Abstract
Our ability to see in bright light depends critically on the rapid rate at which cone photoreceptors detect and adapt to changes in illumination. This is achieved, in part, by their rapid response termination. In this study, we investigate the hypothesis that this rapid termination of the response in red cones is dependent on interactions between the 9-methyl group of retinal and red cone opsin, which are required for timely metarhodopsin (Meta) II decay. We used single-cell electrical recordings of flash responses to assess the kinetics of response termination and to calculate guanylyl cyclase (GC) rates in salamander red cones containing native visual pigment as well as visual pigment regenerated with 11-cis 9-demethyl retinal, an analogue of retinal in which the 9-methyl group is missing. After exposure to bright light that photoactivated more than ∼0.2% of the pigment, red cones containing the analogue pigment had a slower recovery of both flash response amplitudes and GC rates (up to 10 times slower at high bleaches) than red cones containing 11-cis retinal. This finding is consistent with previously published biochemical data demonstrating that red cone opsin regenerated in vitro with 11-cis 9-demethyl retinal exhibited prolonged activation as a result of slowed Meta II decay. Our results suggest that two different mechanisms regulate the recovery of responsiveness in red cones after exposure to light. We propose a model in which the response recovery in red cones can be regulated (particularly at high light intensities) by the Meta II decay rate if that rate has been inhibited. In red cones, the interaction of the 9-methyl group of retinal with opsin promotes efficient Meta II decay and, thus, the rapid rate of recovery.
INTRODUCTION
The detection of changes in our visual environment occurs through the photoactivation of rod and cone photoreceptors located in the retina. Under scotopic conditions (dim light), such as those that exist during night, this function is subserved by the rods. In the brighter conditions of daylight, this takes place through the photoactivation of cones. For the retina to process the dynamic changes that occur in the visual environment as intensities of light change and as images travel across the retina, it is critical that both rods and cones be capable of faithfully signaling the onset (increase) as well as the termination (decrease) of light.
Detection of the onset of light occurs by the activation of a series of biochemical reactions collectively referred to as the phototransduction cascade. In both receptor types, it begins with the absorption of a photon by the visual pigment (Yoshizawa and Wald, 1963; Wald, 1968). The visual pigment is composed of the chromophore 11-cis retinal bound covalently to a G protein–coupled receptor. The receptor consists of seven α-helical transmembrane domains to which the chromophore is covalently attached by a Schiff base linkage at lysine 296 in the seventh transmembrane segment. The visual pigment is tightly packed in membranes of the outer segments of both rod and cone photoreceptors. Photon absorption leads to an almost instantaneous isomerization of the chromophore to its all-trans configuration and to the eventual formation of an activated form of the pigment, metarhodopsin (Meta) II. For rhodopsin, it is known that Meta II catalyzes the exchange of guanosine triphosphate (GTP) for guanosine diphosphate on the α subunit of the G protein transducin. Each transducin activated in this way can then bind to the γ subunit of cyclic guanosine monophosphate (cGMP) phosphodiesterase (PDE) to produce the activated form that catalyzes the hydrolysis of the intracellular messenger, cGMP. The lowered cytosolic concentration of cGMP that results from light absorption thus triggers the closure of cation cGMP-gated channels in the outer segment membrane, membrane hyperpolarization, and, finally, a decrease in neurotransmitter release by synaptic processes onto secondary retinal neurons (for recent reviews of rod phototransduction see Fain et al., 2001; McBee et al., 2001; Burns and Arshavsky, 2005).
The recovery of responsiveness during and after exposure to light requires the efficient and timely termination of the multiple excitation processes discussed above. One of the most important of these mechanisms is the initial step, the deactivation of Meta II. Meta II in rods has a spectroscopically measured lifetime in intact retina that is on the order of minutes to tens of minutes (Baumann, 1972; Brin and Ripps, 1977; Kibelbek et al., 1991). However, physiological measurements show that its effective lifetime is no longer than a few seconds and is possibly much shorter (Pepperberg et al., 1992; Lyubarsky and Pugh, 1996; Matthews et al., 1996; Murnick and Lamb, 1996). This shortness of Meta II effectiveness in rods is caused by its rapid phosphorylation by rhodopsin kinase (GRK1) and subsequent binding to arrestin (Arr1), a capping protein that effectively terminates activation (Hofmann et al., 1992; Arnis et al., 1994). Thus, response termination of rods after exposure to dim light is relatively rapid, occurring within about a second after light termination. However, the exposure of rods to brighter lights that photoactivate (bleach) a significant fraction of the visual pigment are very slow to recover, and the complete recovery of sensitivity requires tens of minutes (for reviews see Fain et al., 2001; Lamb and Pugh, 2004). This slowness of recovery has been attributed to the persistence of Meta II and Meta II–like photoproducts of bleaching that sustain the activity of the transduction cascade at high levels (Leibrock et al., 1998; for review see Lamb and Pugh, 2004). Eventual recovery occurs as Meta II decays (the Schiff base linkage between retinal and opsin is hydrolyzed), and all-trans retinal is reduced to all-trans retinol (Firsov et al., 2005).
The factors that regulate Meta II lifetime in cones and the effects of phosphorylation and arrestin binding are less well studied. The decay of Meta II measured spectroscopically is considerably more rapid in cones compared with rods. For instance, recent microspectrophotometric measurements of metapigment decay in salamander red and blue cones show that Meta product decay in cones is >70-fold faster than that in red rods (Ala-Laurila et al., 2006). Meta II in cones has been shown to be rapidly multiphosphorylated (Kennedy et al., 2004). Cones contain two different but related kinases, GRK1 and GRK7, depending on the species (Weiss et al., 2001; Liu et al., 2005). Recently, GRK7 has been shown to be dominate in zebrafish cones (Wada et al., 2006). The kinases present in the salamander cone have not been identified. The physiological importance of the phosphorylation of cone pigment has been demonstrated by measurements of photoresponses of S and M cones in Nrl −/− /GRK −/− mice in which the recovery of flash responses was considerably slowed compared with responses measured in cones of wild-type mice in which the kinase was normal (Nikonov et al., 2005). The salamander cone arrestin has been identified (Smith et al., 2000) and crystallized (Sutton et al., 2005), although its role has not been extensively studied. It has been shown that cone arrestin does bind to the phosphorylated cone opsins in the Nrl −/− mouse after photoactivation (Zhu et al., 2003). However, in wild-type mice, the light-dependent movement of cone arrestin has been found not to be dependent on the association of arrestin with the pigments, as is true in mouse rods (Zhang et al., 2003a). Thus, some differences in the rod and cone transduction pathways are being identified
Cones exposed to bright light recover their responsiveness rapidly, within a few seconds of the termination of bright light, regardless of its intensity. Indeed, this effect is so robust that response recovery occurs even in the presence of bright background lights that bleach in excess of 90% of their visual pigment (Kenkre et al., 2005). Were it not so, our cone system could not operate effectively in bright daylight conditions.
The reasons for the large differences in the rate of response recovery between rods and cones are not well understood. One possible mechanism that may contribute to rapid response recovery in cones exposed to bright light is a rapid rate of Meta II decay. A recent study by Das et al. (2004) has suggested that this may occur as a result of interactions between the particular opsin and specific structural elements of retinal. In these studies, visual pigment was constituted from red cone opsin expressed in COS cells and 11-cis 9-demethyl retinal, an analogue of retinal in which the 9-methyl group has been deleted. Bleaching of this visual pigment was shown to activate transducin normally, but the termination of this reaction was several-fold slower than when the 9-methyl group was present, as assessed by the slower rate at which visual pigment could be regenerated. From these data, the authors suggested that the 9-demethyl red cone Meta II, unlike the other cone opsins or the rod opsin, was abnormally slow to decay.
The experiments presented here were designed to test the hypothesis that the 9-methyl group of the ligand is critical for normal pigment decay and, thus, response termination. We measured the recovery of step responses to light in salamander red cones bleached and regenerated to contain either native 11-cis retinal (A1)–based visual pigment or analogue visual pigment in which the 9-methyl group on retinal was missing. Our results show that the termination of light responses in salamander red cones containing 9-demethyl visual pigment are slower to recover responsiveness than red cones containing the native chromophore. Interestingly, at dim flash intensities, this was not observed. These data provide evidence that different mechanisms may be operative for high bleaches.
MATERIALS AND METHODS
Animals and Preparation
Experiments were performed on intact red-sensitive cone photoreceptor cells that were isolated from retinae of larval tiger salamanders (Ambystoma tigrinum). Animals were purchased from Charles D. Sullivan, Inc. All experiments were performed according to procedures approved by the Institutional Animal Care and Use Committee at the Boston University School of Medicine and in accordance with the Guide for the Care and Use of Laboratory Animals and the Animal Welfare Act.
Salamanders were dark adapted overnight, killed by decapitation, and double pithed under dim red illumination. Eyes were enucleated and hemisected in physiological Ringer solution under infrared illumination, to which the cone photoreceptors were insensitive. The Ringer solution for all dissections and measurements contained 110 mM NaCl, 2.5 mM KCl, 1.6 mM MgCl2, 1.0 mM CaCl2, 10 mM dextrose, 10 mM HEPES, and 100 mg/L BSA. The pH was adjusted to 7.8. The retinae were separated from the retinal pigment epithelium by dissection with fine forceps. Photoreceptors were isolated by chopping the retina with a small piece of razor blade. The resulting suspension of retinal debris and intact cells in Ringer solution was transferred to a gravity-fed perfusion chamber located on the stage of an inverted microscope (Invertescope D; Carl Zeiss MicroImaging, Inc.). Individual cells were viewed using an infrared television camera (LCL-902K; Watec American Corp.) and monitor fitted to the microscope. All of these methods have been described previously (Cornwall et al., 1983, 1990, 2000).
Cones were distinguished from rods by their outer segment morphology (Cornwall et al., 1984; Mariani, 1986; Makino and Dodd, 1996; Sherry et al., 1998). Red cones were distinguished from blue and UV cone types electrophysiologically by measuring their relative sensitivity to test flashes of 620-, 440-, and 380-nm light.
