Abstract
Hemoglobin has been a long-standing paradigm for understanding protein allostery. Here, the x-ray structures of two chemically crosslinked, fully liganded hemoglobins, α2β82CA82β and α2β82ND82β, are described at 2.3 Å and 2.6 Å resolution, respectively. Strikingly, these crosslinked hemoglobins assume intermediate conformations that lie between those of R and the controversial liganded hemoglobin state R2 rather than between R and T. Thus, these structures support only a T ↔ R ↔ R2 allosteric pathway and underscore the physiological importance of the R2 conformation.
The quaternary end-state structures of human hemoglobin have long been accepted as the unliganded T and liganded R conformations (1–7). However, the appearance of a second fully liganded conformation, R2 (8–10), has stirred debate as to whether this conformation is an intermediate that lies between T and R (8, 11), an off-pathway structure (12), or the physiologically relevant end state (11, 13). On the basis of the dislocation of the imidazole side chain of residue β2His-97 from the α1C-helix, the R2 conformation was first proposed as an intermediate between the R and T states because it suggested a mechanism by which this residue switches from its T to R state position (8). However, the results of the calculated trajectory of the atomic coordinates in transiting from the T to R2 structure casted doubt on the validity of this proposal (13). Specifically, that trajectory was shown to pass close to the R conformation and thereby suggested R2 might be the physiologically relevant liganded end-state conformation. Clearly, the relevance of the R2 conformation and its position along hemoglobin’s allosteric pathway is critical to our complete understanding of the function of hemoglobin. Here, we present the structures of two chemically crosslinked, fully liganded hemoglobins that capture R ↔ R2 conformational intermediates and thus clarify the relevance of the R2 state.
MATERIALS AND METHODS
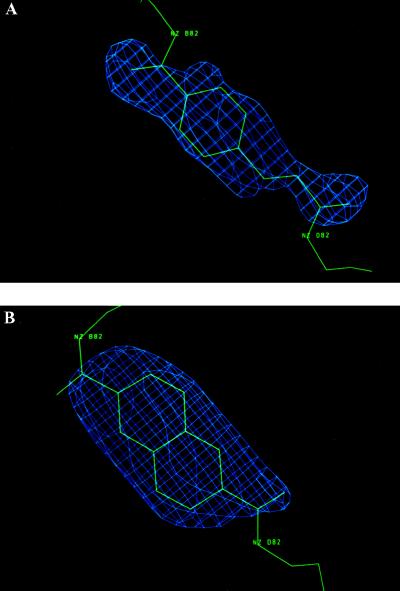
The crosslinked hemoglobins, which display only slightly lower oxygen affinities than uncrosslinked hemoglobin and normal cooperativities (14, 15), were prepared by reacting human deoxyhemoglobin with the bis(methylphosphate) derivatives (15, 16) of either 4-carboxycinnamic acid (CA) or 2,6-napthalene dicarboxylic acid (ND), respectively, and saturated with carbonmonoxide (CO) before their crystallization. Both α2β82CA82β and α2β82ND82β are crosslinked between the Nζ atoms of β1Lys-82 and β2Lys-82. Although crystallized under the high phosphate conditions that result in the tetragonal crystals of unmodified carbonmonoxy hemoglobin (COHbA) (17), α2β82CA82β and α2β82ND82β assume the orthorhombic space group P212121 (Table 1). The structure of α2β82CA82β was solved by molecular replacement (18) and found to contain a tetramer in the asymmetric unit. Refinement converged to a final R factor of 18.4% at 2.3 Å resolution (20) (Table 1). This structure served as the starting model, minus the crosslinker and water molecules, for refinement of the α2β82ND82β structure, which converged to a final R factor of 15.4% at 2.6 Å resolution (Table 1). Fig. 1 shows the results of Fobs-Fcalc omit maps in the vicinity of the crosslinkers.
Table 1.
