Abstract
Acid-sensing ion channels (ASICs) are thought to trigger some forms of acid-induced pain and taste, and to contribute to stroke-induced neural damage. After activation by low extracellular pH, different ASICs undergo desensitization on time scales from 0.1 to 10 s. Consistent with a substantial conformation change, desensitization slows dramatically when temperature drops (Askwith, C.C., C.J. Benson, M.J. Welsh, and P.M. Snyder. 2001. PNAS. 98:6459–6463). The nature of this conformation change is unknown, but two studies showed that desensitization rate is altered by mutations on or near the first transmembrane domain (TM1) (Coric, T., P. Zhang, N. Todorovic, and C.M. Canessa. 2003. J. Biol. Chem. 278:45240–45247; Pfister, Y., I. Gautschi, A.-N. Takeda, M. van Bemmelen, S. Kellenberger, and L. Schild. 2006. J. Biol. Chem. 281:11787–11791). Here we show evidence of a specific conformation change associated with desensitization. When mutated from glutamate to cysteine, residue 79, which is some 20 amino acids extracellular to TM1, can be altered by cysteine-modifying reagents when the channel is closed, but not when it is desensitized; thus, desensitization appears to conceal the residue from the extracellular medium. D78 and E79 are a pair of adjacent acidic amino acids that are highly conserved in ASICs yet absent from epithelial Na+ channels, their acid-insensitive relatives. Despite large effects on desensitization by mutations at positions 78 and 79—including a shift to 10-fold lower proton concentration with the E79A mutant—there are not significant effects on activation.
INTRODUCTION
Acid-sensing ion channels (ASICs) are members of the DEG/ENaC (degenerin/epithelial sodium channel) family of sodium-selective ion channels (Waldmann et al., 1997; Kellenberger and Schild, 2002; Krishtal, 2003). In rats, there are four asic genes, two of which form splice variants. Of the six proteins, only four are activated by low pH when expressed alone: ASIC1a, 1b, 2a, and 3. The channel subunit proteins are proposed to have two transmembrane domains, with a large extracellular loop. Several ASIC proteins come together to form a functional channel and the various homomeric and heteromeric channels have clearly distinct kinetic properties (Benson et al., 2002; Hesselager et al., 2004).
Desensitization rate varies greatly between different ASIC subtypes and slows dramatically upon cooling, arguing that it involves a large conformation change (Askwith et al., 2001). Two papers indicate that the region in and around the first transmembrane domain (TM1) is relevant to desensitization. Chimera and mutation studies showed that three residues extracellular to TM1 confer differences in desensitization rates between rat and toadfish ASIC1 (Coric et al., 2003). A mutation within TM1 of rat ASIC1a, R43C, led to slower desensitization rates when channels were treated with Cd2+ (Pfister et al., 2006).
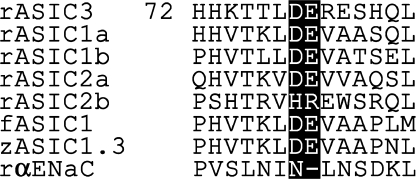
Strikingly, there are 27 strongly acidic residues, glutamates and aspartates, conserved in the extracellular domain of acid-gated rat ASICs. Given that at least three ASIC subunits form a functional channel, the extracellular surface is highly charged. It seems possible that these titrateable residues play a role in proton-dependent gating because all but one of the conserved acidic residues are absent from epithelial Na+ channels, which are relatives of ASICs that are not gated by protons. In this paper, we focus efforts on an adjacent pair of acidic residues, D78 and E79, because (a) they are absent from all epithelial Na channels; (b) they are present in virtually every ASIC yet notably absent from those few ASICs that do not generate acid-gated currents as homomers (Fig. 1); (c) being immediately next to each other, they might provide a local negative environment that could shift the pKa from 4.5, the value in solution, toward the physiological range of ASIC gating (pH 7–6). To our surprise, our results argue that these residues are critical to acid-induced desensitization, but not to activation.
Figure 1.
Alignment of protein sequence near to D78-E79. The DE pair is absent from the epithelial Na channel (rαENaC) and absent from rASIC2b, which is the one ASIC in the list that fails to make acid-gated current when expressed alone.
