Abstract
The prokaryotic K+ channel KcsA is activated by intracellular protons and its gating is modulated by transmembrane voltage. Typically, KcsA functions have been studied under steady-state conditions, using macroscopic Rb+-flux experiments and single-channel current measurements. These studies have provided limited insights into the gating kinetics of KcsA due to its low open probability, uncertainties in the number of channels in the patch, and a very strong intrinsic kinetic variability. In this work, we have carried out a detailed analysis of KcsA gating under nonstationary conditions by examining the influence of pH and voltage on the activation, deactivation, and slow-inactivation gating events. We find that activation and deactivation gating of KcsA are predominantly modulated by pH without a significant effect of voltage. Activation gating showed sigmoidal pH dependence with a pKa of ∼4.2 and a Hill coefficient of ∼2. In the sustained presence of proton, KcsA undergoes a time-dependent decay of conductance. This inactivation process is pH independent but is modulated by voltage and the nature of permeant ion. Recovery from inactivation occurs via deactivation and also appears to be voltage dependent. We further find that inactivation in KcsA is not entirely a property of the open-conducting channel but can also occur from partially “activated” closed states. The time course of onset and recovery of the inactivation process from these pre-open closed states appears to be different from the open-state inactivation, suggesting the presence of multiple inactivated states with diverse kinetic pathways. This information has been analyzed together with a detailed study of KcsA single-channel behavior (in the accompanying paper) in the framework of a kinetic model. Taken together our data constitutes the first quantitative description of KcsA gating.
INTRODUCTION
Several decades of exhaustive studies by electrophysiology and patch-clamp recordings of eukaryotic voltage-gated K+ channels have culminated in well accepted models that, fairly adequately, describe the kinetic behavior of these channels (Bezanilla et al., 1994; Sigworth, 1994; Zagotta et al., 1994; Schoppa and Sigworth, 1998; Armstrong, 2003; Bezanilla, 2005). These functional insights complement direct structural findings on selectivity, ion permeation, gating, toxin binding, and pore blocking, mostly derived from the high resolution structures of their bacterial counterparts (Doyle et al., 1998; Morais-Cabral et al., 2001; Zhou et al., 2001; Jiang et al., 2002, 2003; Kuo et al., 2003). KcsA, a proton-activated channel from Streptomyces lividans, is the most widely studied member of the bacterial potassium channel family and is accepted to enclose a prototype of potassium- selective pores (Schrempf et al., 1995; Cuello et al., 1998; Doyle et al., 1998). Despite being a simple protein to express and purify, and being fairly amenable to structural studies by X-ray crystallography (Doyle et al., 1998; Zhou et al., 2001) and spectroscopic analyses (Perozo et al., 1998, 1999; Liu et al., 2001; Tatulian et al., 1998; Gross et al., 1999; Kelly and Gross, 2003; Chill et al., 2006; Lange et al., 2006; Takeuchi et al., 2007), there have been only few reports that attempt a thorough functional characterization of KcsA (Cuello et al., 1998; Heginbotham et al., 1998, 1999; LeMasurier et al., 2001). A reason for this gap between structural and functional studies may be attributed, at least in part, to the observed low open probability of KcsA even under the most acidic conditions tested. Although electron paramagnetic resonance (EPR) measurements showed large movements in the lower gate of KcsA in response to intracellular acidification (Perozo et al., 1999; Liu et al., 2001), these findings were in contrast with evidences indicating that, at steady-state, the channel resides predominantly in the nonconductive state. To resolve this issue, strategies have been designed to increase the open probability of KcsA, but none has provided a system that reliably generated high channel activity (Irizarry et al., 2002). Recently, two studies demonstrated the presence of an inactivation mechanism in KcsA that offered the first evidence as to why this channel opened so rarely under steady-state conditions (Gao et al., 2005; Cordero-Morales et al., 2006a). After a pH jump, KcsA was found to activate quickly (time constant ∼15 ms) and subsequently enter into an inactivated state (time constant 1–3 s) from which the recovery was fairly low (as seen from low steady-state currents, which represents only ∼10% of the peak amplitude). Since all of the previous studies were done under steady-state conditions (Cuello et al., 1998; Heginbotham et al., 1999; LeMasurier et al., 2001), the reported low open probabilities were a reflection of channels recovering from this deep inactivated state. The steady-state open probability of KcsA has also been shown to be modulated by voltage (Cuello et al., 1998; Heginbotham et al., 1999). We are now aware that the basis of this modulation comes mostly from the effect of voltage on the rate of entry and exit into inactivation (Cordero-Morales et al., 2006b). This inactivation mechanism shares many features with C-type inactivation in Kv channels. First, decreasing the extracellular concentration of K+ leads to a dramatic increase in the rate of inactivation (Lopez-Barneo et al., 1993; Baukrowitz and Yellen, 1995; Levy and Deutsch, 1996; Cordero-Morales et al., 2006a); second, permeant ions, like Rb+, which have long residence time in the selectivity filter, decrease the rate of inactivation (Swenson and Armstrong, 1981; Spruce et al., 1989; Shapiro and DeCoursey, 1991; Demo and Yellen, 1992; Lopez-Barneo et al., 1993; LeMasurier et al., 2001); and third, mutations in residue Thr449, located close to the filter in Shaker (equivalent to residue Y82 in KcsA), modify C-type inactivation (Lopez-Barneo et al., 1993; Cordero-Morales et al., 2006a). Although, the precise molecular mechanism that underlies C-type inactivation is still unclear, ample evidences suggest a concerted conformational change at the selectivity filter that occludes ion permeation (Ogielska et al., 1995; Panyi et al., 1995; Liu et al., 1996; Loots and Isacoff, 1998).
Studies based on alanine scanning mutagenesis and single-channel analysis in KcsA showed that the glutamate residue located in the pore helix (Glu71) is the key entity that governs inactivation mechanism in KcsA (Cordero-Morales et al., 2006a). High resolution structures of KcsA have shown that this residue participates in a carboxyl–carboxylate interaction with position Asp80 (Zhou et al., 2001). Eliminating this interaction by an alanine substitution at position Glu 71 resulted in a mutant that essentially removed inactivation. In macroscopic recordings by pH jump experiments, E71A showed wild type (WT)–like activation with no visible decay of current for tens of seconds. In steady-state single- channel recordings, the long closures, characteristic of WT KcsA, completely disappeared and the open probability was found to be ∼1.0 (Cordero-Morales et al., 2006a). The E71A mutant, therefore, serves as an excellent tool to study true activation gating in the absence of interference from inactivation events.
In light of this new evidence, we sought to perform a systematic study of the different gating steps and to establish a working kinetic model that would help to correlate structural snapshots of KcsA with various gating conformational states. Furthermore, a quantitative model would also serve as a mechanistic framework to understand the molecular events that underlie alterations in gating behavior as a consequence of structural perturbations. In this and the accompanying paper (see Chakrapani et al. on page 479 of this issue), we document several fundamental properties of the activation, deactivation, and inactivation gating mechanisms of KcsA. Here we focus on the measurement of macroscopic currents to analyze the effect of pH and voltage on different gating events while in the accompanying paper we use single-channel recording and burst analysis under stationary conditions. Together, we aim towards developing a model that can account for the salient features of KcsA gating.
