Abstract
Small conductance calcium-gated potassium (SK) channels share an overall topology with voltage-gated potassium (Kv) channels, but are distinct in that they are gated solely by calcium (Ca2+), not voltage. For Kv channels there is strong evidence for an activation gate at the intracellular end of the pore, which was not revealed by substituted cysteine accessibility of the homologous region in SK2 channels. In this study, the divalent ions cadmium (Cd2+) and barium (Ba2+), and 2-aminoethyl methanethiosulfonate (MTSEA) were used to probe three sites in the SK2 channel pore, each intracellular to (on the selectivity filter side of) the region that forms the intracellular activation gate of voltage-gated ion channels. We report that Cd2+ applied to the intracellular side of the membrane can modify a cysteine introduced to a site (V391C) just intracellular to the putative activation gate whether channels are open or closed. Similarly, MTSEA applied to the intracellular side of the membrane can access a cysteine residue (A384C) that, based on homology to potassium (K) channel crystal structures (i.e., the KcsA/MthK model), resides one amino acid intracellular to the glycine gating hinge. Cd2+ and MTSEA modify with similar rates whether the channels are open or closed. In contrast, Ba2+ applied to the intracellular side of the membrane, which is believed to block at the intracellular end of the selectivity filter, blocks open but not closed channels when applied to the cytoplasmic face of rSK2 channels. Moreover, Ba2+ is trapped in SK2 channels when applied to open channels that are subsequently closed. Ba2+ pre-block slows MTSEA modification of A384C in open but not in closed (Ba2+-trapped) channels. The findings suggest that the SK channel activation gate resides deep in the vestibule of the channel, perhaps in the selectivity filter itself.
INTRODUCTION
Ion channels are the primary regulators of membrane excitability in nervous and muscle tissue. To precisely control a cell's excitability, ion channels must open and close in response to appropriate stimuli such as voltage or ligand binding, the process of gating. Voltage-gated potassium (Kv) channels contain a densely charged voltage sensor domain that allows them to gate in response to changes in membrane potential. Small conductance calcium-gated potassium (SK) channels, on the other hand, open in response to increases in intracellular Ca2+, such as those generated by action potentials. In this way, SK channels serve to dampen cell excitability by exerting a hyperpolarizing influence during periods of neuronal activity (Bond et al., 2005). There are three highly homologous SK channel subunits (SK1, SK2, and SK3), each containing six putative transmembrane (TM) domains with predicted topologies similar to Kv channels (Kohler et al., 1996). The fourth domain, TM4, contains positively charged residues but single channel and macroscopic current studies showed that SK channel open probability is independent of membrane voltage (Hirschberg et al., 1998). SK channel gating is accomplished through an association with coassembled calmodulin (CaM) that is constitutively bound to a succinct domain (CaMBD) in the membrane-proximal region of the intracellular C terminus of the channel. CaM functions as the Ca2+ sensor for SK channels, transducing the Ca2+ gating signal through the CaMBD to a yet unidentified activation gate (Xia et al., 1998; Keen et al., 1999; Schumacher et al., 2001).
The activation gate of Kv channels has long been thought to reside at the intracellular end of the pore. Using native Kv channels in the squid giant axon, it was shown that cytoplasmically applied quaternary amine such as TEA not only blocked conduction through K+ channels, they could inhibit channel closure (Armstrong, 1971, 1974; Armstrong and Hille, 1972). This “foot-in-the-door” effect could be explained if TEA derivatives bound to a site in the channel pore and prevented closure of an intracellular gate. For cloned Shaker Kv channels, functional experiments using the substituted cysteine accessibility method (Liu et al., 1997; del Camino et al., 2000; del Camino and Yellen, 2001), cross-linking methodologies (Holmgren et al., 1998), and “trapping” of pore blockers (Holmgren et al., 1997) strongly suggest that the activation gate is located at the intracellular end of the pore. Indeed, the use of intracellular activation gates appears to be widespread among voltage-gated ion channels (Shin et al., 2001; Rothberg et al., 2002; Zhao et al., 2004; Xie et al., 2005), and intracellular activation gates are seen in crystal structures of two types of K channels (Doyle et al., 1998; Jiang et al., 2002b; Kuo et al., 2003, 2005).
We have previously reported that the lower TM6 region of SK channels does not form an effective barrier to 2-aminoethyl methanethiosulfonate (MTSEA) or tetrabutylammonium (TBuA) (Bruening-Wright et al., 2002). In this report, Cd2+ was used to test for state-dependent access to a position just intracellular to the region that forms the activation gate in other K channels. The state dependence of MTSEA access to sites deeper in the inner vestibule was also tested, and Ba2+ was used to test for state dependence at the level of the selectivity filter. The results show that Cd2+ access to V391C and MTSEA modification of A384C, sites predicted to reside intracellular to the TM6 bundle crossing, are not strongly state dependent. In contrast, Ba2+ applied to the intracellular side of the membrane blocks only open SK2 channels, and Ba2+ can be trapped in closed channels. Moreover, Ba2+ hinders the ability of MTSEA to modify A384C in the open but not the closed state. Together the results suggest that the SK2 channel gate resides deep within the vestibule of the channel from the intracellular side, perhaps at the selectivity filter.
MATERIALS AND METHODS
Molecular Biology
DNA constructs were subcloned into the expression vector pJPA5 for transient expression in mammalian cells. Site-directed mutagenesis was performed using PFU polymerase (Stratagene) and the overlap PCR technique (Ho et al., 1989). The complete nucleotide sequence of the coding region of mutated molecules was verified by standard double-stranded DNA sequencing technique before expression studies.
