Abstract
Although inhibition of voltage-gated calcium channels by RGK GTPases (RGKs) represents an important mode of regulation to control Ca2+ influx in excitable cells, their exact mechanism of inhibition remains controversial. This has prevented an understanding of how RGK regulation can be significant in a physiological context. Here we show that RGKs—Gem, Rem, and Rem2—decreased CaV1.2 Ca2+ current amplitude in a dose-dependent manner. Moreover, Rem2, but not Rem or Gem, produced dose-dependent alterations on gating kinetics, uncovering a new mode by which certain RGKs can precisely modulate Ca2+ currents and affect Ca2+ influx during action potentials. To explore how RGKs influence gating kinetics, we separated the roles mediated by the Ca2+ channel accessory β subunit's interaction with its high affinity binding site in the pore-forming α1C subunit (AID) from its other putative contact sites by utilizing an α1C•β3 concatemer in which the AID was mutated to prevent β subunit interaction. This mutant concatemer generated currents with all the hallmarks of β subunit modulation, demonstrating that AID-β–independent interactions are sufficient for β subunit modulation. Using this construct we found that although inhibition of current amplitude was still partially sensitive to RGKs, Rem2 no longer altered gating kinetics, implicating different determinants for this specific mode of Rem2-mediated regulation. Together, these results offer new insights into the molecular mechanism of RGK-mediated Ca2+ channel current modulation.
INTRODUCTION
Voltage-gated Ca2+ channels are the signature feature of excitable cells, transducing electrical activity into increased intracellular [Ca2+] that mediates specific cellular effects such as muscle contraction, hormone secretion, and release of neurotransmitters. Thus, many regulatory mechanisms have evolved to fine tune Ca2+ channel activity and the resultant Ca2+ influx, mostly by protein–protein interactions with, or posttranslational modifications of, the pore-forming α1 subunit. Some are rapid, such as Ca2+-dependent inactivation of L-type (CaV1.2) channels (Budde et al., 2002); others occur after the activation of signaling pathways, such as PKA potentiation of CaV1.2 channels or G protein inhibition of N-type (CaV2.2) channels (Catterall, 2000). In contrast, mechanisms that result in finely graded responses to changes in the cellular environment developing over longer time scales have not been well described.
RGK GTPases (Rad, Rem, Rem2, Gem/Kir), the most recently characterized group within the Ras family of GTP-binding proteins (Reynet and Kahn, 1993; Maguire et al., 1994; Finlin and Andres, 1997; Finlin et al., 2000), have received special attention because they are potent inhibitors of Ca2+ channels and candidates for Ca2+ channel regulators under transcriptional control that can therefore integrate the influence of multiple extracellular signals. Experiments in a variety of cell types have shown a drastic reduction of peak current amplitude for multiple Ca2+ channels after expression of Gem/Kir (Beguin et al., 2001, 2005b; Murata et al., 2004; Ward et al., 2004), Rem, Rad (Finlin et al., 2003; Crump et al., 2006), and Rem2 (Chen et al., 2005; Finlin et al., 2005). Among Ras family members, RGKs differ by having extended variable N-terminal regions and conserved C-terminal extensions lacking the CAAX motif for fatty acylation, and containing binding motifs for calmodulin and 14-3-3 proteins (Kelly, 2005). Individual RGKs have nonoverlapping patterns of expression, and are transcriptionally induced and repressed by different factors. For example, Gem and Rem2 transcription has been reported to be stimulated by glucose in insulin-secreting pancreatic cells but follow a different time course (Ohsugi et al., 2004; Finlin et al., 2005); Rad is overexpressed in muscle of type II diabetics (Reynet and Kahn, 1993), and Rem transcription is repressed by lipopolysaccharide exposure (Finlin and Andres, 1997). RGKs also vary in their downstream targets. Gem inhibits the Rho/RhoA kinase pathway (Ward et al., 2002) and induces neuroblastoma morphological and ganglionic differentiation (Leone et al., 2001). Expression of both Gem and Rem2 has been shown to decrease glucose-stimulated insulin secretion (Beguin et al., 2001; Finlin et al., 2005).
Models for how RGKs potently inhibit Ca2+ channels are controversial. A two-hybrid experiment identified Ca2+ channel β subunits as a Gem-interacting protein in the insulin-secreting MIN6 cell line (Beguin et al., 2001). Since β subunits have been implicated in trafficking α1 subunits to the plasma membrane, this led to the hypothesis that RGKs prevent β subunits from interacting with α1 subunits, thereby preventing membrane targeting and resulting in reduced channels at the cell surface (Beguin et al., 2001, 2005a,b). A number of recent studies suggest instead that RGKs inhibit channels already resident at the cell surface (Chen et al., 2005; Finlin et al., 2005). Moreover, though it is their potency that has earned them interest, it is a more subtle and tunable response that likely has physiological ramifications. It has already been established that changes in Ca2+ channel currents less severe than the near complete reduction observed when RGKs are expressed in heterologous systems lead to drastic pathophysiological consequences (Splawski et al., 2004). It is difficult to understand how RGK expression could result in a finely graded response.
In this study, we provide new insights into how Gem and Rem2 regulate Ca2+ channels. Exploiting the Xenopus oocyte system to control levels of expression (Canti et al., 2001), we found that Gem and Rem2 drive a dose-dependent inhibition of Ca2+ currents. Rem2, but not Gem, also modulated both the kinetics of channel activation and inactivation in a manner that was dependent on β subunit interaction with the α1 interaction domain (AID). Together, these results suggest that specific RGKs contribute to the fine tuning of Ca2+ influx by different mechanisms.
