Abstract
The transport activity of the glutamine/neutral amino acid transporter SNAT3 (former SN1, SLC38A3), expressed in oocytes of the frog Xenopus laevis is associated with a non-stoichiometrical membrane conductance selective for Na+ and/or H+ (Schneider, H.P., S. Bröer, A. Bröer, and J.W. Deitmer. 2007. J. Biol. Chem. 282:3788–3798). When we expressed SNAT3 in frog oocytes, the glutamine-induced membrane conductance was suppressed, when carbonic anhydrase isoform II (CAII) had been injected into the oocytes. Transport of substrate, however, was not affected by CAII. The reduction of the membrane conductance by CAII was dependent on the presence of CO2/HCO3 −, and could be reversed by blocking the catalytic activity of CAII by ethoxyzolamide (10 μM). Coexpression of wild-type CAII or a N-terminal CAII mutant with SNAT3 also reduced the SNAT3- associated membrane conductance. The catalytically inactive CAII mutant V143Y coexpressed in oocytes did not affect SNAT3-associated membrane conductance. Our results reveal a new type of interaction between CAII and a transporter-associated cation conductance, and support the hypothesis that the transport of substrate and the non-stoichiometrical ion conductance are independent of each other. This study also emphasizes the importance of carbonic anhydrase activity and the presence of CO2-bicarbonate buffers for membrane transport processes.
INTRODUCTION
The principle substrate of the system N-type sodium- dependent neutral amino acid transporter isoform 3 (SNAT3, SLC38A3) is glutamine, the main nitrogen carrier between cells. The transfer of glutamine plays a pivotal role in the amino acid and nitrogen metabolism in liver, muscle, kidney, and brain (Bröer and Brookes, 2001; Hertz, 2004; Zwingmann and Butterworth, 2005). Glutamine is involved in the synthesis of purines and pyrimidines, in the metabolism of ammonia, and in the glutamate/glutamine cycle between neurons and glial cells in the brain, where transmitter resynthesis is necessary for proper function of excitatory and inhibitory neurotransmission (Bak et al., 2006). A number of pathologies result from impaired glutamine transport and metabolism, such as, e.g., hepatic encephalopathy and hyperammonemia (Butterworth, 2002; Jalan et al., 2003). Recently, increased expression of SNAT3 was suggested to be a marker for malignant gliomas (Sidoryk et al., 2004).
The glutamine transporter SNAT3 (formerly SN1, system N) has been cloned, expressed, and analyzed molecularly and heterologously expressed in Xenopus oocytes (Chaudhry et al., 1999, 2001; Fei et al., 2000; Gu et al., 2000; Bröer et al., 2002). The transport of the neutral amino acid involves an exchange of 1 Na+ and 1 H+ and is therefore electroneutral. Hence, transport via SNAT3 is Na+ dependent, and there is no glutamine uptake under Na+-free conditions (Fei et al., 2000). SNAT3 transport activity is also H+ sensitive with an optimum near pH 8.0 (Bröer et al., 2002). The Km value for the substrate glutamine ranges between 0.7 and 1.6 mM, near the value for glutamine concentration in vivo conditions (Bode, 2001). However, currents that were reported to be associated with the transport are presumably due to a stoichiometrically uncoupled H+ conductance with some dependence on Na+ (Chaudhry et al., 2001; Bröer et al., 2002). Recently, we could show that this current is carried by monovalent cations at pH 7.4, and more selectively by H+ at pH 8.4 (Schneider et al., 2007).
In the present study, we have expressed rat SNAT3 in Xenopus oocytes with and without injected or coexpressed carbonic anhydrase II (CAII) in order to test if CAII may form a transport metabolon with SNAT3 to enhance its transport activity, as suggested for other acid/base- coupled membrane transporters (Sterling et al., 2001; McMurtrie et al., 2004; Becker and Deitmer, 2007). Our results suggest that CAII has no effect on the transport activity, and hence does not form a transport metabolon with SNAT3, but, unexpectedly, suppresses the SNAT3-associated membrane conductance by means of its enzymatic activity. This might be of considerable interest for evaluating the independence and molecular mechanism of substrate transport and ion conductance.
MATERIALS AND METHODS
Molecular Biology
Rat SNAT3-DNA was isolated from Escherichia coli XL-1 blue transfected with pGEM-He-Juel-SNAT3 (see Bröer et al., 2002). Isolation of the plasmid was performed using the Plasmid MiniPrep Kit II (PeqLab Biotechnologie GmbH). Isolated plasmids were linearized with NotI, and cleaned up with the QIAquick PCR purification kit (QIAGEN GmbH), followed by in vitro transcription (mMessage mMachine, Ambion Inc.) and another clean-up (RNeasy MinElute-Cleanup). The cRNA was stored at −70°C. CAII-DNA was isolated from E. coli DH5α either transfected with pGEM-He-Juel-CAII-WT (wild type), pGEM-He-Juel-CAII-HEX (N-terminal mutant, obtained from R. Reithmeier, University of Toronto, Toronto, Canada) or pGEM-He-Juel-CAII-V143Y (catalytically inactive mutant, obtained from C. Fierke, University of Michigan, Ann Arbor, MI). Isolation to transcription followed the protocol for SNAT3-DNA.
Oocytes
Female frogs (Xenopus laevis) were supplied by Horst Kähler, (Bedarf für Forschung und Lehre, Hamburg, Germany). Oocytes were operatively isolated as described before (Becker et al., 2005), separated by collagenase treatment (collagenase A, Roche) and left to recover over night. Oocytes of the stages V and VI were selected and injected with 11 ng SNAT3-cRNA (23 nl) using glass micropipettes and a microinjection device (Nanoliter 2000, World Precision Instruments). Control oocytes were injected with an equivalent volume of DEPC-H2O.
For experiments with CAII, either the protein (isolated from bovine erythrocytes, C3934, Sigma-Aldrich) was injected the day before experiments (46 ng) in SNAT3-expressing oocytes as well as in control oocytes, or the cRNA of CAII-wild type (WT), the catalytically inactive mutant of CAII (V143Y), or the mutant with an exchange of amino acids H for P, H for Q, L for A, H for K, H for Q, and H for S at the position 3, 4, 9, 10, 15, and 17, respectively, in the N terminus of CAII (CAII-HEX), was coinjected with SNAT3-cRNA (7 ng SNAT3-cRNA and 11 ng CAII-cRNA). Oocytes were stored in HEPES-buffered salt solution (NaCl 82.5 mmol, KCl 2.5 mmol, Na2HPO4 1 mmol, HEPES 5 mmol, MgCl2 + 6 H2O 1 mmol, CaCl2 + 2 H2O 1 mmol; pH 7.8, Gentamycin) at 18°C, and the medium was exchanged every day. This buffer was also used in the experiments as the nonbicarbonate buffer.
Electrophysiology
Experiments were performed with microelectrodes impaled into oocytes as reported previously (Bröer et al., 1998; Becker et al., 2004). Current- and ion-sensitive double-barrelled microelectrodes were connected to HS-2A headstages (AxoClamp 2B amplifier, Axon Instruments), gain 10× MG and 1× LU, respectively, or home-build headstages. For pH-sensitive electrodes, two borosilicate glass capillaries (1 mm and 1.5 mm in diameter) were twisted together and pulled to a micropipette. The tips of the micropipettes were backfilled with a mix of tri-N-butylchlorosilane and pure carbon tetrachloride and baked on a hotplate for 4 min 45 s at 450°C. For filling of electrodes see Becker et al. (2004). Calibration of pH-sensitive electrodes was accomplished by changing the pH of extracellular solution by one pH unit (7 to 6 or 7 to 8), the electrodes responded with 52–54 mV/one pH unit change.
