Abstract
Plasmon-waveguide resonance (PWR) spectroscopy is an optical technique that has been developed in our laboratories and applied to the study of membrane-associated proteins, especially GPCRs. It has high sensitivity and requires no labeling of materials, and can monitor changes in proteolipid mass density and conformation in real time using plasmon excitation by light polarized both perpendicular and parallel to the resonator surface. Direct measurements will be described of the association of ligands and G-proteins to GPCRs incorporated into a self-assembled lipid bilayer deposited on the silica surface of a PWR resonator. These studies have provided new insights into the functioning of this important class of signaling proteins.
Introduction
One of the central mechanisms of cell signaling [1] involves modulation by G-proteins of the activity of membrane-bound effectors such as adenyl cyclase and phospholipase C, resulting in changes in intracellular second messenger concentrations (cAMP and phosphoinositols, respectively). Such modulation proceeds via the activation by agonists of members of a superfamily of integral membrane proteins known as G-protein coupled receptors (GPCRs). There are three main groups of these receptors: Family A (rhodopsin/β2 adrenergic-like); Family B (glucagon/calcitonin-like); and Family C (metabotropic neurotransmitter/calcium receptor-like), each of which consists of a seven transmembrane-helix domain linked together by a series of extra- and intracellular loops and having N- (extracellular) and C-terminal (intracellular) extramembrane extensions of varying size and complexity. GPCRs interact with the environment via ligand binding from the extracellular side (e.g. epinephrine in the case of the adrenergic receptors and endorphins/enkephalins in the case of the opioid receptors) or by light absorption in an already bound ligand in the case of rhodopsin. This results in the activation of a relatively weakly membrane-associated intracellular G-protein, leading to the exchange of bound GDP by GTP, dissociation of the heterotrimeric G-protein, and interactions of the α-, β- and γ-subunits with downstream effectors resulting in activation or inhibition and corresponding changes in second messenger concentrations.
Classically, GPCR activity has been monitored by displacement of a radiolabeled ligand by an unlabeled ligand, or, in the case of G-protein activation, either by the binding of enzymatically stable radiolabeled GTP (e.g. GTPγ35S) or indirectly by various second messenger assays [2]. Although this allows a direct observation of the association of a second ligand with an already-liganded GPCR, it does not provide information about the unliganded receptor. Furthermore, it does not allow a direct determination of the binding of the G-protein to the receptor, either liganded or unliganded. In this review, we will describe an optical spectroscopic method (plasmon-waveguide resonance spectroscopy; PWR spectroscopy) that does allow a direct determination of these tasks with high sensitivity, using a membrane-bound receptor, and without requiring ligand or G-protein labeling (either radioactive or chromophore/fluorophore).
Plasmon-waveguide resonance spectroscopy [3,4]
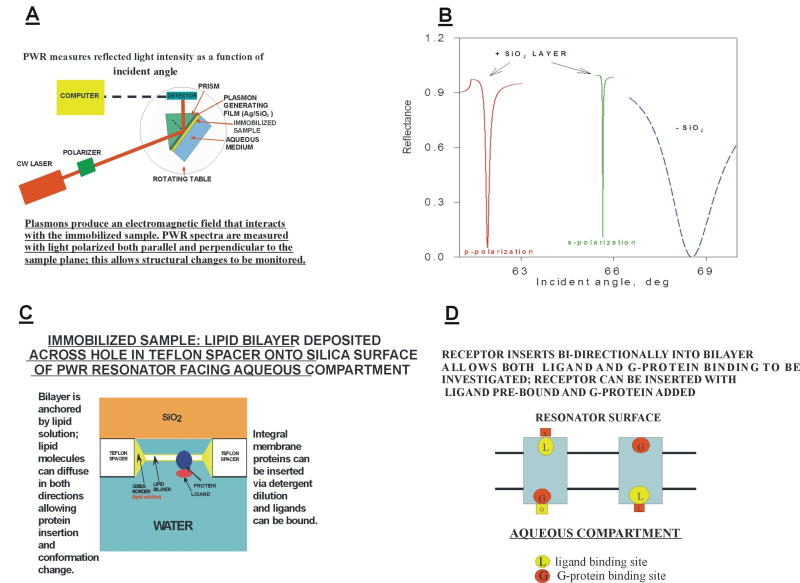
PWR involves the optical excitation of plasmons in a thin metal film (usually silver), overcoated by a thicker dielectric film (usually silica), and deposited onto the surface of a right angle prism. A typical PWR spectrometer is shown schematically in Figure 1A. Plasmons are oscillations of the conduction electrons in the metal film, and their excitation is a resonance process occurring at the expense of the exciting light energy. Resonance occurs at an incident angle slightly above the critical angle for total internal reflection. This generates an evanescent electromagnetic field localized at the interface between the outer surface of the silica layer and the external aqueous medium, thereby decreasing the intensity of the reflected light. A sample to be analyzed is immobilized at that surface, interacts with the evanescent field and changes the characteristics of the resonance. By placing the resonator on a rotating table, a PWR spectrum can be measured, which corresponds to a plot of the reflected light intensity as a function of incident angle. If the silica layer has a thickness comparable to the wavelength of the exciting light, waveguide modes can also be generated. Plasmon excitation in the metal film requires