Electrophysiology
CHO or CosM6 cells were transiently transfected with 1 μg channel DNA, a 10-fold dilution (100 ng) of calmodulin (to increase channel expression), and CD-4 antigen using Polyfect reagent (QIAGEN). Cells were plated on Fisherbrand Growth coverslips (Fisher Scientific) and labeled with CD-4 antibody-coated polystyrene beads (Dynabeads M-450, Dynal) and currents were recorded 24–48 h post-transfection. Bath solution contained 150 mM KOH titrated with methanesulfonic acid to pH 7.2 to form K methanesulfonate (KMES), 10 mM HEPES, 1 mM EGTA, and CaOH and BaOH to yield the desired free Ca2+ and Ba2+ concentrations (Fabiato and Fabiato, 1979). The pipette solution contained either 2 mM KMES and 148 mM Na+ methanesulfonate (NaMES) or 150 mM KMES, 10 mM HEPES, and ∼100 μM free Ca2+. All recordings were performed at room temperature (22–25°C). For closed state experiments, no Ca2+ was added (0 Ca2+), yielding a free Ca2+ level <2 nM. For closed state experiments with Cd2+, EGTA was omitted. For preliminary experiments, Ca2+ was removed from these solutions using Calcium Sponge S (Invitrogen), which is a polystyrene conjugate of BAPTA. The solutions were mixed with the Calcium Sponge S reagent overnight, and the beads removed before recording. More consistent results were obtained by following the same protocol, but replacing the Calcium Sponge S with Chelex-100 (Bio-Rad Laboratories). MTSEA was purchased from Toronto Research Chemicals and dissolved in purified distilled water at a concentration of 100 mM, and aliquots were kept frozen until the day of use at which point they were thawed and kept on ice until diluting into recording solution immediately before use. Inside-out patches were pulled using borosilicate glass patch electrodes (TW150 F-4, Warner Instrument Corp.) pulled to resistances between 1.0 and 3.0 MΩ. Rapid solution changes were performed using an RSC-200 (Molecular Kinetics). Currents were measured and digitized with an EPC9 (Heka) and currents sampled and filtered at 1 kHz.
Data Analysis
Analysis was performed using Pulse (Heka) and Igor (Wavemetrics), software. All values are reported as the mean ± SEM of n experiments. Statistical significance was evaluated using a Student's t test, and a P value ≤0.05 considered significant. The dose–response relationship for Ba2+ was obtained by measuring current amplitudes in control solution and at the indicated concentrations over the average of the final 1 s of a 10-s voltage step to 0 mV. Currents were normalized to the control amplitude and fit with a single binding isotherm using a nonlinear least square procedure. The binding equation used was Icontrol*[X]/([X] + IC50), where [X] is the concentration of Ba2+, Icontrol is the current amplitude before application of blocker, and IC50 represents the concentration at which macroscopic current is half blocked.
RESULTS
Cd2+ Access to a Site above the Canonical Activation Gate Is Weakly State Dependent
Previous experiments on SK channels showed state-independent MTSEA access and TBuA protection of position T387C (Fig. 1 A), suggesting that, different from K channels, there was not an activation gate between T387 and the cytoplasm (Bruening-Wright et al., 2002). Given the surprising nature of these results, we wanted to test for state-dependent access to this region (V391C) using a more rapidly reacting, smaller probe. Cd2+ is thought to bind to the thiol side chain of cysteine residues, can coordinate between cysteine residues (or between cysteine and histidine residues), and has been used to help localize the activation gate in cyclic nucleotide-gated (CNG) channels, Shaker K+ channels, and in hyperpolarization-activated cyclic nucleotide-modulated (HCN) channels (Liu and Siegelbaum, 2000; del Camino and Yellen, 2001; Rothberg et al., 2002). 5 μM Cd2+ applied to 391C channels in the open state (Fig. 1 B) caused a rapid reduction in current amplitude that was well described by a single exponential with a time constant of 1.22 ± 0.14 s (n = 7) that was only partially and slowly reversible and not seen in WT SK2 channels (unpublished data). Similarly, 5 μM Cd2+ applied to closed 391C channels (Fig. 1 C) rapidly reduced the current amplitude (time constant = 2.52 ± 0.50 s; n = 6), an effect that was not seen in WT SK2 channels (unpublished data). The average modification rate in the open state was 1.77 ± 0.20 × 105 M−1s−1 (n = 7), compared with 0.97 ± 0.15 × 105 M−1s−1 (n = 6) in the closed state. The open and closed state rates are significantly different (P < 0.05). Therefore, Cd2+ can rapidly access position 391C in the inner vestibule of SK channels whether they are open or closed, but the modification rate is approximately twofold slower in the closed state (Fig. 1 D).
Figure 1.
Cd2+ modification of V391C channels. (A) Sequence alignments of TM6 and proximal cytoplasmic residues of rSK2, Shaker B, and the proton-gated bacterial K+ channel KcsA. The homologous glycine representing the glycine gating hinge in MthK is indicated by the asterisk. Numbered lines indicate residues of particular importance (see Results). (B and C) 5 μM Cd2+ was applied five times (dashes, 2s duration each application) to the inside face of an inside-out patch containing V391C channels in either the open state (B, 2 μM free Ca2+) or closed state (C, nominally Ca2+-free solution) at 0 mV. Lines above the traces indicate when channels were open (O) or closed (C), and the dashed lines below the traces indicate the zero current level. (D) Current amplitude after each 2-s application was measured, normalized to the maximum current before application, and plotted versus time to determine the modification time course for n > 5 patches in the open (open circles) and closed (closed circles) states.
MTSEA Modifies a Site Deep in the Pore in Open and Closed Channels
To probe for a gate deeper in the pore, cysteines were individually substituted at positions 380–385 in TM6 and their availability to MTSEA was assessed in the open state. One mutation (G383C), homologous to the glycine gating hinge in MthK (Jiang et al., 2002b), did not give currents, and only one of the mutations, A384C, was modified by MTSEA as assessed by reduction in current amplitude. After closing the channels in 0 Ca2+, channels were reopened in the presence of 2 μM Ca2+ and open channels were exposed to MTSEA (200 μM; Fig. 2 A). MTSEA irreversibly blocked the current with a time constant of 3.45 s. Closed channel modification was assessed by repeatedly (11 times) closing the channel for 8 s and applying MTSEA for 5 s, and then reopening in the absence of MTSEA to assess current reduction (Fig. 2 B). The reduction in current amplitude normalized to control plotted versus the cumulative exposure to MTSEA was well fit by a single exponential with a time constant of 5.4 s (Fig. 2 C). Fig. 2 D shows the average modification rates in the open and closed states. Open state modification was approximately threefold faster than closed state modification. Modification rates, in M−1s−1, were as follows: open state 1449 ± 68 (n = 3), and closed state 462 ± 41 (n = 5). Therefore, there is a significant (P < 0.05) state dependence to the MTSEA modification rate, but MTSEA has access to A384C in both closed and open channels.