Oocytes were voltage clamped at −40 mV and perfused with different concentrations of glutamine (0.5 mM, 3 mM or 10 mM) added to HEPES-buffered oocyte saline or to 5% CO2/HCO3 −-buffered saline (containing 24 mM HCO3 − at pH 7.4, and 77 mM HCO3 − at pH 7.9 instead of HEPES; NaCl was replaced with NaHCO3 maintaining 84.5 mM Na+). Intracellular pH, pHi, and membrane current, Im, were recorded at a holding potential of −40 mV online with software based on the program LabView (National Instruments Corporation). Changes of the membrane potential in steps of 20 mV from −100 to +20 mV were performed to obtain current/voltage relationships of native oocytes and SNAT3-expressing oocytes with injected CAII protein or DEPC-H2O, or of SNAT3 and CAII- coexpressing oocytes.
Flux Measurements
For glutamine uptake measurements, SNAT3-expressing oocytes injected with CAII protein or DEPC-H2O were used (n = 7). Each sample was washed twice with HEPES- or with 5% CO2/24 mM HCO3 −-buffered solution, pH 7.4, respectively. After wash, 90 μl radioactive uptake solution was added to the oocytes, containing 1–3 μl radio-labeled glutamine (L-14C-glutamine, GE Healthcare) per 100 μl glutamine solution (depending on the substrate concentration in normal solution). Uptake was stopped after 5 min by adding 4 ml ice-cold saline or 5% CO2/24 mM HCO3 −-buffered saline to the samples and washed three times. Single oocytes were placed in individual scintillation vials adding 200 μl SDS (10%) and thoroughly vortexed. Standards were prepared by pipetting 10 μl of each radio-labeled uptake solution into individual vials, without adding SDS. After lysis, 3 ml scinitillation cocktail was added to each vial, including standards, and put into scintillation counter.
Mass Spectrometry
CA activity was determined by measuring the 18O exchange from doubly labeled 13C18O2 to water caused by the bidirectional conversion to CO2 and HCO3 −. During the reaction H2 18O is produced, which is diluted infinitely with H2 16O. The concentration of gaseous CO2 species was determined by measuring the signals of m/z = 45 (13C16O2), m/z = 47 (13C18O16O) and m/z = 49 (13C18O2) (Sültemeyer et al., 1990). Graphical representation shows the logarithmic enrichment of 18O atom fraction, calculated as 18O atom fraction = log (13C18O2) × 100/13C16O2 = (49) × 100/ 45 + 47+ 49. For measurements, SNAT3+H2O- and SNAT3+CAII- (protein) injected oocytes, SNAT3+CAII-WT-expressing oocytes as well as SNAT3+CAII-HEX-expressing, SNAT3+CAII-V143Y (catalytically inactive mutant), and native oocytes were prepared. Samples of 20 oocytes were used for experiments. CAII protein was injected into oocytes (SNAT3, native) the day before measurements. Amounts of 46 ng per oocyte result in a total CAII concentration in the cuvette with 20 oocytes of ∼1 μg/8 ml after lysis of cells by mechanical force. Coexpressing oocytes were prepared as described for electrophysiological measurements. Expression of SNAT3 was tested in electrophysiological experiments.
Immunohistochemistry
Oocytes were prepared by injecting the same amounts of RNA as used for eletrophysiology. For antibody staining experiments, native and CAII-WT-, CAII-HEX-, and CAII-V143Y-, as well as SNAT3-, and SNAT3+CAII-WT-, -HEX-, and -V143Y-(co)expressing cells were used. Samples of two to four oocytes for each probe were fixed for 30 min in paraformaldehyde, washed 3× with PBS, incubated in 100% methanol for 20 min at −20°C and washed again 3× with PBS. Nonspecific binding sites were blocked with PBS containing 4% BSA and 0.1% Triton-X-100. Primary antibodies against SNAT3- and CAII-protein were used (Goat polyclonal IgG SNAT3, Santa Cruz, and Rabbit RBX, Chemicon International), and incubated overnight at 4°C in PBS containing 1% BSA and 0.1% Triton-X-100. After 3× wash with PBS, secondary antibodies (1:200) were given to the probes for 2 h in the dark (donkey anti–goat-IgG, Alexa 488, Invitrogen; goat anti–rabbit-IgG, Alexa 546, Molecular Probes). Double staining was done by incubating cells with secondary antibody against SNAT3 for 2 h, washed 3× with PBS, blocked for 1 h with normal goat serum and incubated with secondary antibody against CAII. Cells were ready for confocal imaging after 3× wash in PBS. As a control for unspecific binding, cells from all samples were incubated only with the fluorophore. Images were taken with a laser scanning microscope (LSM 510, Carl Zeiss MicroImaging Inc. GmbH).
Statistics
In the graphs each data point represents the mean ± SEM, for IV diagrams the mean values of control oocytes (H2O injected) are subtracted from values of each SNAT3-expressing oocyte, thus data points represent the mean ± SEM of differences. For calculation of significance, the Student's t test was used. If appropriate, a paired t test was used. In the figures, a significance level of P < 0.05 is marked with *, P < 0.01 with **, and P < 0.001 with ***.
RESULTS
Identification of SNAT3 and Injection of Carbonic Anhydrase Protein
To evaluate the transport activity and the membrane conductance associated with the glutamine transporter SNAT3, we compared three types of oocytes: (1) injected with SNAT3-cRNA 3–6 d before measurement and with bovine carbonic anhydrase isoform II protein (CAII) 1 d before measurement, (2) injected with SNAT3-cRNA and H2O instead of CAII protein, and (3) injected with H2O instead of SNAT3-cRNA, and with CAII protein 1 d before measurement (Fig. 1, A–C). We measured the intracellular pH, converted to intracellular H+ concentration (Hi +) changes, and the membrane current at a holding potential of −40 mV. The substrate glutamine (Km ∼1,6 mM; Fei et al., 2000) was applied in three concentrations, 0.5, 3, and 10 mM in saline buffered with HEPES in the nominal absence of CO2/HCO3 − at pH 7.4, and in the presence of 5% CO2/24 mM HCO3 − at pH 7.4. Before, during, and after applying the substrate, we changed the membrane holding potential in steps between −100 and +20 mV for obtaining current–voltage (I/V) relationships.
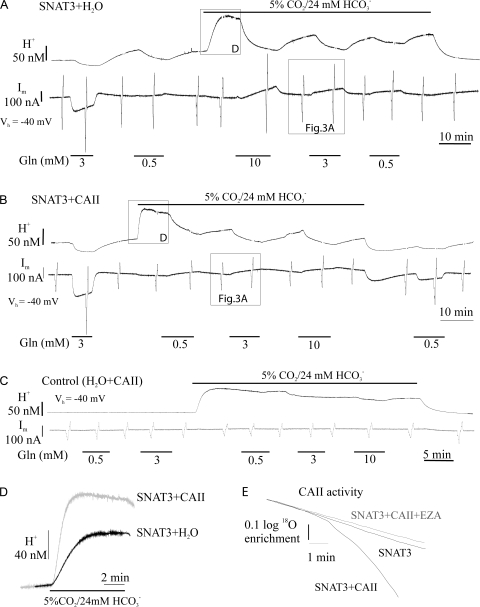
Figure 1.
Effect of injected carbonic anhydrase II (CAII) on transport activity and membrane conductance of SNAT3 expressed in Xenopus oocytes. (A–C) Intracellular H+ changes as measured with pH-selective microelectrodes (H+, top traces) and membrane current in voltage clamp (Im, bottom traces), at a holding potential Vh of −40 mV, during application of glutamine in HEPES-buffered solution (0.5 and 3 mM Gln) and in CO2/HCO3 −-buffered solution (0.5, 3, and 10 mM Gln) in oocytes injected with H2O instead of CAII (A), with CAII protein injected 24 h before the experiment (B), and in native oocytes injected with H2O instead of SNAT3-cRNA and with CAII (C). Injection of CAII accelerates the CO2/HCO3 −-induced rate of intracellular acidification (D) due to its catalytic activity, which is inhibited by ethoxyzolamide (EZA, 10 μM), as measured in mass spectrometry (E).
