Until recently, the two essential aspects of channel function seemed to be clearly distinct: a selectivity filter that discriminates among incoming ions and an intracellular gate that regulates the ion flux. As usual, reality is more complex, and this traditional view of ion channels is changing rapidly. Although a physical gate appears to operate at the intracellular part of the channel and undergo conformational transitions associated with the open-closed transitions of the channels, there is much more going on in the permeation pathway than was initially appreciated and the notion of a fairly “passive” or “rigid” selectivity filter is no longer adequate. Initial observations that different permeant ions could alter channel gating and/or inactivation rates pointed in that direction, but new data provides a compelling demonstration for an extended and more complex function of the pore, beyond its role in selectivity.
Several pieces of evidence, obtained mostly in selective K+ pores, suggest that the selectivity filter can contribute substantially to channel gating, thereby indicating that the selectivity filter itself may also be undergoing conformational transitions. One of the first studies supporting this view (Chapman et al., 1997), which was based on a careful analysis of the subconductance states in DRK1 channels, proposed that ion permeation and channel opening are coupled, meaning that the same structures that control selectivity also participate in channel opening and closing. More recently, Blunck et al. (2006) found that movement of the TM2 helix bundle, forming the inner gate in KcsA channels, is not the main determinant of the open probability. Fluorescence lifetime data led to the conclusion that the open probability of the bacterial channel is controlled by a second gate—likely the selectivity filter—in series with the bundle crossing gate. Opening of the intracellular gate may be a necessary, but not sufficient, condition for ion conduction.
In addition, insights provided by the crystal structure of the first K+ pore (Morais-Cabral et al., 2001) has stimulated extensive theoretical work, including free energy molecular dynamic simulations of ion movement through a K+ selective pore, which subsequently led to the prediction of fluctuations in the selectivity filter structure that likely increase the energy barrier for ion translocation, thereby de facto reproducing a nonconducting state without the involvement of an additional gate (Berneche and Roux, 2005). These “pore events” that underlie different gating mechanisms or inactivation processes (e.g., C-type inactivation) observed in voltage-gated K+ channels are direct consequences of a highly dynamic pore structure as opposed to a more static structure.
Unfortunately, Ca2+ channel researchers do not (yet) enjoy the advantages associated with having a resolved crystal structure, so the molecular details of conduction, selectivity, and ion binding in the Ca2+ channels pore remain hazy. In addition, only limited assistance comes from the K+ pore structure because the Ca2+ pore (selectivity filter) seems to have molecular organization that differs from the K+ channels' K+-binding carbonyl oxygens (provided by the pore TVGYG backbone chain). Rather, Ca2+ channel selectivity seems to rely on the carboxyl side chains of four glutamates (one from each of the four channel domains) that form a highly conserved EEEE locus that characterizes the pore of high voltage–activated channels (CaV1-CaV2 families; for review see Sather and McCleskey, 2003). Nevertheless, well designed experimental strategies can reveal important information about the likely mechanisms that underlie the function of these channels.
In this issue, Babich et al. (p. 461 and 477) contribute two companion articles that report on molecular events that reveal possible structural rearrangement in the selectivity filter of L-type channels (CaV1.2). Babich et al. (2007a,b) cleverly exploited the Gd3+ block of the L-type channels to probe molecular events in the pore that occur during channel activation and inactivation. Submicromolar [Gd 3+] is known to produce both a tonic block and to accelerate decay of the L-type current during depolarizing steps (Biagi and Enyeart, 1990). The acceleration of current decay appears to be very similar to an enhancement of inactivation. Babich et al. (2007a,b) found that both these effects of Gd3+ blocks take place at the same site (which also binds Ca2+ or Ba2+ with a 1-mM affinity), possibly located at the extracellular entrance of the selectivity filter, where the Gd3+ competes with the permeant ions. The use-dependent block (which is typically assumed to be the manifestation of a higher affinity of the blocker for the open, as compared with the closed, state) is reinterpreted to be a consequence of an increased off rate of the permeant ion for the same site. An overall 10-fold decrease in Ca2+ affinity (a necessary step for permeation) seems to occur during channel activation, allowing Gd3+ to gain access to the site, thereby producing the use-dependent block. This transition leads to an apparent Ca2+ affinity of the open channel in the 10-mM range, which is consistent with the observed single-channel conductance. The Gd3+ block thus unmasks a voltage-dependent gating transition in the Ca2+ pore before activation (Biagi and Enyeart, 1990; Babich et al., 2005). What is the molecular basis for this voltage dependence? A definitive answer would require better knowledge about the structural organization of the pore. Inferring from the voltage dependence of the Gd3+ block, which is similar to that of the gating charge movement, a rearrangement of the channels' voltage-sensing regions seems to be a reasonable source for the preopening transition of the selectivity filter (though the measured gating currents are not visibly affected by Gd3+). However, as the Babich et al. (2007a,b) propose, the voltage dependence may also arise from a reorientation of charged residues within the pore.