MATERIALS AND METHODS
Cell Culture
CHO-K1 cells were used in all experiments. To transfect the cells, 0.3–0.5 μg of wild-type or mutant rat ASIC3 cDNA and 5 μg of pCMV-DsRed-Express cDNA (CLONTECH Laboratories, Inc.) was added to 100 μl of cells suspended in HBS (140 mM NaCl, 25 mM HEPES, 2 mM Na2CO3, pH 7.4) (∼106 cells/ml). Cells were then electroporated (380V, 75 μF) in a 0.4-cm gap cuvette, and plated on glass coverslips in a dish containing F12 media with 10% FBS. Transfected cells were identified by their DsRed expression under epifluorescence. Red cells were recorded from 1–2 d after transfection. Nontransfected CHO cells show no detectable acid-evoked current.
Mutagenesis
Mutations were introduced into the rat ASIC3 cDNA clone by PCR as previously described (Weiner et al., 1994) using Pfu DNA polymerase. Mutant constructs were fully sequenced to ensure accuracy of mutagenesis and to confirm the absence of unintended mutations.
Electrophysiology
Whole cell patch clamp recordings were made with an Axopatch 200 amplifier (Axon Instruments). Data was filtered at 2 kHz and digitized at 5 kHz with a Digidata 1322 (Axon Instruments), and acquired using pClamp 8 software (Axon Instruments). Cells were held at −70 mV, and series resistance of 10 MΩ or less was compensated by at least 75%. Solutions were exchanged rapidly with a series of gravity-driven solution lines emptying out of 10-μl pipettes. Solution switching was accomplished with computer-controlled solenoid valves. This system allows for fast (<20 ms in control experiments) solution switching, which is crucial for rapidly desensitizing channels. Acid pulses were separated by 15 s at pH 8, which assures complete recovery from desensitization.
Solutions
Extracellular solutions had 150 mM NaCl, 10 mM pH buffer, 5 mM KCl, and 1 mM CaCl2, with NMG (N-methyl-d-glucamine) added to adjust pH. Appropriate pH buffers were used within their ranges of high buffer capacity: MES for solutions ≤pH 6.7, MOPS for pH 6.5–7.9, HEPES for pH 6.8–8.2, and TAPS for ≥pH 7.7. Internal solution had 120 mM KCl, 25 mM HEPES, 5 mM EGTA, 5 mM NaCl, adjusted to pH 7.3 with KOH. Methanethiosulfonate (MTS) compounds (Toronto Research Chemicals) were dissolved in extracellular solution and kept on ice, when not in use to ensure stability (Vemana et al., 2004). Wild-type ASIC3 showed no detectable changes upon exposure to MTS compounds.
Steady-state desensitization curves were fit with Hill equations: I/IMAX = IMAX*[H]n/(pH0.5 n+[H]n), where n is the Hill slope, and pH0.5 is the pH where half the channels are desensitized. Current decay kinetics were fit with a single exponential: I = I0 + Ae−t/τ, where τ is the time constant of desensitization. Where noted, currents were fit with a double exponential: I = I0 + A1e−t/τ1 + A2e−t/τ2.
RESULTS
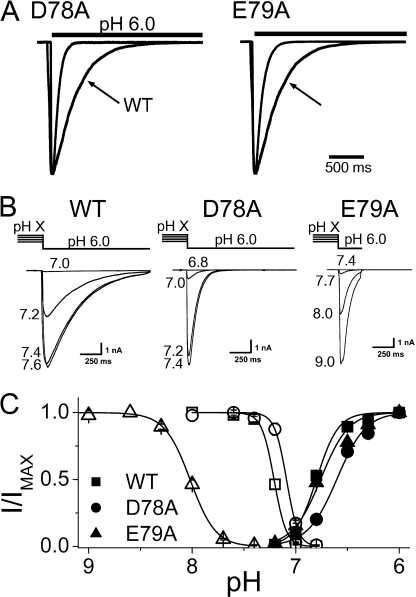
Mutating the acidic amino acids, D78 and E79, to alanine created channels that desensitized notably faster than wild type (Fig. 2 A), and a double mutant (D78A/E79A) was faster still (Table I). We converted five other acidic residues in the extracellular domain to alanine and none of them caused this effect (Table I). Three of these were just outside the second transmembrane domain; E432A and E435A were no different from wild type, and D439A did not express. D107A, nearer the center of the extracellular domain was no different from wild type, and E63A, within a few amino acids of TM1, prolonged the desensitization rate. D78 and E79 are noteworthy for their high conservation in ASICs, but not in ENaCs or in ASICs that cannot by themselves form acid-gated channels (Fig. 1). Alanine mutants of residues surrounding D78 and E79 also desensitized quickly, indicating that these two residues do not uniquely affect desensitization within this region of the protein (Table I).