Figure 2.
MTSEA modification of A384C channels. (A) Open state MTSEA modification. Following channel closing and reopening in 10 μM Ca2+ to verify rapid solution exchange, open channels were exposed to 200 μM MTSEA (thick line) for 60 s and current decay monitored. Holding potential was −80 mV in symmetrical K+. Currents were inverted in A–C. (B) Closed state MTSEA modification. Following channel closing (c) and reopening (o), channels were closed for 2 s, MTSEA applied in the closed state for 5 s, and the channels washed for 2 s in “0” Ca2+ solution before opening for 3 s to monitor the fraction of current modified. This closed state procedure was repeated 11 times to saturate modification. (C) Time course of modification for the representative patches shown in A and B, currents normalized to control and plotted versus time (line, open) or cumulative MTSEA exposure (open symbol, closed). Single exponential fits are overlaid with each plot. (D) Average MTSEA modification rates determined from time course of modification in the open or closed state.
Barium Is an Open Channel Blocker
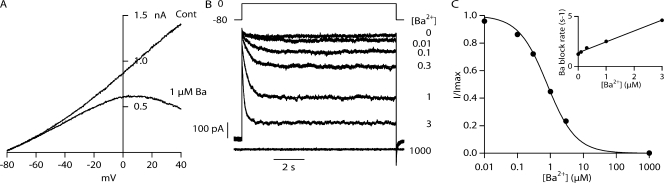
Previous work has shown that intracellular application of Ba2+ blocks SK channels (Soh and Park, 2001, 2002), and Ba2+ is believed to bind at the intracellular base of the selectivity filter of K channels (Jiang and MacKinnon, 2000). Responses to voltage ramp commands (−80 to 40 mV; Fig. 3 A) in the absence or presence (1 μM) of Ba2+ demonstrate the voltage dependence of Ba2+ block of WT SK2 channels for potentials greater than −60 mV. Voltage steps from −80 mV to 0 mV (Fig. 3 B) in the presence of various concentrations of Ba2+ reveal that Ba2+ blocks SK2 channels with an IC50 = 1.00 ± 0.18 μM at 0 mV (n = 4; Fig. 3 C), with readily measurable blocking kinetics that are well described by first order rate dependence (Fig. 3 C, inset). On average, the time constant of block by 1 μM Ba2+ at 0 mV was 353 ± 22 ms (n = 4).
Figure 3.
Ba2+ block of WT rSK2, open state. (A) An inside-out membrane patch containing WT rSK2 channels was voltage clamped and the voltage ramped from –80 to 40 mV (80 mV/s) in asymmetrical K+ (2 Kout, 150 Kin) and 2 μM Ca2+ (fully open) either without (Cont) or with 1 μM Ba2+ (1 μM Ba) to illustrate that block is strongly voltage dependent. (B) Consecutive 10-s voltage steps from −80 to 0 mV in the presence of increasing concentrations of Ba2+ (in μM: 0, 0.01, 0.1, 0.3, 1, 3, 1,000) are overlaid to demonstrate the WT SK2 channel response to Ba2+ applied to the intracellular side of the membrane. (C) The average current amplitude over the final 1 s of each voltage step in B was normalized, plotted against Ba2+ concentration, and the points fitted with a single binding isotherm (see Materials and methods), yielding an apparent dissociation constant (IC50) of 997 nM. Inset, plot of inverse time constant determined from fits of a single exponential to traces in B versus Ba2+ concentration. Line represents a linear fit to data yielding a slope 1.4 × 106 M−1s−1 and an intercept of 1.1 s−1.
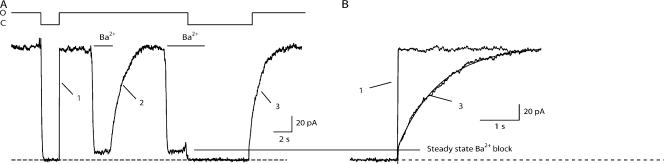
Given that the Ba2+ block rate is >10 times slower than channel opening in 2 μM Ca2+ (time constant 27.1 ± 3.9 ms, n = 4), it is possible to test if Ba2+ can block closed WT SK2 channels by applying Ba2+ in the closed state, then rapidly opening channels in the presence of Ba2+. If Ba2+ can block closed channels, then the channels should open directly to the blocked level. If Ba2+ cannot block closed channels, then the channels should open before being blocked, and the current should transiently overshoot the open-blocked level before decaying to the open-blocked level. Fig. 4 demonstrates that Ba2+ does not block closed channels. Following channel closing, 1 μM Ba2+ was applied for 10 s before opening the channel with 2 μM Ca2+ in the continued presence of Ba2+ (Fig. 4 A). The channel transiently opened to 81% of control followed by a voltage-dependent Ba2+ block to 22% of control (Fig. 4 A, inset). Regardless of the duration of closed state application between 1 and 100 s, opening in the presence of 1 μM Ba2+ always resulted in a transient overshoot to ∼80% of the control (80.5 ± 0.7% of control for the 10-s application, n = 4) followed by a decay to the open-blocked level (Fig. 4, B and C). These data show that Ba2+ cannot access its binding site when the channel gate is closed.
Figure 4.