MATERIALS AND METHODS
Channel Expression and Purification
WT and mutant KcsA, cloned in pQE32 vector, were expressed in Escherichia coli XL1-blue cells (Cortes and Perozo, 1997). Membrane preparations were made by homogenizing the cells and spinning them down at 100,000 g for 1 h. Membrane pellets were then solubilized by incubating them with PBS containing dodecyl maltoside at room temperature and then purified with a Co2+-based metal-chelate chromatography resin (Talon resin, CLONTECH Laboratories, Inc.). The quality of the purified protein was checked by gel-exclusion chromatography using Superdex-200 column.
Electrophysiology and Kinetic Analysis
Electrophysiological measurements were made by patch clamp recordings in channel-reconstituted liposomes prepared as described earlier (Delcour et al., 1989; Cortes et al., 2001). Purified protein was reconstituted in asolectin vesicles by dilution with 200 mM KCl and 10 mM MOPS buffer at pH 7.0. Residual detergent was further removed by incubation with biobeads (Bio-Rad Laboratories). Channel-incorporated liposome suspension was then centrifuged for 2 h at 100,000 g and the pellet was resuspended in 60 μl of KCl/MOPS buffer. A drop of the proteoliposome was placed on a glass slide and dried overnight in a desiccator at 4°C. The sample was then rehydrated with 20 μl of buffer, which yielded giant liposomes. This preparation was suitable for patch clamp recordings after ∼2 h. For macroscopic currents, KcsA was reconstituted in 1:100 (mass:mass) of protein to lipid ratio, while for single channel studies we used a ratio of 1:10,000 (mass:mass). Currents were recorded under symmetrical conditions of 200 mM KCl and 10 mM MOPS buffer unless otherwise specified. Some of the critical experiments were also performed in succinate buffer to ensure that the fundamental gating properties are not affected by MOPS (not depicted). Recording pipettes were pulled from thin-walled borosilicate glass and heat polished such that they had a bath resistance of 1–2 MΩ when filled with 200 mM KCl, 10 mM MOPS solution. All measurements in this study were conducted in the inside-out configuration of the patch clamp technique. Experiments were performed at room temperature (20–22°C). Currents were elicited in response to pH jumps from 8.0 to 3.0–5.5 using an RCS-160 fast solution exchanger (Biologic) fed by gravity. During pH pulses, the membrane was held at either ±100 mV unless specifically noted. Macroscopic currents were sampled at 5 kHz using Axon 200-B patch-clamp amplifier. Macroscopic currents were analyzed using Clampfit (Molecular Devices). Activation time course of KcsA was best described by a sum of two or more exponentials. We therefore used the time required for the current to reach half its maximum value as a measure of dependence of activation on pH. Inactivation time constants were obtained by fitting single-exponential to the decay phase of the current during a prolonged exposure to acidic pH.
Online Supplemental Material
Analysis of KcsA gating under different buffer conditions (of varying pKa) are described in detail in the online supplemental material (available at http://www.jgp.org/cgi/content/full/jgp.200709843/DC1). We find that buffer type does not affect the salient properties of KcsA gating; although there are slight differences in the ensemble behavior that lead us to use MOPS as the preferred buffer system for all our experiments. Fig. S1 shows that in the presence of succinate buffer the rate of inactivation of KcsA is significantly increased along with a simultaneous decrease in the peak amplitude of current. Although the diffusion-related delay is affected by the nature of the buffer, there are no major changes in the activation kinetics. Fig S2 demonstrates that the single-channel properties of KcsA measured in succinate buffer are similar to the measurements in MOPS.
RESULTS
Activation Gating
Activation Kinetics of KcsA is pH Dependent.
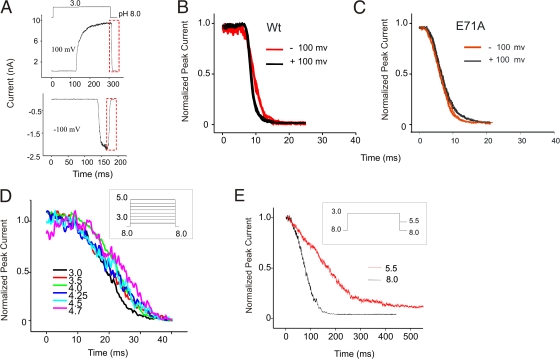
As demonstrated earlier, KcsA is activated by lowering the intracellular pH (Heginbotham et al., 1999). KcsA channels tend to preferentially orient in the reconstituted membranes (Cuello et al., 1998; Heginbotham et al., 1999) such that in a typical pH jump experiment performed on an inside-out patch, the C terminus of most of the channels are exposed to the solution exchanger. In response to a pH jump, the channels are seen to rapidly activate (milliseconds timescale), and in the continued presence of protons, slowly inactivate (seconds time scale) (Fig. 1).
Figure 1.
Macroscopic behavior of KcsA in a pH jump experiment. Upon reconstitution in asolectin liposomes, KcsA is predominantly oriented with its C terminus inside the vesicle. In an inside-out patch, KcsA is activated by pH jumps from 8.0 to 3.0 using a rapid solution exchanger. The channels are seen to activate in ∼15 ms and in the sustained presence of protons inactivate with a time constant of 1–3 s.
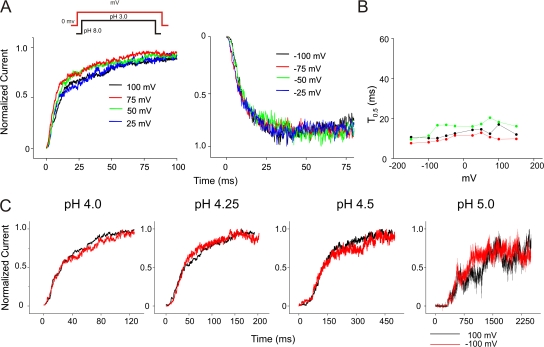
To study the effect of pH on the activation time course of KcsA, we used pH-pulse protocols of duration that were long enough to elicit maximal currents but short enough to close the channels before the currents started to decay due to inactivation (Fig. 2 A). Following a pH jump, the current starts to increase after a delay (from the start of the pH pulse to the foot of the macroscopic), which is possibly mechanical in origin (Fig. 2 A). The extent of this delay can be attributed to a number of experimental factors. These include, first, variable time for the diffusion of protons from the bulk solution to the “binding site” on the channel, which in turn is governed by the position of the pipette with respect to the solution exchanger and the geometry of the patch within the pipette; second, the buffering capacity of the solutions; and, third, from the likely binding of protons to the negatively charged phosphate moieties of the lipid headgroups in the patch membrane. Due to the variable, and presently unquantifiable, nature of this delay it was excluded from our analysis.
Figure 2.
pH-dependent activation of KcsA. (A) Macroscopic activation of KcsA by pH jump. The duration of the pulse was chosen so as to elicit maximal activation and to close the channels before the currents begin to decay due to inactivation. (B) Representative macroscopic current traces elicited in response to jumps of pH from 8.0 to the indicated value. Currents were recorded under symmetrical 200 mM K+ at a holding membrane potential of +100 mV. (C) An expanded view of the first 25 ms of the activation time course. Foot of the sigmoidal macroscopic activation (Boxed area) is evident at relatively more basic pH (4.0–4.5) (D) Time required for the current to reach half its maximal value (T0.5) was measured and plotted as a function of pH and fitted with the Hill equation. (Inset) A plot of normalized T0.5 (measured for six different patches). The pKa of activation and nH was found to be 4.37 ± 0.03 and 2.11 ± 0.33, respectively. (E) Peak amplitude, which is a reflection of the open probability, vs. pH fitted with the Hill equation. (Inset) Normalized peak amplitudes plotted for 10 patches, pKa (4.22 ± 0.02) and nH (1.9 ± 0.2).
