Abstract
HCN pacemaker channels (If, Iq, or Ih) play a fundamental role in the physiology of many excitable cell types, including cardiac myocytes and central neurons. While cloned HCN channels have been studied extensively in macroscopic patch clamp experiments, their extremely small conductance has precluded single channel analysis to date. Nevertheless, there remain fundamental questions about HCN gating that can be resolved only at the single channel level. Here we present the first detailed single channel study of cloned mammalian HCN2. Excised patch clamp recordings revealed discrete hyperpolarization-activated, cAMP-sensitive channel openings with amplitudes of 150–230 fA in the activation voltage range. The average conductance of these openings was ∼1.5 pS at −120 mV in symmetrical 160 mM K+. Some traces with multiple channels showed unusual gating behavior, characterized by a variable long delay after a voltage step followed by runs of openings. Noise analysis on macroscopic currents revealed fluctuations whose magnitudes were systematically larger than predicted from the actual single channel current size, consistent with cooperativity between single HCN channels.
INTRODUCTION
Originally identified for their role in the generation of cardiac sinus rhythm (Brown et al., 1979), HCN channels (also called If, Iq, or Ih channels) are involved integrally in the physiology of many excitable cell types (for review see Robinson and Siegelbaum, 2003). Pioneering work by DiFrancesco produced the first single channel recordings of If in a native sino-atrial node preparation (DiFrancesco, 1986; DiFrancesco and Mangoni, 1994). This work revealed a remarkably small single channel conductance of ∼1 pS, among the smallest known for voltage-dependent cation channels. More recently, nonstationary fluctuation analysis has estimated the conductance of cloned HCN2 to be ∼2.5 pS (Johnson and Zagotta, 2005), and that of channels underlying Ih in neuronal dendrites to be ∼0.7 pS (Kole et al., 2006). Although this very small conductance has prevented single channel recordings of the cloned members of the HCN family to date (see Discussion), such experiments would contribute fundamentally to our understanding of gating in this important class of ion channel.
Here we describe the first detailed single-channel analysis of cloned HCN2 channels. We found a very small single channel conductance of ∼1.5 pS, which is compatible with studies on native channels but in contrast to an earlier report on cloned HCN2 channels (Michels et al., 2005). The recordings revealed unusual gating behavior that suggested some form of cooperativity between channels. We used two quantitative approaches to ask whether gating was, in fact, nonindependent.
MATERIALS AND METHODS
HEK 293 cells (American Type Culture Collection) were transfected by electroporation as described previously (Shin et al., 2001) with mHCN2 channel DNA. Channels were cotransfected with the πH3-CD8 plasmid, which encodes the α-subunit of the human CD8 lymphocyte antigen, allowing detection of transfected cells with antibody-coated beads (Jurman et al., 1994). All experiments were performed at room temperature on excised inside-out patches held under voltage clamp from identified transfected cells 18–72 h after electroporation. Currents were acquired with a 1 kHz low pass filter and digitized at 5 kHz. Traces were baseline adjusted and digitally refiltered to 0.8–0.3 kHz for analysis. The data in Fig. 1 C and Fig. 2 A were refiltered at 0.5 kHz for display, and all other single channel data were refiltered at 0.3 kHz for display. Capacitance transients were subtracted from the traces in Fig. 1 A, and were partially blanked in Fig. 2 for display purposes. The holding potential for all experiments was +10 mV. Bath and pipette solutions were identical and contained 160 mM KCl, 1 mM MgCl2, 10 mM HEPES, 0.1–1 mM EGTA; pH was adjusted to 7.4 with KOH. Where indicated, cAMP was used internally at the saturating concentration of 1 mM. Conductance was calculated by dividing the single channel current by the electrical driving force. All data are reported as mean ± SEM.
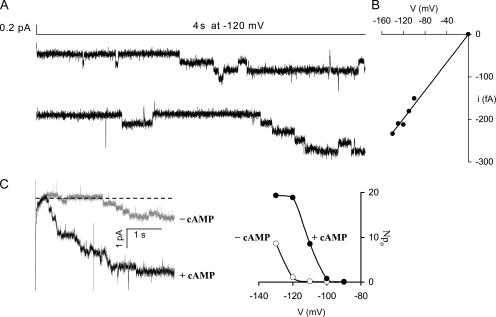
Figure 1.
