Abstract
Many different ion channel pores are thought to have charged amino acid residues clustered around their entrances. The so-called surface charges contributed by these residues can play important roles in attracting oppositely charged ions from the bulk solution on one side of the membrane, increasing effective local counterion concentration and favoring rapid ion movement through the channel. Here we use site-directed mutagenesis to identify arginine residues contributing important surface charges in the intracellular mouth of the cystic fibrosis transmembrane conductance regulator (CFTR) Cl− channel pore. While wild-type CFTR was associated with a linear current–voltage relationship with symmetrical solutions, strong outward rectification was observed after mutagenesis of two arginine residues (R303 and R352) located near the intracellular ends of the fifth and sixth transmembrane regions. Current rectification was dependent on the charge present at these positions, consistent with an electrostatic effect. Furthermore, mutagenesis-induced rectification was more pronounced at lower Cl− concentrations, suggesting that these mutants had a reduced ability to concentrate Cl− ions near the inner pore mouth. R303 and R352 mutants exhibited reduced single channel conductance, especially at negative membrane potentials, that was dependent on the charge of the amino acid residue present at these positions. However, the very low conductance of both R303E and R352E-CFTR could be greatly increased by elevating intracellular Cl− concentration. Modification of an introduced cysteine residue at position 303 by charged methanethiosulfonate reagents reproduced charge-dependent effects on current rectification. Mutagenesis of arginine residues in the second and tenth transmembrane regions also altered channel permeation properties, however these effects were not consistent with changes in channel surface charges. These results suggest that positively charged arginine residues act to concentrate Cl− ions at the inner mouth of the CFTR pore, and that this contributes to maximization of the rate of Cl− ion permeation through the pore.
INTRODUCTION
Ion permeation in several different ion channel types is influenced by the presence of charged amino acid side chains around the entrances to the channel pore (Green and Andersen, 1991). “Surface charge effects” resulting from the presence of these residues act to attract oppositely charged ions from the bulk solution, increasing their effective local concentration, while at the same time repelling ions of like charge. The increase in permeant ion concentration close to the entrance to the pore that results from surface charge effects is thought to increase the unitary conductance in both cation channels (Worley et al., 1986; MacKinnon et al., 1989; Brelidze et al., 2003) and anion channels (Smith et al., 2001; Moorhouse et al., 2002). In addition, because charged pore mouths influence the rate of entry of ions into the pore from the solution on one side of the membrane, surface charges can influence the relative conductance of the channel for ions crossing the membrane in different directions, affecting the linearity of the current–voltage relationship (Brelidze et al., 2003; Haug et al., 2004).
Because surface charges on channel proteins act by electrostatic attraction of permeating ions, they are contributed by negatively charged amino acid side chains in cation channels and by positively charged side chains in anion channels. Indeed, site-directed mutagenesis has been used to identify individual aspartate and glutamate residues that contribute to surface charge effects in cation channels (Imoto et al., 1988; Brelidze et al., 2003; Nimigean et al., 2003; Haug et al., 2004; D'Avanzo et al., 2005; Wang et al., 2005), and arginine and lysine residues acting in an analogous way in anion channels (Smith et al., 2001; Moorhouse et al., 2002).
Fixed positive charges contributed by positively charged amino acid side chains are known to affect the permeation properties of the cystic fibrosis transmembrane conductance regulator (CFTR) Cl− channel (Linsdell, 2006). An arginine residue in the sixth transmembrane (TM) region, R334, appears to be located in the outer mouth of the pore, where it acts to attract negatively charged Cl− ions from the extracellular solution (Smith et al., 2001). Similarly, a lysine residue in TM1, K95, attracts Cl− ions from the intracellular solution (Linsdell, 2005). It was proposed that the fixed positive charges donated by K95 and R334 may be located at the intracellular and extracellular ends, respectively, of a short, narrow section of the pore (Linsdell, 2006). However, this narrow section appears to be located in the mid-to-outer part of the pore (Ge et al., 2004), and current models of the pore postulate that a wide inner vestibule exists between this narrow region and the cytoplasmic solution (McCarty, 2000; Linsdell, 2006). Presently much less is known about the molecular determinants and functional roles of the wide inner pore vestibule. Here we use site-directed mutagenesis and chemical modification to identify positively charged arginine residues that increase the unitary conductance of CFTR by attracting intracellular Cl− ions into the cytoplasmic mouth of the pore by a surface charge effect.
MATERIALS AND METHODS
Experiments were performed on baby hamster kidney cells transiently transfected with wild-type or mutant forms of human CFTR (Gong et al., 2002; Ge et al., 2004). Macroscopic and single channel patch clamp recordings were made from inside-out patches excised from these cells, as described in detail previously (Gong et al., 2002; Gong and Linsdell, 2003a; Ge et al., 2004). After patch excision and recording of background currents, CFTR channels were activated by exposure to PKA catalytic subunit plus MgATP (1 mM) in the cytoplasmic (bath) solution. As in a previous study (Ge et al., 2004), single channel currents were recorded after weak PKA stimulation (1–10 nM), whereas all macroscopic CFTR currents were recorded after maximal PKA stimulation (∼20 nM) and subsequent treatment with sodium pyrophosphate (PPi) to “lock” channels in the open state. Both intracellular (bath) and extracellular (pipette) solutions were based on one containing (in mM): 150 NaCl, 10 N-tris[hydroxymethyl]methyl-2-aminoethanesulfonate (TES), 2 MgCl2. Chloride concentration-dependent effects (Fig. 3 B, and Fig. 10 B) were studied by changing the NaCl concentration symmetrically in both the intracellular and extracellular solutions to 40 or 300 mM. To investigate Cl−:Na+ selectivity from macroscopic currents (Fig. 11), 150 mM NaCl in the pipette solution was replaced with 37.5 mM NaCl plus 225 mM sucrose. For some single channel recordings (Figs. 4, 6, and 10), NaCl in the extracellular solution was replaced by 150 mM Na gluconate. Elevated intracellular Cl− concentration (Fig. 6) was then achieved by increasing both intracellular NaCl and extracellular Na gluconate to 300 mM. All solutions were adjusted to pH 7.4 using NaOH. Methanethiosulfonate (MTS) reagents, sodium (2-sulfonatoethyl) methanethiosulfonate (MTSES), and [2-(trimethylammonium)ethyl] methanethiosulfonate bromide (MTSET) were initially prepared as concentrated stock solutions (160 mM) in distilled water and stored frozen until the time of use; they were then diluted in normal bath solution immediately before use. Given voltages have been corrected for liquid junction potentials calculated using pCLAMP9 software (Axon Instruments) where necessary. All chemicals were from Sigma-Aldrich, except for PKA (Promega) and MTSES and MTSET (Toronto Research Chemicals).