Application of glutamine elicited an intracellular alkalinization, indicated by a decrease of Hi +, and a membrane current accompanied by an increase in the membrane conductance in oocytes injected with SNAT3-cRNA (Fig. 1, A and B). The membrane current was inward in HEPES-buffered solution, but absent or slightly outward in the presence of CO2/HCO3 −, due to the shift in reversal potential of the current (see below). In oocytes with injected CAII (Fig. 1, B and C), the rate of intracellular acidification to CO2/HCO3 − application was significantly increased as compared with oocytes without injected CAII (Fig. 1 D). The catalytic activity of the enzyme, and its sensitivity to ethoxyzolamide (EZA, 10 μM), was confirmed by measurements of CAII activity in oocytes by mass spectrometry (Fig. 1 E). The glutamine-induced increase in membrane conductance of the SNAT3-expressing oocytes in the presence of CO2/HCO3 − was significantly reduced, when CAII had been injected into the oocytes (Fig. 1 B). This decrease in membrane conductance was subject of further experiments, which will be described below.
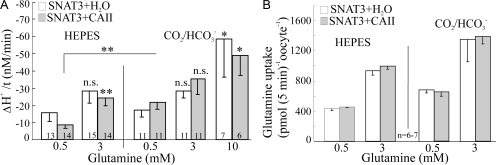
The rate of Hi + decrease in the presence of glutamine, due to the H+ efflux during glutamine uptake, and hence indicative for the transport activity of SNAT3, was dependent on the substrate concentration (Fig. 2 A). It tended to be larger in the presence of CO2/HCO3 −, which was probably due to the lower intracellular pH, and hence larger H+ transmembrane gradient, in CO2/HCO3 −-buffered saline (Table I). This was confirmed by flux measurements of radio-labeled L-14C-glutamine (0.5 and 3 mM) in SNAT3-expressing oocytes (Fig. 2 B). There was no significant difference in either the rate of Hi + decrease or the radio-labeled glutamine uptake in SNAT3-expressing oocytes, when CAII or H2O had been injected into oocytes. We conclude from these experiments that injected CAII had no influence on the transport activity of SNAT3.
Figure 2.
Transport activity of SNAT3 expressed in Xenopus oocytes is dependent on substrate concentration, but is not affected by injected carbonic anhydrase II (CAII). (A) The rate of [H+]i decrease induced by glutamine in SNAT3-expressing oocytes and (B) the rate of uptake of radiolabeled L-14C-glutamine into SNAT3-expressing oocytes, both indicative for the transport activity of SNAT3, were dependent on the glutamine concentration and the buffer used (HEPES and CO2/HCO3 −), but were independent of whether the oocytes had been injected with CAII or H2O. Figures in or between the bars in Fig. 2 give the number of experiments n.
TABLE I.
Measured Intracellular pH, H+ Concentration, and Computed H+ Equilibrium Potential in HEPES and in CO2/HCO3 −-buffered Saline at pH 7.4 and 7.9
| I | SNAT3 + H2O | SNAT3 + CA | SNAT3 + H2O | SNAT3 + CA |
| In HEPES | pHo 7.4 | pHo 7.4 | pHo 7.9 | pHo 7.9 |
| pHi | 7.34 ± 0.03 | 7.25 ± 0.03 | 7.16 ± 0.05 | 7.24 ± 0.03 |
| (n = 14) | (n = 13) | (n = 7) | (n = 9) | |
| [H+]i (nM) | 46 | 56 | 69 | 58 |
| EH+ (mV) | −4 | −9 | −43 | −39 |
| II | SNAT3 + H2O | SNAT3 + CA | SNAT3 + H2O | SNAT3 + CA |
| In CO2/HCO3 − | pHo 7.4 | pHo 7.4 | pHo 7.9 | pHo 7.9 |
| pHi | 6.81 ± 0.04 | 6.73 ± 0.04 | 6.90 ± 0.09 | 6.93 ± 0.07 |
| (n = 15) | (n = 10) | (n = 6) | (n = 9) | |
| [H+]i (nM) | 155 | 186 | 126 | 118 |
| EH+ (mV) | −35 | −39 | −59 | −57 |
| III | SNAT3 + H2O | SNAT3 + CA | SNAT3 + CA | SNAT3 + CA |
| In CO2/HCO3 − | pHo 7.9 | WT pHo 7.9 | V143Y pHo 7.9 | HEX pHo 7.9 |
| pHi | 6.83 ± 0.05 | 6.82 ± 0.05 | 6.79 ± 0.03 | 6.78 ± 0.08 |
| (n = 9) | (n = 13) | (n = 8) | (n = 6) | |
| [H+]i (nM) | 148 | 151 | 162 | 166 |
| EH+ (mV) | −63 | −63 | −65 | −66 |
The Effect of CAII on the SNAT3-associated Membrane Conductance
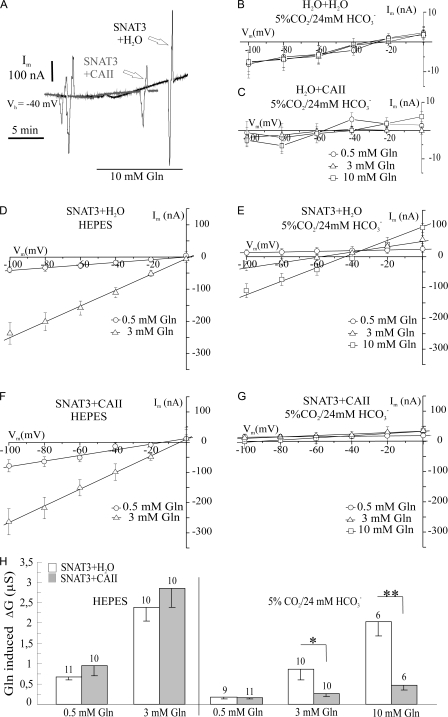
The membrane currents of SNAT3-expressing oocytes with and without injected CAII are shown enlarged in Fig. 3 A before and during application of glutamine to demonstrate the reduced conductance as measured during voltage steps after injection of CAII. I/V relationships in native, control oocytes injected with either H2O (Fig. 3 B) or CAII (Fig. 3 C) with glutamine applied in three different concentrations (0.5, 3, and 10 mM) indicate the background slope conductance of native oocytes of 0.03 ± 0.03 μS (n = 18) without injected CAII protein, and 0.09 ±0.05 μS (n = 19) with injected CAII protein. No endogenous conductances sensitive to glutamine could be observed in these control oocytes. I/V relationships were also plotted for oocytes expressing SNAT3 with and without injected CAII (Fig. 3, D–G) from experiments as shown in Fig. 1 (A and B). The net substrate-dependent currents are shown after steady-state currents in the absence of substrate were subtracted from the currents in the presence of substrate. In SNAT3-expressing oocytes without CAII (SNAT3+H2O), glutamine-induced currents showed nearly linear I/V relationships in HEPES-buffered solution with a reversal potential between 0 and 15 mV (Fig. 3 D). The slope of the I/V curve, indicative for the membrane conductance Gm, increased from 0.7 ± 0.1 to 2.4 ± 0.3 μS, when the glutamine concentration was raised from 0.5 to 3 mM, respectively (Fig. 3 H). In the presence of CO2/HCO3 −, the slope of the I/V curves decreased, and the SNAT3-associated conductance was only 0.2 ± 0.04, 0.9 ± 0.3, and 2.0 ± 0.3 μS in the presence of 0.5, 3, and 10 mM glutamine, respectively (Fig. 3, E and H). The reversal potential for the current induced by 10 mM glutamine shifted to −45 mV in CO2/HCO3 −-buffered solution (Fig. 3 E), presumably due to the lower pHi value in CO2/HCO3 −-buffered solution (Table I). At 3 mM glutamine, the reversal potential of the glutamine- induced current appeared to be shifted to −60 mV; however, with the small change in conductance recorded, the determination of the reversal potential is less accurate.