Fig. 2 B shows representative macroscopic current traces recorded under symmetrical 200 mM K+ at the indicated pH values. The current traces were recorded at a holding membrane potential of +100 mV. The overall time course of activation showed appreciable pH dependence with the rising phase of the current being fastest at pH 3.0 and progressively slowing as the pH increased to 5.0. Beyond pH 5.0 the activation of the channels did not give rise to reliable macroscopic currents. At each pH (notably at more basic pH), the rising phase appears to be sigmoidal (Fig. 2 C), which could be in principle associated with multiple conformational changes of the channel as it progresses through several closed states before reaching the open state. However, determination of the precise number of these closed states may be obscured by the uncertainties arising from the above mentioned delays, which is partly reflected in the varying number of exponentials that can be used to describe the channel activation time course. To quantify the activation kinetics, we therefore used the time required for the current to reach half its maximal value (T0.5). As shown in Fig. 2 D, the T0.5 for activation was regulated by pH, most remarkably in the range of pH 3.0–4.75. We normalized the pH dependence of T0.5 using the maximum (pH 4.75) and minimum values (pH 3.0) from different patches and fitted this data with the Hill equation. The pKa of activation and the Hill coefficient (nH) were found to be ∼4.37 ± 0.03 and 2.11 ± 0.33, respectively. A plot of the peak amplitude, which reflects the peak open probability, as a function of pH also yields similar values for the pKa (4.22 ± 0.02) and nH (1.9 ± 0.2) (Fig. 2 E). These values are in excellent agreement with those previously reported for KcsA expressed in mammalian cells (Gao et al., 2005).
KcsA Activation Kinetics Is Not Intrinsically Voltage Dependent.
The steady-state open probability of KcsA has been shown to increase up to two orders of magnitude between +150 and −150 mV (Cordero-Morales et al., 2006b). The voltage dependence of the open probability is correlated with the movement of an equivalent of ∼0.7 electronic charges. The origin of voltage modulation in steady-state open probability has been proposed to arise, at least partially, from the effect of voltage on inactivation. However, these results do not rule out a voltage effect on the activation kinetics of the channels. Fig. 3 A shows overlapping traces of currents recorded by pH jumps at different membrane potentials and in each case normalized to the peak amplitude. An inspection of the time course of activation reveals that, at depolarizing potentials, there is a fast rising phase followed by a slow increase to the peak (Fig. 3 A, left), but at hyperpolarizing potentials the channels activate with a similar fast rising phase while the slow component of activation is clearly missing (Fig. 3 A, right).
Figure 3.
Effect of voltage on KcsA activation. (A) Overlap of normalized current traces recorded in response to jumps of pH from 8.0 to 3.0 at depolarizing (left) and hyperpolarizing (right) membrane potentials. (B) The time required for half maximal activation (T0.5) plotted against voltage from three datasets. Activation rates at negative potentials are marginally faster (approximately two times) than at positive membrane potentials. (C) Macroscopic activation of E71A elicited by jumps from pH 8.0 to indicated pH. Traces in black and red were recorded at depolarizing and hyperpolarizing potentials, respectively. Currents were normalized to their peak amplitudes. This result shows that there is no change in the activation time constants at positive and negative potentials when inactivation is removed.
Despite these differences in the activation time course, the time constant for half-maximal activation does not show a significant effect of voltage, suggesting that the activation gating of KcsA has a negligible intrinsic voltage dependence. We believe that this effect of negative potentials on the activation phase arises from a relatively fast inactivation rate (accompanied by slow recovery rates) at these potentials, whereby the channels inactivate much before the currents reach the peak. On the other hand, at depolarizing potentials when the inactivation rates are relatively slower, the channels continue to open and populate the conductive pool for a much longer duration, thereby giving rise to the slow component (discussed below). These features of the activation kinetics in the presence of slow inactivation rates are similar to those observed in Na+ channels after removal of the inactivation particle by proteolytic enzymes (Stimers et al., 1985; Gonoi and Hille, 1987).
To further explore the hypothesis that it is indeed the difference in the inactivation rates at depolarizing and hyperpolarizing voltages that underlies the variation in the activation kinetics at these potentials, we studied the effect of voltage on the activation gating in the noninactivating mutant E71A. Currents were recorded from jumps to four different pH 4.0, 4.25, 4.5, and 5.0 from a holding pH of 8.0 at +100/−100 mV membrane potential (Fig. 3 C). An overlap of normalized current traces recorded at the two extreme voltages clearly shows that there is no difference in the activation rates. These results quite convincingly show that the transitions in the activation pathway are not intrinsically modulated by voltage.
Inactivation Gating
Inactivation Gating Is Modulated by Voltage.
We studied the effect of voltage on the inactivation kinetics of KcsA by eliciting macroscopic currents in response to pH jumps from 8.0 to 3.0 at different membrane potentials (Fig. 4 A). The time constant of inactivation was measured by fitting a single exponential to the macroscopic decay. As reported earlier, we find an increase in the time constant of inactivation as the membrane is held more negative (Fig. 4 B) (Cordero-Morales et al., 2006b). The measured voltage dependence of the inactivation time constant for macroscopic current decays resembles the voltage dependence of the steady-state open probabilities, suggesting that most of the voltage effects on open probability at steady-state arise from the voltage dependence of the rates governing inactivation (Cordero-Morales et al., 2006b).
Figure 4.
Effect of voltage on the inactivation gating. (A) Macroscopic currents elicited in response to pH jump from 8.0 to 3.0 and recorded at various membrane potentials. (B) The time constant of inactivation, measured by fitting a single exponential to the decay phase of the macroscopic, was plotted as a function of voltage. KcsA inactivates faster when the membrane is held at hyperpolarizing potentials. (Inset) Shows normalized time constants from three patches.
Inactivation Kinetics of KcsA Is Not Modulated by pH.
The effect of pH on the inactivation gating was studied by recording currents at +100 mV and jumping to various pH values (Fig. 5 A). The rate of inactivation was analyzed by fitting a single exponential to the decay phase of the current. An overlap of the current decay at each pH, normalized to the peak amplitude, shows that there is no significant effect of pH on inactivation. However, the time constant of inactivation measured from several different patches shows considerable variability (ranges from 1.0 to 3.0 s). To quantify the effect of pH on inactivation among patches, we normalized the time constant at different pHs to that measured at pH 3.0. Although, a small decrease in the rates of inactivation at more basic pH can be noted (Fig. 5 A), there is no pronounced effect of pH (in the range 3.0–4.75) on the inactivation gating of the channel.
Figure 5.
Effect of pH on the inactivation gating. (A) Currents were evoked in response to different pH in the presence of 200 mM K+ symmetrical. The lengths of the pH pulse protocol were chosen such that in each case the currents decayed to their steady-state value. The membrane potential was held at +100 mV (left). The decay phases of the macroscopic currents were overlapped upon normalizing to their peak amplitude. The time constant of inactivation was estimated by monoexponential fits to the current decay and plotted against pH (middle). Since there was considerable variability in the inactivation rates among patches, for each experiment the values measured at different pH were normalized to that measured at pH 3.0. Plots show measurements from nine patches (right). Currents were recorded in the presence of (B) 5 mM K+ external/200 mM K+ internal and (C) 200 mM Rb+ symmetrical. When compared with symmetrical K+, the rates of inactivation increased significantly when the external K+ was lowered and dramatically decreased when Rb+ was the permeant ion (note the difference in time scale). Normalized time constants are from eight and three patches for 5 mM K+ external/200 mM K+ internal and 200 mM Rb+ symmetrical, respectively. Under any of these conditions, pH did not significantly affect the inactivation rates.