What does this multivalent cation-binding site represent? As estimated from the high on rate of Gd3+ block, the site's apparent ion affinity seems to drop just before channel activation. Yet, Ca2+ is known to bind to the pore with high affinity, well within the micromolar range. Such a high affinity site would allow only for small Ca2+ fluxes, which is inconsistent with the observed single channel currents (in the pA range).To resolve this quandary, Dang and McCleskey (1998) proposed, based on experimental evidence for an extracellular low affinity Ca2+ binding site in the permeation pathway (Kuo and Hess, 1993), a model for Ca2+ permeation in which a single high affinity ion-binding site is flanked by two lower affinity sites (at both sides of the selectivity filter), which provide potential energy steps to accelerate ion flux (Sather and McCleskey, 2003). The findings of Babich et al. (2007a,b) that the affinity of this site is dramatically reduced before channel opening not only seem to be in full in agreement with the low affinity site of Dang and McCleskey (1998; Sather and McCleskey, 2003) but also offer evidence for the merging of gating and permeation in Ca2+ channels.
A shift in pore affinity for permeant ions is also the focus of one of the articles by Babich et al. (2007b) The conclusion is provocative and challenges the conventional wisdom regarding Ca-dependent inactivation. Almost 30 yr ago, Brehm and Eckert (1978), studying Ca2+ currents in Paramecium, observed that the inward Ca2+ flux quickly “relaxed” during sustained depolarization. If the charge carrier Ca2+ in the extracellular medium was replaced by Ba2+ or Sr2+, the fast decay of the current was greatly attenuated. Brehm and Eckert's (1978) conclusion that “the Ca2+ channel undergoes inactivation as a consequence of Ca2+ entry during depolarization” started the search for the underlying mechanism. Molecular cloning of the first Ca2+ channels (Tanabe et al., 1987; Mikami et al., 1989; Perez-Reyes et al., 1989) provided a physical substrate for molecular and electrophysiological studies to identify the molecular players involved in the inactivation process. Nevertheless, it took another 10 yr and seminal contributions from several laboratories (Lee et al., 1999; Peterson et al., 1999; Qin et al., 1999; Zuhlke et al., 1999) to identify calmodulin (CAM) as the single most important player in the Ca2+-induced inactivation. Ca2+ binding to the C-terminal lobe of CAM (constitutively tethered to the C-terminal domain of the pore-forming Ca2+ channel α subunit) produces a structural rearrangement that ultimately abolishes ion conduction, possibly by removing a “brake” that otherwise impedes inactivation (Soldatov, 2003; Halling et al., 2006). The definite role of the α subunit intracellular I–II loop (connecting domains I and II) in the inactivation process culminated with a comprehensive view by Kim et al. (2004) in which the I–II loop acts as an inactivating gate (reminiscent of a “ball and chain” mechanism), while the whole process is accelerated by Ca2+ binding to CAM.
