PI(4,5)P2 (Fig. 2 A). A fit of the Hill equation to the dose–response curve yielded a value for the maximal shift in V1/2 of +20.9 mV, a K1/2 value of 10.9 μM, and a Hill coefficient (h) of 1.11.
Figure 2.
Action of diC8 PI(4,5)P2 on the gating of HCN2. (A) Dose–response curve for shift in V1/2 (ΔV1/2) as a function of concentration of diC8 PI(4,5)P2. Data fit with the Hill equation. (B) Time constants (τ) of HCN2 current activation (left) and deactivation (right) in absence (squares, solid line) and presence (circles, dashed line) of 25 μM diC8 PI(4,5)P2. The difference between the time constants of activation at −135 mV before (0.731 ± 0.079 s) and after (1.28 ± 0.22 s) diC8 PI(4,5)P2 application were statistically significant (*, P < 0.02, t test). The difference between the time constants of deactivation at −40 mV before (67.6 ± 5.0 ms) and after (136 ± 13 ms) diC8 PI(4,5)P2 application were also statistically significant (*, P < 0.001, t test).
Both full-length PI(4,5)P2 and diC8 PI(4,5)P2 had a variable effect on the peak tail current amplitude after steps to negative voltages where voltage-dependent activation was complete (see Fig. S2). On average, there was a small decrease in peak current amplitude with both 25 μM full-length PI(4,5)P2 (37.1% ± 18.7% decrease; range of 0.7% increase to 88.0% decrease; n = 4) and 25 μM diC8 PI(4,5)P2 (36.2% ± 6.8% decrease; range of 6.8% increase to 73.3% decrease; n = 12). However, these changes were not statistically significant when the effects of PI(4,5)P2 were analyzed over an entire dose–response curve, including control experiments without PI(4,5)P2 application (13.1 ± 1.6% decrease; n = 3; P > 0.35; ANOVA).
We next examined the effects of diC8 PI(4,5)P2 on the activation and deactivation kinetics of HCN2 channels (Fig. 2 B). Currents during hyperpolarizing voltage steps were fit with a single exponential to determine an effective time constant of activation. There was no significant effect of PI(4,5)P2 on the time constant of activation with steps to −115 or −125 mV. However, we observed a significant slowing of activation with steps to −135 mV (P < 0.02; t test). We also observed a significant twofold slowing in the rate of channel deactivation, measured by the time constant of tail current decay upon return of the membrane to −40 mV (P < 0.001; t test).
Specificity Profile of HCN2 for Dioctanoyl Phosphatidylinositol Phosphates
Different PI(4,5)P2 binding domains and PI(4,5)P2-regulated ion channels show varying degrees of specificity for PI(4,5)P2 versus phosphatidylinositides that contain different numbers and positions of phosphates on the inositol headgroup (Rohacs et al., 1999; McLaughlin et al., 2002). To determine the lipid specificity profile for HCN2, we compared the actions of a variety of PI lipids, all applied at the same concentration (25 μM) (Fig. 3 A).
Application of dioctanoyl phosphatidylinositol (diC8 PI), whose inositol headgroup is nonphosphorylated, produced no voltage shift in the HCN2 activation curve (−0.3 ± 1.7 mV, n = 3), indicating the need for one or more phosphate moieties (or alternatively, a requirement for a larger headgroup). A phosphoinositide with a single phosphate, dioctanoyl phosphatidylinositol 4-phosphate (diC8 PI(4)P), produced a small 7.5 ± 2.0 mV (n = 6) positive shift in the V1/2 that was significantly less than the 15.4-mV shift seen with 25 μM diC8 PI(4,5)P2 (P < 0.02; ANOVA). Application of a bisphosphate isomer distinct from PI(4,5)P2, dioctanoyl phosphatidylinositol 3,4-bisphosphate (diC8 PI(3,4)P2), caused a 12.3 ± 1.8-mV depolarizing shift (n = 7), whereas dioctanoyl phosphatidylinositol 3,4,5-trisphosphate (diC8 PI(3,4,5)P3) produced a 9.3 ± 1.8-mV depolarizing shift (n = 8). Although these responses to diC8 PI(3,4)P2 and diC8 PI(3,4,5)P3 were somewhat smaller than the response to diC8 PI(4,5)P2, the differences were not statistically significant. Thus HCN2 is relatively nonselective among the polyphosphoinositides, although the inositol sugar of PI may need to be phosphorylated for modulation to occur.
ATP-sensitive K+ channels (KATP) are the least specific of the inwardly rectifying K+ channels in their activation by phosphoinositides and are uniquely activated by long chain (LC) acyl-CoA, an effect that is thought to reflect this lack of specificity (Rohacs et al., 2003). Given the low phosphoinositide specificity of HCN2, we tested the ability of LC acyl-CoA to alter its gating. Bath application of 1 μM Oleoyl CoA caused a +16.0 ± 1.7-mV shift (n = 4) in the HCN2 activation curve that was complete within 2 min. The magnitude of this effect was similar to the shift seen with 25 μM diC8 PI(4,5)P2 but significantly smaller than the shift seen with 1 μM native PI(4,5)P2 (Fig. 3 B). Thus, HCN2 and KATP appear to define a class of channels that respond to LC acyl-CoA and exhibit a low phosphoinositide selectivity. It is interesting to note that fatty acyl-CoA is a major metabolite of cardiac myocytes, suggesting a possible physiological or pathophysiological role of this modulation.
