Abstract
The current marine pharmacology review that covers the peer-reviewed literature during 2003 and 2004 is a sequel to the authors' 1998-2002 reviews, and highlights the preclinical pharmacology of 166 marine chemicals derived from a diverse group of marine animals, algae, fungi and bacteria. Anthelminthic, antibacterial, anticoagulant, antifungal, antimalarial, antiplatelet, antiprotozoal, antituberculosis or antiviral activities were reported for 67 marine chemicals. Additionally 45 marine compounds were shown to have significant effects on the cardiovascular, immune and nervous system as well as possessing anti-inflammatory effects. Finally, 54 marine compounds were reported to act on a variety of molecular targets and thus may potentially contribute to several pharmacological classes. Thus, during 2003-2004, research on the pharmacology of marine natural products which involved investigators from Argentina, Australia, Brazil, Belgium, Canada, China, France, Germany, India, Indonesia, Israel, Italy, Japan, Mexico, Morocco, the Netherlands, New Zealand, Norway, Panama, the Philippines, Portugal, Russia, Slovenia, South Korea, Spain, Thailand, Turkey, United Kingdom, and the United States, contributed numerous chemical leads for the continued global search for novel therapeutic agents with broad spectrum activity.
Keywords: drug-leads, marine, metabolites, natural products, pharmacology, review, toxicology
1. Introduction
The purpose of this article is to review the 2003-4 primary literature on pharmacological studies with marine natural products using the same format as in our previous reviews of the marine pharmacology peer-reviewed literature (Mayer and Lehmann, 2000), (Mayer and Hamann, 2002, 2004, 2005). Consistent with our previous reviews, only those articles reporting on the bioactivity or pharmacology of 166 marine chemicals whose structures have been established are included in the present review. As in our previous reviews, we have used Schmitz's chemical classification (Schmitz et al., 1993) to assign each marine compound to a major chemical class, namely, polyketides, terpenes, nitrogen-containing compounds or polysaccharides. Those publications reporting anthelminthic, antibacterial, anticoagulant, antifungal, antimalarial, antiplatelet, antiprotozoal, antituberculosis or antiviral properties of 67 marine chemicals have been tabulated in Table 1 with the corresponding structures shown in Fig.1. The articles reporting on 45 marine compounds affecting the cardiovascular, immune and nervous systems, as well as those with anti-inflammatory effects are grouped in Table 2 and the structures presented in Fig. 2. Finally 54 marine compounds targeting a number of distinct cellular and molecular targets and mechanisms are shown in Table 3 and their structures depicted in Fig. 3. Publications on the biological or pharmacological activity of marine extracts or as yet structurally uncharacterized marine compounds have been excluded from the present review, although several promising reports were published during 2003-4: a specific inhibitor of a thyrotropin releasing hormone-specific peptidase (Pascual et al., 2004); antimicrobial activity in sub-Arctic marine invertebrates (Lippert et al., 2003); antifilarial activity of the red alga Botryocladia leptopoda (Lakshmi et al., 2004); antiviral effects of a sulfated exopolysaccharide from the marine microalga Gyrodinium impudicum (Yim et al., 2004) and Sargassuum patens (Zhu et al., 2004); a polyhydroxylated fucophlorethol isolated from the marine brown alga Fucus vesiculosus shown to be bactericidal towards selected Gram-positive and Gram-negative bacteria in vitro (Sandsdalen et al., 2003); and an improvement of “current cytokine-based therapies” by sulphated polysaccharides purified from the green alga Codium fragile, as well as fucoidan and carrageenan, isolated from brown and red algae, respectively (Nika et al., 2003).
Table 1.
Marine pharmacology in 2003-4: Marine Compounds with Anthelmintic, Antibacterial, Anticoagulant, Antifungal, Antimalarial, Antiplatelet, Antiprotozoal, Antituberculosis, and Antiviral Activities.