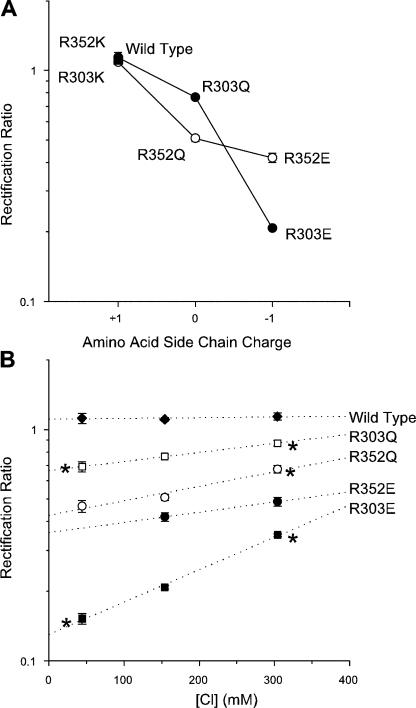
Figure 3.
Charge and chloride dependence of current rectification in mutant forms of CFTR. (A) The degree of rectification depends on the charge of the amino acid side chain present at position 303 or position 352. (B) The degree of rectification in R303E, R303Q, and R352Q is dependent on the Cl− concentration. Rectification was quantified at different symmetrical Cl− concentrations. * indicates a significant difference from the rectification ratio of the same channel variant with 154 mM Cl− (P < 0.05, two-tailed t test). Unfortunately, because of low current density in this mutant, R352E could not be studied at low Cl− concentration. Mean of data from 3–10 patches in both A and B.
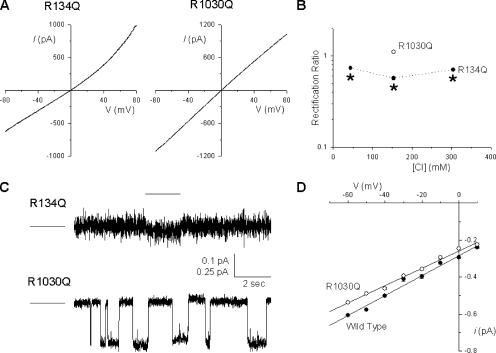
Figure 10.
Effect of mutagenesis of “central arginine” residues. (A) Example leak-subtracted macroscopic I-V relationships from R134Q and R1030Q-CFTR in inside-out membrane patches with symmetrical 154 mM Cl− solutions, after maximal channel activation. (B) Quantification of the rectification of the I-V relationship for these two channel mutants at different symmetrical Cl− concentrations. * indicates a significant difference from wild type at the same Cl− concentration (P < 10−4, two-tailed t test), † indicates a significant difference from R134Q at 154 mM Cl− (P < 0.005, two-tailed t test). Mean of data from three to eight patches. (C) Example single channel currents recorded from these two channel mutants at a membrane potential of −30 mV. For each trace, the line to the left represents the current level when the channel is closed. For R134Q, the tiny current associated with a putative channel opening is identified by a line above it. Note the different scale to the ordinate axis for these two current traces. (D) Mean single channel current–voltage relationships for wild type (filled circles) and R1030Q (open circles). Mean of data from four to five patches.
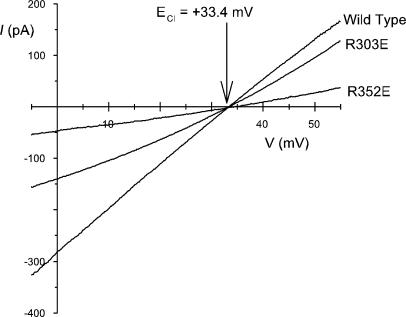
Figure 11.
Anion selectivity of mutant forms of CFTR. Example leak-subtracted macroscopic I-V relationships from different CFTR channel variants in inside-out membrane patches after replacement of 75% of extracellular NaCl with sucrose. Under these ionic conditions, each of the I-V relationships reverses close to the calculated Cl− equilibrium potential (ECl) of +33.4 mV. Examples of similar data obtained from three to four patches for these channel variants and also for R80E, R242E, R352Q, R933E, and R1102E (not depicted).
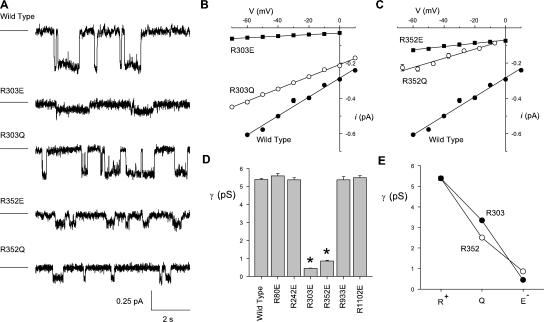
Figure 4.
Mutagenesis of positively charged arginine residues reduces CFTR single channel conductance. (A) Example single channel currents recorded from different channel variants at a membrane potential of −30 mV. For each trace, the line to the left represents the current level when the channel is closed. (B and C) Mean single channel current–voltage relationships constructed from such recordings for wild type (filled circles in each case) and channels bearing mutations at either R303 (B) or R352 (C). (D) Mean unitary conductance for wild-type CFTR and for channels bearing charge-changing mutations at each of six “inner arginines.” Unitary conductance was estimated as the slope of individual single channel current–voltage relationships such as those shown in B and C. * indicates a significant difference from wild type (P < 10−10, two-tailed t test). (E) Unitary conductance depends on the charge of the amino acid side chain present at position 303 (•) or position 352 (◯). Mean of data from three to five patches in B–E.
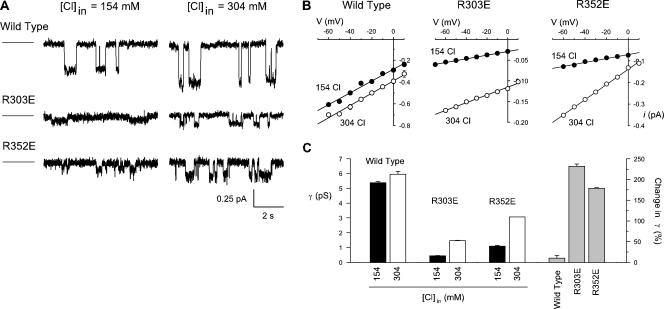
Figure 6.