Interaction of diC8 PI(4,5)P2 and cAMP
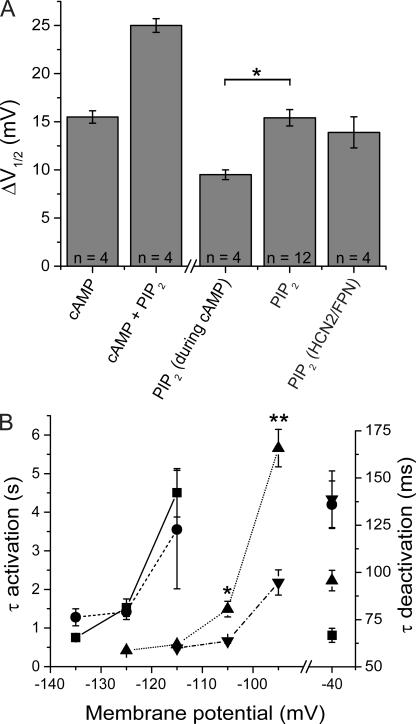
Because the effect of PI(4,5)P2 to shift the activation of HCN2 to positive voltages is qualitatively similar to the modulatory action of cAMP, we explored the possibility that these two compounds act through similar mechanisms by determining whether cAMP application could occlude the response to diC8 PI(4,5)P2 (Fig. 4). Inside-out patches were initially exposed to a saturating concentration of cAMP (10 μM), which resulted in a +15.5 ± 0.6-mV shift in the V1/2 (n = 4). The subsequent addition of 25 μM diC8 PI(4,5)P2 in the continued presence of 10 μM cAMP produced a further shift of +9.5 ± 0.7 mV (n = 4), resulting in a cumulative shift in V1/2 of +25.0 ± 0.7 mV (Fig. 4 A). Thus, application of a saturating concentration of cAMP did not completely block the effect of diC8 PI(4,5)P2. Nonetheless, the effect of the lipid in the presence of cAMP was significantly less than the 15.4-mV shift in the absence of cAMP (P < 0.01; t test). This suggests that the two compounds bind to distinct sites but may recruit a convergent biophysical mechanism to alter the gating of HCN channels.
Figure 4.
Effects of cAMP and diC8 PI(4,5)P2 on V1/2 and channel kinetics are not independent. (A) Interaction of effects of cAMP and diC8 PI(4,5)P2 on V1/2. Bars show the following (from left to right): cAMP, shift in V1/2 in response to 10 μM cAMP; cAMP + PIP2, shift in V1/2 in response to 10 μM cAMP plus 25 μM diC8 PI(4,5)P2; PIP2 (during cAMP), the difference between the shift in V1/2 in response to cAMP plus diC8 PI(4,5)P2 and the shift produced by cAMP alone; PIP2, shift in response to 25 μM diC8 PI(4,5)P2 alone; PIP2 (HCN2/FPN), shift of mutant channel in response to 25 μM diC8 PI(4,5)P2. The shift with diC8 PI(4,5)P2 in the presence of cAMP is less than the shift with diC8 PI(4,5)P2 alone (PIP2) (*, P < 0.01, t test). Error bars show SEM. Number of experiments shown in each bar. (B) Interaction of effects of cAMP and diC8 PI(4,5)P2 on time constants (τ) of HCN2 activation (left axis) and deactivation (right axis). Squares, data obtained in absence of PIP2 and cAMP (solid line, n = 14); circles, data obtained in the presence of 25 μM diC8 PI(4,5)P2 (dashed line, n = 10); triangles, data obtained in presence of 10 μM cAMP (dotted line, n = 4); inverted triangles, data obtained in combined presence of 10 μM cAMP plus 25 μM diC8 PI(4,5)P2 (dash dotted line, n = 4). The difference between the time constant of activation at −105 mV in the presence of cAMP (1.49 ± 0.20 s) versus that in the presence of cAMP and PIP2 (0.664 ± 0.056 s) was statistically significant (*, P < 0.02, t test). The difference between the time constant of activation at −95 mV in the presence of cAMP (5.66 ± 0.48 s) and in the presence of cAMP and PIP2 (2.18 ± 0.33 s) was also statistically significant (**, P < 0.001, t test).
An interaction between cAMP and diC8 PI(4,5)P2 was also seen in their effects on the kinetics of HCN2 tail current deactivation (Fig. 4 B). As discussed above, in the absence of cAMP, diC8 PI(4,5)P2 caused a significant slowing of tail current deactivation at −40 mV. cAMP also slowed the tail current kinetics at −40 mV and, importantly, these effects were occluded by diC8 PI(4,5)P2. Thus, the rate of channel deactivation at −40 mV in the combined presence of cAMP and diC8 PI(4,5)P2 was similar to that observed in the presence of diC8 PI(4,5)P2 alone, indicating that the two modulatory agents share certain common mechanisms of action.
We observed a more complex interaction between cAMP and diC8 PI(4,5)P2 on activation kinetics. As previously described, cAMP application markedly accelerated HCN2 activation kinetics over a wide range of hyperpolarized voltages (Wainger et al., 2001; Fig. 4 B). In contrast, as mentioned above, diC8 PI(4,5)P2 caused a modest slowing of activation kinetics at very negative voltages (−135 mV), with no significant effect on activation kinetics at more positive potentials (see Fig. 2 B). However, when we applied diC8 PI(4,5)P2 in the presence of cAMP, the lipid now produced a significant speeding in the rate of channel activation (P < 0.02 at −105 mV and P < 0.001 at −95 mV; t test; Fig. 4 B) at less hyperpolarized voltages. These results reinforce the notion that cAMP and PIP2 alter channel gating through both shared and unique mechanisms.