the electric vector of the exciting light to be polarized perpendicular to the plane of the sample (referred to as p-polarization), whereas waveguide excitation requires the polarization to be parallel to the sample plane (s-polarization). The waveguide modes couple with the plasmon modes to produce two separate resonances that occur at different incident angles. In conventional surface plasmon resonance (SPR) spectrometry, produced in the absence of the dielectric overcoating, only the p-polarized resonance is excited. An important consequence of the presence of the dielectric waveguide is the narrowing of the resonance lines and an amplification of the evanescent field thereby greatly enhancing signal to noise. This allows a large increase in sensitivity and spectral resolution over SPR. We estimate that we can readily study femtomolar quantities of GPCRs incorporated into a single lipid bilayer approximately 2mm in diameter. These characteristics are shown in Figure 1B. The ability to measure resonance spectra using both polarizations also allows information to be obtained about structural changes that occur in the immobilized sample, in addition to changes in mass density. This will be discussed further below.
Figure 1. Some characteristics of PWR spectroscopy.
A: Schematic illustration of a typical PWR spectrometer.
B: Examples of resonance spectra obtained in PWR and SPR experiments.
C: Schematic view of the surface of a PWR resonator.
D: Illustration of the bi-directional insertion of a GPCR into a lipid bilayer.
Maxwell’s equations applied to thin films allow a theoretical understanding of the plasmon spectra to be obtained. Thus, it can be shown that the PWR spectra are completely determined by three parameters of the resonator and the immobilized sample: the refractive indices in the two polarization directions (np and ns), the optical extinction coefficients at the excitation wavelength in these two directions (kp and ks) and the thicknesses (t) of the various layers (4-6). The resonance spectra can be fit or simulated to obtain values for these parameters, and thereby to obtain information about the structural characteristics of the sample, as well as changes that occur in these properties as a consequence of interactions between the sample and its environment.
Application of PWR spectroscopy to membrane systems [3,5,6]
Immobilization of proteolipid bilayers at the resonator surface allows the use of PWR spectroscopy to obtain new insights into a wide range of biomembrane systems. Here we will focus on applications to GPCRs. Figure 1C shows an enlarged view of the hydrated silica surface of a PWR resonator. Between this surface and the aqueous chamber is a Teflon spacer with a small orifice (approx. 2 mm in diameter). A solution of lipid (usually in a squalene-butanol solvent, sometimes also containing methanol) is deposited across this orifice prior to filling the chamber with a buffer solution. The hydrophilic surface attracts the polar head groups of the lipid, causing a lipid-containing droplet to adhere. Subsequent filling of the chamber allows the excess lipid solvent to be removed and a bilayer to self-assemble and to become anchored to the orifice by an annulus of the remaining lipid solution (Gibbs border). Integral membrane proteins can be inserted into the bilayer by adding a detergent solution of the protein into the aqueous compartment under conditions that dilute the detergent to below its critical micelle concentration (cmc) (The choice of detergent and its proper use to maintain the membrane protein (e.g. GPCR) in a biologically viable state is a critical issue and must be thoroughly investigated before proceeding to its use in biophysical studies). This causes spontaneous transfer of the protein from the detergent micelle into the bilayer, in a manner quite analogous to the reconstitution of integral membrane proteins into lipid vesicles. Octylglucoside has proven to be an excellent detergent for this purpose, both because of its high cmc (25 mM) and its low propensity to interact with lipid bilayers. The Gibbs border allows the bilayer to be quite flexible and to accommodate protein insertion and conformational changes by permitting lipid molecules to transfer back and forth. It is also possible to insert membrane fragments derived from sonicated cells into such bilayers. The lipid composition of the bilayer can be modulated quite readily, allowing systematic studies of the effect of microenvironment on protein functional behavior.