Figure 3.
Membrane conductance of SNAT3 expressed in Xenopus oocytes as affected by injected carbonic anhydrase II (CAII). Injection of CAII suppresses the glutamine-induced membrane conductance in the presence of CO2/HCO3 − as evident in the superimposed traces (A), as indicated by boxes in Fig. 1 (A and B). The current was recorded during voltage steps between −100 and +20 mV from a holding potential Vh of −40 mV, indicating the membrane conductance. (B–G) Current–voltage relationships of glutamine-induced membrane currents, as obtained from experiments shown in Fig. 1 (A–C), in control oocytes without (B) and with (C) CAII-injected, and oocytes expressing SNAT3 with no CAII injected (SNAT3+H2O; D and E) and in oocytes expressing SNAT3 with CAII protein injected (SNAT3+CAII; F and G) in HEPES-buffered (D and F) and in CO2/HCO3 −-buffered solution (B, C, E, and G). Glutamine-induced currents were isolated by subtracting the currents in the presence of, from the currents in the absence of, glutamine. The glutamine-induced membrane conductance, ΔGm (with the conductance measured in the absence of glutamine subtracted), was determined from the slope of individual current–voltage relationships and plotted for SNAT3-expressing oocytes with H2O or CAII injected (H), indicating that, in the presence of CO2/HCO3 −, CAII nearly completely suppressed the SNAT3-associated membrane conductance. Figures above the bars (H) give the number of experiments n.
In oocytes expressing SNAT3 and injected with CAII protein, the reversal potential of the glutamine-induced current in HEPES-buffered solution was −5 and –15 mV for 0.5 and 3 mM glutamine, and the glutamine-induced conductance was 1 ± 0.2 and 2.9 ± 0.5 μS for these glutamine concentrations, respectively (Fig. 3, F and H). In the presence of CO2/HCO3 −, the slope of the I/V curves became nearly horizontal, indicating that the glutamine-induced conductance was suppressed in oocytes, expressing SNAT3 and injected with CAII, being 0.2 ± 0.03, 0.3 ± 0.1, and 0.5 ± 0.1 μS in 0.5, 3, and 10 mM glutamine, respectively (Fig. 3, G and H). Due to these very small conductances, reversal potentials of the glutamine-induced currents could not be determined.
The results show that the intracellular acidification in CO2/HCO3 −-buffered solution causes a shift of the reversal potential to more negative membrane potentials, indicating the contribution of H+ as charge carrier and/or the dependence on pHi, during the glutamine-induced, SNAT3-associated membrane conductance. The reversal potential of the SNAT3-associated currents remained more positive than the H+ equilibrium potential by up to 20 mV (Table I, parts I and II), in line with the previous study, which showed that cations other than H+, such as Na+, contribute to the current at external pH of 7.4 (Schneider et al., 2007). The change from HEPES- to CO2/HCO3 −-buffered solution decreased the SNAT3-associated conductance, and additional injection of CAII removed this conductance completely.
The Catalytic Activity of CAII Is Necessary for Inhibition of the SNAT3-associated Conductance
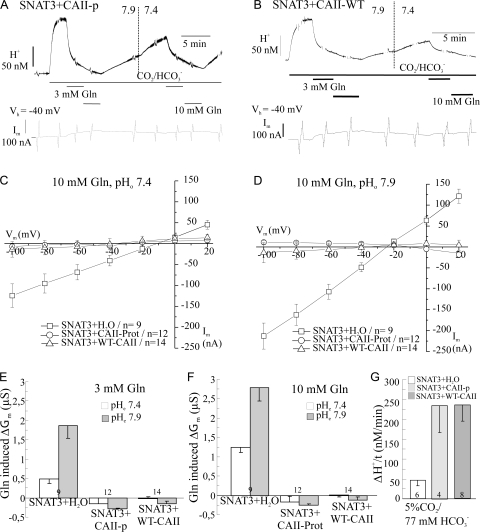
To evaluate the significance of the catalytic activity of the CAII for its effect on the SNAT3-associated membrane conductance, and the reversibility of this effect on the membrane conductance, we blocked the CAII with the sulfonamide EZA (10 μM). These experiments were performed at an external pH of 7.9, when the transporter activity is increased due to the higher pH value (Chaudhry et al., 2001; Bröer et al., 2002; Nakanishi et al. 2001). Fig. 4 A shows the changes in [H+]i and membrane current in a SNAT3-expressing oocyte injected with CAII protein. During the continuous application of 10 mM glutamine, which induced transport activity and an inward current, the solution was changed from HEPES-buffered to 5% CO2/77 mM HCO3 −-buffered solution and back, in the absence and in the presence of EZA. The reduction of the glutamine-induced membrane conductance, Gm, in CO2/HCO3 − was largely abolished, when CAII was inhibited by EZA. The I/V relationships in the different solutions for oocytes expressing SNAT3 with injected CAII show reversibility of the conductance block in CO2/HCO3 −-buffered solution (Fig. 4 B). There was no effect of EZA in SNAT3-expressing oocytes with H2O injected instead of CAII (Fig. 4 C). Hence, the SNAT3-associated membrane conductance was suppressed in CO2/HCO3 −-buffered solution, and recovered in the presence of EZA (Fig. 4 D). In oocytes expressing SNAT3 alone, with no CAII injected, the I/V curves and conductance were similar in HEPES and in CO2/HCO3 −-buffered solution, except for a shift in the reversal potential of the glutamine-induced current by ∼20−25 mV, as discussed above (Fig. 4, C and D; Table I, parts I and II). A similar shift of the reversal potential could be observed for the current recorded in SNAT3+CAII oocytes in the presence of EZA (Fig. 4 B). There was no significant difference between the membrane conductance in SNAT3+CAII-injected oocytes in HEPES-buffered solution and in CO2/HCO3 −-buffered solution in the presence of EZA (Fig. 4 D). In addition, EZA had no apparent effect on the transport activity of SNAT3 (unpublished data). The results show that blocking the catalytic activity of CAII with EZA resulted in the nearly complete recovery of the glutamine-induced membrane conductance.
Figure 4.
Glutamine-induced membrane currents and conductance are restored after blocking the catalytic activity of the carbonic anhydrase. (A) Recording of the intracellular [H+] and the membrane current, at a holding potential Vh of −40 mV, in an oocyte expressing SNAT3 with injected CAII protein, when CO2/HCO3 − was applied and removed in the presence of 10 mM glutamine at external pH 7.9 in the absence and in the presence of 10 μM ethoxyzolamide (EZA) to block the catalytic activity of CAII. (B and C) Current–voltage relationships of the isolated glutamine-induced currents in SNAT3-expressing oocytes with injected CAII protein (B) and with injection of H2O instead of CAII (C) in the absence and presence of CO2/HCO3 −. (D) The glutamine-induced membrane slope conductance, ΔGm, in SNAT3-expressing oocytes with and without injected CAII at pH 7.9 in the absence and presence of EZA in HEPES- and in CO2/HCO3 −-buffered solution.
Coexpression of SNAT3 and Wild-type and Mutant CAII
To elucidate the mechanism of CAII action on the SNAT3 conductance, we compared the effect of injected CAII protein and of coexpressed wild-type and mutant CAII. In similar protocols, oocytes were superfused with 3 and 10 mM glutamine in the presence of CO2/HCO3 −, at two external pH values (7.9 and 7.4; Fig. 5, A and B). The initial application of CO2/HCO3 − induced a cytosolic acidification, the kinetics of which indicated the presence of catalytic CAII activity. The rate of H+ change at an external pH of 7.9 was 234 ± 66 nM/min in SNAT3-expressing oocytes with CAII protein injected (n = 4) and 235 ± 40 nM with CAII wild type coexpressed (n = 8), as compared with 46 ± 13 nM/min in oocytes with no CAII injected or coexpressed (n = 6, P < 0.001; Fig. 5 G). The catalytic activity of CAII was also confirmed with mass spectrometry for all three types of oocytes; SNAT3-expressing oocytes with no CAII injected showed a catalytic activity of 2.08 ± 0.32 U/ml, SNAT3-expressing oocytes injected with CAII protein had a 24-fold higher conversion rate of 50.05 ± 1.6 U/ml, and did not differ significantly from oocytes coexpressing both proteins (49.8 ± 0.49 U/ml).