Summary of selected crystallographic data
| Crosslinked hemoglobin | α2β82CA82β | α2β82ND82β |
| Space group | P212121 | P212121 |
| Cell dimensions, Å | a = 86.8, b = 87.1, c = 97.6 | a = 86.8, b = 87.1, c = 97.6 |
| αβ dimers per ASU | 2 | 2 |
| Data collection | ||
| Resolution, Å | 2.3 | 2.6 |
| Number of observations/reflections | 105,634/34,416 | 65,290/19,979 |
| Rsym, %* | 5.6 | 7.0 |
| Refinement, Å | 10.0–2.3 | 10.0–2.6 |
| Completeness, % | 99 | 78 |
| R factor, %† | 18.4 | 15.4 |
| Number of atoms | 4,576 | 4,578 |
| Number of solvent molecules | 143 | 51 |
| Root-mean-squared deviations | ||
| Bond distances, Å | 0.020 | 0.018 |
| Bond angles, degrees | 2.65 | 2.58 |
α2β82CA82β and α2β82ND82β were crosslinked in their deoxy forms by reacting human deoxyhemoglobin with the bis(methylphosphate) derivatives of either 4-carboxycinnamic acid (CA) or 2,6-napthalene dicarboxylic acid (ND), respectively (15, 16). α2β82CA82β and α2β82ND82β are crosslinked from the Nζ of β1Lys-82 to the Nζ of β2Lys-82. The negative charge of the methyl phosphate leaving groups serves to target the crosslinkers to the cationic 2,3-bis-phosphoglycerate binding pocket where the βLys-82 residues are located. Crystals of the carbonmonoxy form of α2β82CA82β and α2β82ND82β were grown at room temperature by the batch method of Perutz (17) from a solution of 2.4 M sodium-potassium phosphate, pH 6.7. These hemoglobins crystallize in space group P212121, i.e., nonisomorphous with respect to unreacted carbonmonoxy hemoglobin. X-ray intensity data were collected at room temperature with an Area Detector Systems Corporation (ADSC) area detector using a Rigaku RU200-H rotating anode generator as the x-ray source (40 kV, 150 mA). These data were processed with the software provided by ADSC (19). The α2β82CA82β hemoglobin was solved by molecular replacement using the merlot software package (18). The native carbonmonoxy αβ dimer (7), minus waters, was used as the starting model. Two large peaks in both the rotation and translation functions located the molecule and confirmed the presence of a tetramer in the asymmetric unit (ASU). Refinement with TNT (20) and difference Fourier syntheses located the cinnamyl crosslinker, which was fitted using frodo (21). Subsequent cycles of refinement to 2.3 Å followed until convergence. This structure, minus the crosslinker and waters, served as the starting model for the α2β82ND82β structure. Crystals of α2β82ND82β diffract to 2.3 Å resolution; however, they do not grow reproducibly, limiting our current data to 2.6 Å resolution. The napthalenyl crosslinker was located in a difference Fourier map, and the structure was refined to convergence.
RSYM = Σ |Io − 〈I〉|/Io, where Io is the observed intensity, and 〈I〉 is the average intensity from multiple observations of symmetry-related reflections.
R factor = Σ ∥Fobs| − |Fcalc∥/Σ |Fobs|.
Figure 1.
(A) An Fobs-Fcalc omit map of α2β82CA82β in which the cinnamyl crosslinker has been omitted from the model refinement. The contour level is 4.0 σ. (B) An Fobs-Fcalc omit map of α2β82ND82β in which the napthalenyl crosslinker has been omitted from the model refinement. The contour level is 3.5 σ.