Basic characterization of single HCN2 channels. (A) Two traces showing multiple openings (downward deflections) in an excised, inside-out patch in response to a voltage step to −120 mV, in the absence of cAMP. Seal resistance was ∼62 GΩ. (B) Single channel current–voltage relationship for openings in a patch with 1 mM cAMP. Linear fit is extrapolated to the origin; fitted slope conductance = 1.65 pS. (C, left) Response of a multichannel patch to a voltage step to −120 mV in presence and absence of 1 mM cAMP. Cyclic AMP increased the activation kinetics and open probability. Dotted line indicates zero current. (C, right) the Npo-V relationship constructed from this same patch in the presence (filled circles) and absence (open circles) of 1 mM cAMP. Channels were activated by hyperpolarization, and 1 mM cAMP shifted the voltage dependence of openings to more positive potentials. These curves are only slightly more left shifted than g-V's from typical excised patch recordings of macroscopic HCN2 currents in 293 cells (midpoints of approximately −115 and −100 mV with and without cAMP), perhaps because of their long excision times.
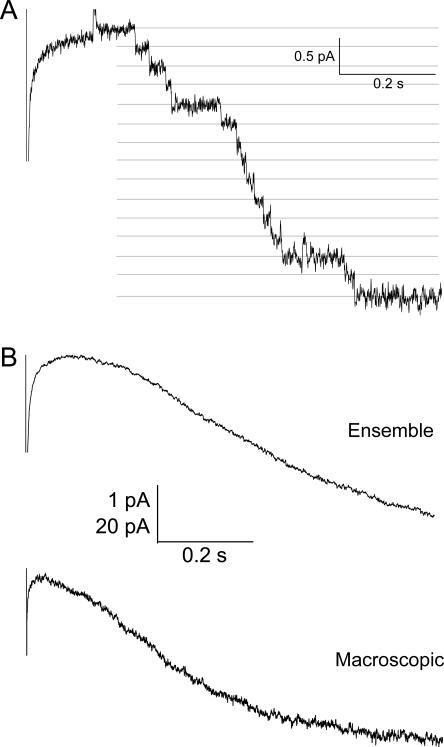
Figure 2.
Ensemble average kinetics for patches with multiple HCN2 channels. (A) Step to −120 mV with 1 mM cAMP in multichannel patch (>20 channels). (B) Ensemble average of traces from the patch in A, compared with macroscopic currents recorded in another patch under same conditions, displayed on same time scale.
For latency analysis, first latency (L1) was defined as the first 0–1 level crossing that persisted for sufficient duration to be reliably distinguished from the noise. We conservatively used 50 ms for this value, which was still short relative to the observed long open state dwell times (often seconds), and captured most of the visible openings. The L12 latency was defined as the time that elapsed between the first opening (as defined above) and the first level 1–2 transition persisting for longer than 50 ms. Macroscopic data for noise analysis were acquired at 1 kHz filtering and 5 kHz sampling, and processed for analysis as described in the text. Simulations were performed in MATLAB using standard time-step simulation methods.
RESULTS
Voltage clamp recordings revealed long-lasting channel openings in excised inside-out membrane patches from HEK293 cells expressing cloned HCN2 (Fig. 1). The observed amplitudes of single openings were very small, yielding currents of 150–230 fA in the activation voltage range (Fig. 1, A and B). The average conductance of these openings at −120 mV was 1.46 ± 0.06 pS (n = 9). To confirm that the observed channels were produced by the expressed HCN2 clone, we compared the cAMP sensitivity, voltage dependence, and ensemble average activation time course of discrete events in multichannel patches with the known properties of HCN2 macroscopic currents. The effect of cAMP on HCN2 macroscopic currents is to speed the activation kinetics and increase the open probability (Ludwig et al., 1998). We found that cAMP similarly increased the activation kinetics and open probability in multichannel patches with distinguishable events (Fig. 1 C). The resolvable long openings were tightly gated by hyperpolarization, and cAMP shifted the voltage dependence of these openings to more positive potentials, comparable with macroscopic effects. The ensemble average activation time courses constructed from multichannel patches with distinguishable events were commensurate with macroscopic kinetics (Fig. 2). Together, these measures confirm that the identified openings were produced by channels from the HCN2 clone, and that the single channels had the average properties expected from the behavior of macroscopic currents.