Figure 5.
Comparison of the effect of injected CAII protein and of coexpressed wild-type CAII on SNAT3 activity at different external pH. (A and B) Recordings of intracellular [H+] and membrane current (Im) at a holding potential Vh of −40 mV, in SNAT3-expressing oocytes during application of 3 and 10 mM glutamine to activate SNAT3 in the presence of CO2/HCO3 − at pH 7.9 and 7.4 with injected CAII protein (SNAT3+CAII-p; A), and with wild-type CAII coexpressed (SNAT3+CAII-WT; B). (C and D) The current–voltage relationships reveal prominent glutamine-induced currents (background currents taken in the absence of glutamine subtracted) only in oocytes with no injected or coexpressed CAII (SNAT3+H2O) at both external pH 7.4 and pH 7.9. (E and F) The isolated glutamine-induced membrane slope conductance, dependent on substrate concentration and external pH, is suppressed equally by injected and coexpressed CAII. (G) Rate of cytosolic acidification (ΔH+/t) induced by addition of CO2/HCO3 − in SNAT3-expressing oocytes without CAII (SNAT3+H2O), with injected CAII protein, and with CAII-wild type coexpressed.
The current–voltage relationships are shown for 10 mM glutamine at pH 7.4 (Fig. 5 C) and at pH 7.9 (Fig. 5 D), indicating that SNAT3-associated membrane current was suppressed, when CAII was injected or coexpressed in SNAT3-expressing oocytes. The glutamine- induced membrane conductances at both pH values and two glutamine concentrations (3 and 10 mM) were suppressed similarly by injected CAII and by coexpressed CAII (Fig. 5, E and F). A measurable conductance and reversal potential sensitive to glutamine were only observed in SNAT3- expressing oocytes, in which CAII was neither injected nor coexpressed.
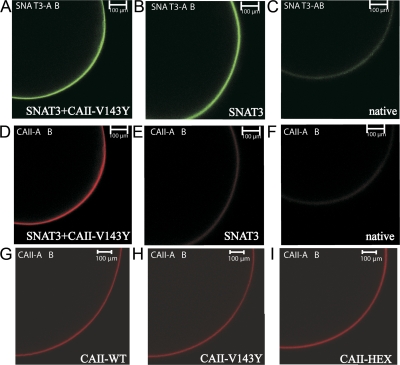
In another set of experiments, we coexpressed the catalytically inactive CAII mutant V143Y, a mutant that shows <10% of its catalytic activity by a single site mutation at V143 (Alexander et al., 1991; Fierke et al., 1991). The expression of the catalytically inactive mutant V143Y was confirmed by immunohistochemistry (Fig. 6). SNAT3+CAII-V143Y–coexpressing oocytes were labeled twice (Fig. 6, A and D). Staining of oocytes expressing SNAT3 alone showed labeling against SNAT3 protein, but barely against CAII protein (Fig. 6, B and E). Native, control, oocytes showed very little or no staining, neither for SNAT3 nor for CAII protein (Fig. 6, C and F). The staining of unlabeled oocytes was presumably due to some unspecific binding of the antibodies and differs in intensity from CAII-expressing oocytes. Antibody staining for CAII-WT, for the catalytically inactive mutant CAII-V143Y, and for the N-terminal mutant CAII-HEX also shows localization of the CAII protein near the cell membrane, as observed in SNAT3-CAII–coexpressing oocytes. Thus, coexpression of CAII with SNAT3 is not required for the CAII to be localized near the cell membrane.
Figure 6.
Immunohistochemistry for wild-type and CAII mutants. SNAT3+CAII-V143Y–coexpressing (A and D), SNAT3-expressing (B and E), and native oocytes (C and F) were stained with antibodies against SNAT3 protein coupled to Alexa 488 dye (green) and against CAII protein coupled to Alexa 546 dye (red), showing the expression of both proteins near the cell membrane, and of oocytes expressing SNAT3 alone and background fluorescence as observed in native oocytes, respectively. (G–I) Oocytes expressing wild-type CAII (CAII-WT; G), catalytically inactive mutant CAII-V143Y (H); or N-terminal mutant (CAII-HEX, I) alone were also stained with the CAII antibody coupled to Alexa 546 dye (red), and show that these CAII proteins are also localized near the cell membrane when expressed alone.
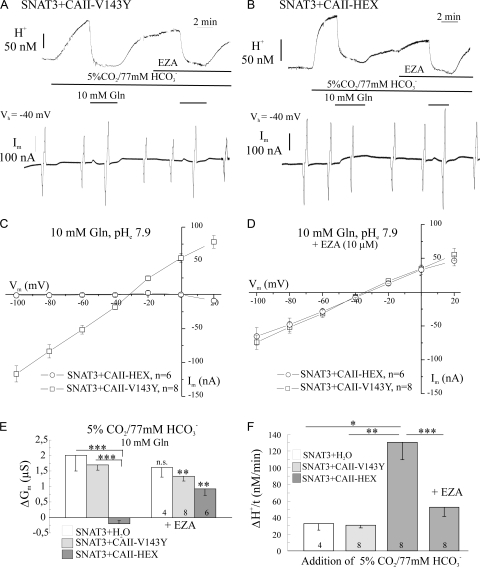
In the presence of CO2/HCO3 −, 10 mM glutamine still induced a prominent membrane conductance, when SNAT3 was coexpressed with the catalytically inactive mutant CAII-V143Y (Fig. 7 A), but not when coexpressed with the N-terminal mutant CAII-HEX (Fig. 7 B). The latter was produced as a mutant, in which binding to acidic residues in anion exchanger 1 (AE1) (Vince and Reithmeier, 2000) and sodium-bicarbonate cotransporter (NBC) (Pushkin et al. 2004) is suppressed. We conclude from our experiments that the mutated binding motif in the N terminus of the CAII is not required to suppress the SNAT3-associated membrane conductance. The transport of substrate by SNAT3, as evaluated by the rate of change of [H+]i, was similar, when either CAII mutant or no CAII had been coexpressed with SNAT3 (not depicted), as has been shown for oocytes with injected CAII protein (Fig. 2). The catalytic activity of the CAII mutant HEX was indicated by the high rate of cytosolic acidification upon introduction of CO2/HCO3 − (Fig. 7, B and F), and was also confirmed by mass spectrometry (unpublished data). In contrast, oocytes expressing SNAT3 alone and oocytes coexpressing the catalytically inactive mutant CA-V143Y (Fig. 7, A and F) showed virtually no catalytic CA activity (Fig. 7, A and F). In the presence of EZA (10 μM), the rate of cytosolic [H+] change was reduced in oocytes coexpressing SNAT3 and the CAII mutant HEX to values obtained for oocytes expressing SNAT3 alone, or coexpressing SNAT3 and the CA mutant V143Y (Fig. 7 F).
Figure 7.