Figure 2.
Properties of D78A and E79A. (A) Faster rate of desensitization compared with wild-type (WT) channels. Channels are opened by a step to pH 6.0 from pH 8.0. (B) Currents from the indicated channels evoked by a step to pH 6.0 from a bath solution of the indicated pH. Wild-type and D78A channels are half desensitized near pH 7.2/7.1, whereas E79A channels are half desensitized at pH 8. (C) Steady-state desensitization curves (open symbols) and activation curves (closed symbols) for the indicated channels. E79A channels have identical activation curves to wild type, but greatly shifted desensitization. D78A channels are little changed from wild type for either curve. Desensitization curves plot the normalized peak currents against the conditioning pH, as in B. Activation curves plot normalized peak current against the test pH (raw data not depicted).
TABLE I.
Desensitization Time Constants of ASIC3 Mutants
| Mutant | τ (ms) |
|---|---|
| WT | 385 ± 8 (27) |
| E63A | 698 ± 31 (5) |
| H73A | 371 ± 6 (6) |
| T75A | 178 ± 7 (4) |
| L77A | 53 ± 5 (14) |
| D78A | 77 ± 3 (17) |
| D78C | 173 ± 6 (17) |
| D78E | 401 ± 16 (15) |
| D78N | 39 ± 2 (12) |
| D78R | 22 ± 2 (11) |
| E79A | 97 ± 3 (31) |
| E79C | 119 ± 4 (16) |
| E79D | 36 ± 1 (9) |
| E79P | 18 ± 1 (9) |
| E79Q | 153 ± 6 (8) |
| R80A | 35 ± 1 (9) |
| R80C | 175 ± 9 (5) |
| R80E | 41 ± 1 (3) |
| E81A | 140 ± 6 (14) |
| S82A | 1192 ± 166 (15) |
| R99A | 394 ± 17 (3) |
| R102A | 371 ± 8 (3) |
| D107A | 338 ± 10 (4) |
| E432A | 412 ± 12 (11) |
| E435A | 395 ± 17 (8) |
| D439A | * |
| D78A/E79A | 22 ± 1 (13) |
Results are given as mean ± SEM (n). * indicates D439A mutants did not express.
Steady-state desensitization of E79A shifts to 10-fold lower proton concentrations than wild type and the slope of the desensitization curve is halved (7.3 to 3.5 Hill slope) (Fig. 2, B and C). In contrast, the activation curves (closed symbols) of wild type and E79A channels are indistinguishable. D78A mutants, although they share the same increased speed of desensitization as E79A, do not exhibit the dramatic shift in steady- state kinetics.