Unusual Gating of Single Channels
During the course of our initial experiments, we were struck by some very unusual features of gating in patches with multiple channels. In response to a voltage step, recordings showed variable (sometimes long) delays with no channel openings followed by multiple openings that appeared highly correlated in time (Fig. 3). These types of events would appear to be statistically improbable if individual channel proteins operated independently, a common assumption on which the quantitative analysis of macroscopic currents is based. Rather, the qualitative behavior seemed to imply the possibility of some form of communication among channels, so that the opening of one channel directly influenced the probability that its neighbors would open.
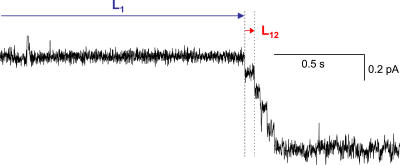
Figure 3.
Unusual gating behavior. Response of a multichannel patch to a voltage step to −120 mV in 0 cAMP. In this example, there are no resolvable openings for ∼1.3 s after the voltage jump, followed abruptly by five openings in the following 250 ms. Seal resistance was ∼80 GΩ. The definitions of L1 and L12 used in the latency analysis are shown in this example (see text).
Cooperativity Is Suggested by Fluctuation Analysis of Many-Channel Currents
We asked whether we could detect a macroscopic manifestation of this possible microscopic cooperativity. Previous theoretical work has demonstrated that cooperative gating of ion channels can produce anomalously large stochastic fluctuations in macroscopic currents (Sigworth, 1980; Liu and Dilger, 1993). For channels that gate in a strictly independent manner, the amplitude of the elementary macroscopic fluctuation inferred from the mean–variance relationship (ifluct) should be equal to the single channel current (itrue). Cooperative gating, however, can produce an elementary fluctuation larger than the single channel current (ifluct > itrue). This can be appreciated by considering the extreme case of a population of channels whose gating is coupled strictly in pairs, such that gating partners always open and close simultaneously. In this case, it is apparent that the elementary noise fluctuation will be twice the single channel current (ifluct = 2 itrue).
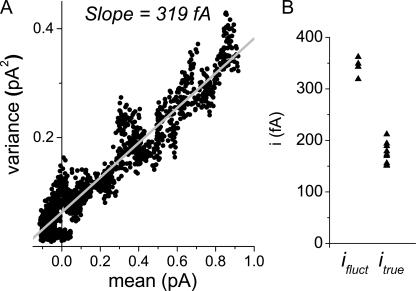
We therefore attempted to detect excess fluctuations in macroscopic currents using nonstationary fluctuation analysis (NSFA). This technique, which quantifies time-dependent channel gating fluctuations after a voltage step, was chosen because simple time-homogeneous excess noise from other sources does not affect the determination of ifluct. Gating-independent noise was minimized by baseline-adjusting traces to correct for changes in leak, and traces with evidence of seal degradation were manually removed. Additionally, mean–variance relationships were constructed by calculating variance from all pairs of successive traces (except those removed due to seal degradation) to minimize the effects of kinetic rundown (Heinemann and Conti, 1992). We calculated ifluct from the initial slope of the mean–variance relationship and compared it with itrue, the directly observed single channel current measured under the same conditions (Fig. 4). The mean ifluct was 343 ± 9 fA (n = 4), corresponding to a conductance of 2.9 pS; the mean itrue was 175 ± 2 fA (n = 9), corresponding to a conductance of 1.5 pS. This measured twofold larger elementary fluctuation compared with the single channel current is consistent with the hypothesis of substantial cooperative gating of channels. Although kinetic rundown or channel loss can lead to an overestimation of the elementary fluctuation event, Monte-Carlo simulations showed that the amount of actual rundown seen in the experiments would produce only a small difference in the fluctuation estimates (unpublished data).
Figure 4.
Nonstationary fluctuation analysis systematically overestimates the single channel conductance. (A) Example of ifluct determination. Mean–variance relationships were constructed from the response of macroscopic currents to repeated steps to −120 mV in 1 mM cAMP. The best linear fit to the initial 10–15% of the mean variance was used for the initial slope. (B) Plot of summary data of ifluct and itrue. Mean ifluct = 343 ± 9 fA (n = 4), conductance = 2.86 pS; mean itrue = 175 ± 2 (n = 9), conductance = 1.46 pS.