Pigment Regeneration
11-cis retinal and 11-cis 9-demethyl retinal, an analogue of retinal in which the 9-methyl group has been deleted (Fig. 1 B), were synthesized and purified in the Department of Ophthalmology at the Medical University of South Carolina (Crouch et al., 2002). In all experiments involving the regeneration of visual pigment with 11-cis retinal, the A1 form was used. Before each experiment, a retinoid solution was prepared by adding 3 μl ethanol and 300 μg retinoid to 3 ml of Ringer (a 1,000-fold dilution). The exact retinoid concentration of this solution was determined spectrophotometrically (the molar extinction coefficient for 11-cis retinal is 24,900 M−1 cm−1 at 380 nm [Brown and Wald, 1956], and that for 11-cis 9-demethyl retinal is 30,000 M−1 cm−1 at 376 nm [Kono, M., personal communication]). Retinoid solutions were adjusted to a final concentration of 35 μM.
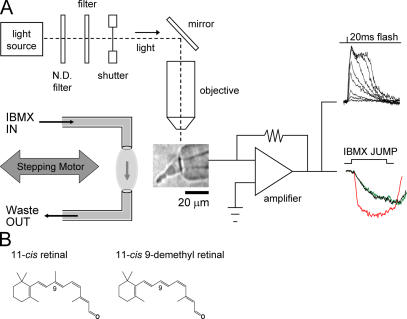
Figure 1.
Schematic representation of the experimental set-up and retinals used in this study. (A) A photographic image of a salamander red cone photoreceptor, drawn inner segment first into a glass pipette, is shown in the middle of the figure. The pipette is connected to a patch clamp amplifier, the output of which measures electrical responses of the cell to light stimulus (superimposed flash responses, top right). The light source used for test flashes and bleaching is shown at the top left. GC rates were determined by measuring current changes when cells were briefly exposed to a solution containing 500 μM IBMX, a PDE inhibitor. This device (shown to the left of the cone) consisted of two pipettes mounted to a micromanipulator and connected to a computer-driven stepping motor. The three superimposed traces to the right of the amplifier are shown for illustration sake only and represent typical jump recordings from a cell in the dark-adapted (black), bleached (red), and regenerated states (green; see Fig. 7 for further details). ND, neutral density. (B) Structure of retinals are shown. Left, 11-cis retinal; right, 11-cis 9-demethyl retinal is an analogue lacking the methyl group at the 9-carbon position.
All electrophysiological experiments involving visual pigment replacement were performed according to the following procedure. A cone was exposed to a light that bleached 99% of its visual pigment and allowed 10 min to recover its sensitivity to a steady state. Then, under infrared illumination, 1 ml of 35-μM 11-cis 9-demethyl retinal was added directly to the experimental chamber (bath volume of ∼1 ml) using a pipetter. The final concentration of retinoid in the bath was measured to be between 10 and 15 μM. The analogue retinoid remained in the bath for 5 min before being washed out. The time course of visual pigment regeneration was subsequently monitored by measuring the sensitivity and response amplitude to saturating test flashes over the course of 1 h or until the cell reached a steady state and full recovery was observed.
Electrophysiological Measurements
Extracellular membrane current recordings were performed by methods that have been described previously (Cornwall et al., 1983, 2000) and are similar to those pioneered by Baylor et al. (1979). A diagram of the experimental apparatus is shown schematically in Fig. 1.
An individual intact cone photoreceptor was drawn, inner segment first, into a tight-fitting fire-polished capillary glass pipette (∼10-μm inner diameter) that was filled with Ringer solution (Fig. 1 A, middle image). The solution in the pipette was connected via a silver/silver chloride junction to the head stage of a patch-clamp amplifier (EPC-7; List Electronic). Light-induced changes in membrane current were converted to voltage, amplified, low-pass filtered, digitized, and stored on a computer hard drive. Subsequent analysis was performed using data acquisition (pClamp6; Axon Instruments, Inc.) and analysis software (Origin 6.1; Microcal Software, Inc.).
Light Stimulation
Test flashes and bleaching lights were provided by an optical stimulator (Fig. 1 A, top left) as described previously (Cornwall et al., 1990). Output from the light source was passed through interference filters (half-band of 10 nm) and neutral density filters before entering a 10× microscope objective that focused a 930-μm–diameter spot at the plane of the preparation. An electronic shutter was used to provide 20- or 40-ms flashes as well as 4-s periods of bleaching light. The light intensity was set before the beginning of each experiment using a calibrated photometer (model 80X; United Detector Technology). The absolute intensity of the unattenuated light at 620 nm was 2.87 × 108 photons μm−2s−1. The wavelength of light stimulation (bleaching and test flashes) for dark-adapted red cones containing native visual pigment was 620 nm. For red cones regenerated with 11-cis retinal or 11-cis 9-demethyl retinal, the stimulation light was at 560 or 520 nm, respectively.
Visual Pigment Bleaching
The fraction of visual pigment bleached after exposure to bright light was either estimated by calculation or measured directly by microspectrophotometry. The calculation was made according to the relation F = 1 − exp(−IPt), where F is the fraction of pigment bleached, I is the bleaching light intensity in photons μm−2s−1, and P is the photosensitivity of cones at the wavelength of peak absorbance (6.0 × 10−9 μm2; Jones, 1995). Microspectrophotometric estimates of the extent of visual pigment bleaching were made by comparing the absorbance spectra of photoreceptor outer segments before and after the bleach as described in the next paragraph.
Microspectrophotometry
A photon-counting microspectrophotometer designed and built by E.F. MacNichol (MacNichol, 1978) was used to characterize in situ spectral absorbance properties over the range of 400–800 THz (375–750 nm) of red cones containing native and analogue visual pigment (Cornwall et al., 1984; Jones et al., 1993). Measurements were obtained in darkness, before and after bleaching, and after pigment regeneration with 11-cis retinal or 11-cis 9-demethyl retinal. The absorption spectrum was calculated at 80 points over this range using the equation OD = log10(Io/It), where Io is the transmitted light in a cell-free space adjacent to the photoreceptor and It is the transmitted light through the outer segment of the photoreceptor. Up to 15 such baseline and sample scans were averaged to produce a final spectrum. Individual scans of the outer segments were determined to have bleached <0.2% of the visual pigment content of dark-adapted outer segments (Cornwall et al., 1984). Correction for nonspecific absorbance and light scattering was made by fitting a straight line by the method of least squares to the long wavelength portion of the spectrum where absorbance caused by the visual pigment is negligible. Differences between these derived values and zero absorbance were then subtracted from the raw spectra to produce a baseline-corrected spectrum. A second order polynomial was fitted to the data points (Ala-Laurila et al., 2002). λmax values were determined from the peak of the best-fitting polynomial, and spectra were normalized to maximum OD.
Measurement of the GC Rate
To estimate changes in the enzymatic rate of guanylyl cyclase (GC) in intact red cones under different conditions of adaptation and pigment content, we made use of a technique that was first developed by Hodgkin and Nunn (1988) and was later used by Cornwall and Fain (1994). For a detailed description of the theory and analysis of these measurements, see Cornwall et al. (2000).
The cytosolic concentration of free cGMP (i.e., [cGMP]) is controlled by the balance between its synthesis by GC and its hydrolysis by PDE. Thus, the cGMP economy can be completely accounted for by
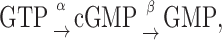 |
(1) |
where α and β are the rate constants of GC and PDE for the production and hydrolysis of cGMP, respectively. Based on equation 1, the change in [cGMP] can be deduced as
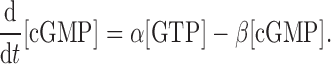 |
(2) |
Furthermore, the relation between [cGMP] and light-sensitive membrane current (i) is given by Pugh and Lamb (1990):
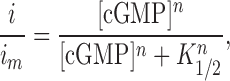 |
(3) |
where i m is the maximum possible membrane current, K 1/2 is the Michaelis constant for binding cGMP to the light-sensitive channels, and n is the Hill coefficient for this cooperative binding. Combining equations 2 and 3 yields a relation between the membrane current (i) and the rate constants of cGMP production (α) and its hydrolysis (β) by GC and PDE, respectively:
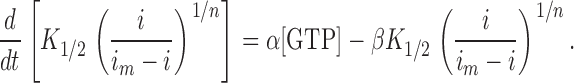 |
(4) |
According to this equation, the sudden block of GC (α = 0) allows an analytical estimation of the PDE rate constant, β. Sudden block of PDE (β = 0) allows an analytical estimate of the GC rate constant, α. When measured under steady-state conditions of light or dark adaptation, these two rates must be equal, as has been demonstrated previously (Cornwall and Fain, 1994), and it suffices to measure either rate to obtain an estimate of the transduction cascade activity under given experimental conditions.
In our experiments, cone outer segments were briefly exposed to a Ringer solution containing 500 μM 3-isobutyl-1-methylxanthine (IBMX), a potent PDE inhibitor (Lipton et al., 1977). Under this condition, the hydrolysis of cGMP by PDE is inhibited, yielding β = 0. Thus, equation 4 becomes
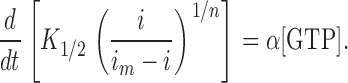 |
(5) |
We make the following further assumptions (for discussion about the validity of the assumptions, see Hodgkin and Nunn, 1988; Cornwall and Fain, 1994; Kefalov et al., 1999; Cornwall et al., 2000): (1) i m ≫ i, yielding i m − i ≈ i m; (2) i m, K 1/2, and [GTP] are essentially unchanged (i.e., are constants during the IBMX exposure); and (3) the Hill coefficient is n = 3 (Yau and Baylor, 1989). Furthermore, i can be replaced by the normalized current, J = i /i D, where i D is the dark current (i.e., amplitude of a saturating light response). Thus, by making these aforementioned assumptions, replacing i with J, and solving equation 5 for the GC rate (α), one gets
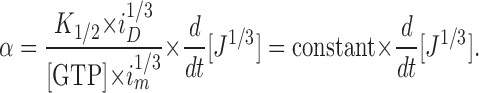 |
(6) |
Thus, the rate of cGMP production by GC is directly proportional to the time derivative of the cube root of the normalized current. Equation 6 was used in this study for comparisons of GC rates under different conditions (e.g., in the dark-adapted state and after bleaching) in cells containing different visual pigment chromophores. The construction of the microperfusion system for rapidly delivering test solutions of IBMX to cells has been described previously (Cornwall and Fain, 1994; Cornwall et al., 2000). A schematic diagram of the perfusion system used for exposure of the cones to steps of IBMX solution as well as examples of changes in membrane current that are measured using this technique are illustrated in Fig. 1 A.