MATERIALS AND METHODS
Construction of cDNA Plasmids
Constructs for α1C (pCARDHE), α2δ, and the α1C C-terminal deletion (amino acids 1670–2171), and the GST I-II loop have been previously reported (Zühlke et al., 2000; Kim et al., 2004). β3 (GenBank/EMBL/DDBJ accession no. NM_000725) was cloned into the pGEM-HE oocyte expression vector using standard molecular biology techniques. Gem full-length (accession no. BC018219) was obtained as an EST and cloned into the pCS2+ oocyte expression vector (gift from D. McKinnon, State University of New York, Stony Brook, NY). Rem2 full-length (AY916790), a gift from D. Andres (University of Kentucky, Lexington, KY), was digested out of the original pCDNA3.1 vector and ligated into compatible sites in pCS2+. The α1C N-terminal deletion (amino acids 2–139) was generated by a PCR-based strategy. The α1C•β3 concatemer included amino acids 1–2134 from α1C and the entire β3 with a valine linker between them. The mutant α1C Y/W and corresponding concatemer included mutations Y467S and W470A created by Quikchange (Stratagene). The KChiP2b clone was a gift from P. Pfaffinger (Baylor College of Medicine, Houston, TX).
Electrophysiological Recordings and Analysis
In vitro cRNA transcription and microinjection into Xenopus oocytes has been previously reported (Kim et al., 2004). The following amounts of cRNA were injected: α1C (1 ng), α2δ (1 ng), β3 (0.22 ng). The amount of RGKs and KChIP2b cRNA injected is indicated in specific experiments. Two-electrode voltage clamp recordings were performed as previously described (Kim et al., 2004). During recordings, oocytes were constantly superfused with a solution containing 40 mM Ba(OH)2 (or 40 mM Ca(OH)2 in experiments recording Ca2+ currents), 50 mM NaOH, 1 mM KOH, and 10 mM HEPES (adjusted to pH 7.4 with methanesulfonic acid). Recordings were performed with a standard two-electrode voltage clamp configuration using an oocyte clamp OC-725C amplifier (Warner Instrument Corp.) connected through a Digidata 1322A A/D interface (Axon Instruments, Inc.) to a personal computer. Ionic currents were filtered at 1 kHz by an integral 4 pole Bessel filter and sampled 10 kHz and analyzed with Clampfit 9.2. Steady-state inactivation was analyzed with a two-pulse protocol in which a 5-s conditioning pulse (P1) from −60 mV to +50 mV was followed by a 100-ms test pulse (P2) at +10 mV. Normalized P2 values were fitted with a Boltzmann equation (I/Ipeak = (1 − Io)/[1 + exp((V − V1/2)/k)] + Io). Activation time constants were estimated by fitting the activating component of the current trace to the following equation: I = I o + Afastexp(−t/τfast) + Aslowexp(−t/τslow). Bursts of pancreatic β cell action potentials were simulated by a 5-s depolarization to −40 mV from −70 mV followed by a series of 26 100-ms voltage-clamp depolarizations between −40 and 0 mV at 5 Hz (Kanno et al., 2002). All values are given as means ± SEM, with statistical comparisons performed with a Student's t test.
Protein Expression/GST Pull-Down Assays
Protein expression/GST pull-down assays were performed as previously described (Maltez et al., 2005).
Immunoblotting
Oocytes were injected with either 1,000 pg of Gem cRNA, 1,000 pg Gem cRNA with 1,000 pg cRNA CaM, or 4,000 pg of Gem cRNA. Control oocytes were injected with RNase-free water. Oocytes were incubated at 17°C for 24 h, lysed in ice-cold oocyte extraction buffer (20 mM HEPES, 5 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 10% glycerol, 0.1% NP-40, and Roche protease inhibitor tablets), and then solubilized in SDS. The equivalent of ∼0.2 oocytes was loaded in each lane. Purified bacterial GST and Gem-GST were used in the control lanes. Immunoblotting was performed with an anti-Gem antibody (Abcam).
RESULTS
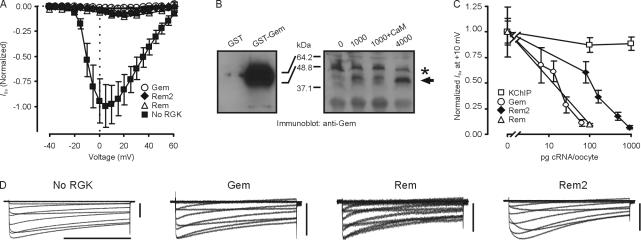
To explore the mechanisms by which RGKs inhibit Ca2+ channel currents, we expressed CaV1.2 channels (α1C, α2δ, and β3) with Gem, Rem, or Rem2 in Xenopus oocytes and recorded the resulting currents by two-electrode voltage clamp. As observed previously, expression of any of these RGKs drastically reduced the I Ba peak current amplitude (Fig. 1 A, exemplar traces shown in Fig. 1 D). To distinguish among the possible models by which RGKs inhibit Ca2+ currents and to explore the physiological implications, we took advantage of the Xenopus oocyte system, in which it has been shown that expression levels of proteins can be accurately titrated in a monotonic fashion (Canti et al., 2001), in order to test whether inhibition were dose dependent. We confirmed this relationship for Gem. Fig. 1 B demonstrates a monotonic increase in Gem protein with increasing amounts of Gem cRNA injected over the range that we studied. Moreover, coinjection of cRNA for another unrelated protein (calmodulin) did not affect Gem protein levels, suggesting that cRNA amounts in this range did not exceed the protein synthesis capacity in oocytes. With this confirmation, Fig. 1 C shows that Gem, Rem, and Rem2 inhibit I Ba peak current amplitude at 10 mV in a dose-dependent manner. This was not a nonspecific effect of increasing the amounts of cRNA injection; coexpression of cRNA for KChIP2b, a protein that modulates K+ currents and is of similar mass to the RGKs (An et al., 2000), did not alter CaV1.2 current amplitude. Examination of individual current traces also revealed differences in the mechanisms by which Gem and Rem2 affect CaV1.2 currents. While coexpression of all three RGKs decreased current amplitude, coexpression of Rem2 also appeared to affect kinetics of activation and inactivation (Fig. 1 D). Effects of Gem and Rem appeared similar, so we focused on Gem in further studies.