Correlated Latencies to Channel Opening
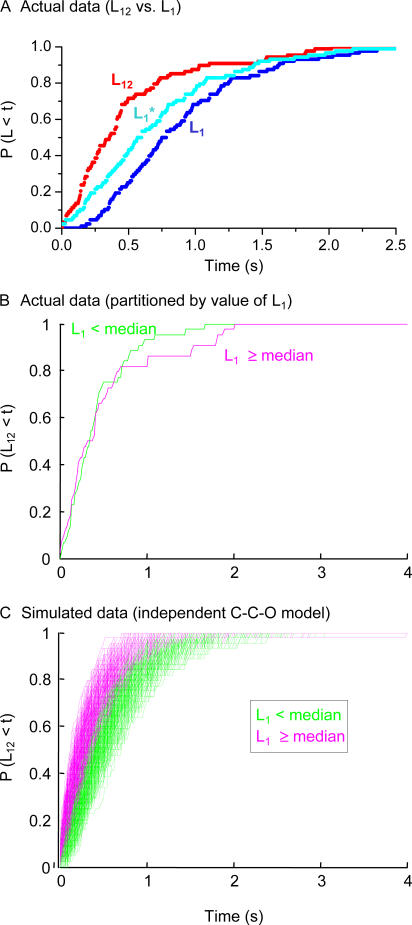
Our qualitative impression from the single channel records was that there was variability in the time to the first opening in a multichannel patch, but that once the first channel opened, the second opening followed quickly. To quantify this tendency, we measured the distribution of first latency times (L1) and the distribution of latencies between first and second openings (L12, as defined in Fig. 3; also see Materials and methods). As expected from the appearance of traces like that in Fig. 3, we found that the average L12 was markedly faster than L1 (Fig. 5 A).
Figure 5.
Fast second openings (short L12) are independent of first latency for the actual data, but not for an independent C-C-O model. (A) Experimental L1 and L12 cumulative distribution measured from 176 latencies in a patch (steps to −120 mV, 0 cAMP). L1* distribution is the L1 distribution shifted by an absolute delay. (B) The L12 distribution for each half of the actual data from the largest dataset, split according to the first latency. (C) The same for a simulated independent model; short first latencies systematically give rise to longer second latencies. These data are for 1,000 simulated datasets of the same size as the actual dataset (88 traces), with 20 independent channels with forward rate constants of 0.4 s−1 for both steps (producing a reasonable approximation to the actual data). Only 2% of the simulated datasets had as small a vertical distance (Kolmogorov-Smirnov criterion) between the two cumulative distributions as the actual dataset (and the largest difference for the actual dataset was in the opposite direction).
Macroscopic Ih currents show a pronounced sigmoid delay in channel opening (for review see Robinson and Siegelbaum, 2003), and this can be seen here as a delay in the L1 distribution. Such a delay is generally explained by supposing that a large number of closed states must be traversed before opening (Cole and Moore, 1960). This type of process would produce a delay in the first latencies, but because all of the channels are simultaneously traversing these hidden closed states, it will not produce a comparable waiting time between the first and second openings. At the time when the first channel opens, many of the remaining closed channels will have completed their passage through the delay states and will be ready to traverse the final opening step.
To the extent that the delay consists of a very large number of states and is nearly identical for all channels, this will simply produce a constant additive offset to the first latency distribution that will be absent from the L12 distribution. In our L1 data, there is a clear delay of ∼200 ms before any channels open. The curve marked L1* shows the original L1 distribution shifted by the absolute delay, for comparison with L12. The L12 distribution not only lacks this delay, it remains faster even than the shifted L1* distribution, consistent with positive cooperativity. Experimentally, the ratio of time constants for monoexponential fits for L12/L1* was 0.70 in one patch (n = 176 latencies) and 0.64 in another (n = 60 latencies). Such behavior could not be produced by a model with a fixed deterministic delay. But if the delay is not quite so distinct from the body of the first latencies, then it can, using a simulated multiple closed state model, give first latencies that are slower than L12 even in the absence of channel cooperativity.