Effect of different CAII mutants coexpressed on SNAT3-associated membrane conductance. (A and B) Recordings of intracellular [H+] and membrane current (Im) at a holding potential Vh of −40 mV, in SNAT3-expressing oocytes during application of 10 mM glutamine to activate SNAT3 in the presence of CO2/HCO3 − before and after blocking CAII with EZA (10 μM) at pH 7.9 with the catalytically inactive CAII mutant V143Y coexpressed (SNAT3+CAII-V143Y; A), and with the N terminus CAII mutant HEX coexpressed (SNAT3+CAII-HEX; B). Current–voltage relationships of the isolated glutamine-induced membrane currents in SNAT3-expressing oocytes with coexpressed CAII mutants V143Y or HEX in the absence (C) and in the presence of EZA (D). (E) Glutamine-induced membrane conductance changes (ΔGm) of SNAT3-expressing oocytes without CAII coexpressed (SNAT3+H2O), and with the mutant CAII-V143Y or CAII-HEX coexpressed in the absence and presence of EZA. Note the recovery of the membrane conductance in oocytes expressing SNAT3-CAII-HEX in the presence of EZA. (F) Rate of cytosolic acidification (ΔH+/t) induced by CO2/HCO3 − in oocytes without CAII (SNAT3+H2O), and with the mutants CAII-V143Y or CAII-HEX coexpressed.
The glutamine-induced membrane conductance was restored after inhibition of CAII with EZA (Fig. 7, B and E). The current–voltage relationships of the SNAT3-expressing oocytes with N-terminal mutant CA-HEX coexpressed show that mutation of the N terminus did not prevent the suppression of the SNAT3-associated membrane conductance by CAII, but could be recovered by inhibiting the catalytic activity of CAII-HEX by EZA (Fig. 7, C and D). The current–voltage relationships of oocytes expressing SNAT3 and either CAII mutant was hence nearly identical in the presence of EZA (Fig. 7 D). The glutamine-induced currents were not suppressed, however, by the catalytically inactive CAII mutant V143Y (Fig. 7, A and C). The reversal potential for the glutamine-induced current, when present, was near −40 mV in all types of oocytes, which is 20–25 mV more positive than the H+ equilibrium potential as calculated by the Nernst equation (Table I, part III), indicating the contribution of Na+ to the stoichiometrically uncoupled membrane conductance associated with SNAT3 also at pH 7.9 (see Schneider et al., 2007).
DISCUSSION
Carbonic anhydrase supports transport of a variety of different acid/base-coupled membrane carriers, such as the AE, the NBC, and the sodium-hydrogen exchanger (NHE) by forming a “transport metabolon,” which requires the enzymatic activity of carbonic anhydrase (Vince and Reithmeier, 1998; Li et al., 2002; Alvarez et al., 2003; Loiselle et al., 2004; Pushkin et al., 2004). It was suggested that the CAII isoform binds to the membrane transporter, and thereby enhances its transport activity. Recently, we reported another type of interaction of the carbonic anhydrase with the monocarboxylate transporter isoform 1 (MCT1; Becker et al., 2005), in which the enhancement by CAII of lactate transport via MCT1 was independent of the catalytic activity of CAII. In the present study we have shown yet another type of interaction between CAII and the H+-coupled glutamine transporter SNAT3, where CAII suppresses the SNAT3-associated nonstoichiometrically coupled cation conductance in the presence of CO2/HCO3 −. However, neither in HEPES- nor in bicarbonate-buffered solution, an enhancement of substrate transport via SNAT3 could be observed, when CAII was either injected or coexpressed. Neither the CAII-binding motif described for NHE1 (Ser 796, Asp 797; Li et al., 2002), the carrier with a counter transport of Na+ and H+ ions, as found in SNAT3, in the C terminus of the protein, nor the acidic sequence shown for AE1 (Vince and Reithmeier, 2000) and NBC (Pushkin et al., 2004) suggested to bind CAII were found in the SNAT3 sequence as tested by homology comparison. One should consider that the interaction of CAII on other SLC proteins, like NBC or NHE1, involves binding of CA to the C terminus of these membrane proteins (Li et al., 2002; Loiselle et al., 2004). However, SLC 38 proteins have been predicted to locate their C terminus extracellularly, which would not likely be accessible by intracellular CA (Mackenzie and Erickson, 2004). Thus, the effect of CAII on SNAT3 is clearly different from that described for the membrane transporters of the SLC4 family, which form a transport metabolon with CA.
Mechanisms of the Interaction between SNAT3 and CAII
Similar to the interaction of other acid/base-coupled membrane transporters with CA isoforms is that it is the catalytic activity of CAII that is required to suppress the membrane conductance associated with SNAT3. First, the effect could only be observed in the presence of CO2/HCO3 −, the substrate of the enzyme; second, the catalytically inactive CAII mutant V143Y did not affect the membrane conductance; and third, blocking the catalytic CA activity with EZA restored the membrane conductance. The N-terminal mutant CAII-HEX, however, reduced the SNAT3-associated membrane conductance similar to CAII-WT, indicating that the mutated motif of the N terminus is not involved in suppressing this conductance. This suggests that either binding of CAII to SNAT3 is not needed at all or binding occurs at other CAII motifs than reported for other acid/ base-coupled transporters (Vince and Reithmeier, 2000; Pushkin et al., 2004).
The cytosolic buffering capacity of oocytes we determined with pH-sensitive microelectrodes is the global buffer capacity of the large oocyte and not significantly altered in SNAT3-expressing cells with or without CAII (injected or coexpressed). The effect of CAII on the membrane conductance might nevertheless be due to the creation of microdomains at the inner surface of the membrane, in which CAII might increase the buffer capacity. Since the membrane conductance is dependent on extra- and intracellular pH, a local change in buffer capacity might help to shape the changes in pH and hence this conductance.
There are arguments, however, that do not support a local pH microdomain effect associated with the suppression of SNAT3-associated membrane conductance by CAII. First, if the intracellular pH change induced by SNAT3 activity resulted in a reduction of the SNAT3 conductance, an initial current/conductance would be expected before SNAT3 transport activity had produced a significant pH change at the inner face of the cell membrane, which was not observed. Second, intracellular acidification, e.g., after the removal of glutamine, when efflux of glutamine is associated with H+ influx, or during application of a weak acid, impairs neither transport activity nor the membrane conductance of SNAT3. Third, in a solution buffered with CO2/HCO3 − (Erev = −36 mV) and/or at an extracellular pH value of 7.9 (Erev = −35 mV), where the H+ equilibrium potential is close to the membrane holding potential of −40 mV, little H+ flux would be expected due to the reduced H+ gradient; under these conditions, however, not even transient currents, indicative for a time-dependent change in conductance, were observed. Therefore, we used the slope conductance associated with SNAT3 activity and not absolute currents, because they may be small or even absent, depending on the difference between the reversal potential of the current and the holding potential. The fact that the rate of H+ change following addition of substrate was not affected by CAII, neither in HEPES- nor in CO2/HCO3 −-buffered solution (see Fig. 2 A), supports our conclusion that the currents associated with SNAT3 activity do not contribute significantly to the pH changes measured. Furthermore, pH changes near the inner cell membrane produced by the conductance would also be expected to affect the pH-dependent substrate transport, which was, however, not altered by CAII.
The dependence or independence of substrate transport and ion conducting pathway have been discussed for the glutamate transporter, which is not only electrogenic, but also shows a stoichiometrically uncoupled anion conductance (Fairman et al., 1995; Wadiche et al., 1995). While interaction of the homomeric transporter subunits was suggested for this anion conductance (Torres-Salazar and Fahlke, 2006), two recent studies reported that glutamate and chloride permeation occur independently by each subunit (Koch et al., 2007; Leary et al., 2007). The fact that CAII suppresses the SNAT3-associated membrane conductance, but leaves glutamine transport completely unaffected, can be taken to support the independence of substrate transport and ion conductance of SNAT3.
There is the question of whether degradation of glutamine in oocytes might affect the SNAT3-associated membrane conductance. In Xenopus oocytes there is very little metabolic activity, which makes substantial degradation of glutamine during the relatively short time of application unlikely. Furthermore, the complete reacidification upon glutamine removal, which brings the pHi back to the value before glutamine application, is in favor of the notion that no significant degradation of glutamine had taken place, which could lead to the production of other ion and hence additional conductances. Several independent studies have suggested that the SNAT3-associated, substrate-dependent conductance is selective for monovalent cations, such as Na+ and H+ (Fei et al., 2000; Chaudhry et al., 2001; Bröer et al., 2002; Schneider et al., 2007). It is unlikely, therefore, that HCO3 − as an anion would contribute to this conductance. In native oocytes, neither glutamine nor CO2/HCO3 − changed the membrane conductance, and in oocytes expressing SNAT3, the introduction of CO2/HCO3 − did not induce a membrane conductance. Thus, the conductance studied here was dependent on the expression of SNAT3 and the presence of substrate (see also Schneider et al., 2007).