Figure 1.
Dose-dependent inhibition of CaV1.2 channels by Gem and Rem2. (A) Normalized I-V relationships of I Ba from oocytes injected with α1C/β3/α2δ without or with Gem (130 pg) or Rem2 (936 pg). n = 5. (B) Immunoblots with anti-Gem antibody. On left is shown GST and GST-Gem, demonstrating specificity of the antibody. The right panel shows an increase in the amount of Gem protein detected with an increasing amount (as indicated) of cRNA injected per oocyte. Gem is indicated by an arrow; a nonspecific band seen even without injection of Gem cRNA is shown by an asterisk. (C) Dose–response of Gem-, Rem-, or Rem2-mediated inhibition of I Ba. KChIP2b cRNA was injected as a negative control. n = 5–7. (D) Exemplar traces of CaV1.2 channels expressed without a RGK, with Gem (20 pg), with Rem (100 pg), or with Rem2 (468 pg). Bars: 1 s and 1 μA.
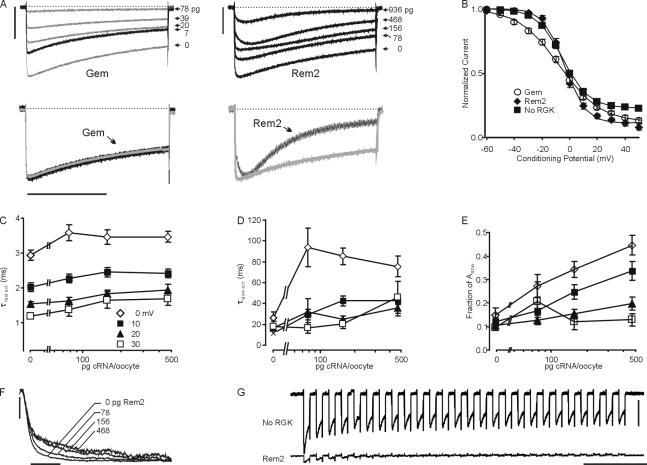
Coexpression of Gem appeared to inhibit Cav1.2 currents by a direct scaling effect, while Rem2 altered the kinetics of activation and inactivation (Fig. 2 A) in a dose-dependent manner. These different effects upon channel activation and inactivation are better appreciated by examining scaled traces from CaV1.2 channels compared with traces from CaV1.2 channels coexpressed with Gem (26 pg) or Rem2 (936 pg). Rem2 both slowed activation and accelerated inactivation during a 2-s test pulse. Quantitative analysis of the kinetics of inactivation for CaV1.2 channels coexpressed with Rem2 was complicated because the decay phases of currents were contaminated by overlapping slow activation. Thus, we analyzed steady-state inactivation with a two-pulse protocol in which the normalized residual peak current during a +10-mV test pulse (P2) was plotted against the voltage of a 5-s inactivating prepulse (P1) and found that both Gem (26 pg) and Rem2 (936 pg) affected steady-state inactivation (Fig. 2 B). The data were fitted to a Boltzmann function with a nonzero pedestal. Rem2 mainly affected the pedestal from 0.23 ± 0.02 to 0.12 ± 0.02, (n = 11–12, P < 0.0001) but Gem reduced the slope to −14.1 ± 0.6 from −9.0 ± 0.8 (n = 10–12; P < 0.0001).
Figure 2.
Rem2 affects channel activation and inactivation. (A) Top panels show exemplar current traces during a 2-s test pulse at +10 mV for Gem and Rem2 at the indicated doses. Bars, 1 μA. The bottom panels show scaled exemplar traces of α1C/β3/α2δ (gray) coinjected with Gem (65 pg) or Rem2 (936 pg) (black) during a 2-s test pulse to +10 mV. (B) Steady-state inactivation for α1C/β3/α2δ without or with Gem (26 pg) or Rem2 (936 pg). n = 10–12 (C–E) Dose–response of Rem2-mediated effects upon kinetics of activation (τfast, fraction Aslow, and τslow, respectively, at the indicated test potentials—see legend in C—and with the amounts of cRNA injected as indicated on the x-axis), n = 7–14. (F) Scaled exemplar traces of activation phases at +10 mV for α1C/β3/α2δ coexpressed with the indicated Rem2 doses. Bar, 10 ms. (G) Ca2+ currents recorded during a simulated burst of action potentials in a pancreatic β cell (see Materials and methods) with no RGK or Rem2 (468 pg). Bars: 200 nA, 1 s.
Rem2 also affected CaV1.2 channel activation in a dose-dependent and voltage-dependent manner (Fig. 2, C–F). In the absence of Rem2 (Fig. 2 C, 0 pg), the activating phase for currents elicited with test potentials from 0 to +30 mV was best fitted with two exponentials (I = Afaste−τ-fast/t + Aslowe−τ-slow/t + C) and the dominant component was τfast (fraction Afast was 80–90% at all test potentials; Fig. 2, C and D). The τfast decreased with more depolarizing test potentials (Fig. 2 C), but was unaffected by increasing the dose of coexpressed Rem2. Instead, the slower activation of CaV1.2 channels induced by higher doses of coexpressed Rem2 could be explained by two effects upon τslow: τslow became longer with increasing doses of Rem2 and the fraction of Aslow increased. These effects were most prominent at test potentials near the peak of the I-V curve (Fig. 2, D and E). The overall consequences of these dose-dependent effects upon activation induced by Rem2 are illustrated by the overlaid traces in Fig. 2 F.