Regardless of the model proposed, the cytoplasmic side of the Ca2+ channel is a major site for regulation, with the I–II loop, the C-terminus, and CAM acting in concert to produce channel inactivation. In addition to this established body of literature, Babich et al. (2007a,b) now invite us to look up a bit higher, into the selectivity filter, for an additional site of regulation. Again using Gd3+ to probe the inactivation in L-type channels, it is found that Gd3+ block and Ca2+-dependent inactivation are mutually exclusive: Gd3+ block prevents Ca2+-dependent inactivation and inactivated channels become resistant to Gd3+ block (Babich et al., 2007a,b). These findings are interpreted to suggest that Ca2+ is binding strongly to the same site Gd3+ binds to, thereby preventing the binding of Gd3+ (Babich et al., 2007a,b). If this view is correct, it would follow that the affinity of the pore for Ca2+ increases dramatically during Ca2+-dependent inactivation, literally trapping ions in the pore and stopping conduction. Thus, the final step of the inactivation process may be a (possibly relatively small) structural rearrangement that sets the selectivity filter in a “sticky” conformation. This proposed novel mechanism does not conflict with the present knowledge about channel inactivation, in particular the well-characterized “CAM-centered” cytoplasmic events. On the contrary, the aforementioned cytoplasmic events may directly trigger or allosterically modulate the pore events. According to this newly proposed interpretation of Ca2+ inactivation, ion flux of Ca2+-inactivated channels is abolished not by an intracellular steric gate but rather by the subtle tuning of a binding site in the pore.
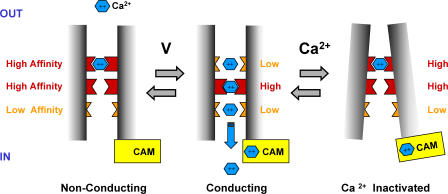
As summarized in Fig. 1, Babich et al. (2007a,b) introduce a more “pore-centered” viewpoint for understanding Ca2+ channel regulation. The linkage between channel gating and changes in the binding of permeant ions within the pore will undoubtedly inspire new experiments that will increase our appreciation of how channels regulate their own activity.
Figure 1.
Voltage- and Ca2+-dependent gating in the Ca2+ channel pore. In addition to the opening of the gate at the intracellular mouth, activation also involves voltage-dependent preopening pore transitions that lower the affinity of an external Ca2+ binding site at the pore entrance. Inactivation abolishes Ca2+ conduction by increasing Ca2+ affinity of this site. Ca2+ binding to intracellularly located CAM stabilizes the inactivated site with Ca2+ bound to the pore, accelerating inactivation.
Abbreviation used in this paper: CAM, calmodulin.
References
- Babich, O., V. Matveev, A.L. Harris, and R. Shirokov. 2007. a. Ca2+-dependent Inactivation of CaV1.2 Channels Prevents Gd3+ Block: Does Ca2+ Block the Pore of Inactivated Channels? J. Gen. Physiol. 129:477–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babich, O., J. Reeves, and R. Shirokov. 2007. b. Block of CaV1.2 Channels by Gd3+ Reveals Preopening Transitions in the Selectivity Filter. J. Gen. Physiol. 129:461–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babich, O., D. Isaev, and R. Shirokov. 2005. Role of extracellular Ca2+ in gating of CaV1.2 channels. J. Physiol. 565:709–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berneche, S., and B. Roux. 2005. A gate in the selectivity filter of potassium channels. Structure. 13:591–600. [DOI] [PubMed] [Google Scholar]
- Biagi, B.A., and J.J. Enyeart. 1990. Gadolinium blocks low- and high-threshold calcium currents in pituitary cells. Am. J. Physiol. 259:C515–C520. [DOI] [PubMed] [Google Scholar]