cAMP and diC8 PI(4,5)P2 Shift HCN2 Activation through Distinct Transduction Mechanisms
To further investigate the relation between the effects of diC8 PI(4,5)P2 and cAMP on HCN2 gating, we next examined a mutant HCN2 channel in which a tripeptide sequence—Q450, E451, K452—in the first α helix of the C-linker region (which couples the transmembrane domain to the cyclic nucleotide binding domain) was replaced with a tripeptide sequence, FPN, present at the corresponding region of a cyclic nucleotide-gated channel β subunit. This mutation was previously found to reverse the normal polarity of cyclic nucleotide gating of HCN2 so that cAMP binding shifts voltage-dependent opening of the mutant in the hyperpolarizing direction (Zhou et al., 2004). Thus, if PI(4,5)P2 acts through a similar molecular mechanism as does cAMP, FPN mutant channels should show a hyperpolarizing response to phospholipid. In fact, we found that diC8 PI(4,5)P2 facilitated the opening of HCN2/FPN channels, producing a normal-sized +13.9 ± 1.6-mV shift (n = 4; Fig. 4 A). Thus, PI(4,5)P2 must regulate HCN2 gating through a transduction mechanism distinct from that used by cAMP, although the effects of the two modulators clearly interact at several different levels.
Rundown of HCN2 Due to Dephosphorylation of Endogenous PIP2
The above results clearly show that HCN2 is modulated by exogenous PI(4,5)P2. We next asked whether HCN2 is also modulated by endogenous PI(4,5)P2 and whether the rundown upon patch excision may be due to hydrolysis of endogenous PI(4,5)P2 by membrane-bound lipid phosphatases, as observed for other channels (Logothetis and Zhang, 1999; Wu et al., 2002).
As a first test of this hypothesis, we examined rundown in the presence of the nonspecific phosphatase inhibitors fluoride (Fl−) and orthovanadate (VO4 3−), which should block or delay any rundown due to lipid dephosphorylation. Patches containing HCN2 were excised into normal bath solution or bath solution supplemented with 0.1 mM Fl− and 0.2 mM VO4 3− (FV bath solution; Fig. 5). 1 min after patch excision into normal bath solution, the V1/2 of HCN2 was −120.6 ± 0.5 mV (n = 5). The V1/2 then remained relatively constant over the next 10 min, indicating that rundown was normally complete within 1 min. However, 1 min after patch excision in FV solution, the V1/2 was significantly more depolarized than in normal solution (−112.8 ± 0.5 mV; n = 4; P < 0.00001; t test). The V1/2 then gradually shifted to more hyperpolarized potentials over the next 5–10 min. The V1/2 for HCN2 in patches excised in FV solution remained significantly positive to the V1/2 observed in normal bath solution up to 3 min after patch excision (P < 0.04; t test). However, by 5 min after patch excision, the difference was no longer statistically significant. Thus the phosphatase inhibitors fluoride and orthovanadate delayed but did not prevent HCN2 channel rundown, suggesting that phosphatase activity in the excised patch may contribute to HCN2 channel rundown.
Figure 5.
Phosphatase inhibitors delay the rundown in V1/2. V1/2 values were assessed after inside-out patches were excised into normal bath solution (circles) or FV bath solution to inhibit phosphatase activity (squares). The differences in respective V1/2 values measured 1 and 3 min after patch excision were statistically significant (*, P < 0.05, t test).
Previous studies suggest that lipid kinases remain associated with the membrane in cell-free patches (e.g., Huang et al., 1998; Rohacs et al., 2002). Thus, as a further test of the hypothesis that endogenous PI(4,5)P2 contributes to the regulation of HCN2, we next examined whether the effects of rundown on the V1/2 could be reversed through lipid kinase-mediated phosphorylation. After allowing channel rundown in FV bath solution to reach completion, patches were perfused with FV bath solution plus 2 mM MgATP to promote phosphorylation. Under these conditions, the HCN2 activation curve gradually shifted to more depolarized potentials (Fig. 6 A), reaching an average maximal shift of +9.3 ± 1.4 mV (n = 4) with a t1/2 of 3.8 min (Fig. 6 B).
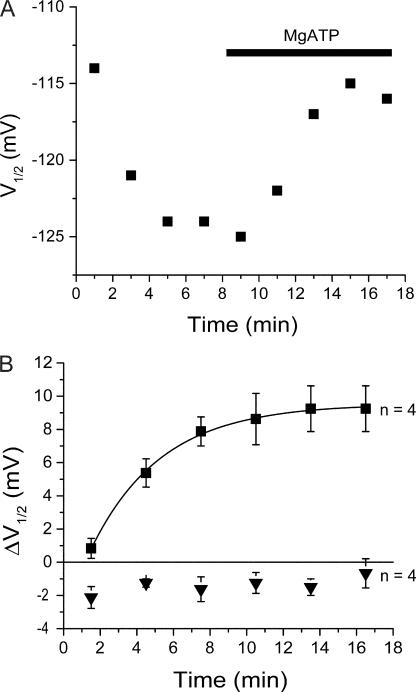
Figure 6.
Application of MgATP to inside-out patches shifts HCN2 activation to more positive voltages. (A) Time course of V1/2 of HCN2 in a single inside-out patch experiment showing rundown and response to MgATP. Experiment performed in FV solution to inhibit endogenous phosphatase activity. (B) Time course of average V1/2 shift after bath application of MgATP in absence (squares) or presence (inverted triangles) of 15 μM wortmannin to block PI kinases. For data in response to MgATP alone, the single exponential fit yielded values for the steady-state shift in V1/2 of +9.5 mV and a t1/2 of 3.8 min.