Strong chloride concentration dependence of unitary conductance in mutant channels. (A) Example single channel currents recorded from wild type, R303E, and R352E at different intracellular Cl− concentrations (154 or 304 mM, as indicated), at a membrane potential of −20 mV. For each trace, the line to the left represents the current level when the channel is closed. (B) Mean single channel current–voltage relationships constructed from such recordings demonstrate that increasing intracellular Cl− concentration has a greater effect on these two mutants than on wild type. This is further shown from the mean unitary conductances estimated from the slope of individual single channel current–voltage relationships (C). Bars on the left in this panel illustrate the mean conductance estimated under different ionic conditions, while gray bars on the right show the mean percent change in conductance comparing 304 mM with 154 mM Cl−. Mean of data from three to five patches in B and C.
Current traces were filtered at 50 Hz (for single channel currents) or 100–200 Hz (for macroscopic currents) using an 8-pole Bessel filter, digitized at 250 Hz to 1 kHz, and analyzed using pCLAMP9 software (Axon Instruments). Macroscopic current–voltage (I-V) relationships were constructed using depolarizing voltage ramp protocols (Linsdell and Hanrahan, 1996, 1998). Background (leak) currents recorded before the addition of PKA were subtracted digitally, leaving uncontaminated CFTR currents (Linsdell and Hanrahan, 1998; Gong and Linsdell, 2003a). Rectification of the I-V relationship was quantified as the “rectification ratio,” the slope conductance at −50 mV as a fraction of that at +50 mV, as in previous studies (Gong and Linsdell, 2003b; Linsdell, 2005). The macroscopic current reversal potential (Fig. 11) was estimated by fitting the I-V curve with a polynomial function. Experiments were performed at room temperature, 21–24°C. Mean values are given as mean ± SEM.
RESULTS
Effects of Mutagenesis of Arginine Residues at the Intracellular Mouth of the Pore
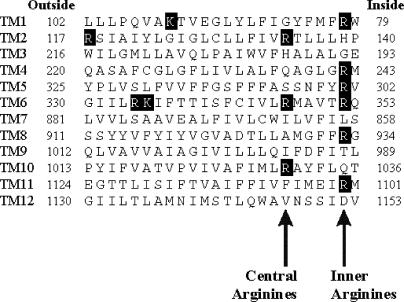
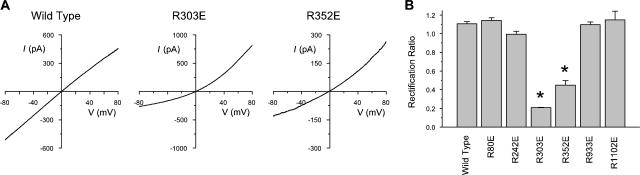
Previous studies have demonstrated the roles of positively charged amino acid side chains located in the mid-to-outer region of the CFTR pore (K95, R334) in attracting Cl− ions into the pore (Smith et al., 2001; Linsdell, 2005). However, the ability of other positive charges in the pore to fulfill a similar role has not been reported. Sequence alignment of the 12 TM regions of CFTR shows that positively charged arginine residues are found at the intracellular ends of TMs 1, 4, 5, 6, 8, and 11 (Fig. 1, Inner Arginines), as well as more centrally in TMs 2, 6, and 10 (Fig. 1, Central Arginines). To investigate if any of these “inner” arginine residues are involved in attracting intracellular Cl− ions into the pore, each was mutated individually to a negatively charged glutamate. As shown in Fig. 2, four of these mutants, like wild type, gave almost linear macroscopic I-V curves under symmetrical ionic conditions. In contrast, both R303E and R352E were associated with strong outward rectification (Fig. 2 A), which is quantified in Fig. 2 B. Since channels were locked open with PPi, any voltage dependence of channel gating (Cai et al., 2003) should be removed, and the open channel I-V shape observed should be a reflection of the underlying unitary current–voltage relationship. In fact, in all cases, the shape of the I-V curve was unaltered by treatment with PPi (unpublished data). To investigate the role of the charge on these residues in controlling I-V shape, these two arginines were also mutated to neutral glutamine residues; as shown in Fig. 3, R303Q and R352Q were also associated with outward rectification, although in both cases the degree of rectification observed was significantly less than with the charge-changing glutamate substitutions. Charge-conservative R303K and R352K mutations were associated with wild type like linear I-V relationships (Fig. 3 A).
Figure 1.
Location of positively charged amino acid residues within the CFTR TM regions. The CFTR protein includes 12 TM regions, which are aligned as described previously (Riordan et al., 1989; Dawson et al., 1999; McCarty, 2000). Positively charged lysine and arginine residues are shaded. The present study introduced mutations of a number of arginine residues predicted to lie close to the intracellular ends of TM1 (R80), TM4 (R242), TM5 (R303), TM6 (R352), TM8 (R933), and TM11 (R1102) (“Inner Arginines”), as well as more centrally located residues closer to the center of TM2 (R134) and TM10 (R1030) (Central Arginines).
Figure 2.
Mutagenesis of positively charged arginine residues induces CFTR channel current rectification. (A) Example leak subtracted macroscopic I-V relationships from different CFTR channel variants in inside-out membrane patches, after maximal channel activation with ATP, PKA, and PPi. In contrast to wild-type CFTR, mutants R303E and, to a lesser extent, R352E showed outward rectification of the I-V relationship with symmetrical 154 mM Cl− solutions. (B) Mutagenesis-induced rectification was specific for these two positions; charge-changing mutations at four other arginine residues did not significantly alter I-V shape. The degree of I-V rectification is quantified as the rectification ratio, as defined in Materials and methods. Mean of data from three to four patches. * indicates a significant difference from wild type (P < 0.0001, two-tailed t test).
The charge-dependent effect of substitutions at these two positions suggests that these two arginine residues normally play an electrostatic role in attracting intracellular Cl− ions into the pore. If this is the case, then it should be possible to overcome the effects of removing these positive charges by increasing the intracellular Cl− concentration. As a result, the I-V relationship should become less rectified as Cl− concentration is increased symmetrically. As shown in Fig. 3 B, macroscopic I-V rectification was indeed highly sensitive to symmetrical Cl− concentration in both R303E and R303Q, suggesting that increasing the Cl− concentration partially reverses the effects of removing these positive charges. Rectification in R352 mutants also appeared to be somewhat sensitive to the Cl− concentration (Fig. 3 B), although noticeably less so than R303 mutants. In contrast, the shape of the I-V curve for wild-type CFTR remained near linear at all symmetrical Cl− concentrations studied (Fig. 3 B).