RESULTS
Postbleach Flash Response Recover
The top panel in Fig. 2 A illustrates a recording of extracellular membrane current made from the inner segment of a dark-adapted red cone before and after a 4-s exposure to a bright light that bleached in excess of 90% of the visual pigment. Here, it can be seen that the onset of the light is associated with an early transient peak response followed by a plateau phase during which the cell was refractory to flashes of any incremental flash intensity. This plateau phase was followed by an additional recovery (the increase in dark current is plotted downward in Fig. 2 A) during which responsiveness recovered. The time course of this recovery phase was monitored by stimulating the cell with 20-ms saturating test flashes at 10-s intervals (Fig. 2 A, top; light monitor). From this record, it is clear that although the cell had experienced photoactivation of >90% of its visual pigment, the recovery of responsiveness commenced within 10 s of the end of light exposure; more than half of its prebleach response amplitude recovered within 20 s. This occurred even though the cell was unable to regenerate visual pigment as a result of its isolation from the retinal pigment epithelium. This illustrates one of the fundamental properties of cone photoreceptors that distinguish them from rods: namely, their ability to continue to respond to light in the face of a multilog unit reduction in sensitivity caused by pigment bleaching.
Figure 2.
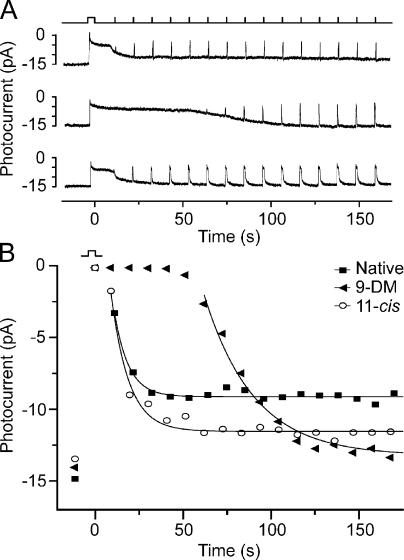
The effect of 11-cis 9-demethyl retinal (9-DM) on the time course of flash response recovery after a bleach. (A) Current recordings were made from the same red cone under three different conditions: the cell contained native dark-adapted pigment (top), the cell contained 11-cis 9-demethyl pigment (middle), and the cell contained 11-cis retinal pigment (bottom). In each recording, the cell was exposed to a 4-s step of 620-nm light that bleached in excess of 90% of the visual pigment. Recovery of responsiveness was monitored by exposure to 20-ms saturating test flashes at 10-s intervals (see light monitor). (B) Time course of the recovery of photocurrent (increasing amplitude plotted downward) as determined from the responses shown in A. Solid lines indicate data fitted with a single exponential function. The experiment was performed on a total of nine cells with similar results; mean time constants are presented in Table I.
The middle panel of Fig. 2 A shows the result of a repetition of this experimental protocol on this same cell after regeneration with the analogue retinoid 11-cis 9-demethyl retinal to form 9-demethyl visual pigment. The method for replacing the native visual pigment with analogue visual pigment in a single red cone is described in Materials and methods. It is clear from an inspection of these two records that deletion of the 9-methyl group from retinal in the visual pigment caused an approximately fivefold delay in termination of the plateau phase of the response as well as a considerable slowing in the recovery of responsiveness to test flashes. To ensure that this slowed recovery was caused by the absence of the 9-methyl group and not to confounding effects of the regeneration protocol, the cell was bleached multiple times, and the visual pigment was regenerated with exogenous 11-cis retinal. The bottom panel of Fig. 2 A shows that under this condition, the flash response recovery after the 4-s bleach was restored to the original dark-adapted rate, as seen in the top panel of Fig. 2 A. Together, these traces show that the replacement of native pigment with the analogue visual pigment was fully reversible in a single experiment.
Fig. 2 B plots the recovery of responsiveness observed in the three conditions of Fig. 2 A as assessed by plotting the maximum amplitudes of the saturating flash responses as a function of time after the bleach (increasing amplitude plotted downward). Here, it can be seen that the time course for the recovery of responsiveness is similar whether the cell contained its native pigment (consisting of a mixture of A1 and 11-cis 3,4-dehydroretinal [A2] chromophores; Fig. 2 B, closed squares) or the exogenous A1-based 11-cis retinal pigment alone (Fig. 2 B, open circles). In contrast, when the cell contained 9-demethyl visual pigment, the recovery of responsiveness after bleaching was considerably delayed. The time constants for recovery were estimated by the fitting of single exponential functions to the data points, as illustrated by the smooth curves.
The experiment in Fig. 2 A was repeated in nine other red cones, and the results are tabulated in Table I. Results in all of these experiments were consistent with those illustrated in Fig. 2. However, complete reversibility of the effect, as illustrated in Fig. 2 A (bottom) with 11-cis retinal, was achieved in only three of these as a result of technical difficulties of the experimental protocol; not all cells survived long enough to be rebleached and regenerated with 11-cis retinal. The delay before the commencement of flash response recovery, t0, corresponds to the plateau phase of the response and represents the time between the termination of the bleaching light and the beginning of flash response recovery, as predicted by the exponential curve fitted to the data points. Means are shown in the second column of Table I, and the mean time constant for the recovery of responsiveness during the recovery phase, τ, is shown in the third column. Based on these mean values, red cones that contained 9-demethyl visual pigment had an ∼10 times longer plateau phase (t0), during which no responses could be elicited, and had an approximately three times slower time constant of recovery (τ) compared with cells containing native visual pigment. Together, these data indicate that the 9-methyl group of retinal is required in the rapid recovery of red cones containing the native chromophore after intense light exposure.
TABLE I.
Mean Rates for Flash Response Recovery and GC Recovery in Red Cones after >90% Bleach
| Pigment type |
t0 for flash recovery |
τ for flash recovery |
τGC for GC recovery |
|---|---|---|---|
| s | s | s | |
| Native | 3.93 ± 0.76 (n = 9) |
11.69 ± 1.44 (n = 9) |
8.52 ± 1.24 (n = 5) |
| 9-demethyl retinal |
38.64 ± 3.87 (n = 9) |
33.80 ± 2.71 (n = 9) |
37.00 ± 6.48 (n = 5) |
| 11-cis retinal | 6.79 ± 4.44 (n = 3) |
13.27 ± 1.85 (n = 3) |
ND |
t0 is the mean time between the end of the light exposure (>90% bleach) and the time at which flash response recovery commenced. τ is the mean time constant for the recovery of maximum amplitude flash responses after >90% bleach. τGC is the mean time constant for the recovery of steady state GC activity after >90% bleach. Both t0 and τ were averaged from exponential curves fitted to individual cells as illustrated in Fig. 2 B. τGC was averaged from exponential curves as illustrated in Fig. 8 (main panel). All data are the mean ± SEM.
Photoreceptor cells that are mechanically isolated from the retina and separated from the retinal pigment epithelium, as is the case in Fig. 2 A (top), are unable to spontaneously regenerate their bleached visual pigment. Therefore, the recovery of dark current after bleaching is only partial (Fig. 2, A [top] and B [closed squares]). This is because there is no 11-cis retinal present and there can be no visual pigment regeneration. Thus, the flash responses elicited subsequently to the bleach had reduced amplitude and accelerated response kinetics that are characteristic of cells containing a large amount of free opsin. However, when a photoreceptor has been regenerated with exogenous chromophore, as was the case in the two bottom traces in Fig. 2 A, total pigment regeneration occurs, and sensitivity and responsiveness recover more completely to dark-adapted levels (Fig. 2 A, middle and bottom; and see corresponding data in B). Thus, the recovery of dark current subsequent to treatment with 11-cis 9-demethyl retinal and 11-cis retinal is more complete than when no exogenous retinoid was present. This also explains the slower time course of flash responses after pigment regeneration in the bottom trace of Fig. 2 A compared with the top trace. The reason for the fast time course of flash responses in the middle panel (Fig. 2 A), even though dark current had substantially recovered, is not known, but it may have resulted from a slower recovery of response kinetics in the analogue-containing cone.
Microspectrophotometric Measurements of Native and Analogue Visual Pigment
A fundamental assumption that is implicit in our explanation for the results shown in Fig. 2 is that treatment of bleached cells with native and analogue chromophores resulted in regeneration of their visual pigment. The experiments illustrated in Fig. 3 were designed to demonstrate that this is the case. Fig. 3 shows normalized red cone visual pigment spectra measured under the three conditions illustrated in Fig. 2 A: when the cells contained native pigment, when the cells contained 11-cis 9-demethyl pigment, and when the cells contained 11-cis retinal (A1) pigment. Measurements were made in isolated photoreceptors using a microspectrophotometer as described in Materials and methods.
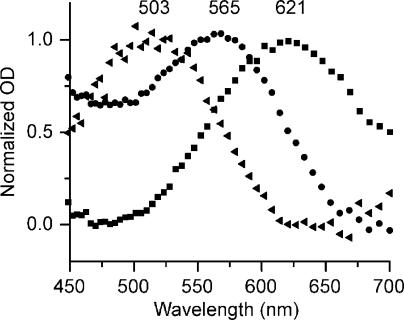
Figure 3.
Absorbance spectra of salamander red cones containing different visual pigments. All spectra were measured microspectrophotometrically as described in Materials and methods. Normalized mean absorbance (optical density) is plotted as a function of wavelength for red cones in their native dark-adapted state (containing a mixture of A1 and A2 retinal [squares]; λmax = 621 and n = 18), regenerated with 11-cis retinal (A1 [circles]; λmax = 565 and n = 14), or regenerated with 11-cis 9-demethyl retinal (triangles; λmax = 503 and n = 8).
The mean absorbance spectra measured in dark-adapted red cones had a λmax at 621 nm (n = 18). These cells contain native visual pigment, which is a mixture of two chromophores: 11-cis retinal (A1) and A2. By fitting the dark-adapted spectra with a combination of A1/A2 visual pigment templates (Govardovskii et al., 2000), it was determined that these cells contained, on average, ∼90–95% A2.
After characterizing dark-adapted spectra, an aliquot of solution containing dark-adapted cells was exposed to long wavelength light (wratten #25 filter; Kodak; transmittance of 590–700 nm) for 10 min in the presence of 11-cis 9-demethyl retinal. In this way, the native pigment was exhaustively bleached by light that was not absorbed by the analogue chromophore (λmax = 380 nm). The analogue visual pigment was allowed to regenerate completely, and the visual pigment spectra of a population of these cells were measured. The resulting mean spectrum of red cones containing 9-demethyl visual pigment is indicated by the filled triangles in Fig. 3. This spectrum peaks at λmax = 503 nm (n = 8). A third aliquot of cells was bleached and regenerated under identical conditions with 11-cis retinal (A1). The resulting mean spectra indicated by the filled circles in Fig. 3 exhibited λmax = 565 nm (n = 14). Our λmax values for dark-adapted red cones as well as red cones regenerated with 11-cis retinal are in agreement with a previous study (Makino et al., 1999), and our λmax of 503 nm for red cones regenerated with 11-cis 9-demethyl retinal corresponds well with the λmax of 509 nm reported by Das et al. (2004) on pigments formed with recombinant opsin and pigments solubilized in 0.1% dodecylmaltoside.