This effect of MgATP was due to lipid phosphorylation, as opposed to protein phosphorylation, since we found that the depolarizing shift with MgATP was blocked by the lipid kinase inhibitor wortmannin (15 μM). In the presence of wortmannin, MgATP produced only a −1.0 ± 1.0 mV (n = 4) shift in the V1/2. In the presence of DMSO alone (the vehicle for wortmannin), addition of MgATP shifted the V1/2 by +7.8 ± 1.1 mV (n = 4) (Fig. 6 B and Fig. 8), which was not significantly different from the MgATP-induced shift in the absence of DMSO (+9.3 mV; P > 0.5; ANOVA). The shifts with MgATP in the absence or presence of DMSO were both significantly greater than the shift in the presence of wortmannin (P < 0.05; ANOVA).
Figure 8.
Summary of effects of altering PI(4,5)P2 levels on HCN2 gating. Average shifts in V1/2 shown in response to various agents using either inside-out patches (gray bars) or whole oocytes (open bars). From top to bottom: bath only (no treatment); 2 mM MgATP (in FV solution, 0.05% DMSO); 2 mM MgATP plus 15 μM wortmannin (in FV solution, 0.05% DMSO); 25 μg/ml poly-d-lysine; 30.3 μg/ml anti-PI(4,5)P2 antibody (αPIP2 Ab); 30.3 μg/ml heat-inactivated anti-PI(4,5)P2 antibody; 30.3 μg/ml anti-granulocyte macrophage colony stimulating factor antibody (αGM-CSF Ab); V1/2 of HCN2 coexpressed with PI(4)P 5-kinase minus V1/2 of HCN2 expressed alone (PIP5K); V1/2 of HCN2 after 30–40-min preincubation in 15 μM wortmannin and 0.05% DMSO minus V1/2 after preincubation in 0.05% DMSO alone (Wortmannin); V1/2 after 2-h preincubation in 20 μM LY294002 and 0.1% DMSO minus V1/2 after preincubation in 0.1% DMSO alone (LY294002). Error bars show SEM. Number of experiments shown next to each bar (n).
Next we examined the effect of reducing endogenous PI(4,5)P2 levels by applying to inside-out patches an anti-PI(4,5)P2 antibody (Huang et al., 1998). After rundown had reached completion in normal solution, application of 30.3 μg/ml anti-PI(4,5)P2 antibody caused an additional 8.8 ± 1.3 mV hyperpolarizing shift with a time course characterized by a t1/2 of 3.6 min (Fig. 7 B). Control experiments performed with a heat-inactivated anti-PI(4,5)P2 antibody or an unrelated antibody failed to produce a significant hyperpolarizing shift (Fig. 8). A further test of the role of endogenous PI(4,5)P2 came from experiments in which we applied to inside-out patches polylysine, a polycation that reduces the effective negative charge associated with lipid headgroups (Rohacs et al., 2002). Polylysine (25 μg/ml) caused an additional 11.5 ± 1.3- mV hyperpolarizing shift in the HCN2 activation curve (Fig. 8), similar to the effects of the anti-PI(4,5)P2 antibody, consistent with a regulatory function of endogenous phospholipid.
Figure 7.
Reducing endogenous PI(4,5)P2 levels shifts HCN2 activation to more negative voltages. (A) Time course of V1/2 in a single inside-out patch experiment in response to bath application of 30.3 μg/ml of anti-PI(4,5)P2 antibody. (B) Time course of average V1/2 shifts after bath application of anti-PI(4,5)P2 antibody (squares) or heat-inactivated antibody (inverted triangles).
In addition to manipulating PI(4,5)P2 levels in inside-out patches, we also examined the effects of reducing PI(4,5)P2 levels in the plasma membrane of intact oocytes. Oocytes were incubated in 15 μM wortmannin for 30–40 min to block the final step in the biosynthetic pathway for PI(4,5)P2 (Doughman et al., 2003). As a control, other oocytes were incubated in 0.05% DMSO alone. Subsequently, the V1/2 values for HCN2 in the intact oocytes were assessed under these two conditions by two-microelectrode voltage clamp. Incubation in wortmannin caused a significant hyperpolarizing shift in the V1/2 of −7.9 ± 1.0 mV relative to the control values (n = 12 pair). As this concentration of wortmannin inhibits both PI-3 kinase and PI-4 kinase, we tested the effects of LY294002, a more specific inhibitor of PI-3 kinase (Vlahos et al., 1994; Browaeys-Poly et al., 2000; Song and Ashcroft, 2001). There was no significant effect on the V1/2 when 20 μM LY294002 was applied to intact oocytes (−0.1 ± 1.9 mV shift relative to DMSO control), indicating a role for PI-4 kinase. Thus, the inhibitory action of wortmannin most likely indicates a role for basal levels of endogenous PI(4,5)P2 in regulating HCN2 gating in the intact oocyte (Fig. 8).
Finally, we attempted to elevate levels of PI(4,5)P2 in intact oocytes by overexpressing PIP5K (Winks et al., 2005). However, paired two-microelectrode voltage-clamp recordings showed no effect of PIP5K overexpression (0.9 ± 0.9 mV difference), perhaps indicating that endogenous PIP5K is not rate limiting for PI(4,5)P2 production in the oocytes (Fig. 8).
