Abstract
The amyloid hypothesis of Alzheimer's toxicity has undergone a resurgence with increasing evidence that it is not amyloid fibrils but a smaller oligomeric species that produces the deleterious results. In this paper we address the mechanism of this toxicity. Only oligomers increase the conductance of lipid bilayers and patch-clamped mammalian cells, producing almost identical current–voltage curves in both preparations. Oligomers increase the conductance of the bare bilayer, the cation conductance induced by nonactin, and the anion conductance induced by tetraphenyl borate. Negative charge reduces the sensitivity of the membrane to amyloid, but cholesterol has little effect. In contrast, the area compressibility of the lipid has a very large effect. Membranes with a large area compressibility modulus are almost insensitive to amyloid oligomers, but membranes formed from soft, highly compressible lipids are highly susceptible to amyloid oligomer-induced conductance changes. Furthermore, membranes formed using the solvent decane (instead of squalane) are completely insensitive to the presence of oligomers. One simple explanation for these effects on bilayer conductance is that amyloid oligomers increase the area per molecule of the membrane-forming lipids, thus thinning the membrane, lowering the dielectric barrier, and increasing the conductance of any mechanism sensitive to the dielectric barrier.
INTRODUCTION
Alzheimer's is a progressive neurological disease affecting a large fraction of the aging population. It may well be the most “costly and disruptive disease in the developed world” (Dobson, 2003), and thus investigation of its causes and possible cures is of some interest to us all. Although the amyloid hypothesis in its original form appeared headed for trouble when correlations between fibril density and clinical symptoms appeared very slim, it has undergone something of a resurgence with increasing evidence that it is not the fibrils or the monomers of the amyloid that exert the toxic effect, but the micelles or aggregates (Walsh et al., 2002; Kayed et al., 2003; Kayed et al., 2004; Walsh et al., 2005). But the mechanism by which these aggregates or micelles might kill or injure living cells has remained obscure.
There are, however, a number of clues that an oligomer-induced conductance mechanism is involved. We showed in a previous paper (Kayed et al., 2004) that soluble oligomers of Aβ peptides and other related peptides increased the conductance of planar lipid bilayers, and the same oligomers increase calcium entry in a number of cell types (Demuro et al., 2005). In each case the observed conductance increase is a property of the oligomers and does not depend on the primary sequence of the peptides that form the oligomers, but on their physical properties. In this paper we report the results of experiments that suggest that amyloid oligomers break down or reduce the normal dielectric barrier to ion translocation provided by the hydrocarbon region of the lipid bilayer. We propose that Aβ oligomers increase membrane conductance and permeability to charged species by spreading the lipid head groups apart and consequently thinning the bilayer and lowering the permeability barrier (Neumcke and Läuger, 1969; Parsegian, 1969). We also show that oligomers (and only oligomers) produce precisely the same conductance increase in the membrane of patch-clamped rat basophilic leukemia (RBL) cells as in lipid bilayer preparations. We did not see ion channels of the sort reported in a number of papers (for reviews see Pollard et al., 1995; Kagan et al., 2002), and we discuss possible reasons for this and the connections between our work and amyloid channel formation.
MATERIALS AND METHODS
Preparation of Aβ Oligomers
Lyophilized peptides were resuspended in 50% acetonitrile in water and relyophilized. Soluble oligomers were prepared by dissolving 1.0 mg of peptide or protein in 200–400 μl hexafluoroisopropanol (HFIP) for 10–20 min at room temperature. 100 μl of the resulting seedless solution was added to 700 μl DD H2O in a siliconized Eppendorf tube. After 10–20 min incubation at room temperature, the samples were centrifuged for 15 min at 14,000 g and the supernatant fraction (pH 2.8–3.5) was transferred to a new siliconized tube and subjected to a gentle stream of N2 for 5–10 min to evaporate the HFIP. The samples were then stirred at 500 RPM using a Teflon-coated micro stir bar for 24–48 h at room temperature. Aliquots were taken at 6–12-h intervals for testing with anti-oligomer antibody (Kayed et al., 2003); the samples were also tested using electron microscopy (EM) and size exclusion chromatography (SEC). The time at which the oligomer state is most populated depends on several factors, such as speed of stirring and the concentration, so it is important to check size and homogeneity with SEC. The maximum population of the oligomeric state occurs between 12 h and 4 d.
Bilayers
Bilayers were formed at room temperature by the union of two monolayers formed from appropriate mixtures of phosphatidyl choline (PC), phosphatidyl serine (PS), phosphatidyl ethanolamine (PE), sphingomyelin (SM), or cholesterol (chol), as previously described (Kayed et al., 2004). The lipid acyl chains were oleolyl in all cases except for sphingomyelin where the chain types were the natural mixture resulting from isolation from egg. In brief, lipid monolayers were opposed over a hole ∼150 μm in diameter in a 15-μm-thick Teflon partition dividing the two aqueous phases. The hole, punched by electric spark, was precoated with a 2.5% solution of squalane in n-pentane. In some cases as noted in the body of the text, decane was used instead of squalane. Lipids were purchased from Avanti Polar Lipids. Salt solutions contained various concentrations of KCl or NaCl buffered by 10 mM HEPES-Tris to pH 7.4. Solutions were stirred with magnetic stirring bars for ∼30 s after additions. Bilayer formation was monitored by measuring capacitance. Silver/silver chloride wires were used as electrodes to apply voltages and record currents across the bilayer. The rear chamber potential was taken as ground and additions were made to the front chamber. For measurements of membrane conductance, a voltage ramp protocol (−150 to +150 mV, at 60 mV/s) was used. Voltages were generated and currents digitized at a resolution of 12 bits by an AD Lab ADC/DAC board running software written in the lab or JCLAMP (SciSoft Co.) driving a National Instruments board. Currents were transduced by an Axopatch 200A amplifier (Axon Instruments) connected to one of the boards described above. Ion selectivity was determined by measuring the reversal potential in asymmetrical ionic conditions using fiberglass bridges filled with 2 M KCl to connect the electrolyte solutions to silver-silver chloride wire. The membrane capacitance, Cm, was measured by applying a triangular voltage (28 mV, 20 Hz), measuring the resulting square wave current and calculating the capacitance from the following formula: Cm = Ccal(I − Ich)/Ical, where Ccal is the capacitance of a calibrated capacitor, I the total capacitance current, Ich the capacitance current produced by capacitance of the chamber, Ical the capacitive current through calibrated capacitor (220 pF) connected to the headstage input. Ich was measured under same condition as “I” with the hole in the Teflon film filled with a drop of lipid solution. For measurements of specific capacitance, the membrane area was determined optically with a CCD camera. Decane suspensions were prepared by mixing 0.3 ml decane with 0.3 ml buffer solution and sonicating in an ultrasonic bath for 5–10 min.