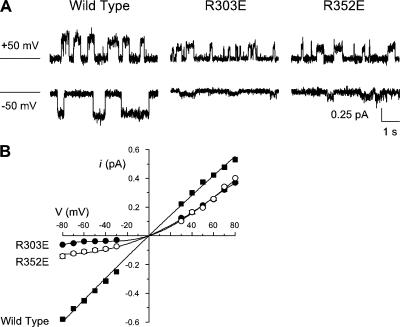
The changes in I-V shape observed after mutagenesis of R303 or R352 suggest that these mutations decrease the unitary conductance, particularly for inward current (Cl− efflux). To test this hypothesis directly, we measured unitary currents carried by Cl− efflux through each of the “inner arginine” channel mutants studied in Fig. 2. Mutations that did not alter I-V shape (R80E, R242E, R933E, and R1102E) also had no significant effect on unitary conductance (Fig. 4 D). In contrast, mutagenesis of either R303 or R352 was associated with significant decreases in unitary conductance, and this effect was dependent on the charge on the amino acid side chain present at these two positions (Fig. 4), again consistent with an electrostatic effect. Single channel currents carried by R303E and R352E under the same symmetrical Cl− solutions used in Fig. 2 are shown in Fig. 5 A. As expected, these mutations cause strong outward rectification of the single channel current–voltage relationship (Fig. 5 B). Nevertheless, both mutations decrease the amplitude not only of inward currents (carried by Cl− efflux) but also of outward currents (carried by Cl− influx), albeit to a much lesser extent. For example, at −80 mV, single channel current amplitude was reduced by 89 ± 0% (n = 4) in R303E and by 75 ± 2% (n = 3) in R352E, while at +80 mV, unitary currents were reduced by 30 ± 1% (n = 4) in R303E and by 25 ± 1% (n = 4) in R352E (Fig. 5 B).
Figure 5.
Mutagenesis of positively charged arginine residues causes rectification of the single channel current–voltage relationship. (A) Example single channel currents carried by Cl− influx or Cl− efflux atthe membrane potentials indicated. For each trace, the line to the left represents the current level when the channel is closed. (B) Mean single channel current–voltage relationships constructed from such recordings for wild type (▪), R303E (•) and R352E (◯). Mean of data from three to five patches.
The Cl− dependence of I-V shape in R303E and R352E is consistent with removal of positive surface charges that normally act to increase the Cl− concentration at the inner mouth of the pore. We therefore hypothesized that increasing intracellular Cl− concentration would partially overcome the effects of these mutations on the rate of Cl− efflux through the channel. As shown in Fig. 6, increasing the intracellular Cl− concentration from 154 to 304 mM led to a dramatic increase in unitary conductance in both of these mutants. This strong Cl− dependence was not observed in wild type, where the same increase in Cl− concentration led to only a slight increase in unitary conductance (Fig. 6, B and C). The ability of high intracellular Cl− concentrations to compensate, albeit only partially, for the effects of these mutations is consistent with the mutations acting by removing surface charges that normally act to concentrate Cl− ions in the inner mouth of the pore.
Modification of Surface Charge In Situ
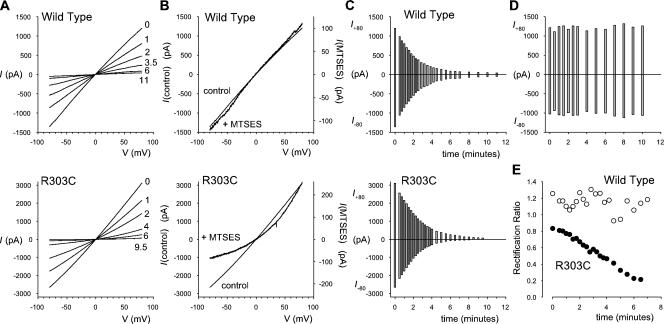
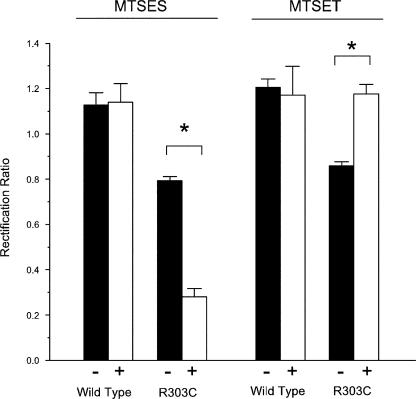
A potential caveat of all site-directed mutagenesis studies is the possibility that any functional effects of a mutation may result from conformational changes in the protein that are propagated some distance from the mutation. To investigate if the charges at positions 303 and 352 were indeed acting to attract Cl− ions into the pore, we investigated the accessibility of these residues and the effects of modification of their charge in situ. Both R303 and R352 were mutated to cysteine, so that their accessibility to intracellular charged MTS reagents could be tested in inside-out patches. Unfortunately, R352C expression did not yield any current in excised BHK cell membrane patches. However, modification of R303C is illustrated in Figs. 7 and 8. Control experiments with wild-type CFTR indicated that application of intracellular MTSES (Fig. 7, A and C) or MTSET (Fig. 8 A) led to rapid rundown of the current. This rundown was apparently caused by the MTS reagents, since addition of vehicle alone did not affect current amplitude (Fig. 7 D) and since current amplitude was always stable for at least 3 min before addition of the MTS reagent (not depicted). This rundown suggests that modification of intracellularly accessible cysteine residues by charged MTS reagents (especially MTSES) strongly inhibits the activity of channels that have been locked open by treatment with ATP and PPi. Nevertheless, the shape of the macroscopic I-V curve was not changed during the rundown process with either MTSES (Fig. 7, B and E; Fig. 9) or MTSET (Fig. 8 B; Fig. 9). Similar rundown was also observed in R303C (Fig. 7, A and C; Fig. 8 A), however, the shape of the I-V curve changed during the rundown process, becoming more outwardly rectifying after addition of MTSES (Fig. 7, B and E; Fig. 9) and more inwardly rectifying after application of MTSET (Figs. 8 and 9). These changes in I-V shape were not observed in R303Q (unpublished data), suggesting that they are specific for the introduction of a reactive cysteine at this position. The results of these experiments are summarized in Fig. 9. Modification of the introduced cysteine residue by negatively charged MTSES leads to pronounced outward rectification, similar to that seen in R303E (Fig. 2 B) and consistent with the deposition of a negative charge at the inner mouth of the pore, while modification by positively charged MTSET resulted in a wild type–like, moderately inwardly rectified I-V relationship. These findings suggest that the amino acid side chain at position 303 is accessible to hydrophilic intracellular molecules, and that modification of the charge at this position is sufficient to alter the permeation properties of the channel by altering the surface charge at the intracellular mouth of the pore.