Regulation of Native HCN Currents in Sinoatrial Node Cells by DiC8 PI(4,5)P2
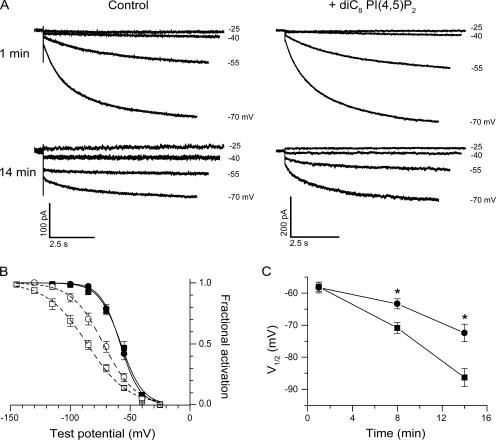
Are native HCN currents in rabbit cardiac sinoatrial cells also regulated by exogenous PI(4,5)P2? To address this question, we evaluated the effect of intracellular dialysis with diC8 PI(4,5)P2 on the normal rundown of sinoatrial HCN currents during whole cell recording. The voltage dependence of activation was assayed by a two-step voltage protocol (see Materials and methods), in the absence or in the presence of diC8 PI(4,5)P2 (200 μM) in the patch pipette solution (Fig. 9 A). Activation curves determined in the presence or absence of diC8 PI(4,5)P2 were identical when measured 1 min after the start of whole-cell recording, with V1/2 values of −58 mV (Fig. 9 B). However, after 14 min of whole cell recording, the two activation curves were clearly different. In the absence of diC8 PI(4,5)P2, the V1/2 shifted to −86 mV, reflecting rundown. In contrast, with diC8 PI(4,5)P2 in the pipette solution, the V1/2 only shifted to −72 mV (Fig. 9 B). A plot of V1/2 versus duration of whole-cell recording revealed the progressive hyperpolarizing shift in the HCN activation curve, in both the absence and presence of diC8 PI(4,5)P2 (Fig. 9 C). However, diC8 PI(4,5)P2 clearly delayed the hyperpolarizing shift, with statistically significant positive shifts observed for the 8- and 14-min time points (P < 0.05).
Figure 9.
Effect of diC8 PI(4,5)P2 on the rundown of the voltage dependence of activation for HCN currents in sinoatrial node cells. (A) Representative whole-cell currents evoked by stepping the membrane from a holding potential of −35 mV to voltages ranging from −25 to −70 mV in 15-mV increments. The recordings were acquired 1 min (top) and 14 min (bottom) after membrane rupture in the absence (left) and in the presence (right) of 200 μM diC8 PI(4,5)P2 in the pipette solution. Hyperpolarizing test voltages indicated at right. (B) Mean tail current activation curves at 1 min (filled symbols) and 14 min (open symbols) in the absence (squares) and in the presence of 200 μM diC8 PI(4,5)P2 (circles). The curves show best fits of the Boltzmann equation. Mean values of parameters from Boltzmann fits of individual activation curves obtained 1 min after patch rupture in absence and presence of diC8 PI(4,5)P2 were, respectively, V1/2 = −58.0 ± 1.5 mV (n = 8) and −58.3 ± 1.7 mV (n = 8, P > 0.05); s = 8.5 ± 0.7 mV and 7.8 ± 0.2 mV (P > 0.05). Mean parameters from Boltzmann fits obtained after 14 min of whole-cell recording in absence and presence of diC8 PI(4,5)P2 were, respectively, V1/2 = −86.4 ± 2.8 mV (n = 6) and −72.4 ± 2.7 mV (n = 6, P < 0.05); s = 16.8 ± 1.3 mV and 12.9 ± 0.7 mV (P < 0.05). (C) Time course of rundown in V1/2 during whole cell recordings in the absence (squares) and in the presence (circles) of 200 μM diC8 PI(4,5)P2. The two curves differ significantly at times indicated by asterisks. Values of V1/2 for 1- and 14-min points from B. For 8-min points, mean activation parameters in absence and presence of diC8 PI(4,5)P2 were, respectively, V1/2 = −70.9 ± 1.7 mV (n = 8) and −63.4 ± 1.6 mV (n = 8, P < 0.05) and s = 14.1 ± 0.3 mV and 10.9 ± 0.6 mV (P < 0.05). Data for B and C obtained from three animals. There was no difference in mean capacitance between the two groups (not depicted).
DISCUSSION
The present study provides strong support for the view that PI(4,5)P2 regulates both recombinant HCN2 and native cardiac HCN channels and that its degradation after patch excision contributes to channel rundown. Thus, application of exogenous PI(4,5)P2 reversed rundown, whereas sequestration of PI(4,5)P2 enhanced rundown. Moreover, rundown was slowed by inhibition of phosphatases and partly reversed when endogenous lipid kinases were supplied with MgATP. Finally, manipulation of PI(4,5)P2 levels in intact oocytes also modulated HCN2 gating.
Comparison of Effects of PIP2 on HCN2 with Other Channels
HCN2 joins a growing list of channels regulated by PI(4,5)P2 (Suh and Hille, 2005). For some channels, such as TRPV1 (Chuang et al., 2001) and mammalian rod cyclic nucleotide-gated channels (Womack et al., 2000), PI(4,5)P2 inhibits channel activity. However, for the majority of channels regulated by PI(4,5)P2, including HCN2, PI(4,5)P2 is permissive for channel activity (Hilgemann et al., 2001). Previous studies that investigated the role of PI(4,5)P2 in channel regulation usually characterized changes in channel function in response to depletion or addition of phosphoinositides to intact membranes. Such studies with the family of inward rectifying K+ channels (Kir) have demonstrated a spectrum of apparent PI(4,5)P2 affinities among different channels. Our experiments indicate that HCN2 falls into the KATP class of channels with relative low specificity for phosphoinositides.
Although HCN2 showed a relatively low selectivity among the polyphosphoinositides, the channel may have a relatively high affinity for PI(4,5)P2. Channels that tightly bind PI(4,5)P2 show a very slow response to application of the anti-PI(4,5)P2 antibody, whereas channels that weakly bind PI(4,5)P2 are rapidly inhibited by the antibody. For example, the high-affinity IRK1 channel is inhibited by anti-PI(4,5)P2 antibody with a half-time of ∼90 s, whereas the low-affinity GIRK1/4 and GIRK2 channels show half-maximal block in <10 s (Huang et al., 1998). Despite our having used slightly higher concentrations of antibody than used in the Kir studies, we found that HCN2 required >200 s for half-maximal inhibition with the anti-PI(4,5)P2 antibody, suggesting that HCN2 has a high affinity for PI(4,5)P2 (although we cannot rule out the possibility that the slow time course of inhibition is due to a particularly slow access of antibody to the inside membrane of the patch in our experiments). A high affinity of HCN2 for PI(4,5)P2 is consistent with the idea that this channel constitutively binds PI(4,5)P2 and that hydrolysis of this phospholipid underlies the ubiquitous rundown of HCN currents.