RBL Cells
RBL cells (Siraganian et al., 1982) were cultured in EMEM and 10% FBS without antibiotics and passaged twice a week. Patch-clamp experiments were performed in whole-cell mode at room temperature using an EPC-9 (HEKA Elektronik) amplifier. A DMZ-Universal puller (Zeitz) was used to manufacture patch pipettes from borosilicate glass capillaries (Garner Glass Company). The patch pipette resistances were in the range of 2–4 MΩ when filled with the standard recording solution. The ground electrode was connected to the bath via an agar bridge. The internal recording solution contained (in mM) 130 Cs-glutamate, 12 EGTA, 0.9 Ca, 8 Na+, 10 HEPES, pH 7.3. The external solution was (in mM) 2 CaCl2, 150 Na-aspartate, 10 HEPES, 2 Cs-methanesulfonate, pH 7.3; Cl− was substituted with aspartate in order to prevent the activation of outward volume-sensitive Cl− channels. Cesium methanesulfonate was included to reduce the endogenous IRK1 current present in RBL cells. The currents were recorded in whole cell mode using −110 to +85 mV voltage ramps (211 ms) applied at 0.5 Hz. Data files were recorded using PULSE/PULSEFIT software (HEKA Elektronik) and processed with Igor Pro (Wavemetrics).
RESULTS
Lack of Single Channel Jumps or Increased Noise
In our experiments we observed no current jumps characteristic of the single channels described in several reports (Pollard et al., 1993; Arispe et al., 1994; Sanderson et al., 1997; Chen et al., 2000; Hirakura et al., 2000; Kourie et al., 2001, 2002; Arispe and Doh, 2002; Lin and Kagan, 2002; Kagan et al., 2002; Quist et al., 2005) nor did we see any increase in noise with increasing conductance, which would be expected if there were channels present having a single-channel conductance below our resolution. To enhance the likelihood of detecting single channels, we increased the salt concentration of the solutions bathing the bilayer to 1–2 M NaCl or KCl. In these solutions, the conductance increased gradually, reaching steady-state level within 3–7 min after the addition of Aβ to either or both sides of bilayer. Fig. 1 shows a series of current records ∼10 s long taken with a voltage of +100 mV applied to a PC/PE lipid bilayer before and after single sided addition to a final concentration of Aβ oligomers 0.1 μM. Even though the conductance increased substantially, there was no apparent single channel activity and no increase in the noise level of the conductance. The variance of the current divided by the mean of the current would give the average current through any Poisson channel as it opens and closes. The variance of the current traces in Fig. 1 is constant and is ∼2 pA2. For a mean current of 75 pA, the variance divided by the mean would be ∼0.026 pA. This is a very conservative upper limit on the unitary current through any putative single channels formed by Aβ oligomers. But the variance of the current does not increase above instrumental noise even as the mean current goes from ∼1.4 pA to >75 pA, the true upper limit on the unitary current is much less. Values of single-channel conductances induced by amyloids reported in the literature range from 3,000 pS in 140 mM KCl (Arispe et al., 1993a) to 112 pS in 40 mM CsCl (Arispe et al., 1993b). The data in Fig. 1 rule out the existence of channels with an equivalent conductance of ∼0.26 pS, so clearly if channels with any of the values published in the literature were present, we would have seen them. This result was confirmed by taking autocorrelation functions of current records obtained from bare membranes and membranes containing 0.1 μM amyloid oligomers giving a conductance of 100 pS. The autocorrelation of both currents was essentially flat at times >0. The autocorrelation of a membrane doped with gramicidin taken under the same conditions showed a typical channel-type autocorrelation function with a decay time constant similar to that obtained by Kolb and coworkers (Kolb et al., 1975; unpublished data). Thus we are confident that failure to see channels is due to their absence, not experimental resolution.
Figure 1.
A high sensitivity recording of the current induced by Aβ oligomers. The current was recorded at 100 mV. The “control” trace was recorded before the addition of Aβ oligomers. Successive traces above the control trace were recorded at 10–20-s intervals in the 2-min period following the addition of Aβ oligomers to a concentration of 0.1 μM. Note that there is no increase in current noise as the current increases to ∼80 pA. Bilayer composition was PC/PE (1/1, molar).
It is of some concern to us that we did not see the channels reported by other investigators. We believe the principal reason for this is the difference in the materials we used and the materials used by other investigators. While the chemical properties of the materials we used correspond well to those of materials used by other investigators, the physical properties may not. The general increase in conductance we see is produced only by the oligomeric form of Aβ or other similar peptides and not by monomers or fibrils. Since the starting material is identical in all cases, it is clearly the difference in physical state that accounts for the difference in conductance properties, and this suggests that differences in the physical states of the material used likely account for differences between our results and those of other investigators. The lifetime of the oligomeric state in our experiments is finite (as it is in vivo), and unless attention is paid to characterizing the physical state of the material it is not possible to reliably observe the conductances we report here.
Concentration Dependence of the Aβ-induced Conductance: A Consistent Dose–Response Curve Over Three Orders of Magnitude
The conductance induced by Aβ depends monotonically on the aqueous concentration of peptide, and the induced conductance increases more than three orders of magnitude over the concentration range of Aβ from 0 to 20 μM. The shape of the induced current–voltage curve is unchanged over this concentration range, indicating that the same species is responsible for the conductance throughout, an important indication that the observed conductance is not due to a trace contaminant. The conductance of the membrane increases with solution conductance but less rapidly than a simple linear relationship. The reasons for this are not clear, but even though we believe the essential conductance of a bare lipid bilayer should be predicted by a simple solubility diffusion mechanism, this is not the case as demonstrated by even very early (but still valid!) measurements of bilayer conductance (Hanai et al., 1965). We should not therefore be too surprised that there are aspects of what is clearly a more complicated system than the bare bilayer that are not consistent with simple theory.