Sensitivity and Kinetics of Flash Responses in Red Cones Containing 9-Demethyl Visual Pigment
The experiments illustrated in Figs. 2 and 3 demonstrate that the 9-demethyl chromophore forms a bleachable and physiologically active visual pigment in red cones but give no information about the absolute levels of sensitivity or the kinetics of dim flash responses in these cells. The experiments illustrated in Figs. 4 and 5 were performed to answer these questions. Fig. 4 A shows a series of flash responses that were recorded from an isolated intact cone in its dark-adapted state. Responses were elicited by 620-nm flashes of 20-ms duration, having intensities that differed by ∼0.5 log unit increments. After exhaustive bleaching, the cell was allowed 10 min to recover to a steady state, and a second set of photoresponses was recorded (Fig. 4 B). As reported previously, it can be seen that the bleach caused a significant reduction in dark current as well as an acceleration of flash response kinetics (Jones et al., 1989). At this point, the visual pigment was regenerated after the addition of 35 μM 11-cis 9-demethyl retinal to the experimental chamber. The analogue chromophore remained in the chamber for 5 min before being washed out. The sensitivity and response amplitude to saturating test flashes were monitored until the cell reached a steady state, and full recovery of the sensitivity was observed. A third set of photoresponses was then recorded (Fig. 4 C). A comparison of responses in Fig. 4 (A and C) shows that full recovery flash response amplitude was achieved.
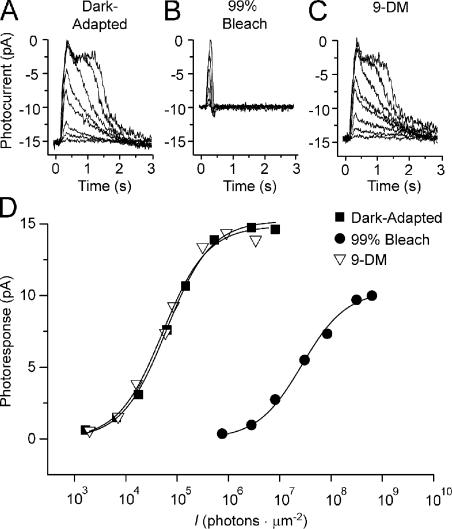
Figure 4.
Families of flash responses and associated intensity response relations of a red cone containing different visual pigments. (A) Series of responses to 20-ms flashes elicited from a dark-adapted red cone. (B) Series of responses elicited 10 min after a 99% bleach. (C) Series of flash responses elicited 1 h after regeneration of the visual pigment with 11-cis 9-demethyl retinal (9-DM). All responses were elicited from the same cell. (D) Intensity response relations were constructed from flash response families in A (dark adapted; squares), B (bleach adapted; circles), and C (9-demethyl retinal regenerated; triangles). All responses were elicited at 620 nm, which is the spectral maximum of dark-adapted cones. The intensity response relation constructed from the data in C was shifted to the left by a factor of 0.95 to account for the difference in spectral sensitivity predicted by absorbance spectra (see Fig. 3), assuming that the extinction coefficients of native and analogue pigment are the same.
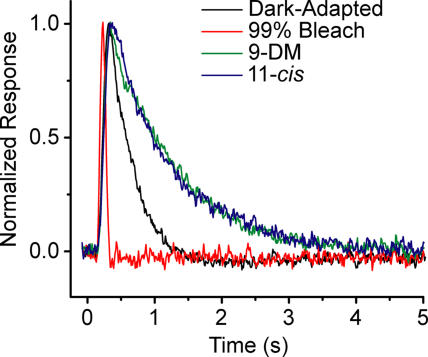
Figure 5.
Comparison of dim flash response kinetics from red cones under different pigment conditions. Normalized average dim flash responses of red cones in the dark-adapted state (black trace), after recovering from a 99% bleach (red trace), and after full pigment regeneration with either 11-cis 9-demethyl retinal (9-DM; green trace) or 11-cis retinal (blue trace). Each trace is the average of recordings from 16 cells, each of which was an average of responses to eight dim flashes elicited in the linear range of the intensity response relation. Mean values for time to peak and integration time are presented in Table II.
The photoresponse amplitudes for each series of responses from Fig. 4 (A–C) were plotted as a function of flash intensity in Fig. 4 D. The response intensity relation from the dark-adapted cell is indicated by closed squares, after bleaching by closed circles, and after regeneration with 11-cis 9-demethyl retinal by open triangles (Fig. 4 D). It can be seen that the bleach caused a significant decrease in sensitivity relative to the dark-adapted state. After regeneration with 11-cis 9-demethyl retinal, the sensitivity recovered completely to the prebleached level. It is important to note that in this experiment, flash responses elicited in all three conditions (dark-adapted, bleached, and regenerated with 11-cis 9-demethyl retinal) occurred with 620-nm light. However, as illustrated in Fig. 3, regeneration with 11-cis 9-demethyl retinal causes a blue shift in the absorption spectrum of the visual pigment and a lower absolute sensitivity at 620 nm. Thus, the intensity response relation constructed from the data in Fig. 4 C was shifted to the left by a factor of 0.95 to account for the difference in the maximum absolute sensitivity calculated for a pigment with λmax = 503 nm. This factor was calculated based on the spectral differences illustrated in the microspectrophotometric records shown in Fig. 3. A comparison of intensity response curves in Fig. 4 D (closed squares and open triangles) shows that full recovery of dark-adapted sensitivity was accomplished. The experiment in Fig. 4 was performed on a total of 14 cells; all produced similar results.
Dim Flash Kinetics
The kinetics of flash responses elicited in the linear range of the response intensity relation reflect the enzymatic activity of the phototransduction cascade (Lamb and Pugh, 1992). For this reason, we compared the dim flash kinetics of flash responses elicited from red cones under the different pigment conditions that prevailed in Fig. 2. Fig. 5 shows a comparison of normalized averaged dim flash responses elicited from red cones in the dark-adapted state (black trace), after bleaching 99% of the pigment (red trace), and after full visual pigment regeneration with either 11-cis 9-demethyl retinal (green trace) or 11-cis retinal (A1; blue trace). Each trace is the average of recordings from 16 cells, each of which was an average of responses to eight dim flashes in the linear range of the intensity response relation.
As expected, the bleach caused a considerable acceleration in the photoresponse kinetics compared with the dark-adapted state. Complete regeneration of visual pigment by both 11-cis 9-demethyl retinal and 11-cis retinal reversed the effect of the bleach and resulted in photoresponses that were even slower than in dark-adapted conditions. This is consistent with the findings of Kefalov et al. (2005), who reported that even in complete dark-adapted conditions, red cones contain ∼10% free opsin (apo-opsin) that produces an approximately twofold desensitization, which is equivalent to that produced by a steady light causing 500 photoisomerizations per second. Thus, it is expected that dark-adapted red cones would exhibit faster dim flash kinetics than red cones regenerated with exogenous retinoids.
The average dim flash response in red cones regenerated with 11-cis 9-demethyl retinal is identical to that regenerated with 11-cis retinal. This observation suggests that, at least in the dim flash regime, lack of the 9-methyl group of retinal has no significant effect on the activation or response recovery rates of red cones. This result was first reported by Corson and Crouch (Corson, D.W., and R.K. Crouch. 2001. Activity of 11-cis 9-demethyl retinal in red-sensitive cones from salamander. Association for Research in Vision and Ophthalmology. 42:S370.).
Table II presents differences in dim flash kinetics calculated from data that were used to construct Fig. 4 as measured by their time to peak, t p, and integration time, ti. tp was determined from individual cells as the time from the middle of the 20-ms stimulating flash until the response reached its maximum amplitude. ti was calculated as described previously by Baylor and Hodgkin (1973) as the time integral of the normalized flash response.
TABLE II.
Mean Time to Peak and Integration Time for Dim Flash Responses of Red Cones
| Red cone pigment condition |
tp ± SEM | ti ± SEM |
|---|---|---|
| s | s | |
| Dark adapted | 0.21 ± 0.01 | 0.41 ± 0.02 |
| 99% bleach | 0.12 ± 0.005 | 0.10 ± 0.004 |
| 9-demethyl retinal | 0.25 ± 0.01 | 0.98 ± 0.06 |
| 11-cis retinal | 0.27 ± 0.02 | 0.95 ± 0.09 |
Time to peak, tp, was taken as the time from the middle of the 20-ms stimulating flash until the response reached maximum amplitude. Integration time, ti, was derived by integration of the normalized dim flash response. Data were collected from individual normalized dim flash responses and were averaged (n = 16). Averaged dim flash responses are shown in Fig. 5.
Intensity Dependence of 9-Demethyl Visual Pigment on Light Responses
The results in Figs. 4 and 5 demonstrate that dim flash responses elicited in red cones containing either 11-cis retinal or 11-cis 9-demethyl retinal are essentially identical and terminate with a similar time course. However, data presented in Fig. 2 show that when red cones containing 9-demethyl visual pigment are exposed to very bright light, response recovery is considerably slower than when native pigment is present. The experiments presented in Fig. 6 were designed to determine the intensity of light (fractional bleach) at which the time course of current recovery in 9-demethyl pigment-containing red cones departs from normal red cones as a result of the absence of the 9-methyl group of retinal.