Another aspect of the protein insertion process is that, in many cases, incorporation proceeds bi-directionally [7]. This is especially significant for GPCRs in that it allows both the ligand binding site and the G-protein binding site to be simultaneously exposed to the aqueous compartment. This is shown schematically in Figure 1D. As will be demonstrated below, this has been quite useful in studying GPCR interactions with G-proteins and with the cognate ligands for these receptors.
Distinguishing mass density and structural changes using PWR
One of the most useful characteristics of PWR spectroscopy is the ability to distinguish between changes in the immobilized sample that result from gain or loss of mass and those that result from changes in mass distribution (i.e. structure/conformation). We will see key examples of this later when we use PWR spectroscopy to examine ligand-GPCR interactions. In many of these cases the mass of the ligand is 1-2% that of the receptor, and yet large changes in the spectra occur for both s and p polarized light. This is a direct consequence of the structural changes that occur in the membrane and can only be properly observed by measurement of both p- and s-polarized resonances in uniaxially oriented materials such as proteolipid bilayers. In such systems, the molecules are anisotropic with respect to the sample plane, i.e. because of their cylindrical shape, their refractive indices (and optical extinction coefficients if they contain chromophores that absorb at the excitation wavelength) differ along the parallel and perpendicular axes. Thus, when the number of such molecules on the resonator surface changes, the percentage changes in the np and ns values are equal. A consequence of this is that the p- and s-shifts in the PWR spectra occur in the same direction (to larger angles for an increase and to smaller angles for a decrease in mass density) and to the same extent (assuming that the sensitivity of the resonator is the same for both polarizations, which may not be the case but which can be calibrated). If the mass density is not changed, but the mass distribution is changed (e.g. by changes in molecular conformation or orientation), then the p- and s-polarized resonances can shift to different extents and even in different directions. This allows information to be obtained about the symmetry of the structural changes. When both mass density and distribution are changed, their relative contributions can be ascertained by either fitting/simulation analysis [8,9] or by graphical methods [10].
Application of PWR spectroscopy to GPCRs
In this section we will present some examples of the application of PWR spectroscopy to the characterization of the functional properties of GPCRs [11,12].
Ligand binding
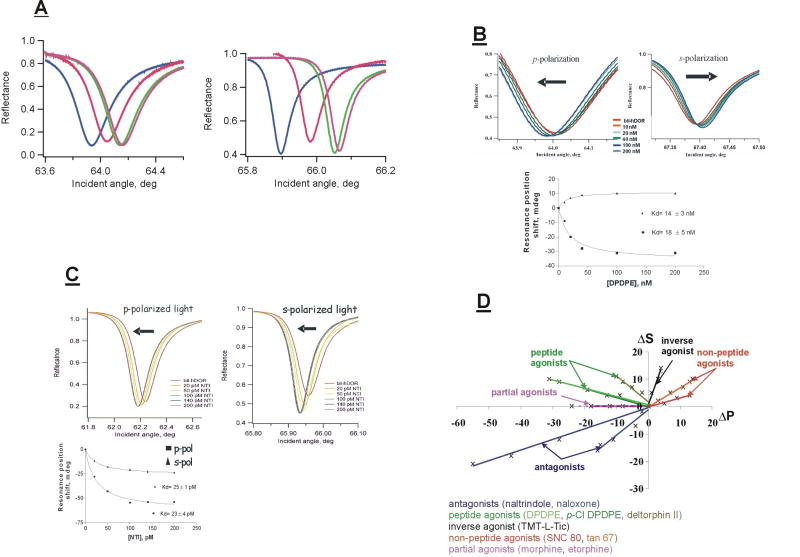
Figure 2A shows p- and s-polarized PWR spectra obtained in a series of measurements in which a lipid bilayer was deposited onto the surface of a resonator, followed by incorporation of the human brain δ-opioid receptor (hDOR; 0.4 nM total concentration) from an octylglucoside solution, and the subsequent addition of a saturating concentration of the small peptide agonist DPDPE [13] (200 nM) to the aqueous compartment [14]. The spectral changes observed are due to 1) the immobilization of an anisotropic mass (the lipid) on the resonator surface forming a bilayer, then 2) the increase in mass and structural changes upon receptor incorporation, and finally 3) the further increase in mass and structural changes upon agonist binding to the receptor.