Fig. 2 shows the membrane current at 150 mV as a function of Aβ concentration in 100 mM KCl + 1 mM HEPES, pH 7.4, 10 mM KCl + 1 mM HEPES, pH 7.4, and in 1 mM HEPES, pH 7.4 alone. The consistency of the observed response over three orders of magnitude and the lack of any effect of monomers or fibrils argue very strongly that it is not produced by a contaminant. The possibility that the observed effect might be due to a contaminant is always a concern in experiments where the activity of only a few channels is evoked. Observing a single channel is at best observing the properties of a few molecules even when a much larger number of molecules is added to the preparation. For example, if nanomolar is the final concentration of the material to be tested in a 1-ml bilayer chamber, the number of molecules present in the chamber is on the order of 1011. If there are trace contaminants present at say one millionth of the concentration of the molecule of interest, there are still 105 contaminating molecules, and something has to be done to rule out that the observed effect is due to contamination. One long-accepted way of addressing this issue is to generate a dose–response curve over several orders of magnitude and to check that the channels in many channel membranes have properties predicted by the single channel properties observed.
Figure 2.
Amyloid-induced conductance depends on the electrolyte concentration of the bathing solution as shown by these three dose–response curves in electrolytes ranging in concentration from 100 mM KCL to 1 mM HEPES-Tris. Bilayer composition was PC/PS (1/1, molar), voltage was 150 mV.
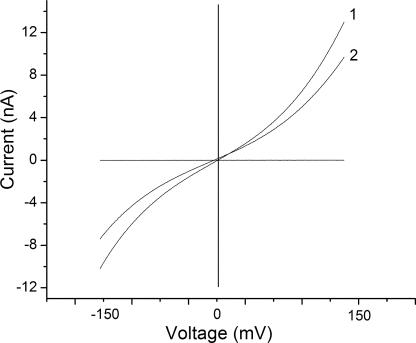
Ion Selectivity of Bilayers Treated with Soluble Oligomers
Aβ increases the conductance of bilayers in approximate proportion to the conductivity of the aqueous phase regardless of the ionic composition of the solution. This suggests that the conductance increase induced by Aβ is not very selective for one ion over another, and bi-ionic potential measurements confirm that this is indeed the case. The reversal potential measured under asymmetrical salt concentration conditions in neutral bilayers was near 0 mV for every pair of solutions we tested. In particular there is essentially no reversal potential in neutral bilayers for 10 to 1 gradients of KCl (100 vs. 10 mM), NaCl, or CaCl2. There is also essentially no reversal potential for 100 mM KCl against 100 mM NaCl and no reversal potential of 100 mM KCl against either 50 or 100 mM CaCl2. Fig. 3 shows one example of these data, the selectivity of KCl versus CaCl2. There is a small degree of rectification or asymmetry in the current–voltage curve even in symmetric solutions. This does not appear to be strongly influenced by the unilateral addition of Aβ, and addition of Aβ to both sides of the membrane increases the conductance over that of unilateral addition but has little effect on the asymmetry.
Figure 3.
The reversal potentials measured for asymmetric solutions of different ionic composition in the presence of 4 μM Aβ oligomers added to one side. Trace 1, 100 mM KCl, cis; 100 mM CaCl2, trans. Trace 2, 100 mM KCl, cis; 50 mM CaCl2, trans. Bilayer composition was PC/PE (1/1, molar).
Aβ Decreases the Electrostatic Barrier of the Lipid Bilayer
The lack of selectivity, the increase of the induced bilayer conductance with solution conductivity for a variety of ions, and the absence of typical current jumps of single channels led us to the hypothesis that Aβ oligomers themselves do not contribute a new intrinsic conductance to the membrane, such as an ion channel or carrier, but instead reduce the height of the dielectric barrier normally provided by the bilayer. For bare bilayers, Aβ simply increases the conductance for the available carriers, namely the anions and cations in the solution bathing the membrane. If our hypothesis is correct, Aβ should also increase the conductance of every conductance mechanism sensitive to the magnitude of the dielectric barrier. Since the dielectric barrier does not discriminate between anions and cations, Aβ oligomers would increase the conductance of both anion carriers and cation carriers more or less to the same degree. We tested this idea by comparing the effect of Aβ on two conductances well studied in lipid bilayers, the potassium-selective cation conductance of nonactin and the anion-selective conductance induced by tetraphenyl borate.
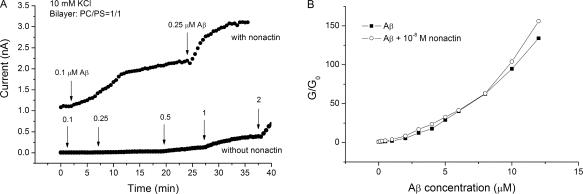
Aβ Increases the Cationic Nonactin Conductance.
Nonactin acts by trapping potassium ions in a hydrophilic cavity and reducing their energy of translocation by increasing the effective ionic radius, thus greatly increasing the conductance of lipid bilayers (for classical reviews of the nonactin carrier-mediated conductance mechanism see Eisenman et al., 1968; Lauger, 1972; Eisenman et al., 1975; Krasne and Eisenman, 1976). Fig. 4 A compares the effect of Aβ on the nonactin-induced conductance of the bilayer to its effect on a bare bilayer in 10 mM KCl. In the presence of 10−8 M nonactin and no Aβ, the conductance of the bilayer is many-fold greater than that of a bare lipid bilayer. Addition of 0.1 μM Aβ to the aqueous phase multiplies the already present nonactin conductance by a factor of two. In the absence of nonactin, the conductance increase induced by 0.1 μM Aβ alone is negligible in comparison. Further additions of Aβ have similar effects. The overall effect of Aβ, then, is to multiply the existing conductance by a factor dependent on the concentration of Aβ. Fig. 4 B drives this point home by plotting G/G0 versus Aβ concentration, where G0 is the conductance of the bilayer in the absence of Aβ for a bare bilayer and for a nonactin doped one. Note that the two normalized curves superimpose almost perfectly even though the nonactin-induced conductance is >200 times that of the bare membrane conductance at every concentration of amyloid tested.