Figure 7.
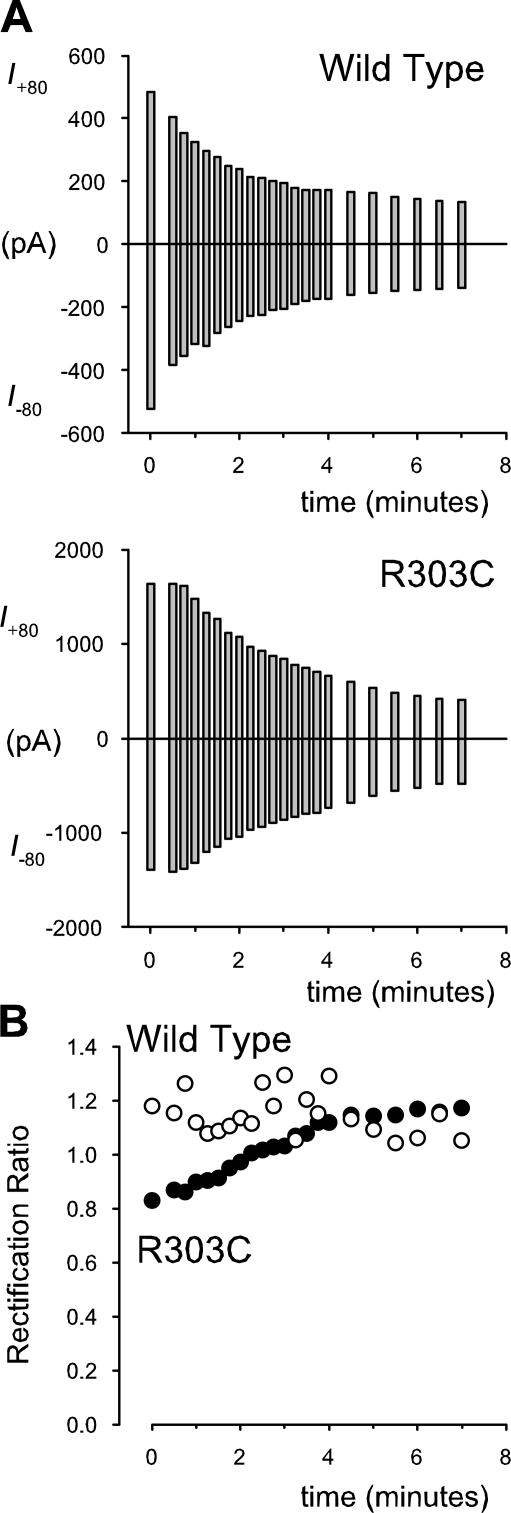
Modification of CFTR by intracellular MTSES. (A) Example leak-subtracted macroscopic I-V relationships in inside-out membrane patches with symmetrical 154 mM Cl− solutions, after maximal channel activation. Currents were recorded at various times before (time zero) and after application of 200 μM MTSES to the intracellular solution. Time after application of MTSES, in minutes, is shown alongside each I-V relationship. (B) MTSES-induced changes in I-V shape. Data from the same patches as in A, recorded before (control) and 6 min after addition of MTSES, are scaled to show the development of outward rectification in R303C but not wild type. (C) Time course of current rundown for these two example patches; current at +80 mV (I +80) and −80 mV (I −80) was monitored continuously after application of MTSES. (D) Lack of rundown over the same timescale on addition of vehicle alone in wild type. (E) Change in rectification ratio with time after addition of MTSES for the two patches used in A–C.
Figure 8.
Modification of CFTR by intracellular MTSET. (A) Individual examples of the time course of MTSET-induced current rundown in wild type and R303C. As described in Fig. 7 for MTSES, current was monitored at different times after addition of 2 mM MTSET to the intracellular solution. (B) Change in rectification ratio with time after addition of MTSET for these two patches.
Figure 9.
Modification of surface charge by intracellular MTS reagents alters I-V shape. Mean I-V rectification was measured before (−) and 4–6 min after (+) application of either MTSES (200 μM) or MTSET (2 mM) to the intracellular solution. * indicates a significant difference from matched, pre-MTS control values (P < 0.05, paired t test). Mean of data from three to five patches.
Effects of Mutagenesis of “Central Arginines”
Additional arginine residues are found in TMs 2, 6, and 10—the “central arginines” identified in Fig. 1. Of these three arginines, we discounted R347 in TM6 as potentially attracting intracellular Cl− ions into the pore, since this residue is known to form a salt bridge with aspartate 924 in TM8 (Cotten and Welsh, 1999). Mutagenesis of either R134 (in TM1) or R1030 (in TM10) to glutamate did not result in functional channel expression in inside-out BHK cell patches. However, both R134Q and R1030Q did yield functional channels (Fig. 10). R134Q was associated with slight outward current rectification with symmetrical 154 mM Cl− (Fig. 10, A and B); however, in contrast to channels bearing mutations at either R303 or R352 (Fig. 3 B), the degree of outward rectification was significantly reduced both at lower and higher symmetrical Cl− concentrations (Fig. 10 B). R1030Q, like wild type, was associated with an almost linear I-V relationship (Fig. 10, A and B). Measurable single channel activity was not observed after R134Q expression; however, Fig. 10 C shows an example of a very small apparent single channel opening frequently observed in a patch excised from an R134Q-expressing cell. This tiny current suggests that R134Q has an extremely small unitary conductance below the accurate resolution of single channel recording. R1030Q single channel currents were readily observed (Fig. 10 C), and these channels showed a slightly, but significantly, reduced single channel conductance (Fig. 10 D) of 4.70 ± 0.04 pS (n = 4) compared with 5.39 ± 0.06 pS (n = 5) for wild type (P < 0.0001, two-tailed t test).