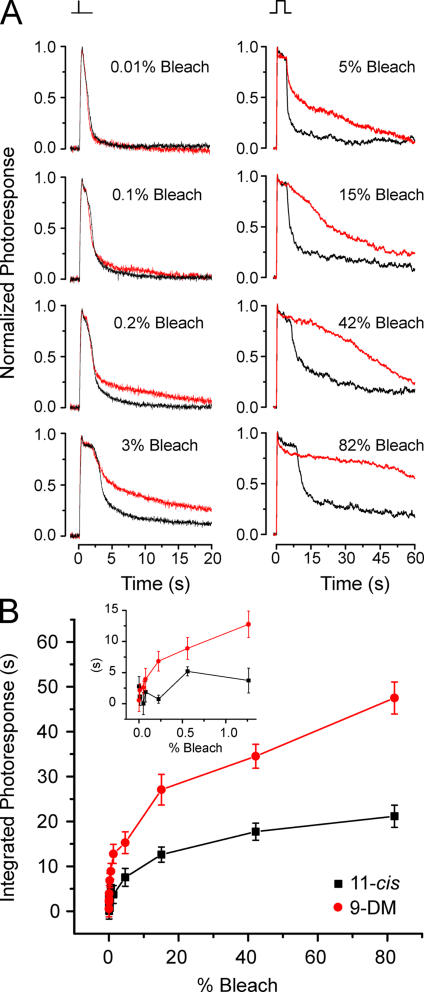
Figure 6.
The intensity dependence of response termination in red cones containing 9-demethyl visual pigment. (A) Comparisons of average normalized flash responses to 40-ms test flashes (left; n = 5 cells for each trace) or 4-s flashes (right; n = 6 cells for each trace) that bleached different pigment fractions in red cones regenerated with 11-cis retinal (black traces) or 11-cis 9-demethyl retinal (red traces). (B) The mean integral of normalized responses (integration time) to 4-s flashes are plotted as a function of percent bleach for the six red cones regenerated with 9-DM (red circles) and the six red cones regenerated with 11-cis retinal (black squares). Error bars represent the SEM. Inset shows the same data as in the main panel plotted on an expanded scale to more clearly illustrate that the first significant difference in integrated photoresponse between 9-demethyl retinal cells and 11-cis cells appears at 0.2% bleach (unpaired two-sample t test; P < 0.02).
Fig. 6 A (left) shows mean normalized photoresponses plotted as a function of time after exposure to a 40-ms flash, which was calculated to have bleached from 0.01 to 3% of the visual pigment. Fig. 6 A (right) also shows data from experiments in which the bleaching range was extended to ∼80% by using 4-s steps of light. In both columns, the black traces represent mean responses from red cones containing 11-cis retinal (A1) pigment, and the red traces represent mean responses from red cones containing 9-demethyl visual pigment (Fig. 6 A; left, n = 5 cells for each trace; right, n = 6 cells for each trace). Cells containing 11-cis retinal (A1) pigment were stimulated with 560-nm light; cells containing 9-demethyl visual pigment were stimulated with 520-nm light. Light intensities were adjusted with neutral density filters so that test flashes at the two wavelengths produced equivalent levels of photoactivation. The exposure of bleached cells at the beginning of the experiments to either 11-cis retinal or 11-cis 9-demethyl retinal resulted in an excess of exogenous retinoid that partitioned within outer segment membranes. The presence of this excess retinoid was used to regenerate the visual pigment bleached by each test flash. Thus, every test flash was measured when the cell contained a full complement of visual pigment. By inspection of these traces, it can be seen that responses produced by cells containing 11-cis retinal and 11-cis 9-demethyl retinal were identical at low flash strengths (bleaching ≤0.1% of the pigment). However, as the light intensity increased (bleaching ≥0.2% of the pigment), the photoresponses deviated; responses from cells containing 11-cis 9-demethyl retinal were slower to recover.
Individual normalized photoresponses from 4-s exposures (i.e., those used to generate the mean responses illustrated in Fig. 6 A [right]) were smoothed by the Savitsky-Golay method (10 points to the left and right). These responses were integrated from 4.1 (the end of the 4-s bleaching exposure) to 80.63 s (the end of the recording). These mean integrals were plotted as a function of percent bleach as shown in Fig. 6 B. The inset shows the same data as in the main panel plotted on an expanded scale to more clearly illustrate the percent bleach at which the difference in integrated responses is significant. This occurred at approximately 0.2% bleach (unpaired two-sample t test; P < 0.02).
Measurements of GC Rates
The data presented thus far illustrate that red cones regenerated with 11-cis 9-demethyl retinal exhibit a slowed recovery of dark current and responsiveness after exposure to bright light that bleached more than ∼0.2% of the visual pigment. These results are based on recordings of light-induced extracellular membrane current changes. However, the possibility exists that our results could be caused by nonspecific effects on the membrane channels independent of the phototransduction cascade. For instance, a recent study has demonstrated direct inhibitory effects of retinoids on membrane CNG channels in salamander rods that are not caused by changes in the activity of the transduction cascade (He et al., 2006). To address this issue of nonspecificity, we performed a series of experiments designed to directly examine reactions of the transduction cascade at the level of cGMP metabolism. We measured the effect of 9-demethyl visual pigment on the rate of recovery of GC activity in red cones after bleaching. The rationale and methods for measuring the rate of GC in single photoreceptor cells are explained in Materials and methods and have been previously described (Hodgkin and Nunn, 1988; Cornwall and Fain, 1994). This technique has been used successfully in previous studies on red cones treated with other analogue retinoids (Kefalov et al., 1999, 2001). Fig. 7 shows an experiment in which the GC activity was measured in the same red cone under different steady-state conditions.
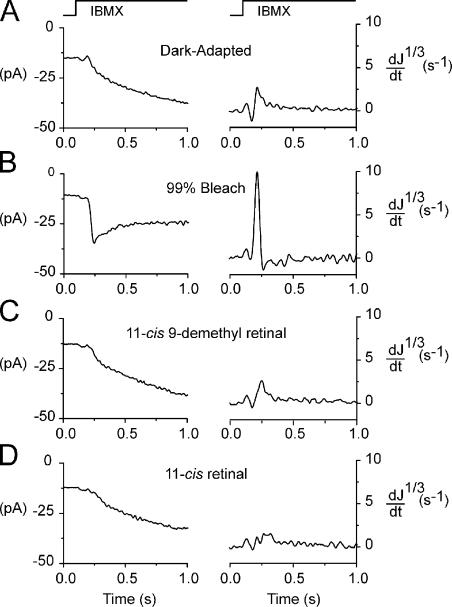
Figure 7.
GC measurements in a red cone regenerated with 11-cis 9-demethyl retinal and 11-cis retinal. The left panels show current recordings from a red cone during steps into 500 μM IBMX solution. The derivative of the cube root of the normalized current, d(J1/3)/dt, is plotted on the right. The time course of the solution step for both panels is indicated at the top. Recordings were made first in the dark-adapted state (A), after a 99% bleach (B; after the cone recovered to a steady state), after full regeneration with 11-cis 9-demethyl retinal (C), and, finally, after a second bleach and full regeneration with 11-cis retinal (D). Each trace is the mean of four measurements. All recordings are from the same cell.
The left panels of Fig. 7 show current recordings made during steps into Ringer solution containing 500 μM IBMX, which inhibits PDE activity (β = 0). Each trace represents the mean of four such stepwise exposures. The corresponding right panels of Fig. 7 show the derivative of the cube root of the normalized current, d(J1/3)/dt, where J = i/id is the current during the solution jump expressed as a fraction of the dark current before the jump. The time course of the solution step is indicated at the top of Fig. 7. As argued in Materials and methods, dJ1/3/dt is proportional to the rate of change in cGMP concentration, and its maximum represents a measure of the rate of synthesis of cGMP by GC.
Measurements were made from the red cone first in the dark-adapted state (Fig. 7 A). As expected (Cornwall and Fain, 1994), bleaching 99% of the pigment caused a persistent and considerable acceleration in the GC rate (Fig. 7 B). This is indicated by the rapid, negative deflection in current and the large spikelike deflection in Fig. 7 B (left and right, respectively). Regeneration of the visual pigment with 11-cis 9-demethyl retinal reversed the acceleration produced by the bleach and restored the cyclase rate to its dark-adapted level (Fig. 7 C). This same effect was observed when the cell was bleached a second time and regenerated with 11-cis retinal (Fig. 7 D). The cyclase rates measured in Fig. 7 (C and D) are virtually identical. These data are consistent with those shown in Figs. 4 and 5 and demonstrate that bleached cells treated with 11-cis 9-demethyl retinal experience a complete recovery of phototransduction activity to dark-adapted levels.
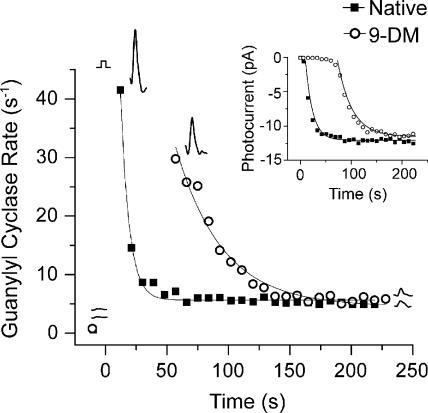
The data presented in Fig. 7 demonstrate that bleached cones in which visual pigment is regenerated with 11-cis 9-demethyl retinal fully recover their dark-adapted GC rate but provide no information as to how fast this occurs. This question is addressed by the experiment illustrated in Fig. 8, in which the time course of recovery of the GC rate is measured after bright light exposure. Each data point in Fig. 8 represents one measurement of the GC rate (i.e., the maximum value of dJ1/3/dt) measured at the time indicated in a single red cone before and after a >90% bleach. The closed squares indicate cyclase activity when the cone contained native dark-adapted visual pigment; open circles indicate cyclase activity when the same cone contained 9-demethyl visual pigment (Fig. 8). In both cases, the cone had a low cyclase activity before the bleach. This is indicated by the two data points before t = 0 and the two adjacent GC rate traces (compare with Fig. 7 A, right). Also, in both cases, the bleach caused an acceleration of cyclase activity, which then declined to a steady state that was still higher than the prebleached level. However, it is clear that recovery of the cyclase activity when the cell contained 9-demethyl visual pigment was considerably delayed compared with when it contained native pigment. These measurements of the recovery of cyclase activity after the bleach can be compared with measurements of the recovery of photocurrent, which was made at the same time on this cell. These data are shown in the inset of Fig. 8. The smooth curves that overlie the datasets are single exponential functions fitted by the method of least squares. This experiment was performed on a total of five cells; all produced similar results. The mean time constant of cyclase recovery measured after the bleaching of cells containing native pigment was 8.5 ± 1.2 s (±SEM). The mean time constant for recovery in cells containing 9-demethyl visual pigment was 37.0 ± 6.5 s (Table I). These data demonstrate that the time constant of recovery of the cyclase rate after bleaching was approximately four times slower in 9-demethyl–containing red cones than that measured in native red cones.