Figure 4.
The effect of Aβ oligomers on the conductance induced by nonactin. (A) The bottom trace shows the current measured at 150 mV induced by Aβ oligomers. Additions of Aβ oligomers and the resulting concentrations (μM) are shown by the arrows. The top trace shows a similar experiment performed in the presence of 10−8 M nonactin but with only two additions of amyloid, corresponding to the two lowest concentrations in the bottom trace. The bath solution contained 10 mM KCl in both cases, lipid composition was PC/PS (1/1, molar). (B) Normalized nonactin conductance and bare bilayer conductance as a function of Aβ oligomer concentration. Conductance at a given Aβ oligomer concentration divided by the conductance at 0 Aβ oligomer concentration is plotted against Aβ oligomer concentration. The initial conductance of the nonactin-K+ membrane was >200 times that of bare bilayer. Note that the dose–response curve for nonactin is essentially a multiple of the bare bilayer dose–response curve.
Aβ Increases the Anionic Tetraphenyl Borate Conductance.
The above results suggest that Aβ micelles reduce the electrostatic component of the energy required to translocate electric charges across the bilayer by increasing the effective dielectric constant or decreasing the thickness of bilayer. If this is so, Aβ micelles should reduce the translocation energy for both positive and negative ions. To test this prediction we used a hydrophobic anion, tetraphenyl borate (TPB), as a probe. The mechanism by which TPB increases bilayer conductance is completely different from the carrier function of nonactin, but should be equally affected by alterations in the electrostatic barrier of the membrane if Aβ oligomers indeed reduce the dielectric barrier.
TPB adsorbs to both sides of the membrane and responds to a voltage pulse redistributing from one side of the membrane to the other. This redistribution produces a capacitance-like current that decays with a time constant depending on the rate at which tetraphenyl borate crosses the membrane (Andersen et al., 1978). This rate depends on voltage and the electrostatic barrier height. If the electrostatic barrier for charge translocation is reduced, the rate of tetraphenyl borate translocation will increase and the time constant of the capacitance-like current will decrease.
Fig. 5 A shows the transition currents induced by TPB and effect of Aβ micelles on them. As predicted, in the presence of Aβ, the decay of the TPB-induced transition current is faster while the steady-state current increases with the increase in Aβ concentration. Fig. 5 B shows a plot of the calculated rate of tetraphenyl borate translocation as a function of Aβ concentration. The effect of Aβ is clearly to increase the rate of translocation of tetraphenyl borate exactly as predicted by its effect on nonactin conductance and bare bilayer conductance.
Figure 5.
The effect of Aβ oligomers on the rate of tetraphenyl borate translocation. (A) The currents induced by tetraphenyl borate in a PC/PS bilayer as a response to voltage pulses of +300 mV. The tetraphenyl borate concentration was 10−6 M on both sides of the membrane in 10 mM KCl. Solid, control; dashed, 4 μM Aβ oligomers; dotted, 16 μM Aβ oligomers. (B) The translocation rate of tetraphenyl borate ions deduced from the time constants as a function of Aβ oligomer concentration.
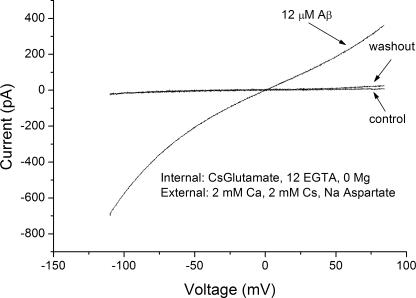
Effect of Aβ on the Conductance of RBL Cells
We tested the effects of all three forms of Aβ on the conductance of patch-clamped RBL cells. In agreement with our findings in lipid bilayer experiments, only the micellar form of Aβ induced a measurable increase in plasma membrane conductance (Fig. 6). Moreover, the induced conductance took precisely the same form as the conductance induced by Aβ in planar lipid bilayers. Fig. 6 shows a current–voltage relation with 12 μM Aβ added to the bathing solution in whole-cell recording mode. Note that the shape of the induced current–voltage curve in an RBL cell is essentially identical to that in a lipid bilayers.
Figure 6.
Amyloids act on mammalian RBL cells in almost exactly the same fashion in which they act on lipid bilayers. Three current voltage curves are shown. A control trace was taken before the addition of Aβ oligomers. The second trace, taken after addition of Aβ oligomers to a concentration of 12 μM, shows a current–voltage curve nearly identical in shape to those obtained after addition of Aβ oligomers to solutions bathing planar lipid bilayers. A final trace was taken after washout.
Washout of the Conductance Increase Is Rapid
The Aβ-induced conductance in both RBL cells and lipid bilayers washes in and out rapidly. This is illustrated in Fig. 7 (A and B). Upon exposure to a solution containing Aβ, the RBL cell conductance rises dramatically, undergoes inactivation with time, and then washes out quite rapidly (Fig. 7 A). Similar results are shown in Fig. 7 B for a lipid bilayer preparation.
Figure 7.
(A) Current recorded in patch-clamped RBL cells during wash in and wash out of 12 mM Aβ oligomers. (B) Increase of bilayer conductivity after addition of 4 μM Aβ oligomers to the cis compartment and its decrease after the perfusion of this compartment with Aβ oligomer-free solution. The break in the record is due to high noise of bilayer current during the perfusion. The current was measured at 150 mV. The bath solution was 10 mM KCl, bilayer composition was PC/PE (1/1, molar).
The Aβ-induced Conductance Is Reduced by a Specific Antibody that Recognizes Only Oligomers
It is worth emphasizing that the conductance induced by Aβ oligomers has a demonstrated specificity. We showed in a previous paper that addition of antibody raised against the oligomeric form of Aβ eliminates the conductance induced by previously added oligomer (Kayed et al., 2004). This antibody recognizes only the oligomeric form and also protects against Aβ oligomer toxicity in a tissue culture based assay (Kayed et al., 2003). Fig. 8 shows that preincubation of an Aβ oligomeric preparation with this same antibody also protects against the conductance increase and greatly reduces the effectiveness of the oligomeric preparation. Thus whatever the nature of the conductance increase we are observing, it is clearly connected to the presence of Aβ oligomers by this immunological specificity.