This means that a simple comparison of the L12 vs. L1 distribution is not adequate to distinguish cooperative from independent models. But we did find a secondary property of the latencies that can in principle distinguish the two types of models. For a range of independent models we have examined, there is a substantial correlation between the L12 and L1 observed in the same trace: a short first latency is typically followed by a long L12, and a long first latency by a short L12. This is because the shorter L12s are produced by channels that accumulate in the later closed states, closer to the open state, and the later the first latency, the more likely it is to find a channel in these more advanced closed states. On the other hand, the cooperative models we have examined that fit the L1 and L12 distributions do not produce such a correlation, because a short L12 is a direct consequence of the first channel's having opened, whenever it opens.
For the dataset analyzed in Fig. 5 A there is hardly any correlation; specifically, if we compare the distribution of L12 values for traces with L1 below the median value for L1 to the distribution of L12 values for traces with L1 above the median, there is hardly any difference in the two (Fig. 5 B). This is in marked contrast to simulations done for the same size dataset with an independent model (three state C-C-O model; Fig. 5 C); only ∼2% of the simulated independent datasets of the same size produce as little correlation of L12 with L1 as seen in the actual dataset. On the other hand, a substantial fraction of the simulated cooperative datasets (∼25%) for this single C-C-O model do show comparably little difference for L12 categorized by short vs. long L1. Thus, for this dataset (the only one large enough to permit a reliable analysis), it is possible to exclude a particular, simple independent model with high confidence, but given the large universe of such models, it is difficult to use latency analysis to confirm the presence of cooperativity without much more extensive datasets.
DISCUSSION
We have presented the first detailed single channel analysis of cloned HCN2 channels. We confirmed the identity of the observed single channels by comparing their voltage dependence, cAMP dependence, and activation kinetics with the known properties of HCN2 macroscopic currents. In the absence of cAMP, the observable openings had slow kinetics and long average open durations (>1 s), as expected from the slow kinetics of macroscopic currents in the absence of cAMP. These long openings were tightly gated by hyperpolarization, and long openings were not observed outside of the negative activation voltage range. The effect of cAMP was to speed the activation kinetics of individual openings and to shift their voltage dependence to more positive potentials. To confirm that average single channel kinetics in the presence of cAMP accorded with the kinetics of macroscopic currents, we constructed ensemble average activation time courses from patches with small numbers of channels. These ensemble averages closely resembled macroscopic currents, with their characteristic sigmoidal delay.
The mean conductance of the observed openings was very small, ∼1.5 pS. This value is comparable with that reported for single native If channels (DiFrancesco, 1986; DiFrancesco and Mangoni, 1994), and with noise analysis estimates for cloned HCN2 (Johnson and Zagotta, 2005), and for Ih in neuronal dendrites (Kole et al., 2006). The values that we observe in symmetric 160 mM K+ are expected to be somewhat larger than those reported for native channels because of cation dependence of the conductance (Moroni et al., 2000). A recent report describes single channel recordings from cells transfected with HCN channel clones (Michels et al., 2005). The single channel openings described by Michels et al. display a very large conductance (∼35 pS for cells transfected with HCN2) and essentially no time-dependent gating after a voltage step, properties that are inconsistent with those expected from macroscopic experiments, and with results of single channel recordings on native If (DiFrancesco, 2005). In contrast, the much smaller channel openings we observed displayed the average properties expected from macroscopic currents produced by the HCN2 clone, and have a single channel conductance consistent with previous estimates. These small conductance channels clearly can account for the voltage-dependent Ih/If currents seen in macroscopic recordings.
Evidence for Nonindependent Gating
Our initial experiments revealed unusual features of gating in patches with multiple channels. This behavior was characterized by variable delays (with no openings) after a voltage jump, followed by runs of apparently correlated openings, features that seemed to indicate some form of unexpected cooperativity between channels.