| Drug Class | Compound/Organisma | Chemistry | Pharmacologic Activity | MMOAb | Countryc | References |
|---|---|---|---|---|---|---|
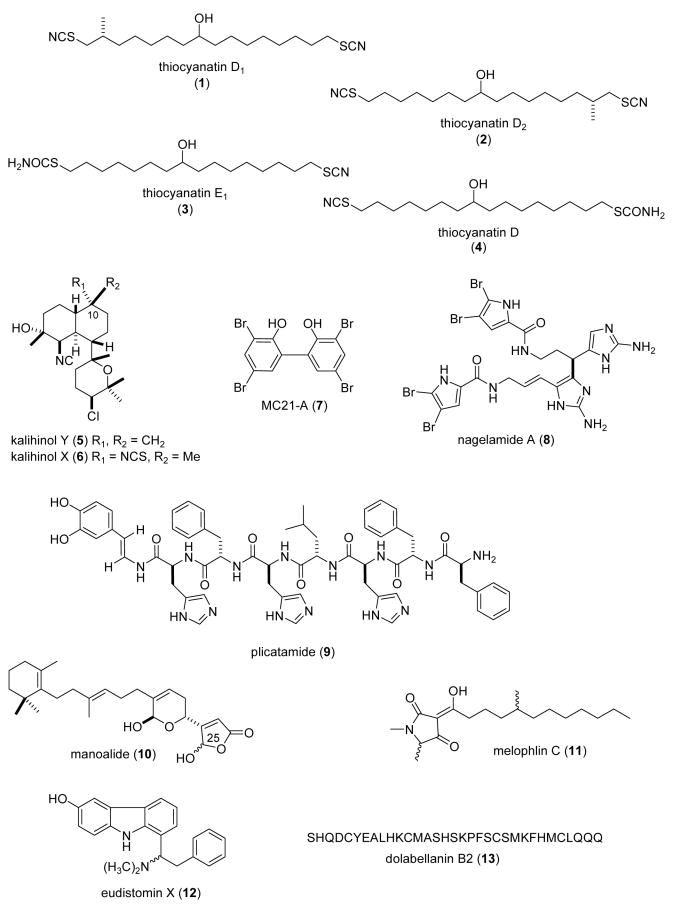
| Anthelminthic | thiocyanatins (1-4)/sponge | Polyketided | Nematocidal activity to Haemonchus contortus | Undetermined | AUS | (Capon, R. J. et al. 2004b) |
| Antibacterial | kalihinol Y & X (5,6)/sponge | Diterpenee | B. subtilis inhibition | Folate biosynthesis inhibition | PHIL, USA | (Bugni, T. S. et al. 2004) |
| Antibacterial | MC21A (7)/bacterium | Bromophenol | Methicillin-resistant S. aureus inhibition comparable to vancomycin | Permeabilization of cell membrane | JAPN | (Isnansetyo, A. et al. 2003) |
| Antibacterial | nagelamide A (8)/sponge | Alkaloidf | M. luteus, B. subtilis & E. coli inhibition | Protein phosphatase 2A inhibition | AUS, JAPN | (Endo, T. et al. 2004) |
| Antibacterial | plicatamide (9)/tunicate | Peptidef | Methicillin-resistant S.aureus, L. monocytogenes, E. coli & P. aeruginosa inhibition | Bind to plasma membrane causing K+ efflux & depolarization | USA | (Tincu, J. A. et al. 2003) |
| Antibacterial | manoalide (10)/sponge | Sesterterpenee | S. aureus inhibition | Undetermined | JAPN | (Namikoshi, M. et al. 2004) |
| Antibacterial | melophlin C (11)/sponge | Polyketided | B. subtilis & S. aureus inhibition | Undetermined | CHI, INDO, GER, NETH | (Wang, C. Y. et al. 2003) |
| Antibacterial | eudistomin X (12)/tunicate | Alkaloidf | S. aureus, B. subtilis & E. coli inhibition | Undetermined | GER | (Schupp, P. et al. 2003) |
| Antibacterial | dolabellanin B2 (13)/sea hare | Peptidef | B. subtilis, H. influenza & V. vulnificus inhibition | Undetermined | JAPN | (Iijima, R. et al. 2003) |
| Antibacterial | pseudopterosin X & Y (14,15)/soft coral | Diterpenee | S. aureus, S. pyogenes, & E. faecalis inhibition | Undetermined | CAN, USA | (Ata, A. et al. 2004) |
| Antibacterial | Sinularia lipids (16-18)/ soft coral | Polyketided | B. subtilis, B. pumilus, E. coli and P. aeruginosa inhibition | Undetermined | RUS | (Dmitrenok, A. S. et al. 2003) |
| Antibacterial | Rhodomela bromophenol (19)/alga | Bromophenol | S. aureus, S. epidermidis and P. aeruginosa inhibition | Undetermined | CHI | (Xu, N. et al. 2003) |
| Antibacterial | cribrostatin 6 (20)/sponge | Alkaloidf | S. pneumoniae inhibition | Undetermined | USA | (Pettit, R. K. et al. 2004) |