Figure 8.
Immunological specificity of the Aβ oligomer–induced conductance. The filled circles show the current increase of a lipid bilayer clamped at 150 mV after the addition of Aβ oligomers to a concentration of 0.25 μM. In this case there was only a single addition. The open circles show the effect of addition of 0.25 μM Aβ oligomers preincubated with an excess (4:1 antibody molar ratio to Aβ) of antibody. Subsequent additions (arrows) of Aβ and antibody in the same ratio raise the aqueous concentration first to 0.5 μM and then to 1.0 μM. This result demonstrates the same immunospecificity of the Aβ oligomer–induced conductance seen when antibody is added after the establishment of an elevated conductance by the addition of oligomer (Kayed et al., 2004).
The Influence of Lipid Composition on the Conductance Induced by Aβ
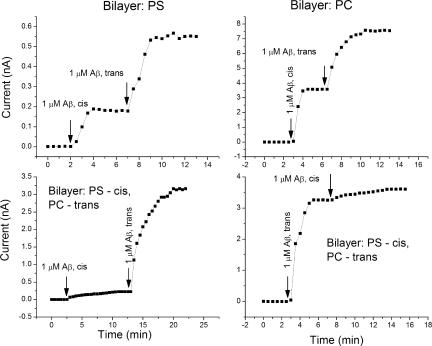
We also studied the effect of the lipid composition of the bilayer on the conductance produced by Aβ oligomers. Fig. 9 illustrates that the inclusion of negatively charged lipid (PC/PS = 1/1 molar) reduces the effect of Aβ compared with the bilayers consisting of neutral PC and PE (1/1 molar). We did not observe any significant effect of cholesterol, using bilayers composed of PC and cholesterol at a 1/1 molar ratio. A further study of the effect of lipid composition on the conductance was performed using asymmetric bilayers (one monolayer was formed with PC and another one with PS). As shown in Fig. 10, the addition of Aβ to the neutral side of the bilayer (PC) increases the bilayer conductance more than its addition to the negatively charged side (PS), a difference that is likely due to electrostatic repulsion (the net charge of Aβ is −3).
Figure 9.
Effect of charge and cholesterol on the amyloid-induced conductance. Currents measured at a voltage of 150 mV in bilayers of different lipid compositions versus Aβ oligomer concentration in 10 mM KCl.
Figure 10.
Effect of lipid composition on the Aβ oligomer–induced conductance measured in symmetric and asymmetric bilayers. The top panel shows the effect of Aβ oligomers additions to the cis and trans sides of bilayers formed with pure PS and PC. Note that both cis and trans addition produced approximately the same increase in conductance. Scales of the left and right figures differ. The bottom panel shows the effect of Aβ oligomers on asymmetric bilayers with one leaflet containing PC and another PS. Note that addition of Aβ to the PC side of bilayer produced a much greater effect.
Role of Bilayer Solvent in Determining the Effects of Aβ
The experiments presented above suggest that Aβ alters the electrostatic barrier of the bilayer in some fashion. But they do not discriminate between two important possibilities, increasing the dielectric constant or reducing the membrane thickness. Both of these would lower the energy barrier for charge translocation and produce the observed effects on bare bilayer conductance, nonactin conductance, and tetraphenyl borate conductance. And both would show the same lack of ionic selectivity and act in the same manner on the conductances of cell membranes. By focusing on these two possibilities, we do not mean to exclude others, for example, increasing the dielectric constant by incorporation of amyloid in the bilayer or increased penetration of water into the bilayer, but only to consider those possibilities that suggest specific experiments.
The rapid washout of the Aβ-induced conductance seen in both bilayers and RBL cells (Fig. 7, A and B) suggests that Aβ acts on the surface of the membrane, possibly by spreading the lipid head groups apart. This would have the effect of forcing the hydrocarbon tails of the lipids to fill in the spaces between the lipids and thus thinning the membrane, lowering the dielectric barrier and increasing the conductance.
This is exactly the effect produced by benzyl alcohol on membranes formed using squalane as the solvent. Addition of benzyl alcohol thins the membrane. But in bilayers formed using decane as the solvent, the addition of benzyl alcohol thickens the membrane (Ebihara et al., 1979). The reason for this difference is easy to understand intuitively. When benzyl alcohol is added to squalane bilayers, it intercalates into the membrane and adds surface area, but not much hydrocarbon volume. Thus the hydrocarbon chains of the host lipids must fill in the excess volume and the membrane thins. In contrast, when benzyl alcohol is added to decane membranes, the empty volume must also be filled, but now plenty of decane is available to do so and the hydrocarbon chains need not fill the void alone. Decane, however, adds a little excess volume and the net result is an increase in membrane thickness. Thus, if Aβ is spreading the lipid head groups, we would expect it to increase the conductance of biological membranes and squalane-formed planar lipid bilayers but not of decane-formed lipid bilayers.
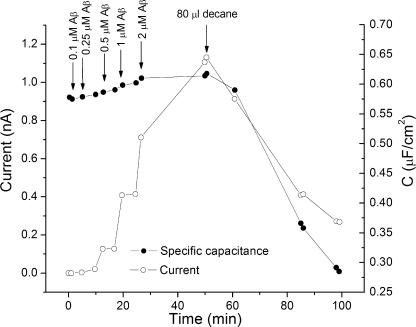
We decided to take advantage of the properties of lipid bilayers formed using different solvents to test the idea that Aβ micelles might act by spreading the head groups, thereby increasing the area per molecule of the lipids. In this case Aβ should thin squalane-formed bilayers, thus increasing their conductance and capacitance. But Aβ should have no effect on the conductance of membranes made with decane as the solvent. This indeed proved to be true. If membranes are formed using decane as the solvent, there is no increase in conductance even in the presence of very high concentrations of Aβ (unpublished data). Moreover, decane reverses the effects of Aβ on the conductance and capacitance of squalane membranes. Fig. 11 shows the effect of adding a sonicated suspension of decane to a lipid bilayer whose conductance has been increased by the addition of increasing amounts of Aβ to the aqueous solution. Note that as the decane partitions into the bilayer, the Aβ-induced conductance drops to control values. Fig. 11 also shows that increasing Aβ concentration increases conductance and capacitance of the membrane in parallel.