Since these effects upon channel kinetics suggested that Rem2 could alter the temporal nature of channel responsiveness during action potentials, we tested whether Rem2 altered simulated Ca2+-dependent action potentials that underlie the rhythmic bursting of electrical activity essential for insulin secretion in pancreatic islet β cells (Mears, 2004). Fig. 2 G shows the resultant Ca2+ currents from CaV1.2 channels (α1C, β3, and α2δ) expressed in Xenopus oocytes during 26 successive 100-ms depolarizations from −40 to 0 mV (at 5 Hz), a protocol that simulates the bursting activity during insulin secretion and has been used in isolated β cells (Kanno et al., 2002). In the absence of RGKs, the peak Ca2+ current amplitude decreased sequentially during the 26 successive depolarizations so that the current amplitude during the last depolarization was 46 ± 4% (n = 8) of the peak current during the first depolarization. Not only were the current amplitudes smaller with coexpression of Rem2 (consistent with the effects of Rem2 presented above), but Rem2 accelerated the decrease in amplitude during the successive depolarizations so that the current amplitude during the last depolarization was 12 ± 7% (n = 7; P = 0.001 compared with no RGK) of the peak current during the first depolarization. This accelerated decrement is likely due to the increased rate of inactivation observed in the presence of Rem2 (Fig. 2 A). In contrast, coexpression of Gem led to decreased current (compared with no RGK), but did not affect the rate of decrement of the current amplitude (unpublished data), consistent with the lack of effect upon channel kinetics shown above. Thus, the presence of Rem2 would decrease the integrated Ca2+ influx and alter its kinetics during a burst of action potentials such as those that drive insulin secretion in pancreatic islet β cells.
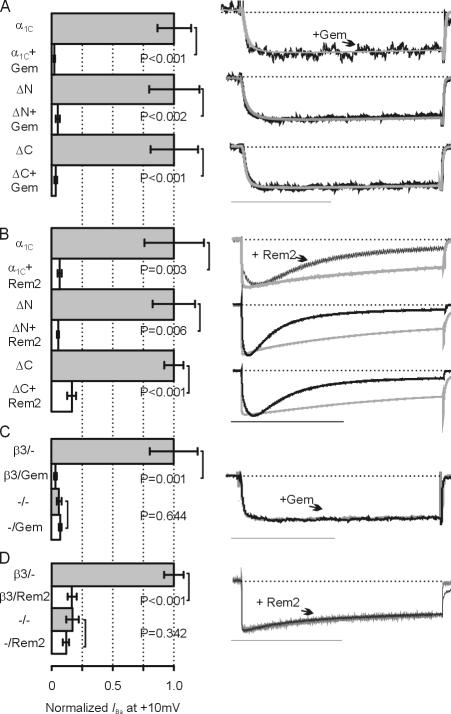
We next tested whether the α1C N or C termini were necessary for these effects by testing whether deletion constructs (Δ2-139 in the N-terminus or Δ1669-2171 in the C terminus) were modulated by Gem or Rem2. These experiments were prompted in part by a recent report suggesting that Rem, another RGK member, required the α1C C terminus, particularly the PKA phosphorylation site at Ser1928, for inhibition of Ca2+ channel currents (Crump et al., 2006). In contrast, we found that Gem (250 pg) and Rem2 (936 pg) consistently reduced peak current amplitude for channels containing intact or truncated α1C subunits (Fig. 3, A and B). Rem2 also maintained its effects upon kinetics of activation and inactivation in the truncated channels, as shown in the scaled exemplar current traces (Fig. 3 B).
Figure 3.
The Ca2+ channel β subunit, but not the α1C N and C termini, are required for Gem- or Rem2-mediated effects. (A and B) Normalized I Ba for the indicated combinations of α1C (WT), the N-terminal truncation (ΔN), or the C-terminal truncation (ΔC), with or with Gem or Rem2, as indicated. All combinations were coexpressed with β3 and α2δ. Scaled exemplar current for each pair with (black) or without (gray) Gem or Rem2 are shown on right. n = 5–9. Bars: 50 ms (A) and 1 s (B). (C and D) Normalized I Ba for the indicated combinations of ΔC and α2δ with or without β3 and Gem or Rem2, as indicated. For the pair without β3, scaled exemplar currents with (black) or without (gray) Gem or Rem2 are shown on right. n = 5. Scale bars as above.
We also used the α1C C-terminal deletion to analyze whether β subunits were necessary for Gem or Rem2 modulation. Truncation of the α1C C terminus produces increased current amplitude in the absence of β subunits (Wei et al., 1994; Klöckner et al., 1995; Gerhardstein et al., 2000; Ivanina et al., 2000), thereby providing a larger baseline current from which to assess RGK inhibition. Fig. 3 (C and D) shows that, in the absence of a coexpressed β subunit, neither Gem nor Rem2 inhibited channel currents. Further, Rem2 modulated neither activation nor inactivation in the absence of a coexpressed β subunit. These results show that RGK modulation is independent of the α1C N or C terminus, but requires β subunits.