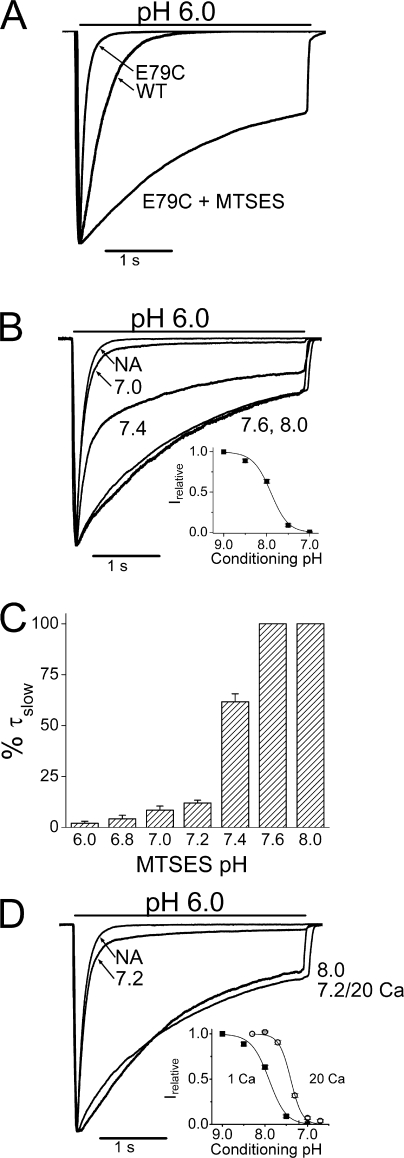
The large shift in steady-state desensitization of E79A suggests that the desensitized state is stabilized (has lower potential energy) in the mutant channel compared with wild type. To explore this possibility, we made cysteine mutants whose terminal sulfhydryl group can react with MTS groups (CH3SO2SX, where X is a variable functional group) (Akabas et al., 1992; Karlin and Akabas, 1998). We tested a variety of these reagents and none affected gating kinetics of wild-type ASIC3 (unpublished data). In contrast, all the MTS reagents slowed desensitization of E79C (MTSEA, MTSES, MTSET, MTSCE, and MTSBn). The most dramatic change occurred with the negatively charged MTSES (Fig. 3 A). The 120-ms desensitization rate of E79C changed to 1,500 ms when MTSES was applied at either pH 8.0 or 7.6. In contrast, there was virtually no change when MTSES was applied at pH 7.0. At pH 7.4 the current had both a fast and a slow component of desensitization (Fig. 3 B). Currents were fit with a sum of two exponentials and the histogram in Fig. 3 C shows the fractions of total current fit by a slow time constant when MTSES is applied at the indicated pH. We take the height of the bar as an indicator of MTS reactivity. MTS reactivity drops off in the range of pH where there is substantial steady-state desensitization (inset).
Figure 3.
E79 accessibility is state dependent. (A) Normalized wild-type currents (WT) and E79C current before (E79C) or after (E79C + MTSES) exposure to 1 mM MTSES for 5 min at pH 8.0. (B) E79C currents before (NA) and after exposure to 1 mM MTSES for 5 min at the indicated pH. Inset shows steady-state desensitization for E79C (pH0.5 of 7.91, n = 9); at pH 7.0, channels are fully desensitized. Currents were fit with a double exponential with fast (∼120 ms) and slow (>600 ms) components. Histograms (C) give the percentage of the current that was fit with a slow time constant against the pH of the MTS incubation solution. (D) MTSES sensitivity depends on desensitization, not pH itself. Cells were exposed to 1 mM MTSES for 5 min at pH 8.0 or pH 7.2 in 1 mM Ca2+, or pH 7.2 in 20 mM Ca2+. The addition of 20 mM Ca2+ shifts the steady-state desensitization curve (hollow circles, inset) so channels are available to open at pH 7.2, thereby rendering them sensitive to MTSES.
The simplest interpretation of Fig. 3 B is that the desensitized channels cannot react with MTSES. However, an alternate possibility is that decreasing pH protonates the thiolate ion on the cysteine, rendering it unreactive to MTS reagents (Roberts et al., 1986). We tested this possibility by shifting the desensitization curve to more acidic pH using increased divalent ion concentration in the extracellular medium (Babini et al., 2002). Fig. 3 C shows that channels reacted readily with MTSES at pH 7.2, 20 mM Ca2+, where desensitization is incomplete (inset), but not at pH 7.2, 1 mM Ca2+, where channels are fully desensitized. We conclude that MTS reactivity is determined by the gating state of the channel, not by pH itself.