Figure 2. Examples of PWR data for ligand binding to GPCRs.
A: PWR spectra obtained upon bilayer deposition, receptor incorporation and ligand binding. Left panel, p-polarization; right panel, s-polarization; blue: no bilayer; red: bilayer deposited (egg PC/POPC; 3:1); green: hDOR inserted; magenta: DPDPE (agonist) added.
B: Spectral shifts obtained at equilibrium upon binding of increasing amounts of the agonist DPDPE to the hDOR incorporated into a lipid bilayer. Lower panel: hyperbolic plots of data (squares: p-polarization; triangles: s-polarization). Arrows show the directions of the shifts.
C: Spectral shifts obtained upon binding of increasing amounts of the antagonist NTI to the hDOR incorporated into a lipid bilayer. Lower panel: hyperbolic plots of data.
D: Plots of spectral shifts obtained with various ligands upon binding to the hDOR. Each data point corresponds to a different ligand concentration.
By adding incremental amounts of the agonist in such an experiment and plotting the shift values at equilibrium vs. added ligand, a binding constant can be determined directly. Figure 2B shows an example of this kind of experiment. Several things are important to note. First, DPDPE binding causes the p- and s-polarized resonances to shift in opposite directions. This is a direct demonstration that the shifts are caused primarily by structural changes in the proteolipid membrane, probably due to both lipid and protein contributions. Second, the values of the dissociation constants (Kd) obtained from a hyperbolic fit to the data are comparable to those obtained in classical pharmacological assays [14], indicating that the receptor is in a native state in the bilayer. It is also important to point out that these Kd values are thermodynamically valid. This is because the spectral shifts are directly proportional to the amount of bound ligand, and the amount of ligand in the medium adjacent to the bilayer is not significantly depleted by the binding process due to the fact that the aqueous volume is much greater (approx. 1000-fold) than the bilayer volume.
The effect of binding an antagonist, naltrindole (NTI), to the hDOR is shown in Figure 2C. Two points are important to note. First, the directions of the spectral shifts are quite different from those obtained with DPDPE, demonstrating that the structural changes occurring upon NTI binding are not the same as with DPDPE. Second, again the Kd values are comparable to those found using classical pharmacological assays [14].
We have done analogous experiments with a wide range of ligands for the hDOR, and the results of these are shown in Figure 2D in the form of plots of the p- vs. s-shifts. It is quite evident that the various classes of ligands represented here all produce distinctive structural changes in the proteolipid bilayer. This indicates a high degree of conformational plasticity of the receptor in its response to ligand binding. As will be shown below, this translates into differing abilities to bind and activate G- proteins. We have obtained similar results with two other GPCRs, the β2-adrenergic [15] and the CB1 cannabinoid receptors [16], although we have not carried out as extensive an examination of ligand binding in these cases.
A further difference in the nature of the interaction between various ligands and GPCRs lies in the binding kinetics. Our results with several GPCRs indicate that agonist binding kinetics are generally more complex and slower than antagonist binding kinetics [12-14]. This is illustrated in Figure 3 with the β2-adrenergic receptor. It is reasonable to attribute this kinetic difference to the fact that agonists need to generate a conformation of the receptor that is able to cause the appropriate structural changes in the G-protein that lead to its activation, whereas antagonists merely have to block agonist binding. Since the former requires information transfer across the lipid bilayer, it seems likely to demand a more extensive structural perturbation that includes both lipid and protein.
Figure 3. Kinetics of agonist and antagonist binding to a GPCR.
Kinetics of the binding of the agonist (-)isoproterenol (10 μM; left panel) and the antagonist alprenolol (10 nM; right panel) to the human β2-adrenergic receptor incorporated into a lipid bilayer. Note the multiphasic character of agonist binding.