State dependence of Ba2+ block. (A) Open WT SK2 channels were closed by removal of Ca2+ from the cytosolic face of an inside-out membrane patch, then 1 μM Ba2+ applied to the closed channels for 10 s. A solution containing 2 μM Ca2+ and 1 μM Ba2+ was then perfused onto the patch, resulting in channel opening and onset of block. Inset, expanded time course of channel opening followed by onset of Ba2+ block. The dashed line below the trace indicates the zero current level. (B) A single patch containing WT SK2 channels was exposed in the closed state to 1 μM Ba2+ according to the protocol outlined in A for 1, 3, 10, or 100 s and the four consecutive recordings overlaid. Ba2+ was washed from the patch between each of the four traces, and overlapping portions of the traces were removed for display. The dashed line is aligned to the peak current amplitude upon opening after the 1-s closed state Ba2+ exposure and does not have any analytical meaning. (C) Average peak current upon opening of channels (Ipost) normalized to the current amplitude before Ba2+ application (Ipre) (see A) plotted against duration of closed state Ba2+ exposure (n = 4). The dashed line is aligned to the average peak current amplitude upon opening after the 1-s closed state Ba2+ exposure and does not have any analytical meaning.
Barium Can Be Trapped in WT Channels
Since Ba2+ can access its binding site in the open state but not the closed state, it may be possible to trap Ba2+ in its binding site by applying Ba2+ to open channels then closing the gate. If Ba2+ is trapped in the pore, it should remain bound even after Ba2+ is washed out from the intracellular face of the channel. Fig. 5 shows, using kinetic measurements during rapid solution exchanges, that Ba2+ can be trapped in closed SK channels. An inside-out patch was excised and held in 10 μM Ca2+ to fully activate the channels, Ca2+ was then removed to close the channels, and the channels were subsequently reopened with a time constant of opening of 5.1 ms (trace 1). After reaching steady state, channels were exposed to 2 μM Ca2+ supplemented with 10 μM Ba2+ to block most of the channels. On average, Ba2+ blocked 89.6 ± 1.1% (n = 5) of the open channel current. Ba2+ was then washed out and the current recovered to its unblock level with a time constant of 1160 ± 79 ms (trace 2). Consistent with Ba2+'s low apparent IC50 (Fig. 2), Ba2+ washout was much slower than channel opening (time constant of channel opening 7.0 ± 1.8 ms, n = 5). To assess if Ba2+ could be trapped in the closed channels, open channels were blocked with the 10 μM Ba2+ (with 10 μM Ca2+) solution, closed with a 0 Ca2+ solution that also contained 10 μM Ba2+, and then the Ba2+ was washed out from the bath for 5 s before reopening the channels with 10 μM Ca2+ (Fig. 5, trace 3). Fig. 5 B shows a time-expanded overlay of trace 1 and trace 3 from the patch shown in Fig. 5 A. During reopening the current recovered in two phases, a fast component corresponding to the opening of the small fraction of unblocked channels and a slow component corresponding to Ba2+ washout from blocked channels. Channel reopening was well described by a sum of two exponentials (Fig. 5 B, trace 3) with time constants of 8.0 ± 0.9 ms and 1096 ± 36 ms (n = 5). The fraction of unblocked current was estimated from the exponential fits as the relative contribution of the fast component and corresponded to 14.5 ± 3.7% (n = 5) of the total current following Ba2+ washout. The difference between the fraction of unblocked current following channel reopening and steady-state current in the presence of Ba2+ before channel closure (10.4%) of 4.1% suggests that some of the Ba2+ may have washed out during the 5-s closed state. This corresponds to an estimated off-rate of Ba2+ in the closed state of 107 s, which is ∼100 times slower than Ba2+ off-rate in the open state. These results are consistent with the hypothesis that Ba2+ can be trapped in the pore of closed SK2 channels.
Figure 5.
Ba2+ trap in WT SK2 channels. (A) An inside-out patch containing WT SK2 channels was voltage clamped to 0 mV and the intracellular solution was altered to open or close the channels (2 μM or 0 μM free Ca2+) and to expose the channels to 10 μM Ba2+. Channels were first closed and then reopened using a rapid exchanger to measure the activation time course of channels in the absence of Ba2+ (trace 1). Ba2+ was then applied in the open state and washed off to approximate the Ba2+ off rate (trace 2). Finally, channels were blocked in the open state and closed in the continued presence of Ba2+. Ba2+ was subsequently washed off for 5 s before reopening the channels to measure the kinetics of current recovery (trace 3). (B) Channel opening in the absence of Ba2+ (trace 1) is overlaid with channel opening after “trap” (trace 3) for the patch shown in A. Superimposed on trace 3 is a double exponential fit to channel reopening and Ba2+ washout.
Barium Protects A384C in Open but Not Closed Channels
The ability of Ba2+, which binds at the selectivity filter, to impede MTSEA modification of A384C was tested. Experimental protocols were similar to those used in Fig. 2 to examine open and closed state MTSEA modification, except the experiments were performed at 0 mV (to eliminate voltage effects on the modification rate) and used 2 μM Ca2+ to open the channel (to avoid pore block by Ca2+) and 2 mM K+ in the extracellular patch pipette solution to shift the reversal potential. Fig. 6 A shows representative traces of MTSEA modification in the open state in the absence (top) and in the presence (bottom) of 100 μM Ba2+. The time constant for the decrease of normalized current versus cumulative MTSEA was 3.6 s (modification rate 1389.9 M−1s−1) in control and 6.7 s (modification rate 746.3 M−1s−1) in Ba2+ (Fig. 6 B). On average, the open state modification rate was 1540.9 ± 148.6 M−1s−1 (n = 5) in the absence of Ba2+ and 828.8 ± 35.4 M−1s−1 (n = 7) in the presence of Ba2+ (P < 0.01, Fig. 6 E). Therefore, Ba2+ can protect A384C from MTSEA modification in the open state. The closed state modification was tested by first blocking with Ba2+ then closing the channel to trap Ba2+. Ba2+ was then washed out, MTSEA was applied for 1 s, and following washout, the channel subsequently reopened (Fig. 6 C). The time constant of decrease of normalized current versus cumulative MTSEA was 16.2 s (modification rate 308.0 M−1s−1) in control and 13.8 s (modification rate 361.6 M−1s−1) in Ba2+ (Fig. 6 D). On average, the modification rate was 372.4 ± 26.4 M−1s−1 (n = 4) in the absence of Ba2+ and 329.8 ± 47.4 M−1s−1 (n = 3) when Ba2+ was trapped in the pore (Fig. 6 E). These values are not statistically different (P = 0.5), and therefore Ba2+ does not protect A384C from MTSEA modification in the closed state.