Figure 8.
The effect of 11-cis 9-demethyl retinal on the time course of GC rate recovery after bleaching a single red cone. Each data point represents one measurement of the cyclase rate plotted as a function of time after >90% bleach. Closed squares represent individual rate measurements when the cone contained native pigment; open circles represent individual rate measurements when the cone contained 11-cis 9-demethyl retinal (9-DM). Data were fitted with single exponential functions. The experiment was repeated in four other cells. The mean time constant for GC recovery in the cells containing native pigment is τGC = 8.50 ± 1.24 s (±SEM; n = 5), and, in cells containing 9-demethyl retinal, the mean is τGC = 37.0 ± 6.48 s (±SEM; n = 5). Inset shows the time course of recovery of flash response amplitude (measured as in Fig. 2 B) for the same cell.
DISCUSSION
Vertebrate cone photoreceptors possess the remarkable ability to respond to incremental light in the face of background light that bleaches a significant fraction of their visual pigment. The goal of the experiments reported in this study was to elucidate mechanisms whereby red cones rapidly quench the transduction cascade that make this possible. Specifically, we have tested the hypothesis that the 9-methyl group of the ligand is critical for normal pigment decay and, thus, rapid response termination in red cones. Our results are summarized in the following paragraphs.
First, red cones that have been bleached and their visual pigment regenerated with 11-cis 9-demethyl retinal recover sensitivity and responsiveness completely to their previous dark-adapted state. Second, light exposures that photoactivate more than ∼0.2% of the analogue visual pigment in these cones result in responses that are slower to recover. Very bright light exposure causes these cones to remain refractory to incremental flash exposure significantly longer than cones containing native pigment. Finally, measurements of the recovery rate of GC activity based on current measurements during this prolonged recovery period demonstrate that persistent activation of the transduction cascade is occurring and that this delay in recovery is not caused by nonspecific effects, such as retinoid binding to the CNG ion membrane channels (Tetreault et al., 2006).
Why does the absence of the 9-methyl group in retinal cause a delay in the termination of light responses in red cones? A recent study by Das et al. (2004) has shown, based on biochemical measurements, that the 9-methyl group in retinal plays a steric role in the efficient hydrolysis of the Schiff base linkage between retinal and red cone opsin. Their data showed that salamander red cone opsins expressed in vitro in COS cells and regenerated with 11-cis retinal lacking the 9-methyl group exhibited a prolonged activation of transducin. They attributed this to a more than fivefold slowing of the Meta II decay rate (Das et al., 2004). This conclusion was based on a slowed rate of visual pigment regeneration, which was assumed to be caused by a slowed rate of Meta II decay. Our results generally support this conclusion by demonstrating that membrane responses induced by bright light (>90% bleach) from 9-demethyl–containing red cones remained in saturation roughly 10 times longer, and the recovery of flash responses occurred more than threefold slower than those in cells containing native visual pigment (Table I).
It is interesting to note that this effect on red cone response termination is in striking contrast to what has been observed in experiments on red rods. In vitro experiments have demonstrated that a stable visual pigment can be formed from 11-cis 9-demethyl retinal and rod opsin and that the spectral maximum of the resulting visual pigment is blue shifted relative to the native pigment (Kropf et al., 1973), as it is in red cones (Fig. 3). However, deletion of the 9-methyl group in rhodopsin appears to shift the Meta I/II equilibrium toward Meta I, causing slowed receptor activation (Meyer et al., 2000; Vogel et al., 2006). In addition, it has been shown that 9-demethyl rhodopsin exhibits reduced light-dependent phosphorylation (Palczewski et al., 1994; Morrison et al., 1995). Collectively, these observations provide an explanation for the slowed recovery of flash responses that has been observed in physiological experiments on rods containing 9-demethyl rhodopsin (Corson et al., 1994a). In contrast to these results on rods, no slowing of the activation of transducin has been observed in biochemical measurements of red cone 9-demethyl pigment (Das et al., 2004). Although no measurements have been obtained on the rate of the Meta I/II transition, it is apparent from an examination of the data in Fig. 5 that the responses to dim flashes in cells containing native and analogue pigments are identical. As a consequence, red rods that have been bleached and their pigment regenerated with 11-cis 9-demethyl retinal exhibit prolonged light responses, even to dim flashes (Corson et al., 1994b), whereas this is not the case in red cones.
Thus, studies on both rods and cones agree that the 9-methyl group acts through steric interactions with opsin (Meyer et al., 2000; Das et al., 2004; Vogel et al., 2006). However, these interactions appear to be very different depending on the opsin type present. In red rods, the 9-methyl group is suggested to sterically assist in the efficient conversion of Meta I to II, whereas in red cones, the 9-methyl group appears to be important for the efficient hydrolysis of the Schiff base linkage between retinal and opsin (decay of Meta II).
If it is correct that the absence of the 9-methyl group in retinal causes slowed Meta II decay, why is the effect on response termination only observed after exposure to light that bleaches greater than ∼0.2% of the visual pigment but not after dimmer light exposure? To answer this question, we consider the principal biochemical mechanisms that are known to quench the transduction cascade in photoreceptors generally.
It is well established that in rods, pigment deactivation occurs through a series of steps, including the phosphorylation of Meta II and its subsequent capping by arrestin (for review see Kennedy et al., 2001). However, recently, Krispel et al. (2006) have shown that the rate-limiting step in the mouse rod is the RGS-mediated GTP hydrolysis of transducin by a complex involving RGS9(Gt-GTP)-PDEγ . It is unclear whether there is a corresponding mechanism operative in the various cones.
Phosphorylation and arrestin binding of rod visual pigment after dim light exposure results in total quenching of the transduction cascade within less than a second of light termination, allowing the total recovery of light responses. Two visual pigment kinases have been identified in cones, GRK1 and GRK7 (Weiss et al., 2001; Liu et al., 2005), depending on the species. Recently, it has been shown that the vmax of the major cone kinase in zebrafish, GRK7, is 32-fold higher than that of the rod kinase, which could produce an enhanced shutoff activity (Wada et al., 2006). However, the specific kinase present in the salamander red cone has not been identified. The opsin phosphorylation patterns in cones are known to be different from those of rods, with multiple phosphorylation occurring rapidly (Kennedy et al., 2004). A study on the carp red cone (Tachibanaki et al., 2005) found that under a large bleach, although levels of phosphorylation increased overall, the actual number of phosphates per opsin decreased. A salamander cone-specific arrestin has been identified (Smith et al., 2000; Zhu et al., 2002), but it is not known whether this arrestin is present in the salamander red cone. There is also some evidence that arrestin distribution in cones is different from that in rods (Zhang et al., 2003a).
A second inactivation mechanism that must be considered is the quenching of the activated transducin–PDE complex that is responsible for the hydrolysis of cGMP. In rods, the normal GTPase activity of transducin is potentiated by the GTPase-activating protein RGS9 in association with its obligatory Gβ5 cofactor (He et al., 1998; Makino et al., 1999). In cones, there is evidence from mouse retina that the inactivation of PDE by RGS9 is required for normal deactivation of cone phototransduction (Chen et al., 2000; Lyubarsky et al., 2001). The rate-limiting RGS-mediated step identified in rods (Krispel et al., 2006) may also have a role in cones. The fact that RGS9-1 expression has been measured to be roughly 10-fold higher in cones than in rods (Cowan et al., 1998; Zhang et al., 2003b) lends support to this notion.
The aforementioned mechanisms are clearly important for the termination of light responses in both rods and cones exposed to dim to moderate light intensities, but, at brighter intensities that result in significant pigment bleaching, other mechanisms must prevail. In rods, exposure to bright light that bleaches >1% of the visual pigment leads to a prolonged activation of the receptor as a result of the persistence of activated Meta II. Many physiological (Donner and Hemilä, 1975; Lamb, 1980; Leibrock and Lamb, 1997; Leibrock et al., 1998) and biochemical (Okada et al., 1989; Jäger et al., 1996) experiments on rods have lent strong support to the notion that transient photoproducts of bleaching play an important role in the decrease in sensitivity after bleaching. This persistence results in a sluggish recovery of responsiveness that can take place over many minutes. As we illustrate in Figs. 2 and 6, recovery of the responsiveness of red cones containing 9-demethyl visual pigment after exposure to all levels of flash intensity (from those that activate only a few hundred of photopigment molecules to those that bleach >90% of the visual pigment) is rapid. We propose a model that incorporates two separate but integrated mechanisms to explain the deactivation of the transduction cascade in cells containing analogue pigment over this broad range of pigment bleaching. According to this model, dim light exposures (bleaching <0.2% of the visual pigment) result in recovery of the light response either through inactivation of the visual pigment by phosphorylation and/or arrestin binding or through RGS9-mediated GTP hydrolysis of transducin. At higher intensities of light exposure, we suggest that the direct decay of Meta II dominates cone recovery.
Whether Meta II decay dominates response recovery in cones containing normal visual pigment exposed to high light levels cannot be determined from these data. However, a possible role for Meta II decay in normal response recovery is suggested by recent microspectrophotometric and microfluorometric experiments of intact salamander red cones in which the rate of metapigment decay and reduction of all-trans retinal to all-trans retinol were measured (Ala-Laurila et al., 2006). This study showed that the mean time constant for A1 cone metapigment decay was ∼7 s, which is close to the mean time constant for the recovery of red cone flash responsiveness (Fig. 2 and Table I). The time constant for the reduction of retinal to retinol as retinal was released from Meta II was 23 s. Thus, based on these data, the deactivation of transduction activation and response recovery would be expected to begin within ∼6–10 s after bright light exposure, assuming that Meta II decay sets its speed. If this is the case, however, the speed of decline could be slowed somewhat as a result of the persistence of unreduced all-trans retinal that may recombine with opsin to prolong receptor activation. However, we wish to stress that under circumstances involving the natural chromophore where Meta II lifetime has not been prolonged, the rate-determining step in cones at any light level is unknown. Whether this mechanism is the same as has recently been identified for rods (Krispel et al., 2006) has yet to be determined.
Finally, we must be careful not to generalize these results to all types of cone opsins. The in vitro results of Das et al. (2004) imply that this analogue will have quite different effects on the blue and UV cone opsins in which no prolongation of the Meta II state was observed. Further experiments are needed to elucidate the role that the 9-methyl group of retinal has on these opsins in these two cones and in the green rod, which contains the SWS2 (blue cone) opsin.