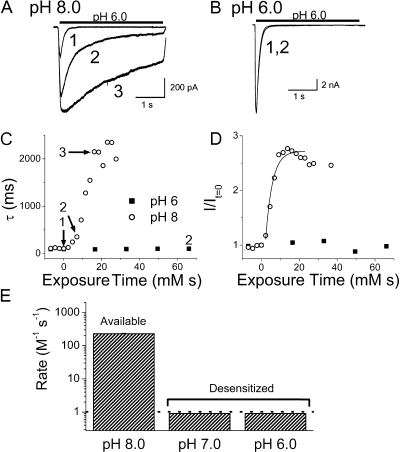
We tried to quantify reaction kinetics of MTSES with residue 79 by varying the concentration and exposure time (Fig. 4). Gradual modification of both desensitization rate and peak amplitude was evident when 200 μM MTSES was applied at pH 8.0 (Fig. 4 A). In contrast to pH 8, no reaction was ever detected at either pH 7.0 or 6 (Fig. 4 B). Exponential fits of the fractional change vs. exposure time (in mM × seconds, Fig. 4, C and D) yielded a reaction rate of 226 M−1s−1 at pH 8, and rates below our detection level (1 M−1s−1) at the two lower pH values (Fig. 4 E). Evidently, MTSES can react with residue 79 if the channel is available to open, but cannot react with desensitized channels.
Figure 4.
MTSES reaction rate at E79C. (A) Currents from E79C channels before application of MTSES (1), and 35 s (2), or 81 s (3) after exposure to 200 μM MTSES at pH 8.0. The increase in amplitude at pH 8.0 was caused by MTSES because it shifts the steady-state desensitization curve of E79C to the right (not depicted). (B) Before and after 5 min in 1 mM MTSES at pH 6; there is no evident change when MTSES is applied to desensitized channels. (C and D) Modification as a function of exposure time (time exposed * concentration MTSES) for E79C. (C) Plot of the time constant (τ) of desensitization; (D) Plot of the relative peak current amplitude, which is fit with a single exponential used to obtain the modification rate constant. (E) Modification rate constants of E79C at pH 8, 7, and 6. The rate at pH 8.0, 226 M−1s−1, could be quantified, whereas rates when channels are desensitized (either pH 6.0 or 7.0) are below our limit of resolution, which was 1 M−1s−1.
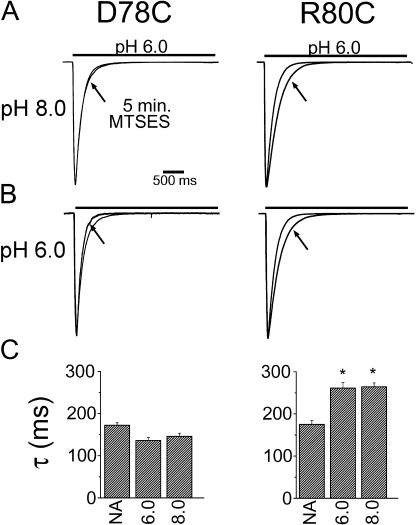
Do residues adjacent to E79 also change accessibility during desensitization? As with E79, the adjacent cysteine mutants, D78C and R80C, exhibited significantly faster desensitization than wild-type channels (Fig. 5); however, they showed clearly different response to MTSES. D78C mutants were not obviously changed by MTSES, whereas R80C was modified by MTSES equally well at pH 8.0 and pH 6.0 (Fig. 5, A and B). The effect of MTSES on R80C—desensitization was slowed by ∼30%—was modest compared with MTS modification of E79C, but it was absolutely consistent and statistically significant (Fig. 5 C). We conclude that, unlike residue 79, residue 80 is accessible to chemical reagents in both the available and desensitized states. Perhaps residue 78 fails to react because it is inaccessible to chemical reagents in both states; alternatively, MTS reagents could react but fail to change desensitization rate.
Figure 5.
Residues adjacent to E79C differ in their apparent state dependency of modification. (A and B) Representative traces from cells transfected with either D78C (left) or R80C (right) without and with (arrows) exposure to 1 mM MTSES for 5 min at pH 8.0 (A) or pH 6.0 (B). (C) Average time constants for desensitization. NA indicates no application of MTSES. A minimum of five cells was tested for each condition. D78C channels failed the statistical test for being modified by MTSES, whereas R80C channels are modified at both pH 6.0 and pH 8.0 (* indicates P < 0.001 vs. WT cells).