Figure 6.
Barium slows MTSEA modification of A384C in the open but not closed state. (A) Ba2+ protection of open state modification. Top traces from control patch in asymmetrical K+ at 0 mV successively exposed to MTSEA for 1 s followed by washout in 2 μM Ca2+. Bottom traces from an experimental patch in which the channel was blocked with 100 μM Ba2+ for 1 s, and subsequently exposed to MTSEA with Ba2+. After MTSEA washout for 1 s Ba2+ was washed out to assess current amplitude. (B) Time course of MTSEA modification in control (closed symbol) and with Ba2+ block (open symbol). Lines represent a single exponential fit with time constant of 3.6 and 6.7 s for control and Ba2+ block modification time course, respectively. (C) Closed state MTSEA modification. Top traces from a control patch in which channels were closed for 2 s, MTSEA applied in the closed state for 5 s, and the channels washed for 2 s in “0” Ca2+ solution before opening to monitor the fraction of current modified. This procedure was repeated until modification was complete. Bottom traces from an experimental patch measuring closed state MTSEA modification with Ba2+ trapped in the pore. Open channels were first blocked with 100 μM barium, closed in the presence of barium, Ba2+ washed out, then 200 μM MTSEA applied for 1 s. MTSEA was washed out in the closed state for 2 s before reopening the channels in 2 μM Ca2+. This procedure was repeated until modification was complete. For clarity the figure shows only the first application, then every fifth application. (D) Time course of MTSEA modification of A384C in the absence (open circles) or presence (closed circles) of Ba2+. Lines represent a single exponential fit with time constant of 13.8 and 16.2 s to control and Ba2+ block modification time course, respectively. (E) Bar graph of mean MTSEA modification rate ± SEM for open and closed state in the absence and presence of Ba2+ block. Asterisk indicates P < 0.01. Concentration of MTSEA in all experiments was 200 μM.
DISCUSSION
A central goal of ion channel research is to understand at the molecular level the mechanisms that underlie channel gating. To this end, considerable progress has been made, particularly for the Kv family of ion channels (Yellen, 2002). Complementing years of functional work on native and cloned Kv channels, a series of remarkable crystal structures of K channels have now been presented. Emerging from these crystallography and functional experiments is a growing consensus that many channels regulate ion flux at a gate formed by the intracellular end of the pore (Armstrong, 1971; Liu et al., 1997; Doyle et al., 1998; Jiang et al., 2002b; Kuo et al., 2003). The gating hypothesis for these channels is that in the closed state the inner helices (the helices that line the inner vestibule) from each of the four channel subunits form a restrictive barrier to permeation by crossing very near the cytoplasmic interface. The bundle crossing forms an inverted tepee with the “smoke hole” comprising the narrowest point, the gate itself.
Remarkably, Kv channels are functionally modular proteins. The voltage sensor domain, formed by TM1–TM4 is essentially self-contained and makes the major contact with the pore domain through the TM4–5 linker. Therefore, in Kv channels the voltage sensor may be analogous to the ligand-binding domain of ligand-gated channels, such as SK channels. SK channels present the modular organization of Kv channels, but they lack a functional voltage sensor, and thus the open probability of the channels has no voltage dependence (Hirschberg et al., 1998). Rather, SK channels open in response to Ca2+ binding to CaM, which is constitutively attached to the channel C terminus. This binding induces conformational changes that are likely transduced through the linker between TM6 and the CaM binding domain to open the channel gate.
For Kv channels, the model that the bundle crossing forms the activation gate is supported by structure–function studies including chemical modification experiments. For example, positions intracellular to the bundle crossing are available for modification only when the channels are in the open conformation (Liu et al., 1997; del Camino and Yellen, 2001). In a previous paper, we probed the intracellular end of TM6 in SK2 channels with MTS reagents to assess if a similar gate is formed in the closed state of SK channels (Bruening-Wright et al., 2002). In contrast to the functional data obtained with the Shaker channel, a position intracellular to the bundle-crossing region (T387C) could be accessed whether the channel was open or closed. In addition, the pore blocker TBuA was shown to protect this position from MTSEA modification in both the closed and open states of the channel. Similar results were observed at position V275 in the intermediate conductance Ca2+-activated K+ channel, IK (KCa3.1), a position equivalent to A384 in SK2 (Klein et al., 2007). Taken together, the data suggested that the activation gate for SK and IK channels could reside in or near the selectivity filter.
In this paper we have probed three positions in the pore, each intracellular to the canonical activation gate region of K channels. Consistent with our previous results, neither Cd2+ access to 391C (near the intracellular end of the pore), nor MTSEA access to A384C (deep in the pore near the “glycine gating hinge” region) was strongly state dependent. Interestingly, Cd2+ access to 391C and MTSEA access to A384C shows weak state dependence (Figs. 1 and 2), suggesting that there may be conformational changes near these regions during gating. Alternatively, there could be an additional “gate” cytoplasmic to these two positions, but in this case the cytoplasmic “gate” would slow, but not completely prevent, access to the pore. This idea is supported by previous data showing state-dependent MTS reactivity at position 392C (Bruening-Wright et al., 2002), which we interpreted to mean that this region was moving during gating. Taken together, the data suggest that the canonical gate region of SK channels does move during gating, but that this region does not close completely in the absence of Ca2+. Further experiments are required to determine the size of the smoke hole in closed SK channels. In any case, these data are incompatible with the gating model for Kv channels, in which the intracellular end of the pore forms a tight gate in the closed state.