G-protein binding and activation
Figure 4A shows a series of PWR spectra for the insertion of a DPDPE-bound hDOR sample into a lipid bilayer, followed by the addition of a saturating amount of a G-protein sample comprised of a mixture of the brain subtypes of the α, β and γ subunits [7, 17]. Again, it is important to point out that the Kd value obtained from the data is consistent with that obtained in classical pharmacological assays, although the latter are more indirect inasmuch as they are based on downstream effects. Also consistent with the pharmacology is the observation (not shown) of lower G-protein affinities for the unliganded receptor (60 nM) and the antagonist-bound receptor (500 nM) [17].
Figure 4. Examples of PWR spectroscopy applied to G-protein interactions with a GPCR.
A: PWR spectra obtained after bilayer deposition, insertion of a DPDPE-bound hDOR and binding of a saturating amount of G-protein. Left panel: p-polarization; right panel: s-polarization. Lower panel: hyperbolic plot of G-protein binding (squares: p-polarization; triangles: s-polarization).
B: Effect of GTPγS addition on the PWR spectra of the ternary complex of agonist-bound hDOR and G-protein.
C: Kd values for binding four G-protein subtypes (left bar) and subsequent binding of GTPγS (right bar) to various liganded forms of the hDOR.
Figure 4B shows the effect of GTPγS addition on the PWR spectrum of the sample shown in Figure 10. Note that the spectral shifts for s- and p-polarized light are both to smaller incident angles. We interpret this as being due to the dissociation of the heterotrimeric G-protein and the mass density decrease resulting from release of the less tightly bound α- subunit from the membrane-bound receptor. Consistent with this is the fact that the shift is approximately one-half of that which occurred when the G-protein was bound, in agreement with the relative masses of the α- vs. βγ-subunits. A binding experiment in which aliquots of the GTPγS solution were added yielded a Kd value of 13 nM (not shown), similar to that for the G-protein. Also consistent with the pharmacology is the lack of binding of GTPγS with either the unliganded or the antagonist-bound receptor (also not shown).
Most striking are the results obtained when individual subtypes of the α-subunits were used to generate the G-proteins. These data are shown in Figure 4C. In the experiments depicted, four brain-specific subtypes were combined individually with a mixture of brain βγ-subunits, and five different agonists plus the unliganded receptor were used. Several important points need to be emphasized:
1- the differences between the values obtained with the various G-protein subtypes are quite large (1-2 orders of magnitude in many cases).
2- different patterns are found for both binding processes, e.g. strong binding of the G-protein does not necessarily correlate with good GTPγS binding (the activation step).
3- these results imply, although they do not prove, that these various liganded forms would have different downstream effects and therefore produce different physiological consequences. This provides a structural basis for the observations of agonist-directed trafficking/functional selectivity found for many GPCRs [18], and yields a possible protocol for selective drug development.
Biological Implications
The implications of these findings to the pharmacology and physiology of GPCRs and their cognate G-proteins, to signaling pathways modulated by GPCRs and their corresponding G-proteins, and to the design of specific agonist and antagonist ligands for the treatment of the many medical disorders modulated by GPCRs (it is estimated that as many as 50% of current drugs have GPCRs as their targets) will be briefly discussed.
A major concern (and potential criticism) of these studies is that they are carried out with a model system and that the results may not correspond to what is happening in a living cellular system. This certainly is a valid concern and we would be the first to point out that the presence of other cellular components that modulate signal transduction via GPCRs may modify both qualitatively and quantitatively the results we have observed. On the other hand, as pointed out above and in our publications utilizing this new biophysical method, the binding affinities of ligands using these artificially prepared proteolipid bilayers are very similar to those reported in the literature using membrane preparations from the brain or from cellular assays [14]. Similarly our GTPγS assay data are quite comparable to those obtained in classical in vitro assays. Thus we suggest that though there are caveats to be considered in PWR experiments with membrane associated proteins such as GPCRs, G-proteins and other membrane associated proteins, PWR spectroscopy provides a powerful and sensitive tool for directly examining binding affinities and kinetics of interactions between ligands and receptors, and their associated G-proteins and other proteins involved in signal transduction processes, as well as interactions of substrates and enzymes with these proteins, under conditions as closely resembling those occurring in vivo as presently possible in a model system and without requiring potentially structure-perturbing labeling.