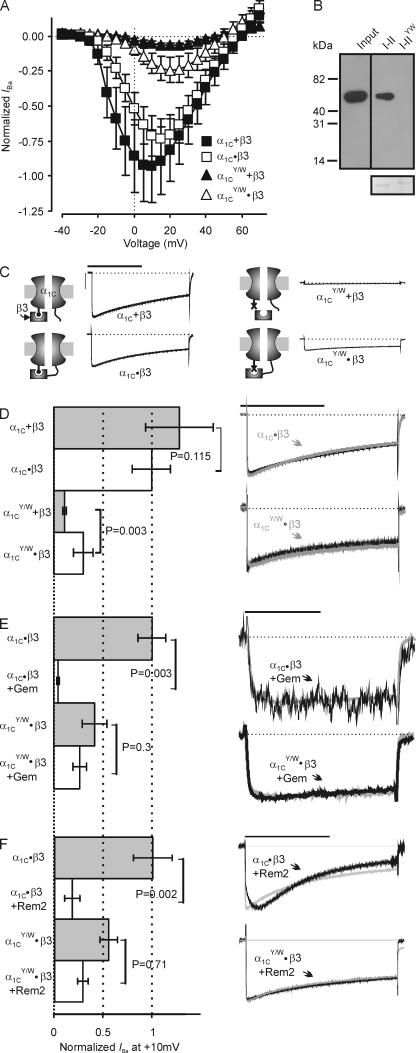
We next tested whether β interaction with the AID was necessary for RGK modulation. Recent models have suggested that RGKs inhibit Ca2+ channels by direct competition with β subunits for this high affinity interaction site on the α1 subunit (Sasaki et al., 2005). Although β subunit interaction with the AID is not required for all aspects of β subunit modulation of Ca2+ channel function (Maltez et al., 2005), AID mutations that block β subunit binding render channel currents too small to accurately assess an inhibitory effect of RGKs (Singer et al., 1991). Building upon a previous hypothesis that the AID–β interaction serves mainly to secure β subunits to α1 so as to allow other, lower affinity regulatory interactions, we covalently tethered β subunits directly to the α1C C terminus after amino acid 2134 (α1C•β3). Currents from this concatemer (expressed with α2δ) were similar to currents from untethered channels (α1C + β3 with α2δ), except that the peak of the I-V curve shifted to more depolarized potentials (Fig. 4 A) as previously reported (Dalton et al., 2005). To prevent β3 interaction with AID we made an α1C with two mutations in AID, Y467S and W470A (α1C YW), either of which has been shown to singly disrupt β subunit interaction and block β subunit modulation (Van Petegem et al., 2004; Leroy et al., 2005). Confirmation of abolished β subunit binding to the mutant α1C I-II loop is shown in a GST pull-down assay (Fig. 4 B). Current amplitudes from an α1C subunit with the same mutations coexpressed with β3 (α1C YW + β3) were very small (Fig. 4, A, C, and D) and indistinguishable from currents from an α1C expressed without β3 (not depicted), which is consistent with an absence of β3 interaction. When β3 was tethered to the AID mutant (α1C YW•β3) however, the resulting current amplitude was significantly larger than from α1C YW coexpressed with untethered β3 (Fig. 4, A, C, and D) Since the requirement for β-AID interaction could be at least partially circumvented by tethering the β subunit to α1, this supported the hypothesis that other interactions between α1 and β are important for β-dependent modulation. Having generated an α1C subunit that was modulated by a β subunit independent of its AID interaction, we therefore could test whether β-AID was required for RGK inhibition. Currents from channels containing α1C•β3 coexpressed with either Gem (250 pg) or Rem2 (628 pg) cRNA showed that they both produced a similar reduction of current as for α1C + β3 (compare Fig. 1 A with Fig. 4, D and E). Although coexpression of Gem or Rem2 also reduced currents from channels containing α1C YW•β3, the reduction was much more modest (Fig. 4, E and F). Moreover, although the effects of Rem2 on activation and inactivation were preserved when coexpressed with α1C•β3, Rem2 did not affect activation or inactivation when coexpressed with α1C YW•β3 (Fig. 4 E). These results show that Rem2-mediated effects upon activation and inactivation require the β-AID interaction while Gem- or Rem2-induced inhibition of current amplitude does not.
Figure 4.
Interaction between the Ca2+ channel β subunits and the AID influence Gem- and Rem2-mediated effects. (A) Normalized I-V relationships of I Ba from oocytes injected with the indicated constructs or combinations, all coinjected with α2δ. n = 31–34. (B) A Hisx6 immunoblot of a pull-down experiment for purified β2 SH3-GK core (Maltez et al., 2005) using a GST-α1C I-II or I-IIYW mutant loop. Coomassie-stained gel below shows equal loading of the GST fusion proteins. (C) Exemplar traces and models of the indicated concatemers or combinations. Bars: 1 s, 4 μA. (D–F) Normalized I Ba for the indicated concatemers or combinations (all expressed with α2δ), with or without Gem or Rem2. Scaled exemplar traces are shown on right. n = 5. Bars: 1 s (D and F) and 50 ms (E).
DISCUSSION
Heterologous overexpression of several RGKs in a variety of systems produces almost complete inhibition of coexpressed or endogenous Ca2+ channel current (Beguin et al., 2001, 2005b; Finlin et al., 2003; Murata et al., 2004; Ward et al., 2004; Chen et al., 2005; Crump et al., 2006) and RGK inhibition of Ca2+ influx has been proposed as a mechanism for physiologic control of Ca2+ channel activity for responses such as regulation of insulin secretion from pancreatic islet cells (Beguin et al., 2001; Finlin et al., 2005) or control of cardiac rhythm (Murata et al., 2004). Lacking a detailed molecular understanding of how RGKs could fine tune Ca2+ influx eclipses how this mode of regulation could shape a specific physiological response; for example, up-regulation of an RGK (Ohsugi et al., 2004; Finlin et al., 2005) and subsequent channel inhibition (Beguin et al., 2001; Finlin et al., 2005) after glucose stimulation might protect islet cells from excessive Ca2+ influx during chronic hyperglycemia, but how would cells retain their ability to secrete insulin with the almost complete loss of Ca2+ currents observed in previous overexpression experiments?
In this study we describe two unexpected means by which RGKs regulate Ca2+ channels, providing a framework for understanding how a wide array of Ca2+ signaling events can be precisely regulated. Exploiting the Xenopus oocyte system to control protein expression levels, we found that RGK inhibition of Ca2+ channel current was dose dependent. The mechanism(s) by which RGKs lead to current amplitude reduction, previously a source of controversy, is not revealed by these experiments; direct effects upon channels resident at the cell surface (Chen et al., 2005; Finlin et al., 2005) or effects upon channel trafficking/assembly (Beguin et al., 2001) cannot be easily distinguished by the experiments presented here. The significant finding, however, is that the suppression of current depends upon the level of RGK expression, thus promising a predictable and titratable attenuation of Ca2+ current by specific RGKs. Thus, our results suggest that the high glucose-stimulated induction of a specific RGK and the resultant down-regulation of the Ca2+ current in pancreatic β islet cells contribute to a protective mechanism against the detrimental effects of an enhanced Ca2+ signal resulting from chronic glucose exposure (Juntti-Berggren et al., 1993); conversely, a reduction of RGK protein in response to elevated glucose would serve to compensate hyperglycemia acutely by increasing Ca2+ channel activity and consequent insulin secretion.