Fixed Positive Charges and Anion:Cation Selectivity
Fixed charges at the pore mouth might not only attract ions of the opposite sign, they may also play a role in repelling like charges. For this reason, fixed charges at pore entrances may be one way in which channels achieve selectivity between anions and cations (Green and Andersen, 1991). Indeed, mutagenesis of one of these arginine residues, R352, has previously been associated with a diminution of anion-over-cation selectivity in CFTR (Cheung and Akabas, 1997; Guinamard and Akabas, 1999), although this suggestion has since been refuted by others (Smith et al., 2001). We measured the Cl−:Na+ selectivity of CFTR channel variants by replacing 75% of the extracellular NaCl with sucrose (Fig. 11). Under these conditions, current carried not only by wild-type CFTR, but also by each of the channel mutants R303E, R352E (Fig. 10), R80E, R242E, R933E, R1102E, and R352Q (not depicted) showed reversal potentials that were not significantly different from the calculated Cl− equilibrium potential (+33.4 mV). Thus we find no evidence for involvement of these fixed positive charges in determining the selectivity of the CFTR pore for anions over cations.
DISCUSSION
Previous work has suggested that positively charged amino acid side chains are involved in attracting negatively charged Cl− ions into a narrow, central region of the CFTR pore where anion selectivity and conductance are predominantly determined (Linsdell, 2006). The fixed positive charges involved in these effects are associated with R334 in TM6 (which attracts extracellular Cl− ions into the pore; Smith et al., 2001) and K95 in TM1 (which exerts a similar attractive influence over intracellular Cl− ions; Linsdell, 2005). However, both of these residues are thought to be located in the mid-to-outer part of the CFTR pore (Ge et al., 2004). Our present results suggest that the intracellular mouth of the CFTR pore is also decorated with positive charges, R303 at the cytoplasmic end of TM5 and perhaps R352 similarly situated in TM6, that act by a surface charge mechanism to attract intracellular Cl− ions into the pore.
Classical surface charge effects are thought to result from fixed charges located near the mouths of ion channel pores that act to attract counterions from the bulk solution, increasing their effective concentration close to the pore mouth and thereby ensuring a ready supply of permeating ions to enter the pore (Green and Andersen, 1991). There are several lines of evidence that this is precisely the mechanism by which R303 and R352 act to influence the functional properties of the CFTR pore. Mutagenesis of either of these positively charged residues reduces unitary currents carried by Cl− efflux (Figs. 4 and 5) in a manner that depends on the charge of the substituted amino acid (Fig. 4 E), consistent with an electrostatic effect. The shape of both the macroscopic (Fig. 2 and Fig. 3 A) and single channel (Fig. 5 B) current–voltage relationships indicate that currents carried by Cl− influx are much less affected by mutagenesis of these residues, consistent with a predominant influence on entry of Cl− ions from the intracellular solution into the pore. Furthermore, the effects of mutagenesis at these sites can be partially overcome by increasing Cl− concentration and are exaggerated at decreased Cl− concentration (Fig. 3 B and Fig. 6), consistent with the predominant effect of the positive charges associated with these residues being to attract Cl− ions electrostatically to the pore.
Perhaps the most direct evidence that R303 contributes directly to a surface charge at the intracellular mouth of the pore is the finding that the shape of the macroscopic current–voltage relationship can be altered by modification of the charge at this position in situ by reaction with negatively or positively charged MTS reagents (Figs. 7–9 ). This demonstrates that the amino acid side chain at this position is accessible to hydrophilic substances in the intracellular solution, and that alteration of the charge at this position has immediate effects on channel permeation properties. Unfortunately, we were unable to confirm the accessibility of the side chain at position 352 in the same manner due to poor expression of the R352C mutant, and as a result it remains uncertain whether this charged amino acid contributes directly to pore surface charge. Qualitatively the effects of mutagenesis at R352 that we have investigated appear similar to those of mutagenesis at R303 (see above), although we note that the effects of mutagenesis at R303 appear more strongly consistent with a pure surface charge effect. Thus, the effects of mutagenesis on both I-V relationship rectification (Fig. 3 A) and unitary conductance (Fig. 4 E) are more strongly dependent on the side chain charge at position 303 than at position 352. Furthermore, rectification in R303Q and R303E appeared more sensitive to the intracellular Cl− concentration than in R352Q and R352E (Fig. 3 B). One possibility is that this difference reflects the surface charge of R303 being closer to the intracellular mouth of the pore than R352, where it could exert a greater electrostatic influence over intracellular Cl− ions entering the pore. Alternatively, mutagenesis of R352 could alter the inner pore architecture, indirectly altering the influence of other residues that form surface charges, for example, R303. We observed poor current expression with all R352 mutants we examined, perhaps consistent with a more major effect of mutagenesis on channel architecture. However, if indirect effects do underlie the observed effect of mutagenesis at R352, then this would appear unique to this position, since mutagenesis of other arginine residues found at the cytoplasmic ends of TMs 1, 4, 8, and 11 did not appear to affect Cl− entry into the pore. Thus, the apparently different functions of the six arginines found at the intracellular ends of different TMs suggest that this apparent arrangement of “inner arginines” (Fig. 1) is not a functionally relevant structural motif within the channel.
Anion permeation in the CFTR pore has previously been modeled using a charged vestibule model, in which it was assumed that the narrow region of the pore is flanked by wide, relatively shallow vestibules containing a net positive charge (Smith et al., 2001). According to this model, a reduction in vestibule charge is associated with a decrease in unitary conductance and a change in current–voltage rectification properties, both of which result from a change in the local concentration of Cl− ions within the vestibule. The effects of mutagenesis at R303 and R352 recapitulate both of these effects and are therefore consistent with a reduction in the electrical potential of the pore inner vestibule of the kind described by Smith et al. (2001).
The reduced unitary conductance associated with mutagenesis of R303 and R352 can be overcome, at least in part, by increasing the intracellular Cl− concentration (Fig. 6). Increasing Cl− concentration from 154 to 304 mM had only a minor effect on wild-type conductance (Fig. 6), consistent with a previous report that the [Cl−]–conductance relationship has an apparent K m of ∼40 mM (Tabcharani et al., 1997). Previously it was shown that in the ClC-0 Cl− channel, mutations that removed important surface charges shifted the [Cl−]–conductance relationship to higher Cl− concentrations, whereas mutations in the selectivity filter reduced the maximum conductance (Chen and Chen, 2003). Unfortunately we were unable to obtain single channel recordings at Cl− concentrations higher than 304 mM; however, the dramatic increase in conductance seen in both R303E and R352E when intracellular [Cl−] was increased from 154 to 304 mM (Fig. 6) suggests that the conductance of these mutant channels is far from saturated at this elevated Cl− concentration, consistent with a large increase in K m.