| Antibacterial | purpuramine L (21)/sponge | Bromotyrosine Alkaloidsf | S. aureus, B. subtilis & C. violaceum inhibition | Undetermined | IND | (Goud, T. V. et al. 2003b) |
| Antibacterial | germacrane (22)/sponge | Sesquiterpenee | S. aureus & B. subtilis inhibition | Undetermined | THAI | (Satitpatipan, V. et al. 2004) |
| Antibacterial | Ptilocaulis guanidine(23)/ sponge | Alkaloidf | S. aureus inhibition | Undetermined | USA | (Yang, S. W. et al. 2003c) |
| Antibacterial | membranolides C & D (24,25) /sponge | Diterpenee | S. aureus & E. coli inhibition | Undetermined | USA | (Ankisetty, S. et al. 2004) |
| Antibacterial | arenicins 1 & 2 (26,27)/ polychaeta | Peptidef | E. coli, L. monocytogenes & C. albicans inhibition | Undetermined | RUS | (Ovchinnikova, T. V. et al. 2004) |
| Antibacterial | perinerin(28)/ polychaeta | Peptidef | Gram-negative, Gram-positive & fungal inhibition | Undetermined | CHI | (Pan, W. et al. 2004) |
| Antibacterial | Hippoglossoides peptide (29)/American plaice | Peptidef | P. aeruginosa & S. aureus inhibition | Undetermined | CAN | (Patrzykat, A. et al. 2003b) |
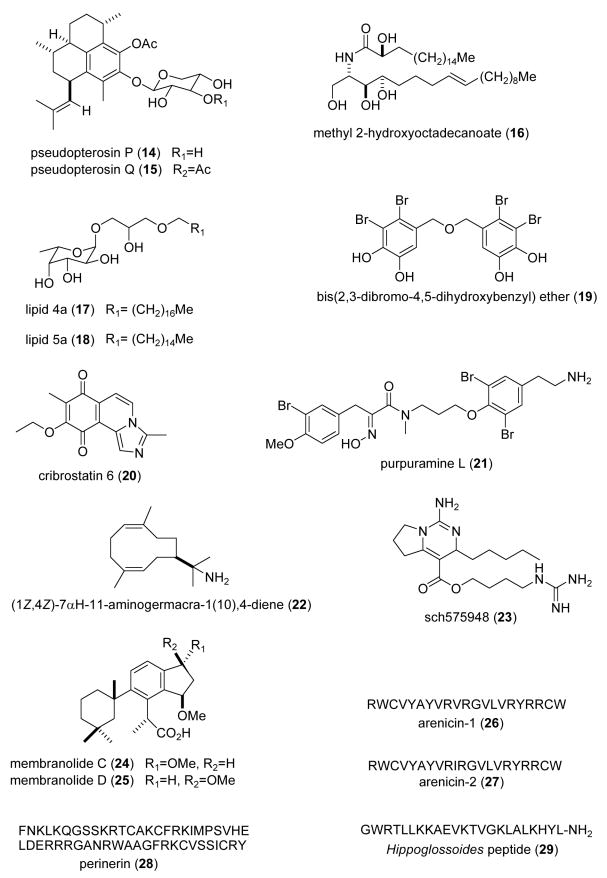
| Anticoagulant | dysinosin C (30)/sponge | Peptidef | Factor VIIa and thrombin inhibition | Two structural motifs contribute to protease binding | AUS | (Carroll, A. R. et al. 2004) |
| Anticoagulant | fucosylated chondroitin sulfate (31)/sea cucumber | Polysaccharideg | Anticoagulant, bleeding and antithrombotic effects in vivo | Accelerated thrombin inhibition | BRA | (Zancan, P. et al. 2004) |
| Anticoagulant | sulfated galactans (32,33)/alga & sea urchin | Polysaccharideg | Antithrombin-mediated anticoagulant activity | Interaction holds antithrombin inactive | BRA | (Melo, F. R. et al. 2004) |
| Antifungal | Astroscleridae sterol (34)/ sponge | Sterol sulfated | S. cerevisiae inhibition | Undetermined | USA | (Yang, S. W. et al. 2003b) |
| Antifungal | Dysidea arenaria sterol (35)/sponge | Terpenee | Fluconazole resistance reversal in C. albicans | MDR1-type efflux pump inhibition | USA | (Jacob, M. R. et al. 2003) |
| Antifungal | massadine (36)/sponge | Alkaloidf | Geranylgeranyltransferase I inhibition | Undetermined | JAPN, NETH | (Nishimura, S. et al. 2003) |