RESULTS AND DISCUSSION
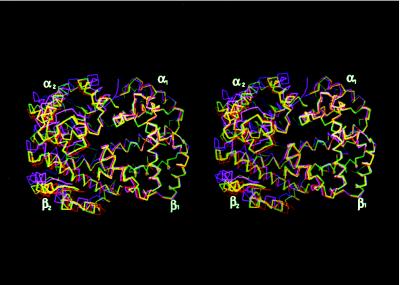
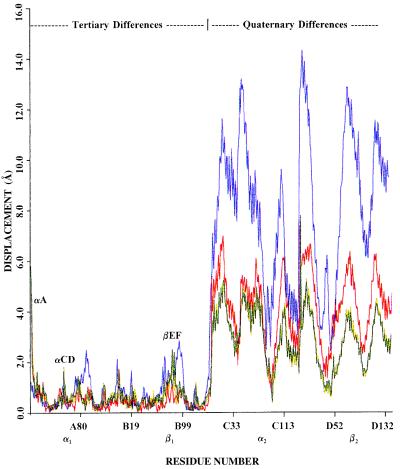
To compare the quaternary structures of α2β82CA82β and α2β82ND82β with those of T, R, and R2 hemoglobin, superimpositions were carried out using the method of Baldwin and Chothia (1). This method involves the superimposition of the appropriate Cα residues of the α1β1 dimers followed by the determinations of the rigid body rotations necessary to align the appropriate Cα residues of the corresponding α2β2 dimers (1). To superimpose the α2β2 dimers of α2β82CA82β and α2β82ND82β onto the α2β2 dimer of the R2 structure requires rotations of 9.0° and 8.4°, respectively (Fig. 2). These are to be compared with rotations of 13.3° for COHbA (R ↔ R2) and 23° for deoxyhemoglobin (T ↔ R2). The tertiary and quaternary differences between the crosslinked hemoglobins and deoxyHbA, COHbA, and R2 hemoglobin were analyzed in more detail by a series of α-carbon coordinate difference plots (CDPs) (Fig. 3). The CDPs confirm the nearly identical tertiary and quaternary structures of α2β82CA82β and α2β82ND82β. Furthermore, they demonstrate that there is little difference in the tertiary structures of the fully liganded forms, i.e., R2, R, and α2β82CA82β and α2β82ND82β, and the slight structural anomalies that are observed are confined to a small region near the site of crosslinker attachment, near the CD turn of the α subunit and the first few residues of the A helix of the α subunit (Fig. 3, labeled βEF, αCD, and αA, respectively). The structural identity also applies to the α and β hemes that are planar in these crosslinked hemoglobins. Finally, although closer to the R structure than to the R2 structure, the CDPs demonstrate unequivocally that the structures of α2β82CA82β and α2β82ND82β lie directly on the path between the R and R2 conformations.
Figure 2.
Stereoview of the overlay of the α1β1 interfaces of α2β82CA82β (yellow Cα trace), α2β82ND82β (green Cα trace), and COHbA (red Cα trace) onto that of R2 hemoglobin (magenta Cα trace), following the method of Baldwin and Chothia (1). In this method the regions in which no structural changes occur in going from T to R are used as a reference frame and include residues α30–α36 (B helix); α102–α113 (G helix); α117–α127 (H helix); β30–β36 (B helix); β51–β55 (D helix); and β107–β132 (G and H helices). After overlaying this region of the α1β1 dimers, a rotation of 13° is required to bring the α2β2 interfaces of the R and R2 structures into coincidence. α2β82CA82β and α2β82ND82β require rotations of 9.0° and 8.4°, respectively. Thus, α2β82CA82β and α2β82ND82β lie between R and R2 in the quaternary pathway.
Figure 3.
Coordinate difference plot (CDP) showing the differences between corresponding Cα atoms of deoxyhemoglobin (blue), COHbA (red), α2β82CA82β (yellow), and α2β82ND82β (green) after their α1β1 interfaces have been superimposed onto that of R2 hemoglobin as described in Fig. 2. The ordinate indicates the displacement of the corresponding Cα atoms, and the abscissa shows the residue number where the A:B dimer corresponds to α1β1 and the C:D dimer corresponds to α2β2. The region labeled tertiary differences (α1β1) corresponds to structural alterations between tertiary elements, such as slight helical displacements. The largest structural difference is confined to the first few residues of the A helix of the α subunit (labeled αA), which, in the R, α2β82CA82β, and α2β82ND82β structures, are identical (see Fig. 2). The region labeled quaternary differences (α2β2) visualizes the large quaternary rotational differences between these structures. From this plot, it is clear that the structures of α2β82CA82β and α2β82ND82β lie directly between those of the R and R2 liganded structures.