Acknowledgments
We thank Drs. Vladimir Kefalov and A.P. Sampath for helpful discussions and for their review of an early version of the manuscript. We also thank Mr. Howard I. Cohen for reviving and maintaining the MacNichol microspectrophotometer used in this study.
This work was supported by National Institutes of Health grants EY-01157 (to M.C. Cornwall), EY-04939 (to R.K. Crouch), and EY14793 (Medical University of South Carolina vision core) and by an unrestricted grant to the Department of Ophthalmology at the Medical University of South Carolina from Research to Prevent Blindness (RPB). R.K. Crouch is an RPB Senior Scientific Investigator.
Olaf S. Andersen served as editor.
Abbreviations used in this paper: cGMP, cyclic guanosine monophosphate; GC, guanylyl cyclase; GTP, guanosine triphosphate; IBMX, 3-isobutyl-1-methylxanthine; Meta, metarhodopsin; PDE, phosphodiesterase.
References
- Ala-Laurila, P., P. Saarinen, R. Albert, A. Koskelainen, and K. Donner. 2002. Temperature effects on spectral properties of red and green rods in toad retina. Vis. Neurosci. 19:781–792. [DOI] [PubMed] [Google Scholar]
- Ala-Laurila, P., A.V. Kolesnikov, R.K. Crouch, E. Tsina, S.A. Shukolyukov, V.I. Govardovskii, Y. Koutalos, B. Wiggert, M.E. Estevez, and M.C. Cornwall. 2006. Visual Cycle: Dependence of Retinol Production and Removal on Photoproduct Decay and Cell Morphology. J. Gen. Physiol. 128:153–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnis, S., K. Fahmy, K.P. Hofmann, and T.P. Sakmar. 1994. A conserved carboxylic acid group mediates light-dependent proton uptake and signaling by rhodopsin. J. Biol. Chem. 269:23879–23881. [PubMed] [Google Scholar]
- Baumann, C. 1972. Kinetics of slow thermal reactions during the bleaching of rhodopsin in the perfused frog retina. J. Physiol. 222:643–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor, D.A., and A.L. Hodgkin. 1973. Detection and resolution of visual stimuli by turtle photoreceptors. J. Physiol. 234:163–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor, D.A., T.D. Lamb, and K.W. Yau. 1979. The membrane current of single rod outer segments. J. Physiol. 288:589–611. [PMC free article] [PubMed] [Google Scholar]
- Brin, K.P., and H. Ripps. 1977. Rhodopsin photoproducts and rod sensitivity in the skate retina. J. Gen. Physiol. 69:97–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, P.K., and G. Wald. 1956. The neo-b isomer of vitamin A and retinene. J. Biol. Chem. 222:865–877. [PubMed] [Google Scholar]
- Burns, M.E., and V.Y. Arshavsky. 2005. Beyond counting photons: trials and trends in vertebrate visual transduction. Neuron. 48:387–401. [DOI] [PubMed] [Google Scholar]
- Chen, C.K., M.E. Burns, W. He, T.G. Wensel, D.A. Baylor, and M.I. Simon. 2000. Slowed recovery of rod photoresponse in mice lacking the GTPase accelerating protein RGS9-1. Nature. 403:557–560. [DOI] [PubMed] [Google Scholar]
- Cornwall, M.C., and G.L. Fain. 1994. Bleached pigment activates transduction in isolated rods of the salamander retina. J. Physiol. 480:261–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwall, M.C., A. Fein, and E.F. MacNichol Jr. 1983. Spatial localization of bleaching adaptation in isolated vertebrate rod photoreceptors. Proc. Natl. Acad. Sci. USA. 80:2785–2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwall, M.C., E.F. MacNichol Jr., and A. Fein. 1984. Absorptance and spectral sensitivity measurements of rod photoreceptors of the tiger salamander, Ambystoma tigrinum. Vision Res. 24:1651–1659. [DOI] [PubMed] [Google Scholar]
- Cornwall, M.C., A. Fein, and E.F. MacNichol Jr. 1990. Cellular mechanisms that underlie bleaching and background adaptation. J. Gen. Physiol. 96:345–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwall, M.C., G.J. Jones, V.J. Kefalov, G.L. Fain, and H.R. Matthews. 2000. Electrophysiological methods for measurement of activation of phototransduction by bleached visual pigment in salamander photoreceptors. Methods Enzymol. 316:224–252. [DOI] [PubMed] [Google Scholar]
- Corson, D.W., M.C. Cornwall, E.F. MacNichol, S. Tsang, F. Derguini, R.K. Crouch, and K. Nakanishi. 1994. a. Relief of opsin desensitization and prolonged excitation of rod photoreceptors by 9-desmethylretinal. Proc. Natl. Acad. Sci. USA. 91:6958–6962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corson, D.W., M.C. Cornwall, and D.R. Pepperberg. 1994. b. Evidence for the prolonged photoactivated lifetime of an analogue visual pigment containing 11-cis 9-desmethylretinal. Vis. Neurosci. 11:91–98. [DOI] [PubMed] [Google Scholar]
- Cowan, C.W., R.N. Fariss, I. Sokal, K. Palczewski, and T.G. Wensel. 1998. High expression levels in cones of RGS9, the predominant GTPase accelerating protein of rods. Proc. Natl. Acad. Sci. USA. 95:5351–5356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouch, R.K., V.J. Kefalov, W. Gaertner, and M.C. Cornwall. 2002. Use of retinal analogues for the study of visual pigment function. In Methods in Enzymology: G Protein Pathways. Vol. 343. R. Ivengar and J.D.H. Hildebrandt, editors. Academic Press, Inc., San Diego. 29–48. [DOI] [PubMed]
- Das, J., R.K. Crouch, J.X. Ma, D.D. Oprian, and M. Kono. 2004. Role of the 9-methyl group of retinal in cone visual pigments. Biochemistry. 43:5532–5538. [DOI] [PubMed] [Google Scholar]
- Donner, K.O., and S. Hemilä. 1975. Kinetics of long-lived rhodopsin photoproducts in the frog retina as a function of the amount bleached. Vision Res. 15:985–995. [DOI] [PubMed] [Google Scholar]
- Fain, G.L., H.R. Matthews, M.C. Cornwall, and Y. Koutalos. 2001. Adaptation in vertebrate photoreceptors. Physiol. Rev. 81:117–151. [DOI] [PubMed] [Google Scholar]
- Firsov, M.L., A.V. Kolesnikov, E.Y. Golobokova, and V.I. Govardovskii. 2005. Two realms of dark adaptation. Vision Res. 45:147–151. [DOI] [PubMed] [Google Scholar]
- Govardovskii, V.I., N. Fyhrquist, T. Reuter, D.G. Kuzmin, and K. Donner. 2000. In search of the visual pigment template. Vis. Neurosci. 17:509–528. [DOI] [PubMed] [Google Scholar]
- He, Q., D Alexeev, M.E. Estevez, S.L. McCabe, P.D. Calvert, D.E. Ong, M.C. Cornwall, A.L. Zimmerman, and C.L. Makino. 2006. Cyclic Nucleotide-gated Ion Channels in Rod Photoreceptors Are Protected from Retinoid Inhibition. J. Gen. Physiol. 128:473–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, W., C.W. Cowan, and T.G. Wensel. 1998. RGS9, a GTPase accelerator for phototransduction. Neuron. 20:95–102. [DOI] [PubMed] [Google Scholar]
- Hodgkin, A.L., and B.J. Nunn. 1988. Control of light-sensitive current in salamander rods. J. Physiol. 403:439–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann, K.P., A. Pulvermuller, J. Buczylko, P. Van Hooser, and K. Palczewski. 1992. The role of arrestin and retinoids in the regeneration pathway of rhodopsin. J. Biol. Chem. 267:15701–15706. [PubMed] [Google Scholar]
- Jäger, S., K. Palczewski, and K.P. Hofmann. 1996. Opsin/all-trans-retinal complex activates transducin by different mechanisms than photolyzed rhodopsin. Biochemistry. 35:2901–2908. [DOI] [PubMed] [Google Scholar]
- Jones, G.J. 1995. Light adaptation and the rising phase of the flash photocurrent of salamander retinal rods. J. Physiol. 487:441–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, G.J., R.K. Crouch, B. Wiggert, M.C. Cornwall, and G.J. Chader. 1989. Retinoid requirements for recovery of sensitivity after visual-pigment bleaching in isolated photoreceptors. Proc. Natl. Acad. Sci. USA. 86:9606–9610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, G.J., A. Fein, E.F. MacNichol Jr., and M.C. Cornwall. 1993. Visual pigment bleaching in isolated salamander retinal cones. Microspectrophotometry and light adaptation. J. Gen. Physiol. 102:483–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kefalov, V.J., M.C. Cornwall, and R.K. Crouch. 1999. Occupancy of the chromophore binding site of opsin activates visual transduction in rod photoreceptors. J. Gen. Physiol. 113:491–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kefalov, V.J., R.K. Crouch, and M.C. Cornwall. 2001. Role of noncovalent binding of 11-cis-retinal to opsin in dark adaptation of rod and cone photoreceptors. Neuron. 29:749–755. [DOI] [PubMed] [Google Scholar]
- Kefalov, V.J., M.E. Estevez, M. Kono, P.W. Goletz, R.K. Crouch, M.C. Cornwall, and K.W. Yau. 2005. Breaking the covalent bond–a pigment property that contributes to desensitization in cones. Neuron. 46:879–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenkre, J.S., N.A. Moran, T.D. Lamb, and O.A. Mahroo. 2005. Extremely rapid recovery of human cone circulating current at the extinction of bleaching exposures. J. Physiol. 567:95–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy, M.J., K.A. Lee, G.A. Niemi, K.B. Craven, G.G. Garwin, J.C. Saari, and J.B. Hurley. 2001. Multiple phosphorylation of rhodopsin and the in vivo chemistry underlying rod photoreceptor dark adaptation. Neuron. 31:87–101. [DOI] [PubMed] [Google Scholar]
- Kennedy, M.J., F.A. Dunn, and J.B. Hurley. 2004. Visual pigment phosphorylation but not transducin translocation can contribute to light adaptation in zebrafish cones. Neuron. 41:915–928. [DOI] [PubMed] [Google Scholar]
- Kibelbek, J., D.C. Mitchell, J.M. Beach, and B.J. Litman. 1991. Functional equivalence of metarhodopsin II and the Gt-activating form of photolyzed bovine rhodopsin. Biochemistry. 30:6761–6768. [DOI] [PubMed] [Google Scholar]
- Krispel, C.M., D. Chen, N. Melling, Y.J. Chen, K.A. Martemyanov, N. Quillinan, V.Y. Arshavsky, T.G. Wensel, C.K. Chen, and M.E. Burns. 2006. RGS expression rate-limits recovery of rod photoresponses. Neuron. 51:409–416. [DOI] [PubMed] [Google Scholar]
- Kropf, A., B.P. Whittenberger, S.P. Goff, and A.S. Waggoner. 1973. The spectral properties of some visual pigment analogs. Exp. Eye Res. 17:591–606. [DOI] [PubMed] [Google Scholar]
- Lamb, T.D. 1980. Spontaneous quantal events induced in toad rods by pigment bleaching. Nature. 287:349–351. [DOI] [PubMed] [Google Scholar]
- Lamb, T.D., and E.N. Pugh Jr. 1992. A quantitative account of the activation steps involved in phototransduction in amphibian photoreceptors. J. Physiol. 449:719–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb, T.D., and E.N. Pugh Jr. 2004. Dark adaptation and the retinoid cycle of vision. Prog. Retin. Eye Res. 23:307–380. [DOI] [PubMed] [Google Scholar]
- Leibrock, C.S., and T.D. Lamb. 1997. Effect of hydroxylamine on photon-like events during dark adaptation in toad rod photoreceptors. J. Physiol. 501:97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibrock, C.S., T. Reuter, and T.D. Lamb. 1998. Molecular basis of dark adaptation in rod photoreceptors. Eye. 12:511–520. [DOI] [PubMed] [Google Scholar]
- Lipton, S.A., H. Rasmussen, and J.E. Dowling. 1977. Electrical and adaptive properties of rod photoreceptors in Bufo marinus. II. Effects of cyclic nucleotides and prostaglandins. J. Gen. Physiol. 70:771–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, P., S. Osawa, and E.R. Weiss. 2005. M opsin phosphorylation in intact mammalian retinas. J. Neurochem. 93:135–144. [DOI] [PubMed] [Google Scholar]
- Lyubarsky, A.L., and E.N. Pugh Jr. 1996. Recovery phase of the murine rod photoresponse reconstructed from electroretinographic recordings. J. Neurosci. 16:563–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyubarsky, A.L., F. Naarendorp, X. Zhang, T. Wensel, M.I. Simon, and E.N. Pugh Jr. 2001. RGS9-1 is required for normal inactivation of mouse cone phototransduction. Mol. Vis. 7:71–78. [PubMed] [Google Scholar]
- MacNichol, E.F., Jr. 1978. A photon-counting microspectrophotometer for the study of single vertebrate photoreceptor cells. In Frontiers in Visual Science: Proceedings of the University of Houston College of Optometry Dedication Symposium. S.J. Cool and E.L. Smith, editors. Springer-Verlag, Berlin. 194–208.