There are two known modulators of inactivation rates: decreasing external K+ concentration increases the rate of inactivation, while replacing the permeant ion with Rb+ decreases this rate. We explored both these conditions to evaluate the effect of pH when the rates of inactivation are shifted by an order of magnitude in either direction. As shown in Fig. 5 (B and C), lowering external K+ (5 mM) increases the inactivation rates by factor of ∼8–10, while replacing all the K+ by Rb+ (200 mM) decreases the rate by a factor of ∼10. Nevertheless, neither of these conditions elicits a clear effect of pH on the inactivation of KcsA. We also looked into the possibility that protons could alter inactivation from the extracellular mouth of the channel. Currents recorded from patches with solution of pH 3.0 and pH 7.0 in the pipette showed no major difference in the inactivation kinetics (unpublished data). These results show that the channel inactivation pathways are primarily modulated by voltage and by the type of permeant ions without a significant contribution from pH.
Deactivation Gating
Deactivation Gating Is pH Mediated and Is Not Modulated by Voltage.
An open channel enters a nonconducting state by two possible pathways; inactivation or deactivation. If at the peak of the current, the intracellular pH is changed to 8.0, the time course of decline of the current is dominated by transitions that reverse the activation process. Deactivation in KcsA is a monotonic decay of current that results from closing of the channels that were open at the end of the pH pulse (Fig. 6 A). We measured the time constant of channel closing from exponential fits to the decay of macroscopic currents as the pH is changed to 8.0. Single-exponential deactivation kinetics and a lack of any noticeable repriming currents suggest that during the closing of the channel by basic pH, the inactivated channels do not pass through the open conducting state. As seen in Fig. 6 B, the deactivation kinetics of KcsA at depolarizing and hyperpolarizing potentials is quite similar except that at −100 mV the rate of deactivation is marginally slower than at +100 mV. Since KcsA inactivates much more rapidly at negative potentials, the slow deactivation kinetics at these potentials could be related to an inactivation-mediated effect. We tested this possibility by evaluating the effect of voltage on deactivation in E71A (Fig. 6 C), and as predicted there was no change in the time course of channel closure once the inactivation was eliminated.
Figure 6.
Effect of voltage and pH on deactivation. (A) Deactivation or channel closing was elicited by jumps to pH 3.0 from pH 8.0. Duration of the activating pulse was chosen such as to evoke maximal channel activation and to terminate before currents appear to decay due to inactivation. (B) Overlap of deactivation measured from four pH pulses at +100 (black) and −100 mV (red) holding potentials. The channel closing at −100 mV was marginally slower than +100 mV. (C) Deactivation kinetics of E71A at both positive and negative potentials was similar. (D) Channels were deactivated by pH 8.0 following activation by different pH pulses. The plot shows an overlap of decay phases of the current normalized to their peak amplitude. The macroscopic channel closing rate is independent of the pH that evoked activation. (E) Deactivation is much slower and biexponential when channel closing is brought about by pH 5.5 compared with pH 8.0.
We then studied the effect of pH on channel closing by two sets of experiments. First, we activated KcsA by jumps to different pH, and at the peak of the macroscopic current, closed the channels by pH 8.0. Overlapping traces of normalized currents at different pH shows a near monoexponential decay in all of the traces (Fig. 6 D). There was no noticeable change in the kinetics of channel closing when channels were activated by jumps to pH values ranging between 3.0 and 4.75, which might suggest that the open state(s) visited by the channel upon activation by different pH are kinetically similar.
In the second set of experiments, we measured the effect of pH on channel deactivation. Fig. 6 E shows representative macroscopic current behavior during jumps to pH 8.0 and 5.5 from a pulse to pH 3.0. Deactivation at pH 8.0 is substantially faster and occurs as a monoexponential decay of peak current, while at pH 5.5 it appears much slower and biexponential. This occurs because at pH 5.5, a population of the channels undergoes reactivation from partly closed states, thereby giving rise to multiexponential decay of the current. Eventually all the channels enter into a nonconducting closed state, as seen from very minimal to completely no steady-state current.
Recovery from Inactivation
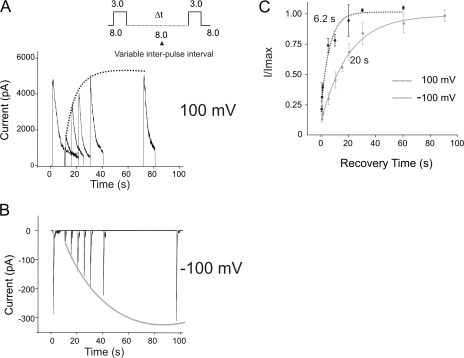
Recovery from Inactivation Occurs via Deactivation and Is Significantly Modulated by Voltage.
Although KcsA recovers from inactivation under equilibrium conditions contributing to a steady-state current, which is ∼10% of the peak amplitude (at +100 mV), the predominant mode of recovery happens upon closure of the lower activation gate (when the intracellular pH is changed to a more basic value). To study the time course of recovery and to determine if there is any effect of voltage on this recovery rate we used a double pH-pulse protocol as shown in Fig. 7. The channels were first switched from pH 8.0 to 3.0 for a duration that allowed the macroscopic current to reach its steady-state value. This was followed by a variable interpulse interval at pH 8.0. The number of channels that recovered during this interval of time in pH 8.0 was estimated by a second pulse to pH 3.0. Fig. 7 (A and B) illustrates the recovery time course at +100 and −100 mV membrane potentials, respectively. Under either condition, interpulse intervals shorter than 500 ms did not elicit currents larger than the steady-state value. For longer durations at pH 8.0, the channels were seen to recover faster at depolarizing potentials than at hyperpolarizing potentials. This is more evident in the plot of the fractional amplitude of currents during the second pulse as a function of the recovery time. Exponential fits yield recovery time constants of 6 and 20 s at +100 and −100 mV, respectively. One interpretation of this result is that voltage directly influences the recovery rate of the channel. Another is that stronger hyperpolarization potentials force the channels to reach more stable inactivation states from which the recovery is slower.
Figure 7.
Recovery from inactivation. Currents were elicited by a double pH pulse protocol with a variable interpulse duration at pH 8.0 to monitor the recovery time course of KcsA. The membrane was held at either +100 mV (A) or −100 mV (B). The amplitude of the current in response to the second pulse starts to increase progressively as the interpulse increases above 500 ms. (C) Fractional recovery, as measured by (I/I max), from four patches was plotted as a function of the interpulse interval and fitted to a single exponential. Recovery of KcsA from inactivation is modulated by voltage and occurs approximately threefold faster at +100 mV than at −100 mV.
Closed-State Inactivation
Inactivation also Occurs from Partially Activated Closed States.
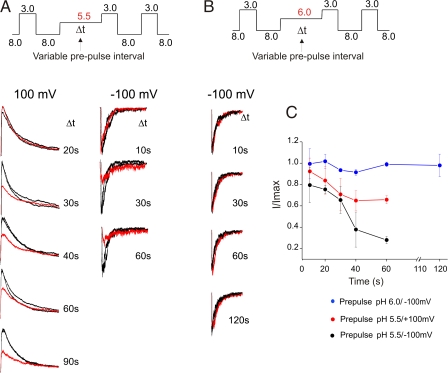
We next addressed the question if inactivation in KcsA is solely a property of the open-conducting channel or whether it can also occur from partially activated closed states. By partially activated closed states, we refer to the nonconducting states in the activation pathway that have some or all of their subunits bound to proton and might represent partial opening of the intracellular gate. We expect that the occupancy of these states can be maximized at intermediate pH. Therefore, experiments were performed at pH 5.5, which elicits minimal to no channel activity in patches that contain several channels, but at which EPR measurements reveal significant changes in the lower gate arising from proton binding (Perozo et al., 1999).