Figure 11.
The effect of decane on the conductance and capacitance increase induced by Aβ oligomers. Time course of both current (open circles) and capacitance (closed circles) with successive additions of Aβ followed by the addition of a suspension containing decane. The current was measured at +150 mV. Bilayer composition: PC/ PE = 1/1 (molar). Bath solution 10 mM KCl.
The Influence of Area Compressibility on the Conductance Induced by Aβ
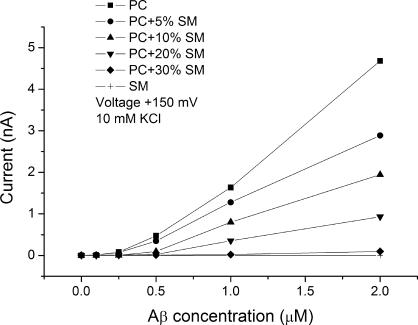
The most dramatic effect of lipid composition is that of area compressibility. If the hypothesis put forth in the previous section is correct, the effect of Aβ oligomers should be greatly affected by the area elastic modulus of the lipids forming the membrane, and this appears to be the case. Fig. 12 shows dose–response curves of membranes formed from lipids ranging from pure sphingomyelin to pure DOPC with a series of intermediate compositions. Pure sphingomyelin membranes are almost insensitive to Aβ oligomers, while pure DOPC membranes are very sensitive to them. The area compressibility modulus of sphingomyelin/cholesterol membranes is 1,734 dynes/cm while that of DOPC is 247 dynes/cm (Allende et al., 2005).
Figure 12.
Aβ oligomers are ineffective in sphingomyelin bilayers. A series of dose–response curves for Aβ oligomers in membranes ranging from pure PC to pure SM. Currents were measured at 150 mV in 10 mM KCl.
If thinning by increase of area per molecule is important, we would expect a saturation in the effect as the area per molecule approaches an upper limit, but we are experimentally limited by the minimum sensitivity of our amplifiers to a concentration of Aβ around 20–30 μM in soft lipids. Dose–response curves do start to show a flattening out at higher concentrations, possibly suggesting the onset of saturation.
DISCUSSION
The results presented above show that Aβ oligomers, and only the oligomers, increase the conductance of lipid bilayers and living cell membranes. In addition, Aβ oligomers proportionally increase the conductance of both cationic and anionic probe species in planar lipid bilayers even though the initial conductance induced by the probes is much higher than that of a bare bilayer. Because the effect is nonspecific and alters the conductance of both probe molecules and the bare bilayer in pretty much the same proportion, it seems that Aβ oligomers are somehow lowering the dielectric barrier for the translocation of charge across the lipid bilayer. Among the possible mechanisms by which Aβ oligomers might lower the dielectric barrier are increasing the membrane dielectric constant, introducing localized defects, and thinning the membrane. These are not mutually exclusive and probably operate simultaneously, each contributing to the overall effect.
If the principal effect is increasing the dielectric constant of the hydrocarbon portion of the membrane, Aβ oligomers would have to intercalate somehow into the hydrocarbon region or water would have to partition more effectively into the bilayer or both. (A simple calculation shows that only about a 10% increase in dielectric constant at a concentration of 20 μM could account for the observed conductance changes.) If amyloid oligomers were intercalated deeply in the hydrocarbon region, it would likely be very difficult to wash out Aβ rapidly, and yet this is exactly what happens experimentally. Thus it is unlikely, but still possible, that amyloid oligomers increase the dielectric constant by inserting into the hydrocarbon portion of the membrane. The rapid washout of the effect in both lipid bilayers and living cells suggests that the reaction of Aβ oligomers with the membrane is a rather superficial one, taking place primarily at the surface of the membrane. If this is so, the mechanisms by which amyloid peptides could lower the dielectric barrier are limited. The effect of Aβ oligomers is nonexistent in decane membranes and can be eliminated by adding a solution of sonicated decane to the membrane. These results suggest that amyloid could act by increasing the area per molecule of the bilayer, and consequently, because the volume of the hydrocarbon moiety is constant, thinning the bilayer. The strain on the bilayer would likely increase the defect density in the hydrocarbon region as well. Thinning would contribute to a reduction in the dielectric barrier by reducing the distance over which image forces can act (Parsegian, 1969).
If one attributes all of the change in specific capacitance seen, for example, in Fig. 10 (0.57 to 0.62 μFd.cm2) to decrease in thickness, the Parsegian expression predicts that the conductance should increase by a factor of three. If on the other hand one attributes all of the capacitance increase to an increase in the dielectric constant, the Born expression for energy of a charge in the membrane predicts the conductance should increase by a factor of 1,000. The actual factor of increase for the given change in capacitance is 300. Because of the very oversimplified assumptions on which these estimates rest, one should not read too much into these numbers, but the comparison does suggest that an increase in dielectric constant is probably the major factor causing the amyloid-induced increase in conductance. We believe the most likely mechanism for this increase is increased partition of water into the membrane consequent to an increase in area per lipid molecule. Thus our model can be viewed as a special way of looking at membrane defects: the more defects, the more water, the more water, the higher the dielectric constant and the more conductance. This view is further supported by the observation that membranes that require considerable energy to increase the area per molecule (those with high area elastic moduli) are far less sensitive to Aβ oligomers than membranes that expand easily (Fig. 11). Whether or not this mechanism is correct remains to be seen, but our data strongly argue that amyloid oligomers act by reducing the dielectric barrier for ion translocation in some fashion.