| Antifungal | naamine G (37)/sponge | Alkaloidf | C. herbarum inhibition | Undetermined | GER, NETH | (Hassan, W. et al. 2004) |
| Antifungal | utenospongin B (38)/sponge | Diterpenee | C. tropicales & F. oxysporum inhibition | Undetermined | NETH, MOR, PORT | (Rifai, S. et al. 2004) |
| Antifungal | (2S,3R)-2-aminododecan-3-ol (39)/ascidian | Polyketided | C. albicans inhibition | Undetermined | BRA, USA | (Kossuga, M.H. et al. 2004) |
| Antimalarial | bielschowskysin (40)/coral | Diterpenee | P. falciparum inhibition | Undetermined | PAN, USA | (Marrero, J. et al. 2004) |
| Antimalarial | briarellins (41-43)/coral | Diterpenee | P. falciparum inhibition | Undetermined | PAN, USA | (Ospina, C. A. et al. 2003) |
| Antimalarial | cembradienes (44)/sea whip | Diterpenee | P. falciparum inhibition | Undetermined | PAN, USA | (Wei, X. M. et al. 2004) |
| Antimalarial | dolostatin 10 (45)/sea hare | Peptidef | P. falciparum FCH5.C2 inhibition | Microtubule & mitotic inhibition | USA | (Fennell, B. J. et al. 2003) |
| Antimalarial | manzamine A (46)/sponge | Alkaloidf | P. falciparum D6 & W2 inhibition | Undetermined | USA | (Rao, K. V. et al. 2003) |
| Antimalarial | trioxacarcin A & D (48,49)/bacterium | Glycoside | P. falciparum strains NF54 & K1 inhibition | Undetermined | NETH, GER | (Maskey, R. P. et al. 2004) |
| Antiprotozoal | renieramycin A (50)/sponge | Alkaloidf | Leishmania amazonensis inhibition | Undetermined | JAPN | (Nakao, Y et al. 2004) |
| Antiprotozoal | euplotin C (51)/ciliate | Sesquiterpenee | Leishmania major & infantum inhibition | Undetermined | ITA | (Savoia, D. et al. 2004) |
| Antiprotozoal | defensins /mussel | Peptidef | T. brucei & L. major inhibition | Undetermined | BEL, FRA | (Roch, P. et al. 2004) |
| Antituberculosis | (+)-8-hydroxymanzamine A (47) /sponge | Alkaloidf | M. tuberculosis inhibition | Undetermined | USA | (Rao, K. V. et al. 2003) |
| Antituberculosis | homopseudopteroxazole (52)/soft coral | Diterpenee | M. tuberculosis inhibition | Undetermined | USA | (Rodriguez, I. I. et al. 2003) |
| Antituberculosis | ingenamine G (53)/sponge | Alkaloidf | S. aureus,E. coli & resistant S. aureus inhibition | Undetermined | BRA, GER | (de Oliveira, J. H. et al. 2004) |
| Antituberculosis | 12-deacetoscalarin 19-acetate (54)/sponge | Sesterterpenee | M. tuberculosis inhibition | Undetermined | THAI | (Wonganuchitmeta, S. N. et al. 2004) |