Despite these caveats, from the studies we have done thus far some of the longstanding textbook concepts of GPCR and G-protein structure-function relationships require revision. Thus, it is especially clear that GPCRs are structurally much more heterogeneous with respect to interactions with ligands than previously appreciated. The textbook concept that the typical GPCR exists in two functional conformations, i.e. an inactive conformation and an active conformation, is inadequate and ultimately misleading. Interactions of GPCRs with agonist, antagonist, and inverse agonist ligands, and with no ligand present (unoccupied state) clearly are all different with regard to the structural states produced. In addition, peptide ligands (the natural ligands for most GPCRs) lead to different conformational states than non-peptide ligands. This may in part explain why many non-peptide ligands for GPCRs often have SAR relationships that bear no close relationship to the SAR of the natural peptide ligand. Furthermore there appears to be a much more subtle relationship between agonist ligand binding to the receptor and G-protein interaction with the receptor, including GDP-GTP exchange (GTPγS binding), than previously recognized. The general assumption has been that there was a one-one to relationship, i.e. stronger ligand binding generated a more potent cellular response. Clearly this does not necessarily seem to be the case based on the results we have obtained thus far. Furthermore, the G-protein that binds most strongly to the receptor in response to an agonist ligand is not necessarily the G-protein that generates the most robust signal, at least in terms of GTPγS binding. It seems that subtle differences in ligand structure/conformation can lead to subtle differences in GPCR conformation, which in turn can lead to subtle differences in interactions with their cognate G-proteins. The true implications of this to GPCR function is still unclear and will require extensive additional studies by PWR spectroscopy and other biochemical and biophysical methods. However, it is clearly consistent with current thinking about functional selectivity (18). In addition, other components of the signal transduction cascade need to be brought into the picture including the effects of protein kinases, inositol phosphates, protein phosphatases, β-arrestins, clathrins, ion channels, etc. PWR spectroscopy can play a vital role in examining further these systems in thermodynamic and kinetic detail.
Conclusions
The implications of these findings to drug design could be quite significant especially considering that ~50% of all drugs address GPCRs for their biological activities, and it is likely this will continue to be the case into the future considering the significant role intercellular signaling plays in virtually all diseases of the peripheral and the central nervous systems. If different structural classes of ligands lead to different transduction pathways this implies differences in downstream biological effects which is one further consideration in drug design. For example, is the generally greater toxicity of non-peptide drugs vs. peptide drugs possibly related to their differential effects on GPCRs and other effector systems? Clearly there is much to learn and PWR spectroscopy can play an important role. We look forward to the opportunity to further develop its potential in these and other important biological problems involving membranes and membrane proteins.
Acknowledgments
This work was supported in the past by grants from the National Science Foundation and the U.S. Public Health, National Institutes of Health, and National Institute of Drug Abuse.
Footnotes
Conflicts of Interest: The authors have patents or patents pending on the instrumentation and applications of PWR spectroscopy.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
-
•
of special interest
-
••
of outstanding interest
- 1.Oldham WM, Hamm HE. Structural basis of function in heterotrimeric G-proteins. Quarterly Rev Biophys. 2006;39:117–166. doi: 10.1017/S0033583506004306. [DOI] [PubMed] [Google Scholar]
- 2.Greasley PJ, Jansen FP. G-Protein-coupled receptor screening technologies. Drug Discov Today: Technologies. 2005;2:163–170. doi: 10.1016/j.ddtec.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 3.Tollin G, Salamon Z, Hruby VJ. Techniques: plasmon-waveguide resonance (PWR) spectroscopy as a tool to study ligand-GPCR interactions. Trends Pharmacol Sci. 2003;24:655–659. doi: 10.1016/j.tips.2003.10.010. • an overview of the application of PWR to GPCR studies.
- 4.Salamon Z, Tollin G. Plasmon resonance spectroscopy: probing molecular interactions at surfaces and interfaces. Spectroscopy. 2001;15:161–175. doi: 10.1016/s0968-0004(99)01394-8. •• a useful review of the general principles of PWR spectroscopy.