The action of amyloids need not be (and almost certainly is not) confined to the effect we describe in this paper. There is considerable literature on formation of ion channels by amyloids in lipid bilayers (Pollard et al., 1995; Hirakura et al., 2000; Zhu et al., 2000; Kourie et al., 2001; Lin et al., 2001; Kagan et al., 2002; Kourie and Henry, 2002; Quist et al., 2005). We do not see such channels in the vast majority of our experiments. We have seen channels after addition of our oligomeric amyloid preparation in no more than three experiments out of more than 100 experiments where oligomers were present. In these instances, the conductance increase we describe here was also present. In our experiments we found the amyloid oligomer–induced conductance increased monotonically (and approximately proportionately) with the aqueous concentration of oligomers. The amyloid channel literature presents very few dose response curves showing an increase in conductance or number of channels with increased aqueous concentrations. The single channel conductances reported vary from 3,000 to 400 pS in 140 mM KCl (Arispe et al., 1993a) to 10 pS in 100 mM KCl (Quist et al., 2005), so clearly there is precedent for the same material producing different results under different conditions. We see conductances on the order of 10−9 to 10−8 S with amyloid oligomer preparations containing concentrations on the order of 1 to 10 μM (concentrations given as moles of monomer, though the majority of material is in the oligomeric form). At similar concentrations of amyloid in the monomer form, the channel literature reports membranes containing only single channels with conductances from tens to hundreds of picosiemens. This leads us to believe that the channels reported in the literature are comparatively rare events with an undetermined number of amyloid monomers forming each channel.
We feel that the lack of a concentration–effect relation is an important omission in the amyloid channel studies we have seen. To our knowledge there has never been a demonstration that the single channel properties determined from studies on a membrane containing only a few amyloid-induced channels explain the properties of a membrane containing many channels. In fact there are almost no reports describing the properties of many-channel membranes. These criticisms aside, the potential role of amyloid channels in Alzheimer's pathology should not be ignored nor should the potential of the mechanism of amyloid action we propose here. The two are clearly not mutually exclusive.
No matter what the mechanism of the conductance increase we observe turns out to be, it has immunological specificity relating it to the presence of a particular oligomeric amyloid form (Fig. 8). Reduction in the dielectric barrier and subsequent increase in membrane conductance can account for many of the pathological symptoms observed in Alzheimer's disease, including the observed increase in cytoplasmic calcium levels. The dielectric energy of translocation increases with the square of the charge. Thus the reduction in energy for the translocation of calcium will be four times that for a monovalent ion. Our mechanism also suggests that in so far as gating charges of voltage-dependent channels are exposed to the lipid environment their normal movements could be perturbed by the presence of amyloid oligomers. Disturbance of voltage-gated calcium channels, essential for proper synaptic function, is a likely consequence of our proposed mechanism. Our proposed mechanism also suggests that Alzheimer's toxicity could be prevented by three different sorts of intervention: breaking up Aβ oligomers or preventing their formation; preventing the surface interaction of oligomer interaction with membranes (as our antibody does; Kayed et al., 2003, 2004); or preventing the thinning of the membrane induced by oligomers as decane does.
Acknowledgments
This work was supported by a grant from the Larry L. Hillblom Foundation and by the Intramural Research Program of the National Institutes of Health and National Institute of Child Health and Human Development (to A. Chanturiya).
Lawrence G. Palmer served as editor.
Abbreviations used in this paper: PC, phosphatidyl choline; PE, phosphatidyl ethanolamine; PS, phosphatidyl serine; RBL, rat basophilic leukemia; TPB, tetraphenyl borate.
References
- Allende, D., S.A. Simon, and T.J. McIntosh. 2005. Melittin-induced bilayer leakage depends on lipid material properties: evidence for toroidal pores. Biophys. J. 88:1828–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen, O.S., S. Feldberg, H. Nakadomari, S. Levy, and S. McLaughlin. 1978. Electrostatic interactions among hydrophobic ions in lipid bilayer membranes. Biophys. J. 21:35–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arispe, N., and M. Doh. 2002. Plasma membrane cholesterol controls the cytotoxicity of Alzheimer's disease AβP (1-40) and (1-42) peptides. FASEB J. 16:1526–1536. [DOI] [PubMed] [Google Scholar]
- Arispe, N., H.B. Pollard, and E. Rojas. 1993. a. Giant multilevel cation channels formed by Alzheimer disease amyloid β-protein [AβP-(1-40)] in bilayer membranes. Proc. Natl. Acad. Sci. USA. 90:10573–10577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arispe, N., E. Rojas, and H.B. Pollard. 1993. b. Alzheimer disease amyloid β protein forms calcium channels in bilayer membranes: blockade by tromethamine and aluminum. Proc. Natl. Acad. Sci. USA. 90:567–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arispe, N., H.B. Pollard, and E. Rojas. 1994. The ability of amyloid β-protein [AβP (1-40)] to form Ca2+ channels provides a mechanism for neuronal death in Alzheimer's disease. Ann. N. Y. Acad. Sci. 747:256–266. [DOI] [PubMed] [Google Scholar]
- Chen, Q.S., B.L. Kagan, Y. Hirakura, and C.W. Xie. 2000. Impairment of hippocampal long-term potentiation by Alzheimer amyloid β-peptides. J. Neurosci. Res. 60:65–72. [DOI] [PubMed] [Google Scholar]
- Demuro, A., E. Mina, R. Kayed, S.C. Milton, I. Parker, and C.G. Glabe. 2005. Calcium dysregulation and membrane disruption as a ubiquitous neurotoxic mechanism of soluble amyloid oligomers. J. Biol. Chem. 280:17294–17300. [DOI] [PubMed] [Google Scholar]