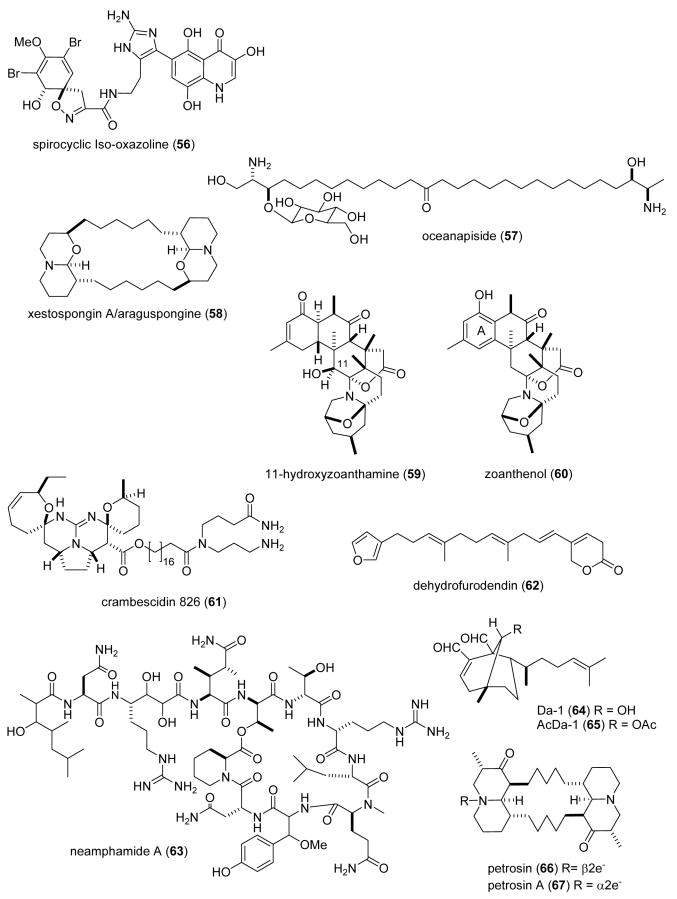
| Antituberculosis | Oceanapiside sp. compounds (55-57)/sponge | Polyketided | Mycothiol-S-conjugate amidase inhibition | Non-competitive inhibition | USA | (Nicholas, G. M. et al. 2003) |
| Antiplatelet | xetospongin A (58) /sponge | Alkaloidf | Collagen-induced platelet aggregation inhibition | Undetermined | PHIL, USA | (Pimentel, S. M. et al. 2003) |
| Antiplatelet | zoanthamine alkaloids (59-60)/zoanthids | Alkaloidf | Agonist-induced platelet aggregation inhibition | Undetermined | BRA, SPA | (Villar, R. M. et al. 2003) |
| Antiviral | crambescidin 826 (61)/ sponge | Alkaloidf | HIV-1 envelope-mediated fusion inhibition in vitro | Undetermined | USA | (Chang, L. et al. 2003) |
| Antiviral | dehydrofurodendin (62)/ sponge | Furanoterpenee | Reverse transcriptase RNA- and DNA-directed DNA polymerase inhibition | Undetermined | ISRA, FRA | (Chill, L. et al. 2004) |
| Antiviral | neamphamide A (63)/sponge | Depsipeptidef | HIV-growth inhibition | Undetermined | USA | (Oku, N. et al. 2004) |
| Antiviral | Dictyota diterpenes (64,65)/alga | Diterpenee | Inhibition of HIV-1 replication in cell line | RNA-dependent DNA-polymerase RT inhibition | BRA | (Pereira, H. S. et al. 2004) |
| Antiviral | petrosins (66,67)/sponge | Alkaloidf | HIV-growth inhibition | Giant cell formation & RT inhibition | IND | (Goud, T. V. et al. 2003a) |
Organism, Kingdom Animalia: flounder (American plaice) and tunicates (Phylum Chordata); polychaeta (Phylum Annelida); sea urchins and cucumbers (Phylum Echinodermata), mussels and sea hares (Phylum Mollusca), sponges (Phylum Porifera); corals, sea whips and zoanthids (Phylum Cnidaria); Kingdom Monera: bacteria (Phylum Cyanobacteria); Kingdom Plantae: algae; Kingdom Protista: ciliates (Phylum Ciliophora).
MMOA: molecular mechanism of action.
Country: AUS: Australia; BEL: Belgium; BRA: Brazil; CAN: Canada; CHI: China; FRA: France; GER: Germany; IND: India; INDO: Indonesia; ISRA: Israel; ITA: Italy; JAPN: Japan; MOR: Morocco; NETH: The Netherlands; NOR: Norway; PAN: Panama; PHIL: The Phillipines; PORT: Portugal; RUS: Russia; SPA: Spain; THAI: Thailand.
Polyketide.
Terpene.
Nitrogen-containing compound.
Polysaccharide.
Figure 1.
Table 2.