We first applied a test pulse to pH 3.0 to estimate the number of channels in the patch followed by prepulses to pH 5.5 for varying time interval. Then we estimated the fraction of channels that were inactivated at the end of the prepulse from the amplitude of the current in response to a subsequent pulse to pH 3.0 (test pulse) (Fig. 8). We found that the amplitude of the current during the third pulse progressively decreased, even though no channels opened during the prepulse. These results suggest that there must be additional inactivation pathway(s) that may be reducing the number of available channels for opening (Fig. 8 A). Further, the extent of this inactivation is dependent on the duration of the conditioning pulse. Since there was no noticeable loss of channels during a 10-s prepulse, inactivation from the closed states might be much slower compared with that from the open state (0.5–3.0 s).
Figure 8.
Closed-state inactivation in KcsA. (A) Inactivation of KcsA from closed states before channel opening was evaluated by measuring currents in response to varying duration of prepulses of intermediate pH (5.5) at positive (left) and negative membrane potentials (right). Currents evoked by flanking pulses of pH 3.0 are shown in black, while the response of the prepulse is shown in red. The amplitude of the peak current progressively decreases as the duration of the prepulse increases. This decrease is attributed to channels inactivating from the partly activated closed states. (B) The above protocol is repeated with prepulses of pH 6.0. There is no visible loss of channels during prepulses of pH 6.0 (C) The amplitude of the peak current after a prepulse is normalized by the peak current value of the first and the third pulse, I/Imax is plotted as a function of the duration of the prepulse from four patches.
To investigate if the inactivation from partially activated closed states showed voltage dependence as the open-state inactivation does, we used the above protocol at hyperpolarized holding potentials (Fig. 8 A, right). The fraction of channels lost by inactivation during the prepulse to intermediate pHs significantly increased. Even though we have not quantified the extent of voltage dependence, our results reveal that as in the inactivation from the open state, closed-state inactivation also occurs to a much larger extent at hyperpolarized potentials (Fig. 8 C).
To assess if inactivation occurs from early closed states (near the most closed state) or it preferentially occurs from states near the open state, we studied the effect of prepulse to pH 6.0. Fig. 8 B shows that there is no change in the amplitude of the peak current recorded at a membrane potential of −100 mV (at which inactivation is expected to be maximal) for different lengths of the prepulse. This suggests that inactivation from states closer to the open state is more probable compared with those farther from the open state. Clearly, these observations imply that in KcsA, inactivation does not entail a complete opening of the permeation pathway or even ion conduction.
Ion Permeation Is Not a Requisite for Inactivation
Inactivation in the Absence of Ion Conduction Is Slower than in the Presence of Ion Conduction.
From the above results we infer that KcsA inactivates even in the absence of ion permeation, although this inactivation appears to occur at a much slower rate than the open-channel inactivation. Because these experiments were done with prepulses to pH 5.5, conditions under which the proton-mediated activation is relatively slow, and since channels need to be partly protonated to inactivate, the slow inactivation rates might be a reflection of slow activation rates at this pH, rather than an intrinsically slow inactivation rate. To distinguish between these possibilities, we used a pulse protocol in which under both conditions (with and without ion conduction) the channels were activated to the same extent (by pH 3.0) (Fig. 9 A). We use a 10-s pH pulse (from 8.0 to 3.0), which is combined with a voltage pulse from 0 to −100 mV, the onset of which varies from 0 to 9 s. Therefore in each case, the channels are activated by protons at time 0, while the ion conduction (a high-throughput, one-directional) per se commences only at the onset of the voltage pulse. The number of channels at each pulse of voltage represents the fraction of channels that did not inactivate without ion conduction. The red trace shows the current elicited by a pH jump from 8.0 to 3.0 at −100 mV, while the black traces show currents where the voltage pulse occurs with a lag (indicated on the time axis) with respect to the pH pulse. Fig. 9 B shows the time course of inactivation with (red) and without ion conduction (black). The time constant of the macroscopic current decay is ∼375 ms, while the time constant of a monoexponential fit to the peak amplitude of the black traces is 2049 ± 309 ms. Further, it is interesting to note that this value is significantly slower than that predicted at 0 mV (832 ± 158 ms) by a linear extrapolation of the time constant of inactivation vs. voltage relationship (Fig. 4 B). These results clearly demonstrate that inactivation that accompanies ion permeation occurs at a much faster rate compared with that when there is no high throughput ion conduction.
Figure 9.
Effect of ion permeation on inactivation. (A) KcsA was activated by a 10-s pulse of pH 3.0 at −100 mV under symmetrical K+ (200 mM). The channels rapidly open and eventually inactivate as shown by red trace. The group of black traces represents conditions in which the voltage pulse is applied with an indicated delay after the pH pulse. During the delay, the channels are fully open but do not conduct due to a lack of the electrochemical driving force. Under these conditions the channels are still seen to inactivate although the time course appears to be slower than when accompanied by ion permeation (red trace). (B) The time course of inactivation with (red) and without ion permeation (black). Inset shows data from three patches.
Evidence for Multiple Inactivated States
Recovery from Inactivation that Occurs from Closed and Nonconducting Activated States Occurs Faster than Recovery from Open-Channel Inactivation.
Based on the finding that membrane potentials and ion permeation alter the rate and extent of inactivation we suggest the presence of more than one inactivated state. Our premise is that these inactivated states have different kinetics and possibly different voltage dependences. Further, we also believe that these different inactivated states can be, at least partially, separated kinetically by recovery measurements. To do so we used pulse protocols to inactivate the channels with and without ion conduction and measured the recovery from inactivation in both cases. Fig. 10 A shows the response of a standard two pulse protocol; in which the channels recover from inactivation (following ion conduction) up to 30 and 70% during an interpulse interval of 5 s (at pH 8.0) when the membrane was held at −100 and +100, respectively. As shown earlier the recovery rate at −100 mV is much slower. Fig. 10 B describes the protocol to evaluate the recovery of the channels upon inactivation without ion conduction. First, we estimated the number of channels in the patch by a pH jump from 8.0 to 3.0 at +100 mV. After recovering from inactivation, the channels were exposed to a second pH jump from 8.0 to 3.0, but this time at 0 mV. Under these conditions the channels were fully activated as in the first pulse but due to lack of an electrochemical gradient, elicited no net current. This was then followed by a voltage pulse to +100 mV to evoke currents that correspond to channels that were not inactivated by pH 3.0 at 0 mV. These experiments were also repeated for membrane potentials at −100 mV (right). As shown in Fig. 10 (and Fig. 7) we find that the channels recovered up to 84 ± 12%, which is to some extent faster than the recovery at +100 mV (with ion conduction, 69 ± 6%) and much faster than the recovery at −100 mV (with ion conduction, 26 ± 5%). These data provide qualitative support for the view that inactivation from partially closed states is kinetically different from inactivation that arises from the open channel, further inactivation that follows the high throughput ion conduction could also be different from inactivation that occurs upon merely opening the channel.