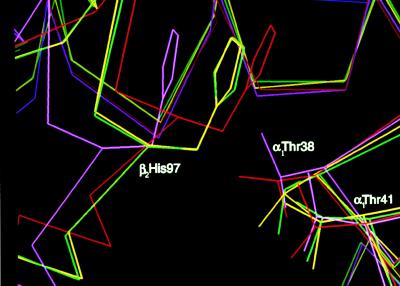
Perhaps the most striking feature that demonstrates the R ↔ R2 intermediate nature of α2β82CA82β and α2β82ND82β is the location of the β2His-97 side chain (Fig. 4). In the R structure the imidazole side chain of β2His-97 is positioned between α1Thr38 and α1Thr41 (7), whereas in the R2 conformation it is disengaged from this pocket (8). For both α2β82CA82β and α2β82ND82β the β2His-97 side chain is found between its R and R2 locations such that the distances between the corresponding Cγ atoms of the imidazole moieties are 1.1 Å for α2β82CA82β to R, and 2.1 Å for α2β82CA82β to R2 (Fig. 4). Thus, the structures of α2β82CA82β and α2β82ND82β provide the first experimental support of the T ↔ R ↔ R2 transitional pathway.
Figure 4.
The switch regions of α2β82CA82β (yellow), α2β82ND82β (green), R2 hemoglobin (magenta), and R hemoglobin (red) after the superimposition of the α1β1 interface as described (1), revealing the intermediate characteristic of the cinnamyl and napthalenyl crosslinked hemoglobins.
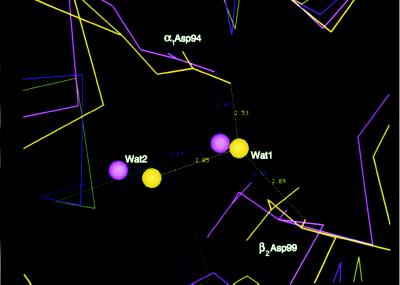
Although clearly more R-like, both α2β82CA82β and α2β82ND82β contain two water molecules in the α1β2 interface that are hallmarks of the R2 structure (8). One of these water molecules (Wat1) maintains the identical hydrogen-bonding network observed in the R2 structure that links the OD2 of α1Asp-94 to the amide nitrogen of β2Asp-99 (Fig. 5), thereby directly supporting the supposition that the α1β2 interface of R2 and its R ↔ R2 intermediates are more solvent accessible than that of R hemoglobin (8, 13). Moreover, that these waters are found in the transitional but more R-like structures of α2β82CA82β and α2β82ND82β (Figs. 2 and 3) suggests that their removal from or addition to the α1β2 interface constitutes a major determinant in the R ↔ R2 pathway.
Figure 5.
The α1β2 interfaces of α2β82CA82β (yellow) and R2 hemoglobin (magenta) after the superimposition of the α1β1 interface as described (1), revealing the presence of two water molecules, Wat1 and Wat2, which are hallmarks of the R2 structure and not found in the R or T quaternary structure. Wat1 is hydrogen bonded to the OD2 of α1Asp-94 (2.53 Å and 2.48 Å for α2β82CA82β and R2, respectively) and the amide nitrogen of β2Asp-99 (2.89 Å and 2.95 Å for α2β82CA82β and R2, respectively), whereas Wat2 is hydrogen bonded to Wat1 (2.85 Å and 3.19 Å for α2β82CA82β and R2, respectively).
Central to the cooperative quaternary transition of hemoglobin are COOH-terminal residues α140-α141 and β145-β146 (1–7). In the unliganded state, these residues participate in interactions that are essential for the stabilization of the T conformation. However, upon ligand binding these T-state interactions are lost, and in the high-phosphate environment (greater than 2 M) of the crystallized R state (17), the COOH-terminal residues are very mobile. The continued transition to the R2 state, crystallized under physiologically relevant anion concentrations (8, 10), results in the repositioning of the β1 and β2His-146 imidazole side chains, which then stack against one another, and the establishment of a salt bridge between the α-carboxylate group of β2His-146 and the β1Lys-82 side chain (8). Thus, the β1Lys-82–β2His-146 salt bridge appears to be an important determinant in the choice between the R and R2 conformations and highlights the importance of the environment on the liganded conformations of hemoglobin. Specifically, we propose that the high-phosphate concentration used to crystallize COHbA (17), which is 20-fold greater than the Kd of orthophosphate for oxyHbA (22, 23), disrupts the Lys-82–His-146 interaction and thereby destabilizes the R2 conformation. In the absence of high-phosphate concentrations, or in the presence of lower ionic strength solutions that are physiologically more relevant, the R2 conformation would be favored. The intermediate nature of the crosslinked hemoglobins is dictated by the opposing forces of a high-phosphate environment, which stabilizes many structural aspects of the R conformation, and the long bridging distances of the crosslinking reagents, which indirectly favor the R2-like α1β2 interface.