- 5.Salamon Z, Brown MF, Tollin G. Plasmon resonance spectroscopy: probing interactions within membranes. Trends Biochem Sci. 1999;24:213–219. doi: 10.1016/s0968-0004(99)01394-8. [DOI] [PubMed] [Google Scholar]
- 6.Tollin G, Salamon Z, Cowell S, Hruby VJ. Plasmon-waveguide resonance spectroscopy: a new tool for investigating signal transduction by G-protein coupled receptors. Life Sci. 2003;73:3307–3311. doi: 10.1016/j.lfs.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Alves ID, Salamon Z, Varga E, Yamamura HI, Tollin G, Hruby VJ. Direct observation of G-protein binding to the human δ-opioid receptor using plasmon-waveguide resonance spectroscopy. J Biol Chem. 2003;278:48890–48897. doi: 10.1074/jbc.M306866200. • the first example of the use of a spectroscopic method to characterize G-protein binding to a GPCR.
- 8.Salamon Z, Devanathan S, Alves ID, Tollin G. Plasmon-waveguide resonance studies of lateral segregation of lipids and proteins into microdomains (rafts) in solid-supported bilayers. J Biol Chem. 2005;280:11175–11184. doi: 10.1074/jbc.M411197200. [DOI] [PubMed] [Google Scholar]
- 9.Alves ID, Salamon Z, Hruby VJ, Tollin G. Ligand modulation of lateral segregation of a G-protein coupled receptor into lipid microdomains in sphingomyelin/phosphatidylcholine solid-supported bilayers. Biochemistry. 2005;44:9168–9178. doi: 10.1021/bi050207a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salamon Z, Tollin G. Graphical analysis of mass and anisotropy changes observed by plasmon-waveguide resonance spectroscopy can provide useful insights into membrane protein function. Biophys J. 2004;86:2508–2516. doi: 10.1016/S0006-3495(04)74306-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salamon S, Cowell S, Varga E, Yamamura HI, Hruby VJ, Tollin G. Plasmon resonance studies of agonist/antagonist binding to the human δ-opioid receptor: new structural insights into receptor-ligand interactions. Biophys J. 2000;79:2463–2474. doi: 10.1016/S0006-3495(00)76489-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salamon Z, Hruby VJ, Tollin G, Cowell S. Binding of agonists, antagonists and inverse agonists to the human δ-opioid receptor produces distinctly different conformational states distinguishable by plasmon-waveguide resonance spectroscopy. J Peptide Res. 2002;60:322–328. doi: 10.1034/j.1399-3011.2002.21060.x. [DOI] [PubMed] [Google Scholar]
- 13.Mosberg HI, Hurst R, Hruby VJ, Gee K, Yamamura HI, Galligan JJ, Burks TF. Bis-Penicillamine Enkephalins Possess Highly Improved Specificity Toward Delta Opioid Receptors. Proc Natl Acad Sci USA. 1983;80:5871–5874. doi: 10.1073/pnas.80.19.5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alves ID, Cowell SM, Salamon Z, Devanathan S, Tollin G, Hruby VJ. Different structural states of the proteolipid membrane are produced by ligand binding to the human δ-opioid receptor as shown by plasmon-waveguide resonance spectroscopy. Mol Pharmacol. 2004;65:1248–1257. doi: 10.1124/mol.65.5.1248. •• the first direct demonstration of the exceptionally high degree of structural plasticity of a GPCR.
- 15.Devanathan S, Yao Z, Salamon Z, Kobilka B, Tollin G. Plasmon-waveguide resonance studies of ligand binding to the human β2-adrenergic receptor. Biochemistry. 2004;43:3280–3288. doi: 10.1021/bi035825a. [DOI] [PubMed] [Google Scholar]
- 16.Georgieva T, Devanathan S, Stropova D, Park CK, Salamon Z, Tollin G, Hruby VJ, Roeske WR, Yamamura HI, Varga E. Unique agonist-bound CB1 receptor conformations indicate agonist specificity in signaling. doi: 10.1016/j.ejphar.2007.11.053. manuscript in preparation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alves ID, Ciano KA, Boguslavski V, Varga E, Salamon Z, Yamamura HI, Hruby VJ, Tollin G. Selectivity, cooperativity and reciprocity in the interactions between the δ-opioid receptor, its ligands, and G-proteins. J Biol Chem. 2004;279:44673–44682. doi: 10.1074/jbc.M404713200. • an excellent illustration of the new insights obtained into GPCR function by PWR spectroscopy.
- 18.Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, Miller KJ, Spedding M, Mailman RB. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Therap. 2007;320:1–13. doi: 10.1124/jpet.106.104463. •• an important review of the physiological basis for the new paradigm of functional selectivity in pharmacology.