Figure 10.
Recovery of channels from an inactivation process that occurs without ion conduction. (A) The fractional recovery of KcsA was measured with a two-pulse protocol with an interpulse duration (pH 8.0) of 5 s at +100 mV (left) and −100 mV (right). (B) To measure recovery of channel from inactivation that occurs without ion conduction, the channels were opened by a jump to pH 3.0 but maintained at 0 mV. This was followed by an interpulse duration of 5 s. The fraction of channels that recovered during this interval was measured from the amplitude of the second pulse of pH 3.0 at +100 mV (left) or −100 mV (right). The extent of recovery is larger when the channels inactivate without ion conduction. (C) To evaluate if channels were indeed inactivated in the pulse of pH 3.0 at 0 mV, the voltage was changed to +100 mV at the end of the pH pulse. The amplitude of the current corresponded to that of the steady-state value of the first test pulse, which clearly shows that most of the channels were inactivated.
DISCUSSION
The present functional characterization of KcsA gating reveals several features that justify its role as an archetypical pore of eukaryotic K+ channels. Although most of the fundamental features of K+ channel gating are recapitulated in KcsA, there are indeed key features that show pertinent differences as discussed below.
KcsA is activated by protons (Cuello et al., 1998), acting on the intracellular side of the channel (Heginbotham et al., 1999). Our experiments with different buffer conditions showed that in practical terms, MOPS was the most reliable buffer system for pH jumps experiments in KcsA, both at the ensemble and single channel levels (Figs. S1 and S2, available at http://www.jgp.org/cgi/content/full/jgp.200709843/DC1). Using the peak amplitude of macroscopic currents as an estimate of the open probability, we determined the apparent pKa of activation to be ∼4.2. Macroscopic activation of KcsA is characterized by a typical sigmoidal rising phase. This feature is reminiscent of activation in eukaryotic K+ channels, and is a consequence of the presence of multiple conformational states in the pathway that leads to channel opening (Sigworth, 1994; Zagotta et al., 1994; Bezanilla, 2000). In Shaker, the activation is believed to occur as independent movements of the voltage sensors, eventually leading to a concerted opening of the conduction pathway (nH ∼ 1), although gating current measurements do suggest some degree of cooperativity in the voltage sensor movement (Bezanilla et al., 1991). In KcsA, there appears to be partial cooperativity in proton mediated activation (n ∼ 2), which would imply that either binding of protons to two subunit is sufficient to open the channel or that the binding of protons to one subunit facilitates the binding to others in a cooperative manner. Further, we also find that the activation of KcsA is not intrinsically modulated by voltage. Though there is a marginally fast activation at negative potentials, this appears to be an apparent effect that arises from its coupling to the fast inactivation rates at these potentials.
Inactivation mechanisms in K+ channels have been the focus of extensive investigations. Although there is a relatively clear understanding of the molecular basis of N-type inactivation, the microscopic events that underlie C-type inactivation are yet to be thoroughly understood. The molecular entity that governs C-type inactivation is thought to be associated with the selectivity filter of the channel (Liu et al., 1996). Perturbations in this region cause enormous effects on the rate of inactivation (Lopez-Barneo et al., 1993). The time course of C-type inactivation varies from tens of milliseconds to seconds in different K+ channels and is sensitive to the extracellular concentration of the permeant ion (Lopez-Barneo et al., 1993; Baukrowitz and Yellen, 1995). In this paper, we show that decreasing K+ concentration in the extracellular part of the channel increases the rate of inactivation while permeating ions like Rb+ with longer residence time in the filter dramatically decrease this rate. The strong similarities in these fundamental features suggest a common molecular basis of C-type inactivation among these channels. An important aspect of difference, however, lies in the inherent voltage dependence of the inactivation process. Based on single-channel analysis, it has been reported that C-type inactivation in Shaker is not particularly sensitive to voltage at least in the range of −25 to +50 mV (Hoshi et al., 1994). While in previous studies, based on macroscopic currents, and from our present work in the single channels (Chakrapani et al., 2007), we find that inactivation of KcsA shows a dependence on voltage, with a z value of 0.7. One reason for this presumably deviant behavior could be attributed to the absence of Glu at position 71 (Val in Shaker), which confers voltage dependence to the inactivation process in KcsA.
In this study, we further report that pH does not significantly modulate the rates of inactivation in KcsA from either the extracellular or intracellular side in the range where the activation is exponential (3.0– 5.0). In the pH range 3.0–4.0 when the channel activation is relatively fast, the inactivation rates are very similar. On the other hand between pH 4.0 and pH 5.0 when the activation rates become rate limiting the channel inactivation shows very small pH dependence. This finding might imply that the key interaction between Glu71 and Asp80 is not being directly modulated by pH but rather by conformational changes that occur during channel opening.
While activation involves sequential transitions through several closed states, as evidenced by the sigmoidal rising phase of the macroscopic conduction, deactivation appears to be a monotonic decay of currents in a pH jump experiment reflecting a single transition from the open to pre-open closed state. Measurements of deactivation time course by jumps from pH 3.0 to pH 8.0 at both depolarizing and hyperpolarizing potentials show no major differences. A comparison of the deactivation time course of macroscopic currents elicited by different pH and closed by pH 8.0 also showed similar time constants, suggesting that the open state(s) visited by the channel upon activation by different pH are kinetically similar.
We find that upon deactivation, closure of the lower gate by basic pH, KcsA recovers from inactivation. During this recovery, the channel appears to bypass the open-conducting state, analogous to Na+ channels, which do not conduct during recovery from inactivation. The time course of recovery measured by double pulse experiments at different membrane potentials showed that KcsA recovered at least threefold faster at depolarizing potentials.
Most models that describe gating in Shaker channels predict that inactivation occurs only upon channel opening, and recovery from inactivation often occurs through open state (Demo and Yellen, 1991). This means that, in principle, inactivation is tightly coupled to the open-conducting state of the channel. This idea is supported by findings that several mutations that shift the voltage dependence of the activation cause a parallel shift in the apparent voltage dependence of the inactivation process; also mutations and blockers that exclusively populate the closed state just before the open state do not increase propensity for inactivation from the closed state (Armstrong and Loboda, 2001; del Camino et al., 2005). In contrast, in Na+ channels, the development of inactivation occurs from both closed and open states and during recovery the channel hardly ever passes through the open state. Conversely, there are reports of a mutation in the S4–S5 linker of Shaker that promotes inactivation to occur from closed state (Ayer and Sigworth, 1997), thereby making it functionally more similar to Na+ channels. Closed-state inactivation, in principle, is also reflected in the presence of null traces in single-channel recordings and has been reported for Shaker channel (Zagotta and Aldrich, 1990; Demo and Yellen, 1991). Further, accessibility assays in Shaker suggest that the recovery of the channel from inactivation at hyperpolarized potentials occurs predominantly through the closed states (Panyi and Deutsch, 2006). Also, a recent study by Claydon et al. (2007) reports the presence of a pH-modulated closed-state inactivation in Shaker-IR that is suggested to be kinetically different from the open-state inactivation. We found that in KcsA, inactivation can occur not only from open states but also from partially activated closed states. Inactivation, however, is not completely independent of activation and does show state dependency, wherein the rate of inactivation from the open state is much higher than that from the closed states. Our finding is further corroborated by single-channel recordings under nonstationary conditions where pH jumps sometimes fail to elicit channel opening (Chakrapani et al., 2007), an indication that inactivation can occur before channel conduction.