CONCLUSION
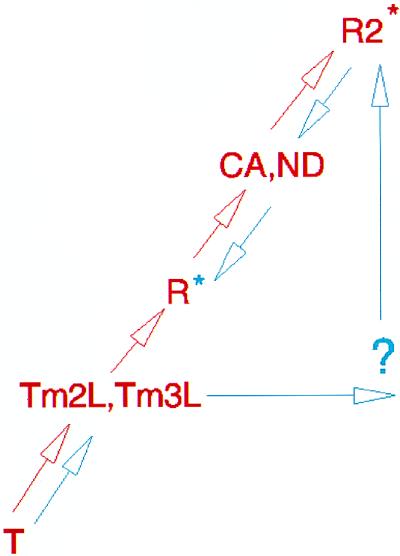
The structures of liganded α2β82CA82β and α2β82ND82β, together with those of crosslinked hemoglobins α2β1Tm82β and α2β1,82Tm82β, which display quaternary structures intermediate to those of T and R (24), support only a T ↔ R ↔ R2 transitional pathway (Fig. 6, red arrows). That is, for the R2 structure to represent a T ↔ R intermediate, an unlikely backward trajectory of the quaternary structure is required that would also have to link the structures of α2β1Tm82β and α2β1,82Tm82β to R2 (Fig. 6, blue arrows). In light of this proposed conformational pathway, there now is a need to reassess the liganded state of hemoglobin; specifically, its increased plasticity (3, 8–10, 25), the effects of environment on the quaternary structure, and the functional importance of these different conformers in a given physiological context.
Figure 6.
Schematic representation of the proposed quaternary pathway of hemoglobin’s transition from the unliganded to liganded state. T, unliganded quaternary state; Tm2L, fully liganded, inter-β-subunit trimesoyl-crosslinked hemoglobin, α2β1Tm82β (22); Tm3L, fully liganded, intra- and inter-β-subunit trimesoyl-crosslinked hemoglobin, α2β1,82Tm82β (22); R, fully liganded, high-phosphate R state hemoglobin; CA, fully liganded, crosslinked hemoglobin, α2β82CA82β (described here); ND, fully liganded, crosslinked hemoglobin, α2β82ND82β (described here); R2, fully liganded R2 state. Two alternative paths are presented: one in which the R state is an intermediate and the R2 state is the final liganded state (red) and a second in which the R2 state is an intermediate and the R state is the final liganded state (blue). For each, an appropriate colored asterisk, blue or red, marks the final liganded state. The quaternary trajectory, as supported by the crystal structures of four intermediate quaternary structures (Tm2L, Tm3L, CA, and ND), is consistent only with the pathway depicted in red.
Acknowledgments
We thank Ms. X. Hong for preparing the crosslinked hemoglobins and determining their oxygen affinities and Hill coefficients. This work was supported in part by the National Institutes of Health (HL-20142), the Department of Defense, the Oregon Health Sciences Foundation, and the Natural Sciences and Engineering Research Council of Canada.
ABBREVIATION
- COHbA
unmodified carbonmonoxy hemoglobin
Footnotes
Data deposition: The atomic coordinates have been deposited in the Protein Data Bank, Chemistry Department, Brookhaven National Laboratory, Upton, NY 11973 [references 1hab (α2β82CA82β) and 1hac (α2β82ND82β)].