Interactions of cAMP and PIP2: Mechanistic Implications
Our results, unfortunately, do not provide a definitive mechanism for how PI(4,5)P2 regulates HCN2 gating. However, a comparison with the modulatory effects of cAMP provides some interesting clues. The binding of cAMP to its C-terminal cyclic nucleotide binding domain is thought to facilitate HCN channel opening by relieving an inhibitory action of the C terminus on gating (Wainger et al., 2001). Moreover, the effect of cAMP binding to the CNBD is transduced into changes in channel opening through the intervening C-linker region (Chen et al., 2001). We find that a C-linker mutation that reverses the polarity of cAMP action on voltage gating (Zhou et al., 2004) causes no change in PI(4,5)P2 action, indicating that phospholipid must act through a distinct mechanism from cyclic nucleotide. However, our finding that the effects of cAMP and PI(4,5)P2 are not completely additive suggests that the actions of these modulators may converge on some final mechanistic step of gating, such as the opening of the channel gate at the inner surface of the membrane (Rothberg et al., 2003).
Potential Physiological Implications of Modulation by PIP2
Our study clearly demonstrates that changes in membrane PI(4,5)P2 concentration can affect the gating of recombinant HCN2, with higher levels of PI(4,5)P2 shifting gating to more positive potentials. In addition, we have shown in cells from rabbit sinoatrial node that diC8 PI(4,5)P2 also delays the onset of native channel rundown in whole cell recordings. These findings suggest that native HCN channels are regulated by PI(4,5)P2 and that the presence of this lipid is required to maintain the relatively depolarized range of activation of HCN channels that is required for their opening at physiologically relevant voltages. Moreover, given that rabbit sinoatrial node cells express primarily HCN4 and HCN1 (Shi et al., 1999), our results further indicate that PI(4,5)P2 is a general modulator of HCN channel function, and does not only target HCN2.
One important remaining question is whether HCN channel function is dynamically regulated in neurons or cardiac myocytes during signal transduction cascades that alter membrane levels of PI(4,5)P2. Receptor-mediated activation of phospholipase C results in a decrease in membrane concentration of PI(4,5)P2 that alters the activity of a number of channels, including GIRK1/4 and KCNQ2/3 (Rohacs et al., 1999; Kobrinsky et al., 2000; Wu et al., 2002; Zhang et al., 2003; Cho et al., 2005). In sinoatrial node cells, a hyperpolarizing shift in HCN channel activation due to reduced levels of PI(4,5)P2 would slow heart rate, similar to the action of nanomolar concentrations of acetylcholine, which cause a hyperpolarizing shift in pacemaker current without affecting other nodal currents (DiFrancesco et al., 1989). However, such regulation is most effective for channels with a relatively low affinity for PI(4,5)P2, which may or may not hold for HCN channels. In addition to acute regulation by receptor activation, basal levels of PI(4,5)P2 may also change during development, times of stress, or in disease states (Suchy et al., 1995; Soares et al., 1997; Pasquare et al., 2004; Suh and Hille, 2005); these changes could lead to more tonic changes in HCN channel function. Lastly, PI(4,5)P2 levels vary among the different membrane compartments of a cell. Thus, a stimulatory effect of PI(4,5)P2 may allow the cell to restrict channel activity as channels are transported from their site of synthesis in the endoplasmic reticulum to final destinations with more permissive phosphoinositide concentrations (Hilgemann et al., 2001; Suh and Hille, 2005). Further studies will be required to determine the physiological importance of the regulation of HCN channels by PI(4,5)P2.
Supplemental Material
Acknowledgments
We thank Gareth Tibbs and Keri Fogle for helpful discussions and sharing unpublished data. We thank John Riley for help with the Xenopus oocyte injections.
This work was partially supported by grants NS-36658 (S.A. Siegelbaum) and HL-28958 (R.B. Robinson) from the National Institutes of Health, and the Howard Hughes Medical Institute (S.A. Siegelbaum).
Olaf S. Andersen served as editor.
Abbreviations used in this paper: HCN, hyperpolarization-activated, cyclic nucleotide-regulated, cation-nonselective; PI(4,5)P2 or PIP2, phosphatidylinositol 4,5-bisphosphate; diC8, dioctanoyl; PI, phosphatidylinositol; LC acyl-coA, long chain acyl-coenzyme A; KATP, ATP-sensitive K+; Kir, inwardly rectifying K+.