DISCUSSION
Using substituted cysteine accessibility, we report evidence that desensitization of ASIC3 involves a conformation change in the extracellular domain. The evidence is that MTSES can react at residue 79 when the channel is closed (pH 8) but not when it is desensitized (pH 7 and below). Thus, residue 79 appears to get buried away from the extracellular space during desensitization. The neighboring residues, 78 and 80, do not exhibit this state-dependent change in accessibility.
Residue 79 is a glutamate immediately next to an aspartate at residue 78, and some 20 residues extracellular to the first transmembrane domain. Mutation of either of these two highly conserved acidic amino acids to a neutral residue speeds desensitization and the double mutant is faster still. This is not a general property of extracellular acidic residues because a variety of others were mutated without effect. It also is not unique to acidic residues because mutation of residues neighboring D78/E79 also sped desensitization rate. This agrees with prior work showing that mutations in this general area affect desensitization rate (Coric et al., 2003).
D78A/C and E79A/C and R80C mutants each desensitize rapidly compared with wild-type channels, yet only E79 mutants have significantly altered steady-state desensitization. In principle, there is no surprise that rate and steady-state occupancy do not predict each other because the two are controlled by different energies. The faster rate indicates a decrease in the transition state energy barrier between the open and desensitized states. The 10-fold lower proton concentrations necessary for steady-state desensitization of E79A indicates stabilization of the desensitized state, a decrease in the energy of the desensitized state. The two unique properties of residue 79—only residue 79 changes accessibility during desensitization and only residue 79 mutations stabilize the desensitized state—seem likely to be related. The steady-state desensitization curve is very steep in wild-type channels (Hill slope = 7) and this decreases by a factor of 2 in the E79A mutant. Even such a large decrease in Hill slope in a mutant cannot be interpreted unambiguously because Hill slope is a function of agonist number, agonist affinity, and the efficiency of gating conformation change (Colquhoun, 1998). Nevertheless, it raises the possibility that E79 contributes to sensing protons that trigger desensitization.
D78 and E79 are present in virtually every ASIC that generates a proton-gated current. (The one exception is an ASIC from zebrafish [zASIC4.1; Paukert et al., 2004] that has an asparagine in place of the aspartate at 78 and does indeed make acid-gated currents.) ASICs that do not generate proton-gated currents (fASIC1.2, fASIC2, zASIC4.2, rASIC2b, and m/r/hASIC4s) all have deviations in the D78-E79 motif. Although D78 and E79 are very highly conserved in ASICs, they are absent in most other DEG/ENaC family members, their acid-insensitive cousins. This pattern suggested to us that the residues might be important for the acid- induced opening of ASICs, so it came as a surprise to find large effects on desensitization without any effect on channel opening. This supports the idea that desensitization can occur without channel opening (Korkushco et al., 1983) and also implies strong evolutionary conservation of residues that are devoted to acid-induced desensitization kinetics. This, in turn, implies an essential biological role for ASIC desensitization. A possibility previously raised in the literature is that, under physiological conditions, desensitization tightly limits the effective range of pH sensitivity to the narrow window of overlap between activation and desensitization curves (Yagi et al., 2006).
Acknowledgments
We thank Chris Bond for helpful comments on the manuscript and for much guidance on mutagenesis.
This work was supported by the National Institutes of Health.
Olaf S. Andersen served as editor.
Abbreviations used in this paper: ASIC, acid-sensing ion channel; DEG, degenerin; ENaC, epithelial sodium channel; MTS, methanethiosulfonate; MTSES, sodium (2-sulfonatoethyl) methanethiosulfonate; MTSET, [2-(trimethylammonium)ethyl] methanethiosulfonate bromide; MTSCE, 2-carboxyethyl methanethiosulfonate; MTSBn, benzyl methanethiosulfonate; MTSEA, 2-aminoethyl methanethiosulfonate hydrobromide; TM, transmembrane; WT, wild-type.