Physiological Significance of Interaction between CAII and SNAT3
Our results also raise the question of whether the SNAT3-associated conductance may only be observed in cells with low or no CA enzymatic activity like frog oocytes, and whether it is of any significance in cells that exhibit normal CA catalytic activity. Since the catalytic activity of CAII is required to suppress this conductance, experimental solutions must be buffered with CO2/HCO3 − even if CAII is present. We showed that in SNAT3-expressing oocytes, the SNAT3-associated membrane conductance was unaffected by injection of CAII in HEPES-buffered, nominally CO2/HCO3 −-free solution (Fig. 1 B and Fig. 3 F). As many experiments, in particular with nonmammalian cells and tissues, including the frequently used Xenopus oocyte as heterologous expression system, are performed in nonbicarbonate buffers, effects attributable to the catalytic activity of CA and or CO2/HCO3 − would be missed. Even if the physiological role of SNAT3-associated membrane conductance can be questioned, our findings are of interest, as they suggest a regulatory role of CA for this SNAT3-associated membrane conductance. Moreover, the suppression of a conductance by a nearly ubiquitous enzyme as the CA may help to identify the conditions under which such a conductance might become significant. In the kidney, e.g., SNAT3 is expressed in the proximal tubule segment 3, but is up-regulated also in segment 2 during chronic acidosis (Moret et al., 2007). SNAT3 also fuels the ammonium production (“ammoniagenesis”) by reabsorbtion of glutamine from the urine, and in the proximal tubule, CO2 is produced following glutamine oxidation. All the processes are dependent on pH, which can be affected by CA. Uptake, deamination, and oxidation of glutamine are supported by system N transporters, glutaminase activity, and entry of carbon skeleton into the tricarboxic cycle, respectively. Interestingly, blocking CA activity with acetazolamide CO2 reduces ammonia production and hence the acid/base balance (Mujais and Zahid, 1992). During acidosis, glutamine reuptake is increased by SNAT3, which helps to reduce the acid overload by outward transport of H+ (George and Solomon, 1981; Wiklund, 1996). Under conditions of CO2/HCO3 − buffering and in the presence of CAII, SNAT3 would not contribute to any changes in membrane potential, since the conductance is inhibited by CAII. However, if CAII is inhibited by acetazolamide, e.g., in patients suffering chronic metabolic acid/base disturbance, a membrane conductance during SNAT3-mediated glutamine transport would be unmasked, which would change the membrane potential, and hence ion channels and electrogenic transporters in the epithelial membrane. Under these conditions, glutamine uptake via SNAT3 would depolarize the cells. This might be considered as a possible side effect during therapeutical treatment with CA inhibitors in the kidney, liver, brain, and other organs where glutamine transport via SNAT3 plays a major role with concomitant expression of CAII.
We conclude that the catalytic activity of CAII affects the SNAT3 conductance, but presumably not by increasing the local H+ buffer capacity. A more direct interaction between the catalytic center of CAII with the cationic conducting pore of SNAT3 may be required. The mode and mechanism of interaction between SNAT3 and CAII needs to be tackled by using different CA isoforms and mutants, together with selected SNAT3 mutants. We have recently described single site SNAT3 mutants, which show a greatly reduced transport activity and membrane conductance (Schneider et al., 2007). These mutants could help to identify the domain that might be affected by CAII interaction with SNAT3. The mechanism of CAII modulation may be a more widespread phenomenon also for other membrane transporters, in particular when transport of acid/base equivalents are involved. Since some membrane carriers have primarily been studied in heterologous expression systems like frog oocytes, where nonbicarbonate buffers are normally used, this would require some reinvestigation in the presence of CO2/HCO3 −-buffered solution and with the injection or expression of CA isoforms.
Acknowledgments
This paper is dedicated to Prof. Roger C. Thomas, Cambridge, UK, on occasion of his 68th birthday.
We are grateful to Carol Fierke and Reinhart Reithmeier for the supply of CAII wild-type and mutant clones, and to Dieter Sültemeyer for helping with the mass spectrometry measurements.
This study was supported by a grant from the Deutsche Forschungsgemeinschaft (DE 231/16-4) and by the DFG-Graduate Research College 845.
David C. Gadsby served as editor.
Abbreviations used in this paper: AE, anion exchanger; CAII, carbonic anhydrase isoform II; EZA, ethoxyzolamide; NBC, sodium-bicarbonate cotransporter; NHE, sodium-hydrogen exchanger.
References
- Alexander, R.S., S.K. Nair, and D.W. Christianson. 1991. Engineering the hydrophobic pocket of carbonic anhydrase II. Biochemistry. 30:11064–11072. [DOI] [PubMed] [Google Scholar]
- Alvarez, B.V., F.B. Loiselle, C.T. Supuran, G.J. Schwartz, and J.R. Casey. 2003. Direct extracellular interaction between carbonic anhydrase IV and the human NBC1 sodium/bicarbonate co-transporter. Biochemistry. 42:12321–12329. [DOI] [PubMed] [Google Scholar]
- Bak, L.K., A. Schousboe, and H.S. Waagepetersen. 2006. The glutamate/GABA-glutamine cycle: aspects of transport, neurotransmitter homeostasis and ammonia transfer. J. Neurochem. 98:641–653. [DOI] [PubMed] [Google Scholar]
- Becker, H.M., and J.W. Deitmer. 2007. Carbonic anhydrase II increases the activity of the human electrogenic Na+/HCO3 − cotransporter. J. Biol. Chem. 282:13508–13521. [DOI] [PubMed] [Google Scholar]
- Becker, H.M., D. Hirnet, C. Fecher-Trost, D. Sültemeyer, and J.W. Deitmer. 2005. Transport activity of MCT1 expressed in Xenopus oocytes is increased by interaction with carbonic anhydrase. J. Biol. Chem. 280:39882–39889. [DOI] [PubMed] [Google Scholar]
- Becker, H.M., S. Bröer, and J.W. Deitmer. 2004. Facilitated lactate transport by MCT1 when coexpressed with the sodium-bicarbonate cotransporter (NBC) in Xenopus oocytes. Biophys. J. 86:235–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode, B.P. 2001. Recent molecular advances in mammalian glutamine transport. J. Nutr. 131:2475S–2485S. [DOI] [PubMed] [Google Scholar]
- Bröer, A., A. Albers, I. Setiawan, R.H. Edwards, F.A. Chaudhry, F. Lang, C.A. Wagner, and S. Bröer. 2002. Regulation of the glutamine transporter SN1 by extracellular pH and intracellular sodium ions. J. Physiol. 539:3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bröer, S., and N. Brookes. 2001. Transfer of glutamine between astrocytes and neurons. J. Neurochem. 77:705–719. [DOI] [PubMed] [Google Scholar]
- Bröer, S., H.P. Schneider, A. Bröer, B. Rahman, B. Hamprecht, and J.W. Deitmer. 1998. Characterization of the monocarboxylate transporter 1 expressed in Xenopus laevis oocytes by changes in cytosolic pH. Biochem. J. 333:167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth, R.F. 2002. Pathophysiology of hepatic encephalopathy: a new look at ammonia. Metab. Brain Dis. 17:221–227. [DOI] [PubMed] [Google Scholar]