- Blunck, R., J.F. Cordero-Morales, L.G. Cuello, E. Perozo, and F. Bezanilla. 2006. Detection of the opening of the bundle crossing in KcsA with fluorescence lifetime spectroscopy reveals the existence of two gates for ion conduction. J. Gen. Physiol. 128:569–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm, P., and R. Eckert. 1978. Calcium entry leads to inactivation of calcium channel in Paramecium. Science. 202:1203–1206. [DOI] [PubMed] [Google Scholar]
- Chapman, M.L., H.M. VanDongen, and A.M. Vandongen. 1997. Activation-dependent subconductance levels in the drk1 K channel suggest a subunit basis for ion permeation and gating. Biophys. J. 72:708–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang, T.X., and E.W. McCleskey. 1998. Ion channel selectivity through stepwise changes in binding affinity. J. Gen. Physiol. 111:185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halling, D.B., P. Aracena-Parks, and S.L. Hamilton. 2006. Regulation of voltage-gated Ca2+ channels by calmodulin. Sci. STKE. 2006:er1. [DOI] [PubMed] [Google Scholar]
- Kim, J., S. Ghosh, D.A. Nunziato, and G.S. Pitt. 2004. Identification of the components controlling inactivation of voltage-gated Ca2+ channels. Neuron. 41:745–754. [DOI] [PubMed] [Google Scholar]
- Kuo, C.C., and P. Hess. 1993. Ion permeation through the L-type Ca2+ channel in rat phaeochromocytoma cells: two sets of ion binding sites in the pore. J. Physiol. 466:629–655. [PMC free article] [PubMed] [Google Scholar]
- Lee, A., S.T. Wong, D. Gallagher, B. Li, D.R. Storm, T. Scheuer, and W.A. Catterall. 1999. Ca2+/calmodulin binds to and modulates P/Q-type calcium channels. Nature. 399:155–159. [DOI] [PubMed] [Google Scholar]
- Mikami, A., K. Imoto, T. Tanabe, T. Niidome, Y. Mori, H. Takeshima, S. Narumiya, and S. Numa. 1989. Primary structure and functional expression of the cardiac dihydropyridine-sensitive calcium channel. Nature. 340:230–233. [DOI] [PubMed] [Google Scholar]
- Morais-Cabral, J.H., Y. Zhou, and R. MacKinnon. 2001. Energetic optimization of ion conduction rate by the K+ selectivity filter. Nature. 414:37–42. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes, E., H.S. Kim, A.E. Lacerda, W. Horne, X.Y. Wei, D. Rampe, K.P. Campbell, A.M. Brown, and L. Birnbaumer. 1989. Induction of calcium currents by the expression of the alpha 1-subunit of the dihydropyridine receptor from skeletal muscle. Nature. 340:233–236. [DOI] [PubMed] [Google Scholar]
- Peterson, B.Z., C.D. DeMaria, J.P. Adelman, and D.T. Yue. 1999. Calmodulin is the Ca2+ sensor for Ca2+-dependent inactivation of L-type calcium channels. Neuron. 22:549–558. [DOI] [PubMed] [Google Scholar]
- Qin, N., R. Olcese, M. Bransby, T. Lin, and L. Birnbaumer. 1999. Ca2+-induced inhibition of the cardiac Ca2+ channel depends on calmodulin. Proc. Natl. Acad. Sci. USA. 96:2435–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sather, W.A., and E.W. McCleskey. 2003. Permeation and selectivity in calcium channels. Annu. Rev. Physiol. 65:133–159. [DOI] [PubMed] [Google Scholar]
- Soldatov, N.M. 2003. Ca2+ channel moving tail: link between Ca2+-induced inactivation and Ca2+ signal transduction. Trends Pharmacol. Sci. 24:167–171. [DOI] [PubMed] [Google Scholar]
- Tanabe, T., H. Takeshima, A. Mikami, V. Flockerzi, H. Takahashi, K. Kangawa, M. Kojima, H. Matsuo, T. Hirose, and S. Numa. 1987. Primary structure of the receptor for calcium channel blockers from skeletal muscle. Nature. 328:313–318. [DOI] [PubMed] [Google Scholar]
- Zuhlke, R.D., G.S. Pitt, K. Deisseroth, R.W. Tsien, and H. Reuter. 1999. Calmodulin supports both inactivation and facilitation of L-type calcium channels. Nature. 399:159–162. [DOI] [PubMed] [Google Scholar]