While these data argue against a lower TM6 gate in SK channels, it is important to consider alternative explanations. For an inwardly rectifying K channel (Kir), for example, it has been proposed that a similar lack of state dependence in apparent MTSEA modification rates could be explained if the channels had a large minimum open probability (approximate minPo > 0.01; for comparison, the Shaker relative minPo is <0.00001 (Soler-Llavina et al., 2003)). In this model, MTSEA was “trapped” in the channels at low Po, serving to increase the apparent modification rate in the “closed” state (Phillips et al., 2003). Although at high concentrations MTS compounds can act as reversible pore blockers in SK channels (Bruening-Wright et al., 2002), we do not see significant block of SK channels by 200 μM MTSEA (Fig. 6; unpublished data), nor do we observe the strong voltage dependence to the modification rate that is seen in Kir channels (compare Figs. 2 and 6). Further, several sets of data lead us to believe that SK channels are tightly closed in EGTA-buffered “0” Ca2+ solution. First, Ba2+ cannot block closed SK channels, even during a prolonged closed state exposure (100 s, >200-fold longer than the open state blocking time constant; Fig. 4). Second, channel opening is not observed in “0” Ca2+ during prolonged (>5 min) single channel recordings of SK2 channels (unpublished data), suggesting a maximum Po of 0.001 in the closed state (and this is almost certainly a high estimate). We therefore believe it is unlikely that the “reagent trap” model can explain the data. Another perhaps remote possibility is that MTSEA can access positions 384C and 387C by diffusing through the lipid membrane and through some “hole” in the channel. This would lead to state-independent modification of these positions by MTSEA, and could explain why barium shows strong state dependence. However, the SK channel activation gate in this model would still have to be deeper in the pore than in Kv channels, since 391C is rapidly modified in both closed and open channels.
Although MTSEA has proved a very useful tool for studying the SK channel pore, it has several limitations. For example, MTSEA is larger and more hydrophobic than a K+ ion, does not react with positions above A384C, and must be used on cysteine-mutated (as opposed to WT) channels. To circumvent these limitations, we probed the WT SK2 pore with Ba2+. Ba2+ is an ideal probe since it is nearly identical to K+ in size (Pauling radii 1.35 Å versus 1.33 Å for K+). Moreover, the binding site for Ba2+ is located very deep in the SK channel pore, presumably at the base of the selectivity filter (Soh and Park, 2002), and it blocks with high affinity (Fig. 3). In Kir6.2 inwardly rectifying K+ channels Ba2+ can access its blocking site in both the closed and open state, indicating that in Kir6.2 the gate is likely within or above the selectivity filter (Proks et al., 2003). In contrast, we show in this paper that Ba2+ is an open channel blocker and can be trapped in the channel pore by closing the channel activation gate. So where is the activation gate located in SK channels? The data suggest that the gate must be located between the selectivity filter and the glycine gating hinge. One hypothesis is that the gate is located in the selectivity filter itself. Consistent with this idea, it is believed that Ba2+ can pass through the selectivity filter and that there are at least three Ba2+ binding sites in and near the selectivity filter, at least in BK and Shaker Kv channels (Neyton and Miller, 1988a,b; Harris et al., 1998; Vergara et al., 1999). Furthermore, crystal structures and functional data suggest that the selectivity filter reorients during gating such that K+ can no longer permeate (Zheng and Sigworth, 1997, 1998; Perozo et al., 1999; Zhou et al., 2001; Kuo et al., 2003). Despite loss of K+ conduction, crystal structures of closed K channels show K+ remains in the selectivity filter. K+ is, in effect, trapped in the closed filter. If Ba2+ acts like K+, it is not unreasonable to imagine Ba2+ trapping by the selectivity filter. Alternatively, the gate could be located at the glycine gating hinge, which clearly undergoes structural reorientation during MthK gating (Doyle et al., 1998; Jiang et al., 2002a,b). In either case, Ba2+ could reduce the rate of MTSEA modification of A384C in the open state by charge repulsion, while in the closed state the effects of Ba2+ may be countered by any number of mechanisms, including a reorientation of the electric field at the Ba2+ binding site, or direct shielding of trapped Ba2+ by the selectivity filter or the glycine gating hinge. One attractive model is that Ba2+ moves further into the selectivity filter in the closed state, thereby increasing the distance between Ba2+ and position A384C and reducing the electrostatic slowing of MTSEA modification. Elucidating the exact mechanism that describes how Ba2+ is trapped in closed channels requires further experimentation.
While it appears that many ion channels have an intracellular gate, there is evidence that some types of ion channels do not. CNG channels, for example, are believed to have a selectivity filter–based activation gate. For these channels, MTS compounds, small ions, and TEA derivatives can access the inner vestibule at equal rates whether the channel is open or closed (Flynn and Zagotta, 2001; Contreras and Holmgren, 2006) and the pore helix undergoes conformational change during activation (Liu and Siegelbaum, 2000). Recent work on BK and KCa3.1 channels suggests that they, too, may lack an intracellular gate (Wilkens and Aldrich, 2006; Klein et al., 2007). It therefore appears likely that the family of intracellular ligand-gated channels, such as SK, IK, CNG, and perhaps BK channels, share a selectivity filter–based gating mechanism that is distinct from Kv channels. Indeed, recent structural and EPR data suggest that the prototype bacterial K channel, KscA, possesses two gates, a bundle crossing gate that efficiently responds to protons and a selectivity filter gate that predominates stationary gating behavior (Cordero-Morales et al., 2006a,b).
Our data may also provide some insight into the overall architecture of the inner vestibule in SK channels. We have reported here that Cd2+ can bridge 391C residues (though we haven't ruled out that Cd2+ may be coordinating between 391C and some other residue such as a histidine). We have also observed that Cd2+ has no effect on A384C and T387C channels when applied in the open state (unpublished data). This suggests that, just as for Kv channels, the pore may be wider around positions 384 and 387, then narrow at the cytoplasmic end, allowing cross-linking of cysteines at position 391. Thus the overall tepee pore structure may be conserved between SK and Kv channels, but the smoke hole must be wider in SK channels.