Furthermore we found that Rem2, but not Gem or Rem, also altered Cav1.2 gating kinetics, slowing activation and enhancing inactivation. These effects upon kinetics suggest that Rem2 must act at least in part upon channels resident at the cell surface. Not only could Rem2 decrease peak Ca2+ influx, it could also impart profound influence on the Ca2+-dependent action potentials that underlie the rhythmic bursting of electrical activity essential for insulin secretion in pancreatic islet cells (Mears, 2004), by shaping the temporal nature of channel responsiveness as suggested by our experiments in Fig. 2 G. Although the molecular basis for the effects of Rem2 upon channel kinetics is not clear, we speculate that its extended N terminus and C terminus that flank the Ras core may be responsible; neither extension is found in Rem or Gem (Fig. 5). The kinetic actions of Rem2 could result from an additional contact between Rem2 and the channel within these nonconserved regions.
Figure 5.
Rem2 has extended termini. CLUSTAL W (1.83) multiple sequence alignment (Thompson et al., 1994) of Rem, Gem, and Rem2. The gray-boxed areas highlight the extended N and C termini in Rem2 that flank the conserved Ras-like core.
These different modes of regulation by Gem and Rem2, in conjunction with their differential temporal patterns of expression, may yield an integrated response to oppose effects of hyperglycemia. Gem, up-regulated in MIN6 cells within 45 min after exposure to glucose (Ohsugi et al., 2004), would diminish Ca2+ influx during acute hyperglycemia; Rem2, induced after 16 h of high glucose (Finlin et al., 2005), would serve to shape Ca2+ responsiveness during chronic hyperglycemia. Although our experiments were not designed to address the relative potency of Gem vs. Rem2—since their comparative levels in pancreatic β cells have not yet been determined—it is intriguing that Rem2 appears to have a broader dose–response range, which supports the proposed role in fine tuning Ca2+ responsiveness over time.
Our study provides several new insights that help clarify the molecular mechanisms by which RGKs inhibit Ca2+ channels. First, we demonstrated that β subunits are necessary for RGK inhibition, corroborating previous reports of β subunit dependence (Beguin et al., 2001). By using α1C subunits with deletions in either the N or C terminus in order to increase basal current amplitude, we avoided the difficulties of accurately measuring the inhibition of an already small signal, which may explain the contrasting result obtained with Rem (Crump et al., 2006). Second, our experiments with the truncated α1C subunits demonstrated that neither the α1C N terminus nor C terminus were required for Gem- or Rem2-mediated inhibition or alteration of channel gating, also in contrast to a recent report (Crump et al., 2006). While these differences may be attributed to Rem- vs. Rem2-specific effects, failure of the C-terminal deletion (Δ1733) in that report to augment Ca2+ currents compared with those from intact α1C subunits, as has been reported previously (Wei et al., 1994; Klöckner et al., 1995; Gerhardstein et al., 2000; Ivanina et al., 2000), point to possible technical discrepancies.
Our results also help clarify a conflict between two recent biochemical studies concerning whether the AID competes with Cavβ for Gem binding (Sasaki et al., 2005) or is present as a complex with Cavβ and Rem (Finlin et al., 2006). Our studies support the latter, where the RGKs function only when the α1 subunit is in association with a β subunit through its high affinity interaction site AID. In this context, our findings offer additional insights into mechanisms by which β subunits modulate Ca2+ channel currents. Coexpression of β subunits with α1 subunits increases current amplitude and affects kinetics of activation and inactivation (Dolphin, 2003). Within α1 subunits, the major interaction site for β subunits is the AID (Pragnell et al., 1994). The AID, however, is not absolutely required for all aspects of β subunit modulation as β2a still modulated channel activation and inactivation for an α1A (Cav2.1) subunit in which the AID had been deleted (Maltez et al., 2005). Because the W→A mutation in the AID completely blocks β subunit interaction (Leroy et al., 2005), our experiments showing that the β subunit– dependent augmentation of current amplitude is partially preserved with the α1C YW•β3 concatemers demonstrate clearly that β subunits can still modulate Ca2+ channels through interactions exclusive of the AID (Walker et al., 1998; Leroy et al., 2005; Maltez et al., 2005; Takahashi et al., 2005). Utilization of these concatemers also elucidated the mechanism of RGK modulation of Ca2+ channels: the partial preservation of the Gem- or Rem2-mediated decrease in current amplitude with the α1C YW–β3 concatemers rules out models in which RGKs compete with β subunits for AID interaction (Beguin et al., 2001; Sasaki et al., 2005). In contrast, the complete loss of Rem2-mediated effects upon activation and inactivation suggest that the β–AID interaction is necessary only for Rem2-mediated effects upon channel gating.
Acknowledgments
We thank D. McKinnon, D. Andres, and P. Pfaffinger for DNA constructs and M.S. George for his input and careful reading of the manuscript.
This work was supported by grants from the National Institutes of Health, the American Heart Association, and the Hirschl Trust. G. Pitt is the Esther Aboodi Assistant Professor of Medicine.
Olaf S. Andersen served as editor.
Abbreviations used in this paper: AID, α1 interaction domain; RGK, Rad, Rem, Rem2, Gem/Kir.