This apparent gating cooperativity was evident in the amplitude of stochastic fluctuations present in macroscopic currents. Previous theoretical work has shown that cooperativity can result in an elementary fluctuation ifluct in macroscopic currents that is larger than the single channel current itrue. We determined ifluct from the initial slope of the mean–variance relationship using NSFA and compared it with itrue measured directly, under the same conditions. The mean elementary fluctuation estimated from NSFA was approximately twofold larger than the actual single channel current, suggesting that single channels gate cooperatively. Additionally, we note that our NSFA estimate of ∼2.9 pS for mHCN2 channels expressed in mammalian HEK 293 cells is consistent with a previous study reporting an NSFA estimate of ∼2.5 pS for the same channels expressed in amphibian Xenopus oocytes (Johnson and Zagotta, 2005).
A quantitative analysis of the microscopic kinetics of openings in these patches revealed that the relative first and second latency distributions could be produced by a variety of models, involving either cooperative or independent gating. However, the absence of a substantial correlation between L1 and L12 appears to arise only for the cooperative models, and even for those models only in a regime where channels that are neighbors to an already open channel constitute a substantial fraction of the second openings. We were unable to collect more than one dataset large enough to perform this analysis, so the conclusion of cooperativity between HCN channels rests mainly on the results of the fluctuation analysis.
The analysis performed in this study suggests that HCN channels gate cooperatively. How unique is this phenomenon? Although independence in gating is often implicitly assumed in the analysis of macroscopic ion channel kinetics and noise fluctuations, several examples of cooperative gating phenomena have been described at the single channel level. Pore forming peptides such as alamethicin (Huang, 2006) as well as ligand-gated channels including nicotinic acetylcholine receptors (Keleshian et al., 1994, 2000) and P2X receptors (Ding and Sachs, 2002) have all been shown to gate nonindependently under certain conditions. Perhaps more relevant to the current study, KcsA, a member of the potassium channel superfamily containing HCN2, has recently been shown to demonstrate cooperative gating modes when expressed at high membrane concentrations (Molina et al., 2006). Finally, a recent analysis of macroscopic action potentials in cortical neurons found evidence suggesting cooperative interactions between voltage-gated sodium channels (Naundorf et al., 2006). Although these latter results have yet to be confirmed at the single channel level, it suggests that cooperativity between voltage-gated channels may play physiologic roles in vivo.
Possible Physical Mechanisms of the Coupling
The high correlation between channel openings might be produced through several physical mechanisms. One possibility is that the correlated channel openings are actually multiple equally spaced subconductance levels produced by a single channel protein. The observed cooperativity would then reflect intersubunit cooperativity rather than interprotein cooperativity. While it is almost impossible to rule out such a model completely, we think it is unlikely because a few patches exhibited only a single open conductance level, apparently produced by a single channel. Gating to a single open conductance level was also seen when multichannel patches were held hyperpolarized for minutes, producing inactivation or rundown of channels that ultimately led to apparent single channel gating lasting for long periods of time (minutes).
If the observed coupled openings indeed represent cooperative gating of multiple channel proteins, what is the physical mechanism coupling the channels? A trivial explanation is that correlated channel gating results from common sensing of a fluctuating exogenous messenger, such as cAMP, PIP2, or calcium. However, since our experiments were performed in excised, cell-free patches, such a signaling system would have to be contained entirely within the membrane patch and remain operative after excision into bulk solution, which seems unlikely. The fact that we observed cooperativity in both zero cAMP and saturating 1 mM cAMP in well-perfused patches rules out a mechanism involving fluctuating (subsaturating) cAMP concentrations. Finally, the fact that the experimental solutions were symmetric, calcium free, and contained 100–1,000 μM EGTA rules out any mechanism involving voltage-driven influx of calcium or another ion through the channels.
Another possible cause of cooperative opening of ion channels is voltage-dependent membrane stretch, which can coordinately activate channels whose open probability is influenced by membrane tension. Magleby and colleagues found that mechanosensitive channels could be activated in an apparent cooperative fashion with voltage in excised membrane patches from Xenopus oocytes (Silberberg and Magleby, 1997; Gil et al., 1999a,b). Video microscopy revealed that this was due, surprisingly, to voltage-dependent movement of the patch membrane in the pipette, apparently resulting in an abrupt change in membrane tension. This study found that such cooperative activation did not occur in whole-cell recording configurations. Recent work has suggested that HCN channels can be activated by membrane stretch (Lin, W., U. Laitko, P.F. Juranka, and C.E. Morris. 2006. Biophysical Society Abstracts. 1544-Plat), and so it is reasonable to ask whether such a mechanism could account for the unusual single channel gating we observed.