The C-type inactivation in Shaker involves a concerted conformational change of all the four subunits leading to the narrowing of the extracellular mouth of the channel, as evidenced by mutational studies in tandem dimer constructs (Ogielska et al., 1995). We therefore hypothesize that during activation by acidic pH, when the proton binding sites are saturated, the channel opening and inactivation from the open states are more so favored. On the other hand, at subthreshold pH, the proton concentration is not sufficient to bring about full activation and therefore we see no conductance. However, partial protonation (partial opening of the lower gate) leads to conformational changes that can trigger inactivation of the channel.
Although it is true that inactivation can occur without ion conduction, our recovery experiments demonstrate that when preceded by high-throughput ion permeation, the inactivation appears to occur faster and to a much deeper extent. Since the rate of C-type inactivation is governed by the occupancy of an ion binding in the external mouth of the pore (Liu et al., 1996; Kiss and Korn, 1998; Loots and Isacoff, 1998; Kiss et al., 1999), we believe that this effect of ion permeation on the inactivation gating might reflect the tendency of the filter to collapse while the ions transition from one binding site to the next. These results suggest that conformational changes that accompany channel opening and ion permeation have a synergistic effect on the rates and extent of inactivation.
In summary, activation of KcsA involves transitions among several states that are governed by pH and does not have intrinsic voltage dependence. Inactivation is modulated by voltage and is not directly altered by protons. Furthermore, inactivation in KcsA is not strictly a property of the fully open conducting channel and can occur from partially activated closed states. The relative proportion of channels inactivating from the closed and open states is determined by pH, and the rate of this inactivation process itself is determined by membrane potential. The entry and recovery rates from these inactivation processes suggest the presence of multiple inactivated states that are linked to the different extent of activation of the channel and to the ion permeation itself.
Supplemental Material
Acknowledgments
We thank L. Cuello, V. Jogini, and F. Bezanilla for insightful discussions; and V. Vásquez, J. Santos, H. Raghuraman, and O. Dalmas for critical reading and comments on the manuscript.
This work was supported by National Institutes of Health grants to E. Perozo and an American Heart Association Postdoctoral Fellowship to S. Chakrapani.
Olaf S. Andersen served as editor.
Abbreviations used in this paper: EPR, electron paramagnetic resonance; WT, wild type.
References
- Armstrong, C.M. 2003. Voltage-gated K channels. Sci. STKE. 2003:re10. [DOI] [PubMed] [Google Scholar]
- Armstrong, C.M., and A. Loboda. 2001. A model for 4-aminopyridine action on K channels: similarities to tetraethylammonium ion action. Biophys. J. 81:895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayer, R.K., Jr., and F.J. Sigworth. 1997. Enhanced closed-state inactivation in a mutant Shaker K+ channel. J. Membr. Biol. 157:215–230. [DOI] [PubMed] [Google Scholar]
- Baukrowitz, T., and G. Yellen. 1995. Modulation of K+ current by frequency and external [K+]: a tale of two inactivation mechanisms. Neuron. 15:951–960. [DOI] [PubMed] [Google Scholar]
- Bezanilla, F. 2000. The voltage sensor in voltage-dependent ion channels. Physiol. Rev. 80:555–592. [DOI] [PubMed] [Google Scholar]
- Bezanilla, F. 2005. Voltage-gated ion channels. IEEE Trans Nanobioscience. 4:34–48. [DOI] [PubMed] [Google Scholar]
- Bezanilla, F., E. Perozo, D.M. Papazian, and E. Stefani. 1991. Molecular basis of gating charge immobilization in Shaker potassium channels. Science. 254:679–683. [DOI] [PubMed] [Google Scholar]
- Bezanilla, F., E. Perozo, and E. Stefani. 1994. Gating of Shaker K+ channels. II. The components of gating currents and a model of channel activation. Biophys. J. 66:1011–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrapani, S., J.F. Cordero-Morales, and E. Perozo. 2007. A quantitative description of KcsA gating II: single-channel currents. J. Gen. Physiol. 130:479–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chill, J.H., J.M. Louis, C. Miller, and A. Bax. 2006. NMR study of the tetrameric KcsA potassium channel in detergent micelles. Protein Sci. 15:684–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claydon, T.W., M. Vaid, S. Rezazadeh, D.C. Kwan, S.J. Kehl, and D. Fedida. 2007. A direct demonstration of closed-state inactivation of K+ channels at low pH. J. Gen. Physiol. 129:437–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero-Morales, J.F., L.G. Cuello, Y. Zhao, V. Jogini, D.M. Cortes, B. Roux, and E. Perozo. 2006. a. Molecular determinants of gating at the potassium-channel selectivity filter. Nat. Struct. Mol. Biol. 13:311–318. [DOI] [PubMed] [Google Scholar]
- Cordero-Morales, J.F., L.G. Cuello, and E. Perozo. 2006. b. Voltage-dependent gating at the KcsA selectivity filter. Nat. Struct. Mol. Biol. 13:319–322. [DOI] [PubMed] [Google Scholar]
- Cortes, D.M., and E. Perozo. 1997. Structural dynamics of the Streptomyces lividans K+ channel (SKC1): oligomeric stoichiometry and stability. Biochemistry. 36:10343–10352. [DOI] [PubMed] [Google Scholar]
- Cortes, D.M., L.G. Cuello, and E. Perozo. 2001. Molecular architecture of full-length KcsA: role of cytoplasmic domains in ion permeation and activation gating. J. Gen. Physiol. 117:165–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuello, L.G., J.G. Romero, D.M. Cortes, and E. Perozo. 1998. pH-dependent gating in the Streptomyces lividans K+ channel. Biochemistry. 37:3229–3236. [DOI] [PubMed] [Google Scholar]
- del Camino, D., M. Kanevsky, and G. Yellen. 2005. Status of the intracellular gate in the activated-not-open state of Shaker K+ channels. J. Gen. Physiol. 126:419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delcour, A.H., B. Martinac, J. Adler, and C. Kung. 1989. Modified reconstitution method used in patch-clamp studies of Escherichia coli ion channels. Biophys. J. 56:631–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demo, S.D., and G. Yellen. 1991. The inactivation gate of the Shaker K+ channel behaves like an open-channel blocker. Neuron. 7:743–753. [DOI] [PubMed] [Google Scholar]
- Demo, S.D., and G. Yellen. 1992. Ion effects on gating of the Ca2+-activated K+ channel correlate with occupancy of the pore. Biophys. J. 61:639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle, D.A., J. Morais Cabral, R.A. Pfuetzner, A. Kuo, J.M. Gulbis, S.L. Cohen, B.T. Chait, and R. MacKinnon. 1998. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 280:69–77. [DOI] [PubMed] [Google Scholar]
- Gao, L., X. Mi, V. Paajanen, K. Wang, and Z. Fan. 2005. Activation-coupled inactivation in the bacterial potassium channel KcsA. Proc. Natl. Acad. Sci. USA. 102:17630–17635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonoi, T., and B. Hille. 1987. Gating of Na channels. Inactivation modifiers discriminate among models. J. Gen. Physiol. 89:253–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross, A., L. Columbus, K. Hideg, C. Altenbach, and W.L. Hubbell. 1999. Structure of the KcsA potassium channel from Streptomyces lividans: a site directed spin labeling study of the second transmembrane segment. Biochemistry. 38:10324–10335. [DOI] [PubMed] [Google Scholar]