References
- An, W.F., M.R. Bowlby, M. Betty, J. Cao, H.P. Ling, G. Mendoza, J.W. Hinson, K.I. Mattsson, B.W. Strassle, J.S. Trimmer, and K.J. Rhodes. 2000. Modulation of A-type potassium channels by a family of calcium sensors. Nature. 403:553–556. [DOI] [PubMed] [Google Scholar]
- Beguin, P., R.N. Mahalakshmi, K. Nagashima, D.H. Cher, N. Kuwamura, Y. Yamada, Y. Seino, and W. Hunziker. 2005. a. Roles of 14-3-3 and calmodulin binding in subcellular localization and function of the small G-protein Rem2. Biochem. J. 390:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beguin, P., R.N. Mahalakshmi, K. Nagashima, D.H. Cher, A. Takahashi, Y. Yamada, Y. Seino, and W. Hunziker. 2005. b. 14-3-3 and calmodulin control subcellular distribution of Kir/Gem and its regulation of cell shape and calcium channel activity. J. Cell Sci. 118:1923–1934. [DOI] [PubMed] [Google Scholar]
- Beguin, P., K. Nagashima, T. Gonoi, T. Shibasaki, K. Takahashi, Y. Kashima, N. Ozaki, K. Geering, T. Iwanaga, and S. Seino. 2001. Regulation of Ca2+ channel expression at the cell surface by the small G-protein kir/Gem. Nature. 411:701–706. [DOI] [PubMed] [Google Scholar]
- Budde, T., S. Meuth, and H.C. Pape. 2002. Calcium-dependent inactivation of neuronal calcium channels. Nat. Rev. Neurosci. 3:873–883. [DOI] [PubMed] [Google Scholar]
- Canti, C., A. Davies, N.S. Berrow, A.J. Butcher, K.M. Page, and A.C. Dolphin. 2001. Evidence for two concentration-dependent processes for β-subunit effects on α1B calcium channels. Biophys. J. 81:1439–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall, W.A. 2000. Structure and regulation of voltage-gated Ca2+ channels. Annu. Rev. Cell Dev. Biol. 16:521–555. [DOI] [PubMed] [Google Scholar]
- Chen, H., H.L. Puhl III, S.L. Niu, D.C. Mitchell, and S.R. Ikeda. 2005. Expression of Rem2, an RGK family small GTPase, reduces N-type calcium current without affecting channel surface density. J. Neurosci. 25:9762–9772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump, S.M., R.N. Correll, E.A. Schroder, W.C. Lester, B.S. Finlin, D.A. Andres, and J. Satin. 2006. The L-type calcium channel α-subunit and protein kinase inhibitors modulate the Rem-mediated regulation of current. Am. J. Physiol. Heart Circ. Physiol. 291:H1959–H1971. [DOI] [PubMed] [Google Scholar]
- Dalton, S., S.X. Takahashi, J. Miriyala, and H.M. Colecraft. 2005. A single CaVβ can reconstitute both trafficking and macroscopic conductance of voltage-dependent calcium channels. J. Physiol. 567:757–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolphin, A.C. 2003. β subunits of voltage-gated calcium channels. J. Bioenerg. Biomembr. 35:599–620. [DOI] [PubMed] [Google Scholar]
- Finlin, B.S., and D.A. Andres. 1997. Rem is a new member of the Rad- and Gem/Kir Ras-related GTP-binding protein family repressed by lipopolysaccharide stimulation. J. Biol. Chem. 272:21982–21988. [DOI] [PubMed] [Google Scholar]
- Finlin, B.S., R.N. Correll, C. Pang, S.M. Crump, J. Satin, and D.A. Andres. 2006. Analysis of the complex between Ca2+ channel β-subunit and the Rem GTPase. J. Biol. Chem. 281:23557–23566. [DOI] [PubMed] [Google Scholar]
- Finlin, B.S., S.M. Crump, J. Satin, and D.A. Andres. 2003. Regulation of voltage-gated calcium channel activity by the Rem and Rad GTPases. Proc. Natl. Acad. Sci. USA. 100:14469–14474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlin, B.S., A.L. Mosley, S.M. Crump, R.N. Correll, S. Ozcan, J. Satin, and D.A. Andres. 2005. Regulation of L-type Ca2+ channel activity and insulin secretion by the Rem2 GTPase. J. Biol. Chem. 280:41864–41871. [DOI] [PubMed] [Google Scholar]
- Finlin, B.S., H. Shao, K. Kadono-Okuda, N. Guo, and D.A. Andres. 2000. Rem2, a new member of the Rem/Rad/Gem/Kir family of Ras-related GTPases. Biochem. J. 347(Pt 1):223–231. [PMC free article] [PubMed] [Google Scholar]
- Gerhardstein, B.L., T. Gao, M. Bunemann, T.S. Puri, A. Adair, H. Ma, and M.M. Hosey. 2000. Proteolytic processing of the C terminus of the α1C subunit of L-type calcium channels and the role of a proline-rich domain in membrane tethering of proteolytic fragments. J. Biol. Chem. 275:8556–8563. [DOI] [PubMed] [Google Scholar]
- Ivanina, T., Y. Blumenstein, E. Shistik, R. Barzilai, and N. Dascal. 2000. Modulation of L-type Ca2+ channels by Gbg and calmodulin via interactions with N and C termini of α1C. J. Biol. Chem. 275:39846–39854. [DOI] [PubMed] [Google Scholar]
- Juntti-Berggren, L., O. Larsson, P. Rorsman, C. Ammala, K. Bokvist, K. Wahlander, P. Nicotera, J. Dypbukt, S. Orrenius, A. Hallberg, et al. 1993. Increased activity of L-type Ca2+ channels exposed to serum from patients with type I diabetes. Science. 261:86–90. [DOI] [PubMed] [Google Scholar]
- Kanno, T., P. Rorsman, and S.O. Gopel. 2002. Glucose-dependent regulation of rhythmic action potential firing in pancreatic β cells by KATP-channel modulation. J. Physiol. 545:501–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly, K. 2005. The RGK family: a regulatory tail of small GTP-binding proteins. Trends Cell Biol. 15:640–643. [DOI] [PubMed] [Google Scholar]