References
- Akabas, M.H., D.A. Stauffer, M. Xu, and A. Karlin. 1992. Acetylcholine receptor channel structure probed in cysteine-substitution mutants. Science. 258:307–310. [DOI] [PubMed] [Google Scholar]
- Askwith, C.C., C.J. Benson, M.J. Welsh, and P.M. Snyder. 2001. DEG/ENaC ion channels involved in sensory transduction are modulated by cold temperature. Proc. Natl. Acad. Sci. USA. 98:6459–6463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babini, E., M. Paukert, H.-S. Geisler, and S. Grunder. 2002. Alternative splicing and interaction with di- and polyvalent cations control the dynamic range of acid-sensing ion channel (ASIC) 1. J. Biol. Chem. 277:41597–41603. [DOI] [PubMed] [Google Scholar]
- Benson, C.J., J. Xie, J.A. Wemmie, M.P. Price, J.M. Henss, M.J. Welsh, and P.M. Snyder. 2002. Heteromultimers of DEG/ENaC subunits form H+-gated channels in mouse sensory neurons. Proc. Natl. Acad. Sci. USA. 99:2338–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun, D. 1998. Binding, gating, affinity and efficacy: the interpretation of structure-activity relationships for agonists and of the effects of mutating receptors. Br. J. Pharmacol. 125:923–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coric, T., P. Zhang, N. Todorovic, and C.M. Canessa. 2003. The extracellular domain determines the kinetics of desensitization in acid-sensitive ion channel 1. J. Biol. Chem. 278:45240–45247. [DOI] [PubMed] [Google Scholar]
- Hesselager, M., D.B. Timmermann, and P.K. Ahring. 2004. pH dependency and desensitization kinetics of heterologously expressed combinations of acid-sensing ion channel subunits. J. Biol. Chem. 279:11006–11015. [DOI] [PubMed] [Google Scholar]
- Karlin, A., and M.H. Akabas. 1998. Substituted-cysteine accessibility method. Methods Enzymol. 293:123–145. [DOI] [PubMed] [Google Scholar]
- Kellenberger, S., and L. Schild. 2002. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol. Rev. 82:735–767. [DOI] [PubMed] [Google Scholar]
- Korkushco, A.O., O.A. Krishtal, and N.I. Chernevskaya. 1983. Steady-state characteristics of the proton receptor in the somatic membrane of rat sensory neurons. Neirofiziologiia. 15:632–638. [PubMed] [Google Scholar]
- Krishtal, O. 2003. The ASICs: signaling molecules? modulators? Trends Neurosci. 26:477–483. [DOI] [PubMed] [Google Scholar]
- Paukert, M., S. Sidi, C. Russell, M. Siba, S.W. Wilson, T. Nicolson, and S. Grunder. 2004. A family of acid-sensing ion channels from the zebrafish: widespread expression in the central nervous system suggests a conserved role in neuronal communication. J. Biol. Chem. 279:18783–18791. [DOI] [PubMed] [Google Scholar]
- Pfister, Y., I. Gautschi, A.-N. Takeda, M. van Bemmelen, S. Kellenberger, and L. Schild. 2006. A gating mutation in the internal pore of ASIC1a. J. Biol. Chem. 281:11787–11791. [DOI] [PubMed] [Google Scholar]
- Roberts, D.D., S.D. Lewis, D.P. Ballou, S.T. Olson, and J.A. Shafer. 1986. Reactivity of small thiolate anions and cysteine-25 in papain toward methyl methanethiosulfonate. Biochemistry. 25:5595–5601. [DOI] [PubMed] [Google Scholar]
- Vemana, S., S. Pandey, and H.P. Larsson. 2004. S4 movement in a mammalian HCN channel. J. Gen. Physiol. 123:21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann, R., G. Champigny, F. Bassilana, C. Heurteaux, and M. Lazdunski. 1997. A proton-gated cation channel involved in acid-sensing. Nature. 386:173–177. [DOI] [PubMed] [Google Scholar]
- Weiner, M.P., G.L. Costa, W. Schoettlin, J. Cline, E. Mathur, and J.C. Bauer. 1994. Site-directed mutagenesis of double-stranded DNA by the polymerase chain reaction. Gene. 151:119–123. [DOI] [PubMed] [Google Scholar]
- Yagi, J., H.N. Wenk, L.A. Naves, and E.W. McCleskey. 2006. Sustained currents through ASIC3 ion channels at the modest pH changes that occur during myocardial ischemia. Circ. Res. 99:501–509. [DOI] [PubMed] [Google Scholar]