We think such an explanation is unlikely for a couple of reasons. First, ensemble average kinetics constructed from single channel events are similar to macroscopic kinetics. Thus, if voltage-induced membrane stretch is the cause of the unusual single channel gating, then it must also play a dominant role in determining macroscopic HCN channel gating kinetics as well, which seems unlikely. Second, comparison of macroscopic kinetics demonstrated no significant difference between cell-attached, excised inside-out, and whole-cell recording configurations. Voltage-dependent membrane stretch activation of macroscopic currents would thus have to be operative in each of these recording configurations, in contrast to the type of behavior seen by Magleby and colleagues for mechanosensitive channels.
A simpler hypothesis is that channels are in direct allosteric communication with one another. Further experiments will be required to determine how this allosteric communication is accomplished. Obvious possibilities include a protein–protein interface between channels, or communication mediated by an adaptor protein. Alternatively, the gating state of one channel might be transmitted to its neighbors through allosteric changes in a linking cytoskeleton. A more radical possibility is that clustered HCN2 channels form interchannel dimers or tetramers by “swapping” their cAMP domains. Any of these possibilities would be compatible with evidence indicating colocalization of HCN channels in lipid microdomains (Barbuti et al., 2004).
Summary
Our single channel analysis of HCN2 channels suggests the presence of interchannel cooperativity. Cooperativity of this sort will have to be taken into account in the interpretation of macroscopic current experiments and argues for caution in inferring single channel properties from noise analysis. It may also have to be incorporated into formal Ih gating models for accurate simulations of cellular physiology. There are many potentially interesting physiologic consequences of gating nonindependence. It is possible that gating cooperativity may contribute to voltage noise in high-impedance cortical dendrites, as has been recently studied (Kole et al., 2006). Additionally, interchannel cooperativity may turn out to be related to the unusually long first latencies and remarkably slow macroscopic kinetics of HCN channels, both of which are of fundamental significance to their physiologic operation in diverse contexts.
Acknowledgments
We thank members of the Yellen lab, particularly Dr. David Prole, for helpful discussions and comments. We are grateful to Tatiana Abramson for her expert technical assistance for transfections, and to Dr. Michael Sanguinetti (University of Utah, Salt Lake City, UT) for supplying the mHCN2 clone.
This work was supported by a grant from the National Institutes of Health/National Heart, Lung, and Blood Institute (HL70320 to G. Yellen).
David C. Gadsby served as editor.
Abbreviation used in this paper: NSFA, nonstationary fluctuation analysis.
References
- Barbuti, A., B. Gravante, M. Riolfo, R. Milanesi, B. Terragni, and D. DiFrancesco. 2004. Localization of pacemaker channels in lipid rafts regulates channel kinetics. Circ. Res. 94:1325–1331. [DOI] [PubMed] [Google Scholar]
- Brown, H.F., D. DiFrancesco, and S.J. Noble. 1979. How does adrenaline accelerate the heart? Nature. 280:235–236. [DOI] [PubMed] [Google Scholar]
- Cole, K.S., and J.W. Moore. 1960. Potassium ion current in the squid giant axon: dynamic characteristic. Biophys. J. 1:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco, D. 1986. Characterization of single pacemaker channels in cardiac sino-atrial node cells. Nature. 324:470–473. [DOI] [PubMed] [Google Scholar]
- DiFrancesco, D. 2005. Letter regarding article by Michels et al, “Single-channel properties support a potential contribution of hyperpolarization-activated cyclic nucleotide-gated channels and If to cardiac arrhythmias”. Circulation. 112:e72–e73. [DOI] [PubMed] [Google Scholar]