- Chaudhry, F.A., D. Krizaj, P. Larsson, R.J. Reimer, C. Wreden, J. Storm-Mathisen, D. Copenhagen, M. Kavanaugh, and R.H. Edwards. 2001. Coupled and uncoupled proton movement by amino acid transport system N. EMBO J. 20:7041–7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry, F.A., R.J. Reimer, D. Krizaj, D. Barber, J. Storm-Mathisen, D.R. Copenhagen, and R. Edwards. 1999. Molecular analysis of system N suggests novel physiological roles in nitrogen metabolism and synaptic transmission. Cell. 99:769–780. [DOI] [PubMed] [Google Scholar]
- Fairman, W.A., R.J. Vandenberg, J.L. Arriza, M.P. Kavanaugh, and S.G. Amara. 1995. An excitatory amino-acid transporter with properties of a ligand-gated chloride channel. Nature. 375:599–603. [DOI] [PubMed] [Google Scholar]
- Fei, Y.-J., M. Sugawara, T. Nakanishi, W. Huang, H. Wang, P.D. Prasad, F.H. Leibach, and V. Ganapathy. 2000. Primary structure, genomic organization, and functional and electrogenic characteristics of human system N 1, a Na+- and H+-coupled glutamine transporter. J. Biol. Chem. 275:23707–23717. [DOI] [PubMed] [Google Scholar]
- Fierke, C.A., T.L. Calderone, and J.F. Krebs. 1991. Functional consequences of engineering the hydrophobic pocket of carbonic anhydrase II. Biochemistry. 30:11054–11063. [DOI] [PubMed] [Google Scholar]
- George, J.P., and S. Solomon. 1981. pH and temperature dependence of glutamine uptake, carbon dioxide and ammonia production in kidney slices from acidotic rats. J. Physiol. 316:251–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu, S., H.L. Roderick, P. Camacho, and J.X. Jiang. 2000. Identification and characterization of an amino acid transporter expressed differentially in liver. Proc. Natl. Acad. Sci. USA. 97:3230–3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz, L. 2004. Intercellular metabolic compartmentation in the brain: past, present and future. Neurochem. Int. 45:285–296. [DOI] [PubMed] [Google Scholar]
- Jalan, R., S.W. Olde Damink, H.F. Lui, M. Glabus, N.E. Deutz, P.C. Hayes, and K. Ebmeier. 2003. Oral amino acid load mimicking hemoglobin results in reduced regional cerebral perfusion and deterioration in memory tests in patients with cirrhosis of the liver. Metab. Brain Dis. 18:37–49. [DOI] [PubMed] [Google Scholar]
- Koch, H.P., R.L. Brown, and H.P. Larsson. 2007. The glutamate-activated anion conductance in excitatory amino acid transporters is gated independently by the individual subunits. J. Neurosci. 27:2943–2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leary, G.P., E.F. Stone, D.C. Holley, and M.P. Kavanaugh. 2007. The glutamate and chloride permeation pathways are colocalized in individual neuronal glutamate transporter subunits. J. Neurosci. 27:2938–2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X., B. Alvarez, J.R. Casey, R.A. Reithmeier, and L. Fliegel. 2002. Carbonic anhydrase II binds to and enhances activity of the Na+/H+ exchanger. J. Biol. Chem. 277:36085–36091. [DOI] [PubMed] [Google Scholar]
- Loiselle, F.B., P.E. Morgan, B.V. Alvarez, and J.R. Casey. 2004. Regulation of the human NBC3 Na+/HCO3 − cotransporter by carbonic anhydrase II and PKA. Am. J. Physiol. Cell Physiol. 286:C1423–C1433. [DOI] [PubMed] [Google Scholar]
- Mackenzie, B., and J.D. Erickson. 2004. Sodium-coupled neutral amino acid (System N/A) transporters of the SLC38 gene family. Pflugers Arch. 447:784–795. [DOI] [PubMed] [Google Scholar]
- McMurtrie, H.L., H.J. Cleary, B.V. Alvarez, F.B. Loiselle, D. Sterling, P.E. Morgan, D.E. Johnson, and J.R. Casey. 2004. The bicarbonate transport metabolon. J. Enzyme Inhib. Med. Chem. 19:231–236. [DOI] [PubMed] [Google Scholar]
- Moret, C., M.H. Dave, N. Schulz, J.X. Jiang, F. Verrey, and C.A. Wagner. 2007. Regulation of renal amino acid transporters during metabolic acidosis. Am. J. Physiol. Renal Physiol. 292:F555–F566. [DOI] [PubMed] [Google Scholar]
- Mujais, S.K., and M. Zahid. 1992. Tubular CO2 production from glutamine in the rat: segmental profile and modulation. Ren. Physiol. Biochem. 15:119–128. [DOI] [PubMed] [Google Scholar]
- Nakanishi, T., R. Kekuda, J.Y. Fei, R. Hatanaka, M. Sugawara, R.G. Martindale, F. Leibach, P. Prasad, and V. Ganapathy. 2001. Cloning and functional characterization of a new subtype of the amino acid transport system N. Am. J. Physiol. Cell Physiol. 281:C1757–C1768. [DOI] [PubMed] [Google Scholar]
- Pushkin, A., N. Abuladze, E. Gross, D. Newman, S. Tatishchev, I. Lee, O. Fedotoff, G. Bondar, R. Azimov, M. Ngyuen, and I. Kurtz. 2004. Molecular mechanism of kNBC1-carbonic anhydrase II interaction in proximal tubule cells. J. Physiol. 559:55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider, H.P., S. Bröer, A. Bröer, and J.W. Deitmer. 2007. Heterologous expression of the glutamine transporter SNAT3 in Xenopus oocytes is associated with four modes of uncoupled transport. J. Biol. Chem. 282:3788–3798. [DOI] [PubMed] [Google Scholar]
- Sidoryk, M., E. Matyja, A. Dybel, M. Zielinska, J. Bogucki, D.J.J. Iski, P.P. Liberski, P. Kowalczyk, and J. Albrecht. 2004. Increased expression of a glutamine transporter SNAT3 is a marker of malignant gliomas. Neuroreport. 15:575–578. [DOI] [PubMed] [Google Scholar]
- Sterling, D., R.A. Reithmeier, and J.R. Casey. 2001. A transport metabolon. Functional interaction of carbonic anhydrase II and chloride/bicarbonate exchangers. J. Biol. Chem. 276:47886–47894. [DOI] [PubMed] [Google Scholar]
- Sültemeyer, D.F., H.P. Fock, and D.T. Canvin. 1990. Mass spectrometric measurement of intracellular carbonic anhydrase activity in high and low Ci cells of chlamydomonas: studies using 180 exchange with 13C/180 labeled bicarbonate. Plant Physiol. 94:1250–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Salazar, D., and C. Fahlke. 2006. Intersubunit interactions in EAAT4 glutamate transporters. J. Neurosci. 26:7513–7522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vince, J.W., and R.A. Reithmeier. 1998. Carbonic anhydrase II binds to the carboxyl terminus of human band 3, the erythrocyte C1−/HCO3− exchanger. J. Biol. Chem. 273:28430–28437. [DOI] [PubMed] [Google Scholar]
- Vince, J.W., and R.A. Reithmeier. 2000. Identification of the carbonic anhydrase II binding site in the Cl−/HCO3− anion exchanger AE1. Biochemistry. 39:5527–5533. [DOI] [PubMed] [Google Scholar]
- Wadiche, J.I., S.G. Amara, and M.P. Kavanaugh. 1995. Ion fluxes associated with excitatory amino acid transport. Neuron. 15:721–728. [DOI] [PubMed] [Google Scholar]
- Wiklund, L. 1996. Carbon dioxide formation and elimination in man. Recent theories and possible consequences. Ups. J. Med. Sci. 101:35–67. [DOI] [PubMed] [Google Scholar]
- Zwingmann, C., and R. Butterworth. 2005. An update on the role of brain glutamine synthesis and its relation to cell-specific energy metabolism in the hyperammonemic brain: further studies using NMR spectroscopy. Neurochem. Int. 47:19–30. [DOI] [PubMed] [Google Scholar]