Mutagenesis of R303 and R352 was associated primarily with a decrease in the conductance of currents carried by Cl− efflux, leading to outward rectification of both the macroscopic (Fig. 2) and unitary current–voltage relationships (Fig. 5) under symmetrical ionic conditions. This is explained by a reduction in attractive forces drawing Cl− ions into the pore from the intracellular solution. Nevertheless, unitary currents carried by Cl− influx were also reduced in both R303E and R352E (Fig. 5). The most likely explanation, as predicted by the charged vestibule model, is that the reduction in the electrical potential of the inner vestibule results in a smaller potential drop across the pore, decreasing the driving force on Cl− ions passing through the pore.
Mutagenesis of R134 (in TM2) and R1030 (in TM10), which are apparently located somewhat more centrally within the TMs, in the “central arginines” (Fig. 1), was associated with some changes in functional channel properties. However, the functional changes we observed in R134Q and R1030Q do not appear consistent with loss of an intracellular surface charge. R134Q is associated with outward rectification (Fig. 10, A and B); however, this effect shows a complex Cl− dependence that, unlike that of R303E, R303Q, and R352Q (Fig. 3 B), is not consistent with a simple surface charge effect. Furthermore, R134Q shows an extremely small apparent unitary conductance (Fig. 10 C). Together, these results suggest that mutagenesis of R134 causes a severe disruption of channel properties. R1030Q did not alter macroscopic current rectification (Fig. 10 B) but was associated with a small decrease in unitary conductance (Fig. 10 D), suggesting that while this residue may play a role in determining pore properties, it does not act as an important inner surface charge. Unfortunately we were unable to identify currents associated with R1030E, although this mutant was previously expressed in another mammalian cell line (Anderson et al., 1991); this previous study did not note any changes in pore properties associated with this mutant. It is possible that other arginine residues not identified in the present study contribute surface charges to the intracellular entrance to the pore. These unidentified arginines could, in principle, be located just cytoplasmic to the TM regions, or in intracellular loops of the CFTR protein that may form part of the inner pore mouth (Seibert et al., 1996).
Simple alignment of the transmembrane regions of CFTR suggests that R303 and R352 are situated at the very cytoplasmic ends of TM5 and TM6, respectively (Fig. 1). While these theoretical alignments should certainly be interpreted with a great deal of caution, these residues appear to be situated considerably more cytoplasmically than the putative narrow pore region (Ge et al., 2004) that is flanked by other positively charged residues (K95 in TM1 and R334 in TM6). The CFTR channel pore is thought to have a wide inner vestibule and to be narrower near the extracellular end, this narrow region being the site at which anion selectivity is determined (Linsdell, 2006). While the dimensions of the inner mouth of the pore are not known, current models suggest the pore will be relatively wide at its intracellular end (Linsdell, 2006); this is consistent with the ability of large organic blockers being able to penetrate as far as K95 when present in the cytoplasmic solution (Linsdell, 2005). Positive surface charges, including those donated by R303 and perhaps R352, may therefore be important in ensuring a sufficiently high local concentration of Cl− ions in this wide intracellular vestibule to allow a ready supply of Cl− ions for the narrow pore region and consequently rapid Cl− permeation through the pore.
The importance of R303 in attracting Cl− ions into the intracellular end of the CFTR pore, as well as the accessibility of the amino acid side chain at this position to intracellular hydrophilic substances, suggests that the intracellular end of TM5 contributes to the cytoplasmic mouth of the pore. Previously we suggested that TMs 1 and 6 play dominant roles in determining the properties of the outer part of the CFTR pore that appears to form its narrowest region (Ge et al., 2004). We suggested that TM5 plays only a minor role in determining the properties of the narrow pore region (Ge et al., 2004), although others have suggested and/or predicted a pore-forming role for TM5 (Gallet et al., 1998; Mansoura et al., 1998; McCarty, 2000). Thus when the full length of the pore is considered, TMs 1, 5, and 6 all appear to play important roles in interacting with permeating Cl− ions to determine the permeation phenotype, with the relative importance of different TMs perhaps changing as Cl− ions pass along the axis of the pore.
Acknowledgments
We thank Kellie Davis, Jeremy Roy, and Elizabeth VandenBerg for technical assistance.
This work was supported by the Canadian Institutes of Health Research.
Olaf S. Andersen served as editor.
C.N. St. Aubin's present address is Department of Pharmacology, University of Alberta, Edmonton, Alberta, Canada.
Abbreviations used in this paper: CFTR, cystic fibrosis transmembrane conductance regulator; MTS, methanethiosulfonate; MTSES, sodium (2-sulfonatoethyl) methanethiosulfonate; MTSET, [2-(trimethylammonium)ethyl] methanethiosulfonate bromide; PPi, pyrophosphate; TM, transmembrane.
References
- Anderson, M.P., R.J. Gregory, S. Thompson, D.W. Souza, S. Paul, R.C. Mulligan, A.E. Smith, and M.J. Welsh. 1991. Demonstration that CFTR is a chloride channel by alteration of its anion selectivity. Science. 253:202–205. [DOI] [PubMed] [Google Scholar]
- Brelidze, T.I., X. Niu, and K.L. Magleby. 2003. A ring of eight conserved negatively charged amino acids doubles the conductance of BK channels and prevents inward rectification. Proc. Natl. Acad. Sci. USA. 100:9017–9022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai, Z., T.S. Scott-Ward, and D.N. Sheppard. 2003. Voltage-dependent gating of the cystic fibrosis transmembrane conductance regulator Cl− channel. J. Gen. Physiol. 122:605–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, M.-F., and T.-Y. Chen. 2003. Side-chain charge effects and conductance determinants in the pore of ClC-0 chloride channels. J. Gen. Physiol. 122:133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung, M., and M.H. Akabas. 1997. Locating the anion-selectivity filter of the cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel. J. Gen. Physiol. 109:289–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotten, J.F., and M.J. Welsh. 1999. Cystic fibrosis-associated mutations at arginine 347 alter the pore architecture of CFTR. Evidence for disruption of a salt bridge. J. Biol. Chem. 274:5429–5435. [DOI] [PubMed] [Google Scholar]
- D'Avanzo, N., H.C. Cho, I. Tolokh, R. Pekhletski, I. Tolokh, C. Gray, S. Goldman, and P. Backx. 2005. Conduction through the inward rectifier potassium channel, Kir2.1, is increased by negatively charged extracellular residues. J. Gen. Physiol. 125:493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson, D.C., S.S. Smith, and M.K. Mansoura. 1999. CFTR: mechanism of anion conduction. Physiol. Rev. 79:S47–S75. [DOI] [PubMed] [Google Scholar]
- Gallet, X., F. Festy, P. Ducarme, R. Brasseur, and A. Thomas-Soumarmon. 1998. Topological model of membrane domain of the cystic fibrosis transmembrane conductance regulator. J. Mol. Graph. Model. 16:72–82. [DOI] [PubMed] [Google Scholar]
- Ge, N., C.N. Muise, X. Gong, and P. Linsdell. 2004. Direct comparison of the functional roles played by different transmembrane regions in the cystic fibrosis transmembrane conductance regulator chloride channel pore. J. Biol. Chem. 279:55283–55289. [DOI] [PubMed] [Google Scholar]
- Gong, X., and P. Linsdell. 2003. a. Mutation-induced blocker permeability and multiion block of the CFTR chloride channel pore. J. Gen. Physiol. 122:673–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong, X., and P. Linsdell. 2003. b. Molecular determinants and role of an anion binding site in the external mouth of the CFTR chloride channel pore. J. Physiol. 549:387–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
-
Gong, X., S.M. Burbridge, E.A. Cowley, and P. Linsdell. 2002. Molecular determinants of
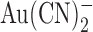 binding and permeability within the cystic fibrosis transmembrane conductance regulator Cl− channel pore. J. Physiol.