- DiFrancesco, D., and M. Mangoni. 1994. Modulation of single hyperpolarization-activated channels (i(f)) by cAMP in the rabbit sino-atrial node. J. Physiol. 474:473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, S., and F. Sachs. 2002. Evidence for non-independent gating of P2X2 receptors expressed in Xenopus oocytes. BMC Neurosci. 3:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil, Z., S.D. Silberberg, and K.L. Magleby. 1999. a. Membrane-pipette interactions underlie delayed voltage activation of mechanosensitive channels in Xenopus oocytes. Biophys. J. 76:3118–3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil, Z., S.D. Silberberg, and K.L. Magleby. 1999. b. Voltage-induced membrane displacement in patch pipettes activates mechanosensitive channels. Proc. Natl. Acad. Sci. USA. 96:14594–14599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemann, S.H., and F. Conti. 1992. Recording of gating currents from Xenopus oocytes and gating noise analysis. Methods Enzymol. 207:131–148. [DOI] [PubMed] [Google Scholar]
- Huang, H.W. 2006. Molecular mechanism of antimicrobial peptides: the origin of cooperativity. Biochim. Biophys. Acta. 10.1016/j.bbamem.2006.02.001 [DOI] [PubMed]
- Johnson, J.P., Jr., and W.N. Zagotta. 2005. The carboxyl-terminal region of cyclic nucleotide- modulated channels is a gating ring, not a permeation path. Proc. Natl. Acad. Sci. USA. 102:2742–2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurman, M.E., L.M. Boland, Y. Liu, and G. Yellen. 1994. Visual identification of individual transfected cells for electrophysiology using antibody-coated beads. Biotechniques. 17:876–881. [PubMed] [Google Scholar]
- Keleshian, A.M., G.F. Yeo, R.O. Edeson, and B.W. Madsen. 1994. Superposition properties of interacting ion channels. Biophys. J. 67:634–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keleshian, A.M., R.O. Edeson, G.J. Liu, and B.W. Madsen. 2000. Evidence for cooperativity between nicotinic acetylcholine receptors in patch clamp records. Biophys. J. 78:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kole, M.H., S. Hallermann, and G.J. Stuart. 2006. Single Ih channels in pyramidal neuron dendrites: properties, distribution, and impact on action potential output. J. Neurosci. 26:1677–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y., and J.P. Dilger. 1993. Application of the one- and two-dimensional Ising models to studies of cooperativity between ion channels. Biophys. J. 64:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig, A., X. Zong, M. Jeglitsch, F. Hofmann, and M. Biel. 1998. A family of hyperpolarization-activated mammalian cation channels. Nature. 393:587–591. [DOI] [PubMed] [Google Scholar]
- Michels, G., F. Er, I. Khan, M. Südkamp, S. Herzig, and U.C. Hoppe. 2005. Single-channel properties support a potential contribution of hyperpolarization-activated cyclic nucleotide-gated channels and If to cardiac arrhythmias. Circulation. 111:399–404. [DOI] [PubMed] [Google Scholar]
- Molina, M.L., F.N. Barrera, A.M. Fernandez, J.A. Poveda, M.L. Renart, J.A. Encinar, G. Riquelme, and J.M. Gonzalez-Ros. 2006. Clustering and coupled gating modulate the activity of KcsA, a potassium channel model. J. Biol. Chem. 281:18837–18848. [DOI] [PubMed] [Google Scholar]
- Moroni, A., A. Barbuti, C. Altomare, C. Viscomi, J. Margan, M. Baruscotti, and D. DiFrancesco. 2000. Kinetic and ionic properties of the human HCN2 pacemaker channel. Pflugers Arch. 439:618–626. [DOI] [PubMed] [Google Scholar]
- Naundorf, B., F. Wolf, and M. Volgushev. 2006. Unique features of action potential generation in cortical neurons. Nature. 440:1060–1063. [DOI] [PubMed] [Google Scholar]
- Robinson, R.B., and S.A. Siegelbaum. 2003. Hyperpolarization-activated cation currents: from molecules to physiological function. Annu. Rev. Physiol. 65:453–480. [DOI] [PubMed] [Google Scholar]
- Shin, K.S., B.S. Rothberg, and G. Yellen. 2001. Voltage-controlled gating at the intracellular entrance to a hyperpolarization-activated cation channel. J. Gen. Physiol. 117:91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigworth, F.J. 1980. The conductance of sodium channels under conditions of reduced current at the node of Ranvier. J. Physiol. 307:131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberberg, S.D., and K.L. Magleby. 1997. Voltage-induced slow activation and deactivation of mechanosensitive channels in Xenopus oocytes. J. Physiol. 505:551–569. [DOI] [PMC free article] [PubMed] [Google Scholar]