- Kim, J., S. Ghosh, D.A. Nunziato, and G.S. Pitt. 2004. Identification of the components controlling inactivation of voltage-gated Ca2+ channels. Neuron. 41:745–754. [DOI] [PubMed] [Google Scholar]
- Klöckner, U., G. Mikala, M. Varadi, G. Varadi, and A. Schwartz. 1995. Involvement of the carboxyl-terminal region of the α1 subunit in voltage-dependent inactivation of cardiac calcium channels. J. Biol. Chem. 270:17306–17310. [DOI] [PubMed] [Google Scholar]
- Leone, A., N. Mitsiades, Y. Ward, B. Spinelli, V. Poulaki, M. Tsokos, and K. Kelly. 2001. The Gem GTP-binding protein promotes morphological differentiation in neuroblastoma. Oncogene. 20:3217–3225. [DOI] [PubMed] [Google Scholar]
- Leroy, J., M.S. Richards, A.J. Butcher, M. Nieto-Rostro, W.S. Pratt, A. Davies, and A.C. Dolphin. 2005. Interaction via a key tryptophan in the I-II linker of N-type calcium channels is required for β1 but not for palmitoylated β2, implicating an additional binding site in the regulation of channel voltage-dependent properties. J. Neurosci. 25:6984–6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire, J., T. Santoro, P. Jensen, U. Siebenlist, J. Yewdell, and K. Kelly. 1994. Gem: an induced, immediate early protein belonging to the Ras family. Science. 265:241–244. [DOI] [PubMed] [Google Scholar]
- Maltez, J.M., D.A. Nunziato, J. Kim, and G.S. Pitt. 2005. Essential Cavb modulatory properties are AID-independent. Nat. Struct. Mol. Biol. 12:372–377. [DOI] [PubMed] [Google Scholar]
- Mears, D. 2004. Regulation of insulin secretion in islets of Langerhans by Ca2+ channels. J. Membr. Biol. 200:57–66. [DOI] [PubMed] [Google Scholar]
- Murata, M., E. Cingolani, A.D. McDonald, J.K. Donahue, and E. Marban. 2004. Creation of a genetic calcium channel blocker by targeted Gem gene transfer in the heart. Circ. Res. 95:398–405. [DOI] [PubMed] [Google Scholar]
- Ohsugi, M., C. Cras-Meneur, Y. Zhou, W. Warren, E. Bernal-Mizrachi, and M.A. Permutt. 2004. Glucose and insulin treatment of insulinoma cells results in transcriptional regulation of a common set of genes. Diabetes. 53:1496–1508. [DOI] [PubMed] [Google Scholar]
- Pragnell, M., M. De Waard, Y. Mori, T. Tanabe, T.P. Snutch, and K.P. Campbell. 1994. Calcium channel β-subunit binds to a conserved motif in the I-II cytoplasmic linker of the α1-subunit. Nature. 368:67–70. [DOI] [PubMed] [Google Scholar]
- Reynet, C., and C.R. Kahn. 1993. Rad: a member of the Ras family overexpressed in muscle of type II diabetic humans. Science. 262:1441–1444. [DOI] [PubMed] [Google Scholar]
- Sasaki, T., T. Shibasaki, P. Beguin, K. Nagashima, M. Miyazaki, and S. Seino. 2005. Direct inhibition of the interaction between α-interaction domain and β-interaction domain of voltage-dependent Ca2+ channels by Gem. J. Biol. Chem. 280:9308–9312. [DOI] [PubMed] [Google Scholar]
- Singer, D., M. Biel, I. Lotan, V. Flockerzi, F. Hofmann, and N. Dascal. 1991. The roles of the subunits in the function of the calcium channel. Science. 253:1553–1557. [DOI] [PubMed] [Google Scholar]
- Splawski, I., K.W. Timothy, L.M. Sharpe, N. Decher, P. Kumar, R. Bloise, C. Napolitano, P.J. Schwartz, R.M. Joseph, K. Condouris, et al. 2004. CaV1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell. 119:19–31. [DOI] [PubMed] [Google Scholar]
- Takahashi, S.X., J. Miriyala, L.H. Tay, D.T. Yue, and H.M. Colecraft. 2005. A CaVβ SH3/guanylate kinase domain interaction regulates multiple properties of voltage-gated Ca2+ channels. J. Gen. Physiol. 126:365–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, J.D., D.G. Higgins, and T.J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Petegem, F., K.A. Clark, F.C. Chatelain, and D.L. Minor. 2004. Structure of a complex between a voltage-gated calcium channel β-subunit and an α-subunit domain. Nature. 429:671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, D., D. Bichet, K.P. Campbell, and M. De Waard. 1998. A β4 isoform-specific interaction site in the carboxyl-terminal region of the voltage-dependent Ca2+ channel α1A subunit. J. Biol. Chem. 273:2361–2367. [DOI] [PubMed] [Google Scholar]
- Ward, Y., B. Spinelli, M.J. Quon, H. Chen, S.R. Ikeda, and K. Kelly. 2004. Phosphorylation of critical serine residues in Gem separates cytoskeletal reorganization from down-regulation of calcium channel activity. Mol. Cell. Biol. 24:651–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward, Y., S.F. Yap, V. Ravichandran, F. Matsumura, M. Ito, B. Spinelli, and K. Kelly. 2002. The GTP binding proteins Gem and Rad are negative regulators of the Rho-Rho kinase pathway. J. Cell Biol. 157:291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, X., A. Neely, A.E. Lacerda, R. Olcese, E. Stefani, E. Perez-Reyes, and L. Birnbaumer. 1994. Modification of Ca2+ channel activity by deletions at the carboxyl terminus of the cardiac α1 subunit. J. Biol. Chem. 269:1635–1640. [PubMed] [Google Scholar]
- Zühlke, R.D., G.S. Pitt, R.W. Tsien, and H. Reuter. 2000. Ca2+-sensitive inactivation and facilitation of L-type Ca2+ channels both depend on specific amino acid residues in a consensus calmodulin-binding motif in the α1C subunit. J. Biol. Chem. 275:21121–21129. [DOI] [PubMed] [Google Scholar]