- Dobson, C.M. 2003. Protein folding and disease: a view from the first Horizon Symposium. Nat. Rev. Drug Discov. 2:154–160. [DOI] [PubMed] [Google Scholar]
- Ebihara, L., J.E. Hall, R.C. MacDonald, T.J. McIntosh, and S.A. Simon. 1979. Effect of benzyl alcohol on lipid bilayers. A comparisons of bilayer systems. Biophys. J. 28:185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenman, G., S.M. Ciani, and G. Szabo. 1968. Some theoretically expected and experimentally observed properties of lipid bilayer membranes containing neutral molecular carriers of ions. Fed. Proc. 27:1289–1304. [PubMed] [Google Scholar]
- Eisenman, G., S. Krasne, and S. Ciani. 1975. The kinetic and equilibrium components of selective ionic permeability mediated by nactin- and valinomycin-type carriers having systematically varied degrees of methylation. Ann. N. Y. Acad. Sci. 264:34–60. [DOI] [PubMed] [Google Scholar]
- Hanai, T., D.A. Haydon, and J. Taylor. 1965. Some further experiments on bimolecular lipid membranes. J. Gen. Physiol. 48:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakura, Y., W.W. Yiu, A. Yamamoto, and B.L. Kagan. 2000. Amyloid peptide channels: blockade by zinc and inhibition by Congo red (amyloid channel block). Amyloid. 7:194–199. [DOI] [PubMed] [Google Scholar]
- Kagan, B.L., Y. Hirakura, R. Azimov, R. Azimova, and M.C. Lin. 2002. The channel hypothesis of Alzheimer's disease: current status. Peptides. 23:1311–1315. [DOI] [PubMed] [Google Scholar]
- Kayed, R., E. Head, J.L. Thompson, T.M. McIntire, S.C. Milton, C.W. Cotman, and C.G. Glabe. 2003. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 300:486–489. [DOI] [PubMed] [Google Scholar]
- Kayed, R., Y. Sokolov, B. Edmonds, T.M. McIntire, S.C. Milton, J.E. Hall, and C.G. Glabe. 2004. Permeabilization of lipid bilayers is a common conformation-dependent activity of soluble amyloid oligomers in protein misfolding diseases. J. Biol. Chem. 279:46363–46366. [DOI] [PubMed] [Google Scholar]
- Kolb, H.A., P. Lauger, and E. Bamberg. 1975. Correlation analysis of electrical noise in lipid bilayer membranes: kinetics of gramicidin A channels. J. Membr. Biol. 20:133–154. [DOI] [PubMed] [Google Scholar]
- Kourie, J.I., and C.L. Henry. 2002. Ion channel formation and membrane-linked pathologies of misfolded hydrophobic proteins: the role of dangerous unchaperoned molecules. Clin. Exp. Pharmacol. Physiol. 29:741–753. [DOI] [PubMed] [Google Scholar]
- Kourie, J.I., C.L. Henry, and P. Farrelly. 2001. Diversity of amyloid β protein fragment [1-40]-formed channels. Cell. Mol. Neurobiol. 21:255–284. [DOI] [PubMed] [Google Scholar]
- Kourie, J.I., A.L. Culverson, P.V. Farrelly, C.L. Henry, and K.N. Laohachai. 2002. Heterogeneous amyloid-formed ion channels as a common cytotoxic mechanism: implications for therapeutic strategies against amyloidosis. Cell Biochem. Biophys. 36:191–207. [DOI] [PubMed] [Google Scholar]
- Krasne, S., and G. Eisenman. 1976. Influence of molecular variations of ionophore and lipid on the selective ion permeability of membranes: I. Tetranactin and the methylation of nonactin-type carriers. J. Membr. Biol. 30:1–44. [DOI] [PubMed] [Google Scholar]
- Lauger, P. 1972. Carrier-mediated ion transport. Science. 178:24–30. [DOI] [PubMed] [Google Scholar]
- Lin, H., R. Bhatia, and R. Lal. 2001. Amyloid β protein forms ion channels: implications for Alzheimer's disease pathophysiology. FASEB J. 15:2433–2444. [DOI] [PubMed] [Google Scholar]
- Lin, M.C., and B.L. Kagan. 2002. Electrophysiologic properties of channels induced by Aβ25-35 in planar lipid bilayers. Peptides. 23:1215–1228. [DOI] [PubMed] [Google Scholar]
- Neumcke, B., and P. Läuger. 1969. Nonlinear electrical effects in lipid bilayer membranes. II. Integration of the generalized Nernst-Planck equations. Biophys. J. 9:1160–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsegian, A. 1969. Energy of an ion crossing a low dielectric membrane: solutions to four relevant electrostatic problems. Nature. 221:844–846. [DOI] [PubMed] [Google Scholar]
- Pollard, H.B., E. Rojas, and N. Arispe. 1993. A new hypothesis for the mechanism of amyloid toxicity, based on the calcium channel activity of amyloid β protein (AβP) in phospholipid bilayer membranes. Ann. N. Y. Acad. Sci. 695:165–168. [DOI] [PubMed] [Google Scholar]
- Pollard, H.B., N. Arispe, and E. Rojas. 1995. Ion channel hypothesis for Alzheimer amyloid peptide neurotoxicity. Cell. Mol. Neurobiol. 15:513–526. [DOI] [PubMed] [Google Scholar]
- Quist, A., I. Doudevski, H. Lin, R. Azimova, D. Ng, B. Frangione, B. Kagan, J. Ghiso, and R. Lal. 2005. Amyloid ion channels: a common structural link for protein-misfolding disease. Proc. Natl. Acad. Sci. USA. 102:10427–10432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson, K.L., L. Butler, and V.M. Ingram. 1997. Aggregates of a β-amyloid peptide are required to induce calcium currents in neuron-like human teratocarcinoma cells: relation to Alzheimer's disease. Brain Res. 744:7–14. [DOI] [PubMed] [Google Scholar]
- Siraganian, R.P., A. McGivney, E.L. Barsumian, F.T. Crews, F. Hirata, and J. Axelrod. 1982. Variants of the rat basophilic leukemia cell line for the study of histamine release. Fed. Proc. 41:30–34. [PubMed] [Google Scholar]
- Walsh, D.M., I. Klyubin, J.V. Fadeeva, W.K. Cullen, R. Anwyl, M.S. Wolfe, M.J. Rowan, and D.J. Selkoe. 2002. Naturally secreted oligomers of amyloid β protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 416:535–539. [DOI] [PubMed] [Google Scholar]
- Walsh, D.M., I. Klyubin, G.M. Shankar, M. Townsend, J.V. Fadeeva, V. Betts, M.B. Podlisny, J.P. Cleary, K.H. Ashe, M.J. Rowan, and D.J. Selkoe. 2005. The role of cell-derived oligomers of Aβ in Alzheimer's disease and avenues for therapeutic intervention. Biochem. Soc. Trans. 33:1087–1090. [DOI] [PubMed] [Google Scholar]
- Zhu, Y.J., H. Lin, and R. Lal. 2000. Fresh and nonfibrillar amyloid β protein(1-40) induces rapid cellular degeneration in aged human fibroblasts: evidence for AβP-channel-mediated cellular toxicity. FASEB J. 14:1244–1254. [DOI] [PubMed] [Google Scholar]