References
- 1.Baldwin J, Chothia C. J Mol Biol. 1979;129:175–220. doi: 10.1016/0022-2836(79)90277-8. [DOI] [PubMed] [Google Scholar]
- 2.Fermi G, Perutz M, Shaanan B, Fourme R. J Mol Biol. 1984;175:159–174. doi: 10.1016/0022-2836(84)90472-8. [DOI] [PubMed] [Google Scholar]
- 3.Shaanan B. J Mol Biol. 1983;171:31–59. doi: 10.1016/s0022-2836(83)80313-1. [DOI] [PubMed] [Google Scholar]
- 4.Perutz M F. Nature (London) 1970;228:726–739. doi: 10.1038/228726a0. [DOI] [PubMed] [Google Scholar]
- 5.Dickerson R E, Geis I. Hemoglobin: Structure, Function, Evolution, and Pathology. Menlo Park, CA: Benjamin & Cummings; 1983. pp. 3–63. [Google Scholar]
- 6.Perutz M F. Annu Rev Physiol. 1990;52:1–25. doi: 10.1146/annurev.ph.52.030190.000245. [DOI] [PubMed] [Google Scholar]
- 7.Derewenda Z, Dodson G, Emsley P, Harris D, Nagai K, Perutz M, Renaud J P. J Mol Biol. 1990;211:515–519. doi: 10.1016/0022-2836(90)90262-k. [DOI] [PubMed] [Google Scholar]
- 8.Silva M M, Rogers P H, Arnone A. J Biol Chem. 1992;267:17248–17256. [PubMed] [Google Scholar]
- 9.Smith F R, Lattman E E, Carter C W. Proteins. 1991;10:81–91. doi: 10.1002/prot.340100202. [DOI] [PubMed] [Google Scholar]
- 10.Smith F R, Simmons K C. Proteins. 1994;18:295–300. doi: 10.1002/prot.340180310. [DOI] [PubMed] [Google Scholar]
- 11.Doyle M L, Lew G, Turner G, Rucknagel D, Ackers G K. Proteins. 1992;14:351–362. doi: 10.1002/prot.340140304. [DOI] [PubMed] [Google Scholar]
- 12.Janin J, Wodak S J. Proteins. 1993;15:1–4. doi: 10.1002/prot.340150102. [DOI] [PubMed] [Google Scholar]
- 13.Srinivasan R, Rose G D. Proc Natl Acad Sci USA. 1994;91:11113–11117. doi: 10.1073/pnas.91.23.11113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones R T, Shih D T, Fujita T S, Sona Y, Xiao X, Head C, Kluger R. J Biol Chem. 1996;271:675–680. doi: 10.1074/jbc.271.2.675. [DOI] [PubMed] [Google Scholar]
- 15.Kluger R, Shen L, Xiao H, Jones R T. J Am Chem Soc. 1996;118:8782–8786. [Google Scholar]
- 16.Kluger R, Grant A S, Bearne S L, Trachsel M R. J Org Chem. 1990;55:2864–2868. [Google Scholar]
- 17.Perutz M F. J Cryst Growth. 1968;2:54–56. [Google Scholar]
- 18.Fitzgerald P M D. J Appl Crystallogr. 1988;21:273–278. [Google Scholar]
- 19.Xuong NH, Nielsen C, Hamlin R, Anderson D. J Appl Crystallogr. 1985;18:342–350. [Google Scholar]
- 20.Tronrud D E, Ten Eyck L F, Matthews B W. Acta Crystallogr A. 1987;43:489–501. [Google Scholar]
- 21.Jones T A. J Appl Crystallogr. 1978;11:268–272. [Google Scholar]
- 22.Bare G H, Alben J O, Bramberg P A, Jones R T, Brimhall B, Padilla F. J Biol Chem. 1974;249:773–779. [PubMed] [Google Scholar]
- 23.Imai K. Allosteric Effects in Haemoglobin. Cambridge, U.K.: Cambridge Univ. Press; 1982. pp. 39–45. [Google Scholar]
- 24.Schumacher M A, Dixon M M, Kluger R, Jones R T, Brennan R G. Nature (London) 1995;375:84–87. doi: 10.1038/375084a0. [DOI] [PubMed] [Google Scholar]
- 25.Kroeger K S, Kundrot C E. Structure. 1997;5:227–237. doi: 10.1016/s0969-2126(97)00181-0. [DOI] [PubMed] [Google Scholar]