References
- Arinsburg, S.S., I.S. Cohen, and H.G. Yu. 2006. Constitutively active Src tyrosine kinase changes gating of HCN4 channels through direct binding to the channel proteins. J. Cardiovasc. Pharmacol. 47:578–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bois, P., B. Renaudon, M. Baruscotti, J. Lenfant, and D. DiFrancesco. 1997. Activation of f-channels by cAMP analogues in macropatches from rabbit sino-atrial node myocytes. J. Physiol. 501(Pt 3): 565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browaeys-Poly, E., K. Cailliau, and J.P. Vilain. 2000. Signal transduction pathways triggered by fibroblast growth factor receptor 1 expressed in Xenopus laevis oocytes after fibroblast growth factor 1 addition. Role of Grb2, phosphatidylinositol 3-kinase, Src tyrosine kinase, and phospholipase Cγ. Eur. J. Biochem. 267:6256–6263. [DOI] [PubMed] [Google Scholar]
- Cerbai, E., R. Pino, F. Porciatti, G. Sani, M. Toscano, M. Maccherini, G. Giunti, and A. Mugelli. 1997. Characterization of the hyperpolarization-activated current, I(f), in ventricular myocytes from human failing heart. Circulation. 95:568–571. [DOI] [PubMed] [Google Scholar]
- Chen, S., J. Wang, and S.A. Siegelbaum. 2001. Properties of hyperpolarization-activated pacemaker current defined by coassembly of HCN1 and HCN2 subunits and basal modulation by cyclic nucleotide. J. Gen. Physiol. 117:491–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho, H., D. Lee, S.H. Lee, and W.K. Ho. 2005. Receptor-induced depletion of phosphatidylinositol 4,5-bisphosphate inhibits inwardly rectifying K+ channels in a receptor-specific manner. Proc. Natl. Acad. Sci. USA. 102:4643–4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang, H.H., E.D. Prescott, H. Kong, S. Shields, S.E. Jordt, A.I. Basbaum, M.V. Chao, and D. Julius. 2001. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature. 411:957–962. [DOI] [PubMed] [Google Scholar]
- DiFrancesco, D. 2006. Funny channels in the control of cardiac rhythm and mode of action of selective blockers. Pharmacol. Res. 53:399–406. [DOI] [PubMed] [Google Scholar]
- DiFrancesco, D., P. Ducouret, and R.B. Robinson. 1989. Muscarinic modulation of cardiac rate at low acetylcholine concentrations. Science. 243:669–671. [DOI] [PubMed] [Google Scholar]
- DiFrancesco, D., A. Ferroni, M. Mazzanti, and C. Tromba. 1986. Properties of the hyperpolarizing-activated current (if) in cells isolated from the rabbit sino-atrial node. J. Physiol. 377:61–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco, D., and M. Mangoni. 1994. Modulation of single hyperpolarization-activated channels (i(f)) by cAMP in the rabbit sino-atrial node. J. Physiol. 474:473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco, D., and P. Tortora. 1991. Direct activation of cardiac pacemaker channels by intracellular cyclic AMP. Nature. 351:145–147. [DOI] [PubMed] [Google Scholar]
- Doughman, R.L., A.J. Firestone, and R.A. Anderson. 2003. Phosphatidylinositol phosphate kinases put PI4,5P(2) in its place. J. Membr. Biol. 194:77–89. [DOI] [PubMed] [Google Scholar]
- Goulding, E.H., J. Ngai, R.H. Kramer, S. Colicos, R. Axel, S.A. Siegelbaum, and A. Chess. 1992. Molecular cloning and single-channel properties of the cyclic nucleotide-gated channel from catfish olfactory neurons. Neuron. 8:45–58. [DOI] [PubMed] [Google Scholar]
- Hilgemann, D.W., S. Feng, and C. Nasuhoglu. 2001. The complex and intriguing lives of PIP2 with ion channels and transporters. Sci. STKE. 2001:RE19. [DOI] [PubMed] [Google Scholar]
- Huang, C.L., S. Feng, and D.W. Hilgemann. 1998. Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gβγ. Nature. 391:803–806. [DOI] [PubMed] [Google Scholar]
- Kobrinsky, E., T. Mirshahi, H. Zhang, T. Jin, and D.E. Logothetis. 2000. Receptor-mediated hydrolysis of plasma membrane messenger PIP2 leads to K+-current desensitization. Nat. Cell Biol. 2:507–514. [DOI] [PubMed] [Google Scholar]
- Logothetis, D.E., and H. Zhang. 1999. Gating of G protein-sensitive inwardly rectifying K+ channels through phosphatidylinositol 4,5-bisphosphate. J. Physiol. 520(Pt. 3):630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin, S., J. Wang, A. Gambhir, and D. Murray. 2002. PIP(2) and proteins: interactions, organization, and information flow. Annu. Rev. Biophys. Biomol. Struct. 31:151–175. [DOI] [PubMed] [Google Scholar]
- Pasquare, S.J., G.A. Salvador, and N.M. Giusto. 2004. Phospholipase D and phosphatidate phosphohydrolase activities in rat cerebellum during aging. Lipids. 39:553–560. [DOI] [PubMed] [Google Scholar]
- Qu, J., A. Barbuti, L. Protas, B. Santoro, I.S. Cohen, and R.B. Robinson. 2001. HCN2 overexpression in newborn and adult ventricular myocytes: distinct effects on gating and excitability. Circ. Res. 89:E8–14. [DOI] [PubMed] [Google Scholar]
- Robinson, R.B., and S.A. Siegelbaum. 2003. Hyperpolarization-activated cation currents: from molecules to physiological function. Annu. Rev. Physiol. 65:453–480. [DOI] [PubMed] [Google Scholar]
- Robinson, R.B., H. Yu, F. Chang, and I.S. Cohen. 1997. Developmental change in the voltage-dependence of the pacemaker current, if, in rat ventricle cells. Pflugers Arch. 433:533–535. [DOI] [PubMed] [Google Scholar]