- Makino, C.L., and R.L. Dodd. 1996. Multiple visual pigments in a photoreceptor of the salamander retina. J. Gen. Physiol. 108:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino, E.R., J.W. Handy, T. Li, and V.Y. Arshavsky. 1999. The GTPase activating factor for transducin in rod photoreceptors is the complex between RGS9 and type 5 G protein beta subunit. Proc. Natl. Acad. Sci. USA. 96:1947–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani, A.P. 1986. Photoreceptors of the larval tiger salamander retina. Proc. R. Soc. Lond. B. Biol. Sci. 227:483–492. [DOI] [PubMed] [Google Scholar]
- Matthews, H.R., M.C. Cornwall, and G.L. Fain. 1996. Persistent activation of transducin by bleached rhodopsin in salamander rods. J. Gen. Physiol. 108:557–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBee, J.K., K. Palczewski, W. Baehr, and D.R. Pepperberg. 2001. Confronting complexity: the interlink of phototransduction and retinoid metabolism in the vertebrate retina. Prog. Retin. Eye Res. 20:469–529. [DOI] [PubMed] [Google Scholar]
- Meyer, C.K., M. Bohme, A. Ockenfels, W. Gartner, K.P. Hofmann, and O.P. Ernst. 2000. Signaling states of rhodopsin. Retinal provides a scaffold for activating proton transfer switches. J. Biol. Chem. 275:19713–19718. [DOI] [PubMed] [Google Scholar]
- Morrison, D.F., T.D. Ting, V. Vallury, Y.K. Ho, R.K. Crouch, D.W. Corson, N.J. Mangini, and D.R. Pepperberg. 1995. Reduced light-dependent phosphorylation of an analog visual pigment containing 9-demethylretinal as its chromophore. J. Biol. Chem. 270:6718–6721. [DOI] [PubMed] [Google Scholar]
- Murnick, J.G., and T.D. Lamb. 1996. Kinetics of desensitization induced by saturating flashes in toad and salamander rods. J. Physiol. 495:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikonov, S.S., L.L. Daniele, X. Zhu, C.M. Craft, A. Swaroop, and E.N. Pugh Jr. 2005. Photoreceptors of Nrl −/− mice coexpress functional S- and M-cone opsins having distinct inactivation mechanisms. J. Gen. Physiol. 125:287–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada, D., T. Nakai, and A. Ikai. 1989. Transducin activation by molecular species of rhodopsin other than metarhodopsin II. Photochem. Photobiol. 49:197–203. [DOI] [PubMed] [Google Scholar]
- Palczewski, K., S. Jager, J. Buczylko, R.K. Crouch, D.L. Bredberg, K.P. Hofmann, M.A. Asson-Batres, and J.C. Saari. 1994. Rod outer segment retinol dehydrogenase: substrate specificity and role in phototransduction. Biochemistry. 33:13741–13750. [DOI] [PubMed] [Google Scholar]
- Pepperberg, D.R., M.C. Cornwall, M. Kahlert, K.P. Hofmann, J. Jin, G.J. Jones, and H. Ripps. 1992. Light-dependent delay in the falling phase of the retinal rod photoresponse. Vis. Neurosci. 8:9–18. [DOI] [PubMed] [Google Scholar]
- Pugh, E.N., Jr, and T.D. Lamb. 1990. Cyclic GMP and calcium: the internal messengers of excitation and adaptation in vertebrate photoreceptors. Vision Res. 30:1923–1948. [DOI] [PubMed] [Google Scholar]
- Sherry, D.M., D.D. Bui, and W.J. Degrip. 1998. Identification and distribution of photoreceptor subtypes in the neotenic tiger salamander retina. Vis. Neurosci. 15:1175–1187. [DOI] [PubMed] [Google Scholar]
- Smith, W.C., E.V. Gurevich, D.R. Dugger, S.A. Vishnivetskiy, C.L. Shelamer, J.H. McDowell, and V.V. Gurevich. 2000. Cloning and functional characterization of salamander rod and cone arrestins. Invest. Ophthalmol. Vis. Sci. 41:2445–2455. [PubMed] [Google Scholar]
- Sutton, R.B., S.A. Vishnivetskiy, J. Robert, S.M. Hanson, D. Raman, B.E. Knox, M. Kono, J. Navarro, and V.V. Gurevich. 2005. Crystal structure of cone arrestin at 2.3A: evolution of receptor specificity. J. Mol. Biol. 354:1069–1080. [DOI] [PubMed] [Google Scholar]
- Tachibanaki, S., D. Arinobu, Y. Shimauchi-Matsukawa, S. Tsushima, and S. Kawamura. 2005. Highly effective phosphorylation by G protein-coupled receptor kinase 7 of light-activated visual pigment in cones. Proc. Natl. Acad. Sci. USA. 102:9329–9334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetreault, M.L., D. Henry, D.M. Horrigan, G. Matthews, and A.L. Zimmerman. 2006. Characterization of a novel cyclic nucleotide-gated channel from zebrafish brain. Biochem. Biophys. Res. Commun. 348:441–449. [DOI] [PubMed] [Google Scholar]
- Vogel, R., S. Ludeke, F. Siebert, T.P. Sakmar, A. Hirshfeld, and M. Sheves. 2006. Agonists and partial agonists of rhodopsin: retinal polyene methylation affects receptor activation. Biochemistry. 45:1640–1652. [DOI] [PubMed] [Google Scholar]
- Wada, Y., J. Sugiyama, T. Okano, and Y. Fukada. 2006. GRK1 and GRK7: unique cellular distribution and widely different activities of opsin phosphorylation in the zebrafish rods and cones. J. Neurochem. 98:824–837. [DOI] [PubMed] [Google Scholar]
- Wald, G. 1968. The molecular basis of visual excitation. Nature. 219:800–807. [DOI] [PubMed] [Google Scholar]
- Weiss, E.R., M.H. Ducceschi, T.J. Horner, A. Li, C.M. Craft, and S. Osawa. 2001. Species-specific differences in expression of G-protein-coupled receptor kinase (GRK) 7 and GRK1 in mammalian cone photoreceptor cells: implications for cone cell phototransduction. J. Neurosci. 21:9175–9184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau, K.W., and D.A. Baylor. 1989. Cyclic GMP-activated conductance of retinal photoreceptor cells. Annu. Rev. Neurosci. 12:289–327. [DOI] [PubMed] [Google Scholar]
- Yoshizawa, T., and G. Wald. 1963. Pre-lumirhodopsin and the bleaching of visual pigments. Nature. 197:1279–1286. [DOI] [PubMed] [Google Scholar]
- Zhang, H., W. Huang, X. Zhu, C.M. Craft, W. Baehr, and C.K. Chen. 2003. a. Light-dependent redistribution of visual arrestins and transducin subunits in mice with defective phototransduction. Mol. Vis. 9:231–237. [PubMed] [Google Scholar]
- Zhang, X., T.G. Wensel, and T.W. Kraft. 2003. b. GTPase regulators and photoresponses in cones of the eastern chipmunk. J. Neurosci. 23:1287–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, X., A. Li, B. Brown, E.R. Weiss, S. Osawa, and C.M. Craft. 2002. Mouse cone arrestin expression pattern: light induced translocation in cone photoreceptors. Mol. Vis. 8:462–471. [PubMed] [Google Scholar]
- Zhu, X., B. Brown, A. Li, A.J. Mears, A. Swaroop, and C.M. Craft. 2003. GRK1-dependent phosphorylation of S and M opsins and their binding to cone arrestin during cone phototransduction in the mouse retina. J. Neurosci. 23:6152–6160. [DOI] [PMC free article] [PubMed] [Google Scholar]