Marine pharmacology in 2003-4: Marine Compounds with Anti-inflammatory activity, and affecting the Cardiovascular, Immune and Nervous Systems.
| Drug Class | Compound/Organisma | Chemistry | Pharmacological Activity | MMOAb | Countryc | References |
|---|---|---|---|---|---|---|
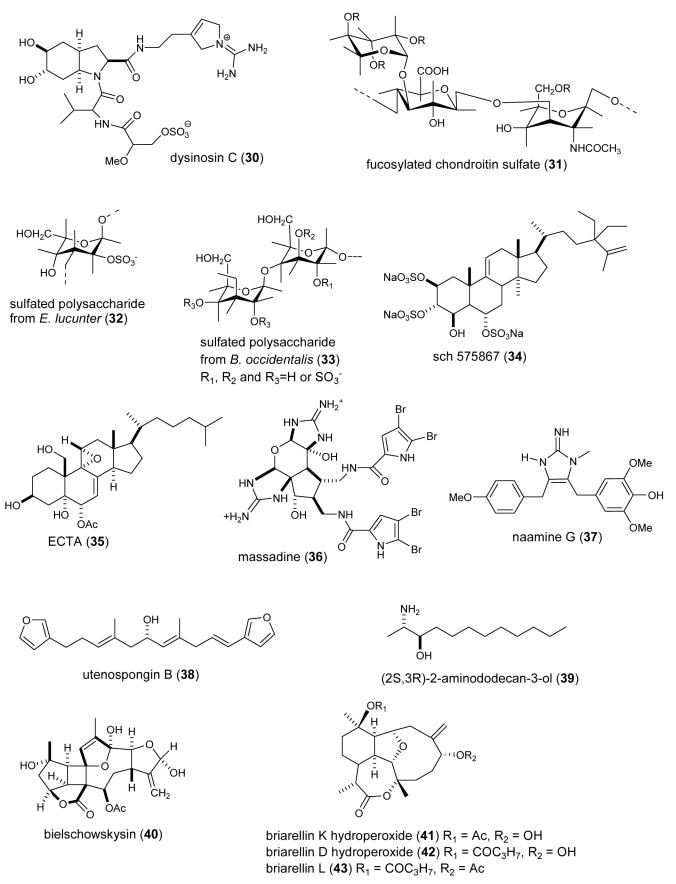
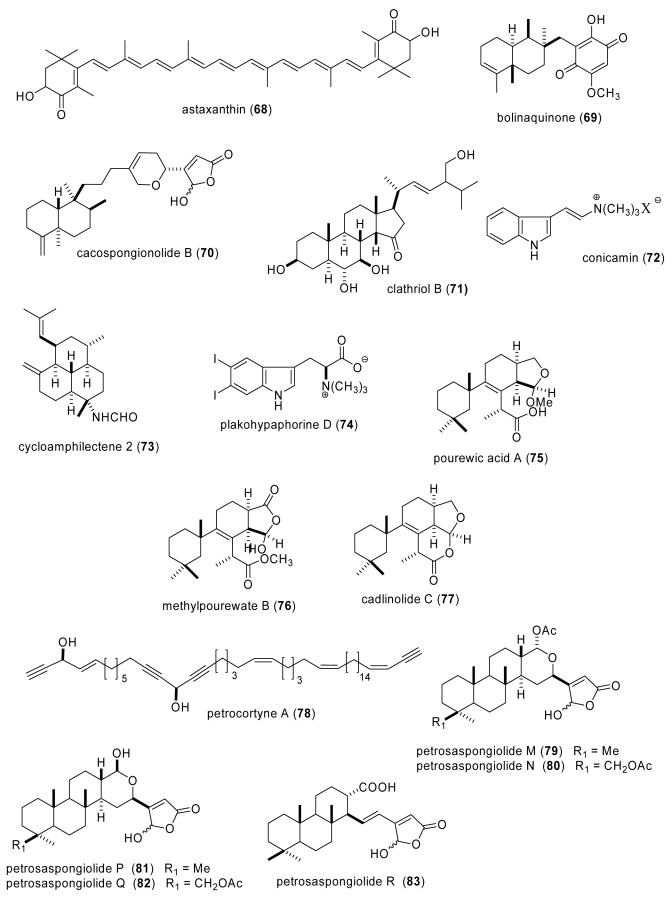
| Anti-inflammatory | astaxanthin (68)/salmon, sea stars | Tetraterpenee | Inhibion of endotoxin-induced uveitis in rats | iNOS, NO, TNF-α & PGE2 inhibition | JAPN | (Ohgami, K. et al. 2003) |
| Anti-inflammatory | bolinaquinone (69)/sponge | Merosesquiterpenee | Inhibition of cytokine, iNOS and eicosanoids | sPLA2 inhibition | SPA, ITA | (Lucas, R. et al. 2003b) |
| Anti-inflammatory | cacospongionolide B (70)/sponge | Sesterterpenee | Nitric oxide, PGE2 & TNF-α inhibition in vitro and in vivo | Nuclear factor-κB inhibition | SPA, ITA | (Posadas, I. et al. 2003a) |
| Anti-inflammatory | clathriol B (71)/sponge | Sterole | Neutrophil superoxide inhibition | Undetermined | NZEL | (Keyzers, R. A. et al. 2003) |
| Anti-inflammatory | conicamin (72)/tunicate | Indole alkaloidf | Histamine antagonist | Undetermined | ITA | (Aiello, A. et al. 2003) |
| Anti-inflammatory | cycloamphilectene 2 (73)/sponge | Diterpenee | Nitric oxide inhibition | Inhibition of NF-κB pathway | ITA, SPA | (Lucas, R. et al. 2003a) |
| Anti-inflammatory | plakohypaphorine D (74)/ sponge | Indole alkaloidf | Histamine antagonist | Undetermined | ITA | (Borrelli, F. et al. 2004) |
| Anti-inflammatory | pourewic acid A & methylpourewate B (75,76)/sponge | Diterpenese | Superoxide inhibition | Undetermined | NZEL | (Keyzers, R. A. et al. 2004) |
| Anti-inflammatory | cadlinolide C (77)/sponge | Diterpenee | Superoxide inhibition | Undetermined | NZEL | (Keyzers, R. A. et al. 2004) |
| Anti-inflammatory | petrocortyne A (78)/sponge | Polyacetylene | Macrophage inflammatory mediator inhibition | NO & TNF-α inhibition | SKOR | (Hong, S. et al. 2003) |