In summary, we have identified an activation gate in SK channels that resides at or very near to the Ba2+ binding site of the selectivity filter. These data add to a growing body of evidence that suggests that an intracellular activation gate is not necessary to close ion channels. Further experiments are required to test the possibility that a selectivity filter–based gate is widely employed in the superfamily of ion channels, perhaps even in channels that possess an intracellular gate.
Acknowledgments
We'd like to thank Peter Larsson for critical reading of the manuscript.
This work was supported by National Institutes of Health grants to J.P. Adelman and J. Maylie, and a pre-doctoral NRSA award to A. Bruening-Wright.
Angus C. Nairn served as editor.
Abbreviations used in this paper: CaM, calmodulin; CNG, cyclic nucleotide-gated; IK, intermediate conductance Ca2+-activated K+; Kv, voltage-gated potassium; MES, methanesulfonate; MTSEA, 2-aminoethyl methanethiosulfonate; SK, small conductance calcium-gated potassium; TBuA, tetrabutylammonium; TM, transmembrane.
References
- Armstrong, C.M. 1971. Interaction of tetraethylammonium ion derivatives with the potassium channels of giant axons. J. Gen. Physiol. 58:413–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong, C.M. 1974. Ionic pores, gates, and gating currents. Q. Rev. Biophys. 7:179–210. [DOI] [PubMed] [Google Scholar]
- Armstrong, C.M., and B. Hille. 1972. The inner quaternary ammonium ion receptor in potassium channels of the node of Ranvier. J. Gen. Physiol. 59:388–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond, C.T., J. Maylie, and J.P. Adelman. 2005. SK channels in excitability, pacemaking and synaptic integration. Curr. Opin. Neurobiol. 15:305–311. [DOI] [PubMed] [Google Scholar]
- Bruening-Wright, A., M.A. Schumacher, J.P. Adelman, and J. Maylie. 2002. Localization of the activation gate for small conductance Ca2+-activated K+ channels. J. Neurosci. 22:6499–6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras, J.E., and M. Holmgren. 2006. Access of quaternary ammonium blockers to the internal pore of cyclic nucleotide-gated channels: implications for the location of the gate. J. Gen. Physiol. 127:481–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero-Morales, J.F., L.G. Cuello, and E. Perozo. 2006. a. Voltage-dependent gating at the KcsA selectivity filter. Nat. Struct. Mol. Biol. 13:319–322. [DOI] [PubMed] [Google Scholar]
- Cordero-Morales, J.F., L.G. Cuello, Y. Zhao, V. Jogini, D.M. Cortes, B. Roux, and E. Perozo. 2006. b. Molecular determinants of gating at the potassium-channel selectivity filter. Nat. Struct. Mol. Biol. 13:311–318. [DOI] [PubMed] [Google Scholar]
- del Camino, D., and G. Yellen. 2001. Tight steric closure at the intracellular activation gate of a voltage-gated K+ channel. Neuron. 32:649–656. [DOI] [PubMed] [Google Scholar]
- del Camino, D., M. Holmgren, Y. Liu, and G. Yellen. 2000. Blocker protection in the pore of a voltage-gated K+ channel and its structural implications. Nature. 403:321–325. [DOI] [PubMed] [Google Scholar]
- Doyle, D.A., J. Morais Cabral, R.A. Pfuetzner, A. Kuo, J.M. Gulbis, S.L. Cohen, B.T. Chait, and R. MacKinnon. 1998. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 280:69–77. [DOI] [PubMed] [Google Scholar]
- Fabiato, A., and F. Fabiato. 1979. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J. Physiol. 75:463–505. [PubMed] [Google Scholar]
- Flynn, G.E., and W.N. Zagotta. 2001. Conformational changes in S6 coupled to the opening of cyclic nucleotide-gated channels. Neuron. 30:689–698. [DOI] [PubMed] [Google Scholar]
- Harris, R.E., H.P. Larsson, and E.Y. Isacoff. 1998. A permanent ion binding site located between two gates of the Shaker K+ channel. Biophys. J. 74:1808–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschberg, B., J. Maylie, J.P. Adelman, and N.V. Marrion. 1998. Gating of recombinant small-conductance Ca-activated K+ channels by calcium. J. Gen. Physiol. 111:565–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho, S.N., H.D. Hunt, R.M. Horton, J.K. Pullen, and L.R. Pease. 1989. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 77:51–59. [DOI] [PubMed] [Google Scholar]
- Holmgren, M., K.S. Shin, and G. Yellen. 1998. The activation gate of a voltage-gated K+ channel can be trapped in the open state by an intersubunit metal bridge. Neuron. 21:617–621. [DOI] [PubMed] [Google Scholar]
- Holmgren, M., P.L. Smith, and G. Yellen. 1997. Trapping of organic blockers by closing of voltage-dependent K+ channels: evidence for a trap door mechanism of activation gating. J. Gen. Physiol. 109:527–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, Y., and R. MacKinnon. 2000. The barium site in a potassium channel by x-ray crystallography. J. Gen. Physiol. 115:269–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, Y., A. Lee, J. Chen, M. Cadene, B.T. Chait, and R. MacKinnon. 2002. a. Crystal structure and mechanism of a calcium-gated potassium channel. Nature. 417:515–522. [DOI] [PubMed] [Google Scholar]
- Jiang, Y., A. Lee, J. Chen, M. Cadene, B.T. Chait, and R. MacKinnon. 2002. b. The open pore conformation of potassium channels. Nature. 417:523–526. [DOI] [PubMed] [Google Scholar]