- Heginbotham, L., L. Kolmakova-Partensky, and C. Miller. 1998. Functional reconstitution of a prokaryotic K+ channel. J. Gen. Physiol. 111:741–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heginbotham, L., M. LeMasurier, L. Kolmakova-Partensky, and C. Miller. 1999. Single Streptomyces lividans K+ channels: functional asymmetries and sidedness of proton activation. J. Gen. Physiol. 114:551–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi, T., W.N. Zagotta, and R.W. Aldrich. 1994. Shaker potassium channel gating. I: Transitions near the open state. J. Gen. Physiol. 103:249–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irizarry, S.N., E. Kutluay, G. Drews, S.J. Hart, and L. Heginbotham. 2002. Opening the KcsA K+ channel: tryptophan scanning and complementation analysis lead to mutants with altered gating. Biochemistry. 41:13653–13662. [DOI] [PubMed] [Google Scholar]
- Jiang, Y., A. Lee, J. Chen, M. Cadene, B.T. Chait, and R. MacKinnon. 2002. Crystal structure and mechanism of a calcium-gated potassium channel. Nature. 417:515–522. [DOI] [PubMed] [Google Scholar]
- Jiang, Y., A. Lee, J. Chen, V. Ruta, M. Cadene, B.T. Chait, and R. MacKinnon. 2003. X-ray structure of a voltage-dependent K+ channel. Nature. 423:33–41. [DOI] [PubMed] [Google Scholar]
- Kelly, B.L., and A. Gross. 2003. Potassium channel gating observed with site-directed mass tagging. Nat. Struct. Biol. 10:280–284. [DOI] [PubMed] [Google Scholar]
- Kiss, L., and S.J. Korn. 1998. Modulation of C-type inactivation by K+ at the potassium channel selectivity filter. Biophys. J. 74:1840–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss, L., J. LoTurco, and S.J. Korn. 1999. Contribution of the selectivity filter to inactivation in potassium channels. Biophys. J. 76:253–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo, A., J.M. Gulbis, J.F. Antcliff, T. Rahman, E.D. Lowe, J. Zimmer, J. Cuthbertson, F.M. Ashcroft, T. Ezaki, and D.A. Doyle. 2003. Crystal structure of the potassium channel KirBac1.1 in the closed state. Science. 300:1922–1926. [DOI] [PubMed] [Google Scholar]
- Lange, A., K. Giller, S. Hornig, M.F. Martin-Eauclaire, O. Pongs, S. Becker, and M. Baldus. 2006. Toxin-induced conformational changes in a potassium channel revealed by solid-state NMR. Nature. 440:959–962. [DOI] [PubMed] [Google Scholar]
- LeMasurier, M., L. Heginbotham, and C. Miller. 2001. KcsA: it's a potassium channel. J. Gen. Physiol. 118:303–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy, D.I., and C. Deutsch. 1996. Recovery from C-type inactivation is modulated by extracellular potassium. Biophys. J. 70:798–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y., M.E. Jurman, and G. Yellen. 1996. Dynamic rearrangement of the outer mouth of a K+ channel during gating. Neuron. 16:859–867. [DOI] [PubMed] [Google Scholar]
- Liu, Y., S.P. Sompornpisut, and E. Perozo. 2001. Structure of the KcsA channel intracellular gate in the open state. Nat. Struct. Biol. 8:883–887. [DOI] [PubMed] [Google Scholar]
- Loots, E., and E.Y. Isacoff. 1998. Protein rearrangements underlying slow inactivation of the Shaker K+ channel. J. Gen. Physiol. 112:377–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Barneo, J.T. Hoshi, S.H. Heinemann, and R.W. Aldrich. 1993. Effects of external cations and mutations in the pore region on C-type inactivation of Shaker potassium channels. Receptors Channels. 1:61–71. [PubMed] [Google Scholar]
- Morais-Cabral, J.H., Y. Zhou, and R. MacKinnon. 2001. Energetic optimization of ion conduction rate by the K+ selectivity filter. Nature. 414:37–42. [DOI] [PubMed] [Google Scholar]
- Ogielska, E.M., W.N. Zagotta, T. Hoshi, S.H. Heinemann, J. Haab, and R.W. Aldrich. 1995. Cooperative subunit interactions in C-type inactivation of K channels. Biophys. J. 69:2449–2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panyi, G., and C. Deutsch. 2006. Cross talk between activation and slow inactivation gates of Shaker potassium channels. J. Gen. Physiol. 128:547–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panyi, G., Z. Sheng, and C. Deutsch. 1995. C-type inactivation of a voltage-gated K+ channel occurs by a cooperative mechanism. Biophys. J. 69:896–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perozo, E., D.M. Cortes, and L.G. Cuello. 1998. Three-dimensional architecture and gating mechanism of a K+ channel studied by EPR spectroscopy. Nat. Struct. Biol. 5:459–469. [DOI] [PubMed] [Google Scholar]
- Perozo, E., D.M. Cortes, and L.G. Cuello. 1999. Structural rearrangements underlying K+-channel activation gating. Science. 285:73–78. [DOI] [PubMed] [Google Scholar]
- Schoppa, N.E., and F.J. Sigworth. 1998. Activation of Shaker potassium channels. III. An activation gating model for wild-type and V2 mutant channels. J. Gen. Physiol. 111:313–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrempf, H., O. Schmidt, R. Kummerlen, S. Hinnah, D. Muller, M. Betzler, T. Steinkamp, and R. Wagner. 1995. A prokaryotic potassium ion channel with two predicted transmembrane segments from Streptomyces lividans. EMBO J. 14:5170–5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro, M.S., and T.E. DeCoursey. 1991. Permeant ion effects on the gating kinetics of the type L potassium channel in mouse lymphocytes. J. Gen. Physiol. 97:1251–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigworth, F.J. 1994. Voltage gating of ion channels. Q. Rev. Biophys. 27:1–40. [DOI] [PubMed] [Google Scholar]
- Spruce, A.E., N.B. Standen, and P.R. Stanfield. 1989. Rubidium ions and the gating of delayed rectifier potassium channels of frog skeletal muscle. J. Physiol. 411:597–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stimers, J.R., F. Bezanilla, and R.E. Taylor. 1985. Sodium channel activation in the squid giant axon. Steady state properties. J. Gen. Physiol. 85:65–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson, R.P., Jr., and C.M. Armstrong. 1981. K+ channels close more slowly in the presence of external K+ and Rb+. Nature. 291:427–429. [DOI] [PubMed] [Google Scholar]
- Takeuchi, K., H. Takahashi, S. Kawano, and I. Shimada. 2007. Identification and characterization of the slowly exchanging pH-dependent conformational rearrangement in KcsA. J. Biol. Chem. 282:15179–15186. [DOI] [PubMed] [Google Scholar]
- Tatulian, S.A., D.M. Cortes, and E. Perozo. 1998. Structural dynamics of the Streptomyces lividans K+ channel (SKC1): secondary structure characterization from FTIR spectroscopy. FEBS Lett. 423:205–212. [DOI] [PubMed] [Google Scholar]
- Zagotta, W.N., and R.W. Aldrich. 1990. Voltage-dependent gating of Shaker A-type potassium channels in Drosophila muscle. J. Gen. Physiol. 95:29–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagotta, W.N., T. Hoshi, and R.W. Aldrich. 1994. Shaker potassium channel gating. III: Evaluation of kinetic models for activation. J. Gen. Physiol. 103:321–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Y., J.H. Morais-Cabral, A. Kaufman, and R. MacKinnon. 2001. Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2.0 Å resolution. Nature. 414:43–48. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.