540:39–47.
[DOI] [PMC free article] [PubMed] [Google Scholar]
binding and permeability within the cystic fibrosis transmembrane conductance regulator Cl− channel pore. J. Physiol.
540:39–47.
[DOI] [PMC free article] [PubMed] [Google Scholar] - Green, W.N., and O.S. Andersen. 1991. Surface charges and ion channel function. Annu. Rev. Physiol. 53:341–359. [DOI] [PubMed] [Google Scholar]
- Guinamard, R., and M.H. Akabas. 1999. Arg352 is a major determinant of charge selectivity in the cystic fibrosis transmembrane conductance regulator chloride channel. Biochemistry. 38:5528–5537. [DOI] [PubMed] [Google Scholar]
- Haug, T., D. Sigg, S. Ciani, L. Toro, E. Stefani, and R. Olcese. 2004. Regulation of K+ flow by a ring of negative charges in the outer pore of BKCa channels. Part I: aspartate 292 modulates K+ conduction by external surface charge effect. J. Gen. Physiol. 124:173–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imoto, K., C. Busch, B. Sakmann, M. Mishina, T. Konno, J. Nakai, H. Bujo, Y. Mori, K. Fukuda, and S. Numa. 1988. Rings of negatively charged amino acids determine the acetylcholine receptor channel conductance. Nature. 335:645–648. [DOI] [PubMed] [Google Scholar]
- Linsdell, P. 2005. Location of a common inhibitor binding site in the cytoplasmic vestibule of the cystic fibrosis transmembrane conductance regulator chloride channel pore. J. Biol. Chem. 280:8945–8950. [DOI] [PubMed] [Google Scholar]
- Linsdell, P. 2006. Mechanism of chloride permeation in the cystic fibrosis transmembrane conductance regulator chloride channel. Exp. Physiol. 91:123–129. [DOI] [PubMed] [Google Scholar]
- Linsdell, P., and J.W. Hanrahan. 1996. Disulphonic stilbene block of cystic fibrosis transmembrane conductance regulator Cl− channels expressed in a mammalian cell line and its regulation by a critical pore residue. J. Physiol. 496:687–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsdell, P., and J.W. Hanrahan. 1998. Adenosine triphosphate-dependent asymmetry of anion permeation in the cystic fibrosis transmembrane conductance regulator chloride channel. J. Gen. Physiol. 111:601–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon, R., R. Latorre, and C. Miller. 1989. Role of surface electrostatics in the operation of a high-conductance Ca2+-activated K+ channel. Biochemistry. 28:8092–8099. [DOI] [PubMed] [Google Scholar]
- Mansoura, M.K., S.S. Smith, A.D. Choi, N.W. Richards, T.V. Strong, M.L. Drumm, F.S. Collins, and D.C. Dawson. 1998. Cystic fibrosis transmembrane conductance regulator (CFTR) anion binding as a probe of the pore. Biophys. J. 74:1320–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty, N.A. 2000. Permeation through the CFTR chloride channel. J. Exp. Biol. 203:1947–1962. [DOI] [PubMed] [Google Scholar]
- Moorhouse, A.J., A. Keramidas, A. Zaykin, P.R. Schofield, and P.H. Barry. 2002. Single channel analysis of conductance and rectification in cation-selective, mutant glycine receptor channels. J. Gen. Physiol. 119:411–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimigean, C.M., J.S. Chappie, and C. Miller. 2003. Electrostatic tuning of ion conductance in potassium channels. Biochemistry. 42:9263–9268. [DOI] [PubMed] [Google Scholar]
- Riordan, J.R., J.M. Rommens, B. Kerem, A. Alon, R. Rozmahel, Z. Grzelczak, J. Zielenski, S. Lok, N. Plasvik, J.-L. Chou, et al. 1989. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 245:1066–1073. [DOI] [PubMed] [Google Scholar]
- Seibert, F.S., P. Linsdell, T.W. Loo, J.W. Hanrahan, J.R. Riordan, and D.M. Clarke. 1996. Cytoplasmic loop three of cystic fibrosis transmembrane conductance regulator contributes to regulation of chloride channel activity. J. Biol. Chem. 271:27493–27499. [DOI] [PubMed] [Google Scholar]
- Smith, S.S., X. Liu, Z.-R. Zhang, F. Sun, T.E. Kriewall, N.A. McCarty, and D.C. Dawson. 2001. CFTR: covalent and noncovalent modification suggests a role for fixed charges in anion conduction. J. Gen. Physiol. 118:407–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabcharani, J.A., P. Linsdell, and J.W. Hanrahan. 1997. Halide permeation in wild-type and mutant cystic fibrosis transmembrane conductance regulator chloride channels. J. Gen. Physiol. 110:341–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y., L. Xu, D.A. Pasek, D. Gillespie, and G. Meissner. 2005. Probing the role of negatively charged amino acid residues in ion permeation of skeletal muscle ryanodine receptor. Biophys. J. 89:256–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worley, J.F., R.J. French, and B.K. Krueger. 1986. Trimethyloxonium modification of single batrachotoxin-activated sodium channels in planar bilayers. Changes in unit conductance and in block by saxitoxin and calcium. J. Gen. Physiol. 87:327–349. [DOI] [PMC free article] [PubMed] [Google Scholar]