- Keen, J.E., R. Khawaled, D.L. Farrens, T. Neelands, A. Rivard, C.T. Bond, A. Janowsky, B. Fakler, J.P. Adelman, and J. Maylie. 1999. Domains responsible for constitutive and Ca2+-dependent interactions between calmodulin and small conductance Ca2+-activated potassium channels. J. Neurosci. 19:8830–8838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein, H., L. Garneau, U. Banderali, M. Simoes, L. Parent, and R. Sauve. 2007. Structural determinants of the closed KCa3.1 channel pore in relation to channel gating: results from a substituted cysteine accessibility analysis. J. Gen. Physiol. 129:299–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler, M., B. Hirschberg, C.T. Bond, J.M. Kinzie, N.V. Marrion, J. Maylie, and J.P. Adelman. 1996. Small-conductance, calcium-activated potassium channels from mammalian brain. Science. 273:1709–1714. [DOI] [PubMed] [Google Scholar]
- Kuo, A., J.M. Gulbis, J.F. Antcliff, T. Rahman, E.D. Lowe, J. Zimmer, J. Cuthbertson, F.M. Ashcroft, T. Ezaki, and D.A. Doyle. 2003. Crystal structure of the potassium channel KirBac1.1 in the closed state. Science. 300:1922–1926. [DOI] [PubMed] [Google Scholar]
- Kuo, A., C. Domene, L.N. Johnson, D.A. Doyle, and C. Venien-Bryan. 2005. Two different conformational states of the KirBac3.1 potassium channel revealed by electron crystallography. Structure. 13:1463–1472. [DOI] [PubMed] [Google Scholar]
- Liu, J., and S.A. Siegelbaum. 2000. Change of pore helix conformational state upon opening of cyclic nucleotide-gated channels. Neuron. 28:899–909. [DOI] [PubMed] [Google Scholar]
- Liu, Y., M. Holmgren, M.E. Jurman, and G. Yellen. 1997. Gated access to the pore of a voltage-dependent K+ channel. Neuron. 19:175–184. [DOI] [PubMed] [Google Scholar]
- Neyton, J., and C. Miller. 1988. a. Discrete Ba2+ block as a probe of ion occupancy and pore structure in the high-conductance Ca2+-activated K+ channel. J. Gen. Physiol. 92:569–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyton, J., and C. Miller. 1988. b. Potassium blocks barium permeation through a calcium-activated potassium channel. J. Gen. Physiol. 92:549–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perozo, E., D.M. Cortes, and L.G. Cuello. 1999. Structural rearrangements underlying K+-channel activation gating. Science. 285:73–78. [DOI] [PubMed] [Google Scholar]
- Phillips, L.R., D. Enkvetchakul, and C.G. Nichols. 2003. Gating dependence of inner pore access in inward rectifier K+ channels. Neuron. 37:953–962. [DOI] [PubMed] [Google Scholar]
- Proks, P., J.F. Antcliff, and F.M. Ashcroft. 2003. The ligand-sensitive gate of a potassium channel lies close to the selectivity filter. EMBO Rep. 4:70–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberg, B.S., K.S. Shin, P.S. Phale, and G. Yellen. 2002. Voltage-controlled gating at the intracellular entrance to a hyperpolarization-activated cation channel. J. Gen. Physiol. 119:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher, M.A., A.F. Rivard, H.P. Bachinger, and J.P. Adelman. 2001. Structure of the gating domain of a Ca2+-activated K+ channel complexed with Ca2+/calmodulin. Nature. 410:1120–1124. [DOI] [PubMed] [Google Scholar]
- Shin, K.S., B.S. Rothberg, and G. Yellen. 2001. Blocker state dependence and trapping in hyperpolarization-activated cation channels: evidence for an intracellular activation gate. J. Gen. Physiol. 117:91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soh, H., and C.S. Park. 2001. Inwardly rectifying current-voltage relationship of small-conductance Ca2+-activated K+ channels rendered by intracellular divalent cation blockade. Biophys. J. 80:2207–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soh, H., and C.S. Park. 2002. Localization of divalent cation-binding site in the pore of a small conductance Ca2+-activated K+ channel and its role in determining current-voltage relationship. Biophys. J. 83:2528–2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler-Llavina, G.J., M. Holmgren, and K.J. Swartz. 2003. Defining the conductance of the closed state in a voltage-gated K+ channel. Neuron. 38:61–67. [DOI] [PubMed] [Google Scholar]
- Vergara, C., O. Alvarez, and R. Latorre. 1999. Localization of the K+ lock-in and the Ba2+ binding sites in a voltage-gated calcium-modulated channel. Implications for survival of K+ permeability. J. Gen. Physiol. 114:365–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkens, C.M., and R.W. Aldrich. 2006. State-independent block of BK channels by an intracellular quaternary ammonium. J. Gen. Physiol. 128:347–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia, X.M., B. Fakler, A. Rivard, G. Wayman, T. Johnson-Pais, J.E. Keen, T. Ishii, B. Hirschberg, C.T. Bond, S. Lutsenko, et al. 1998. Mechanism of calcium gating in small-conductance calcium-activated potassium channels. Nature. 395:503–507. [DOI] [PubMed] [Google Scholar]
- Xie, C., X.G. Zhen, and J. Yang. 2005. Localization of the activation gate of a voltage-gated Ca2+ channel. J. Gen. Physiol. 126:205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellen, G. 2002. The voltage-gated potassium channels and their relatives. Nature. 419:35–42. [DOI] [PubMed] [Google Scholar]
- Zhao, Y., V. Yarov-Yarovoy, T. Scheuer, and W.A. Catterall. 2004. A gating hinge in Na+ channels; a molecular switch for electrical signaling. Neuron. 41:859–865. [DOI] [PubMed] [Google Scholar]
- Zheng, J., and F.J. Sigworth. 1997. Selectivity changes during activation of mutant Shaker potassium channels. J. Gen. Physiol. 110:101–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, J., and F.J. Sigworth. 1998. Intermediate conductances during deactivation of heteromultimeric Shaker potassium channels. J. Gen. Physiol. 112:457–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Y., J.H. Morais-Cabral, A. Kaufman, and R. MacKinnon. 2001. Chemistry of ion coordination and hydration revealed by a K+ channel- Fab complex at 2.0 Å resolution. Nature. 414:43–48. [DOI] [PubMed] [Google Scholar]