- Rohacs, T., J. Chen, G.D. Prestwich, and D.E. Logothetis. 1999. Distinct specificities of inwardly rectifying K+ channels for phosphoinositides. J. Biol. Chem. 274:36065–36072. [DOI] [PubMed] [Google Scholar]
- Rohacs, T., C. Lopes, T. Mirshahi, T. Jin, H. Zhang, and D.E. Logothetis. 2002. Assaying phosphatidylinositol bisphosphate regulation of potassium channels. Methods Enzymol. 345:71–92. [DOI] [PubMed] [Google Scholar]
- Rohacs, T., C.M. Lopes, T. Jin, P.P. Ramdya, Z. Molnar, and D.E. Logothetis. 2003. Specificity of activation by phosphoinositides determines lipid regulation of Kir channels. Proc. Natl. Acad. Sci. USA. 100:745–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberg, B.S., K.S. Shin, and G. Yellen. 2003. Movements near the gate of a hyperpolarization-activated cation channel. J. Gen. Physiol. 122:501–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro, B., B.J. Wainger, and S.A. Siegelbaum. 2004. Regulation of HCN channel surface expression by a novel C-terminal protein-protein interaction. J. Neurosci. 24:10750–10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, W., R. Wymore, H. Yu, J. Wu, R.T. Wymore, Z. Pan, R.B. Robinson, J.E. Dixon, D. McKinnon, and I.S. Cohen. 1999. Distribution and prevalence of hyperpolarization-activated cation channel (HCN) mRNA expression in cardiac tissues. Circ. Res. 85:e1–e6. [DOI] [PubMed] [Google Scholar]
- Soares, J.C., C.S. Dippold, and A.G. Mallinger. 1997. Platelet membrane phosphatidylinositol-4,5-bisphosphate alterations in bipolar disorder–evidence from a single case study. Psychiatry Res. 69:197–202. [DOI] [PubMed] [Google Scholar]
- Song, D.K., and F.M. Ashcroft. 2001. ATP modulation of ATP-sensitive potassium channel ATP sensitivity varies with the type of SUR subunit. J. Biol. Chem. 276:7143–7149. [DOI] [PubMed] [Google Scholar]
- Suchy, S.F., I.M. Olivos-Glander, and R.L. Nussabaum. 1995. Lowe syndrome, a deficiency of phosphatidylinositol 4,5-bisphosphate 5-phosphatase in the Golgi apparatus. Hum. Mol. Genet. 4:2245–2250. [DOI] [PubMed] [Google Scholar]
- Suh, B.C., and B. Hille. 2005. Regulation of ion channels by phosphatidylinositol 4,5-bisphosphate. Curr. Opin. Neurobiol. 15:370–378. [DOI] [PubMed] [Google Scholar]
- Vlahos, C.J., W.F. Matter, K.Y. Hui, and R.F. Brown. 1994. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). J. Biol. Chem. 269:5241–5248. [PubMed] [Google Scholar]
- Wainger, B.J., M. DeGennaro, B. Santoro, S.A. Siegelbaum, and G.R. Tibbs. 2001. Molecular mechanism of cAMP modulation of HCN pacemaker channels. Nature. 411:805–810. [DOI] [PubMed] [Google Scholar]
- Winks, J.S., S. Hughes, A.K. Filippov, L. Tatulian, F.C. Abogadie, D.A. Brown, and S.J. Marsh. 2005. Relationship between membrane phosphatidylinositol-4,5-bisphosphate and receptor-mediated inhibition of native neuronal M channels. J. Neurosci. 25:3400–3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womack, K.B., S.E. Gordon, F. He, T.G. Wensel, C.C. Lu, and D.W. Hilgemann. 2000. Do phosphatidylinositides modulate vertebrate phototransduction? J. Neurosci. 20:2792–2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, L., C.S. Bauer, X.G. Zhen, C. Xie, and J. Yang. 2002. Dual regulation of voltage-gated calcium channels by PtdIns(4,5)P2. Nature. 419:947–952. [DOI] [PubMed] [Google Scholar]
- Yu, H., J. Wu, I. Potapova, R.T. Wymore, B. Holmes, J. Zuckerman, Z. Pan, H. Wang, W. Shi, R.B. Robinson, et al. 2001. MinK-related peptide 1: A β subunit for the HCN ion channel subunit family enhances expression and speeds activation. Circ. Res. 88:E84–E87. [DOI] [PubMed] [Google Scholar]
- Yu, H.G., Z. Lu, Z. Pan, and I.S. Cohen. 2004. Tyrosine kinase inhibition differentially regulates heterologously expressed HCN channels. Pflugers Arch. 447:392–400. [DOI] [PubMed] [Google Scholar]
- Zhang, H., L.C. Craciun, T. Mirshahi, T. Rohacs, C.M. Lopes, T. Jin, and D.E. Logothetis. 2003. PIP(2) activates KCNQ channels, and its hydrolysis underlies receptor-mediated inhibition of M currents. Neuron. 37:963–975. [DOI] [PubMed] [Google Scholar]
- Zhang, H., C. He, X. Yan, T. Mirshahi, and D.E. Logothetis. 1999. Activation of inwardly rectifying K+ channels by distinct PtdIns(4,5)P2 interactions. Nat. Cell Biol. 1:183–188. [DOI] [PubMed] [Google Scholar]
- Zhou, L., N.B. Olivier, H. Yao, E.C. Young, and S.A. Siegelbaum. 2004. A conserved tripeptide in CNG and HCN channels regulates ligand gating by controlling C-terminal oligomerization. Neuron. 44:823–834. [DOI] [PubMed] [Google Scholar]
- Zhou, Z., and S.L. Lipsius. 1993. Effect of isoprenaline on If current in latent pacemaker cells isolated from cat right atrium: ruptured vs. perforated patch whole-cell recording methods. Pflugers Arch. 423:442–447. [DOI] [PubMed] [Google Scholar]
- Zong, X., C. Eckert, H. Yuan, C. Wahl-Schott, H. Abicht, L. Fang, R. Li, P. Mistrik, A. Gerstner, B. Much, et al. 2005. A novel mechanism of modulation of hyperpolarization-activated cyclic nucleotide-gated channels by Src kinase. J. Biol. Chem. 280:34224–34232. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.