| Anti-inflammatory | petrosaspongiolide M (79)/sponge | Sesterterpenee | Nitric oxide, PGE2 & TNF-α inhibition in vitro and in vivo | Nuclear factor-κB inhibition | ITA, SPA | (Posadas, I. et al. 2003b) |
| Anti-inflammatory | petrosaspongiolides M-R (79-83)/sponge | Sesterterpenese | Macrophage inflammatory mediator inhibition | PLA2 inhibition | ITA | (Monti, M. C. et al. 2004) |
| Anti-inflammatory | pseudopterosin N (84)/sea whip | Diterpenee | Inhibition of mouse ear inflammation | Undetermined | USA | (Ata, A. et al. 2003) |
| Anti-inflammatory | seco-pseudopterosin E (85)/sea whip | Diterpenee | Inhibition of mouse ear inflammation | Undetermined | USA | (Ata, A. et al. 2003) |
| Anti-inflammatory | elisabethadione(86)/sea whip | Diterpenee | Inhibition of mouse ear inflammation | Undetermined | USA | (Ata, A. et al. 2003) |
| Anti-inflammatory | pseudopterosin R (87)/sea whip | Diterpenee | Microglia thromboxane B2 inhibition | Undetermined | USA | (Rodriguez, I. I. et al. 2004) |
| Cardiovascular | callipeltin A (88)/sponge | Depsipeptidef | Affected resting aorta contraction | Na+-ionophore action | ITA | (Trevisi, L. et al. 2004) |
| Immune system | Codium fragile polysaccharide/alga | Polysaccharideg | Binding to IL-2, IL-7 and INF-γ | Undetermined | UK | (Nika, K. et al. 2003) |
| Immune system | sulfircin (89)/sponge | Sesterterpenee | Mobilization of T cells and dendritic cells | CCR7 chemokine receptor binding | USA | (Yang, S. W. et al. 2003d) |
| Immune system | mucins/sea star | Polysaccharideg | Inhibition of neutrophil adhesion | Undetermined | UK | (Bavington, C.D. et al. 2004) |
| Immune system | perybysins A-D (90-93)/fungus | Sesquiterpenese | Inhibition of human leukemia cell adhesion | Undetermined | JAPN | (Yamada, T. et al. 2004) |
| Immune system | phycarine/alga | Polysaccharideg | Stimulation of macrophage phagocytosis | IL-1, IL-6 & TNF-α synthesis | FRA, USA | (Vetvicka, V. et al. 2004) |
| Immune system | sulfated PMG (94)/alga | Polysaccharideg | Inhibition of HIV virus infection of lymphocytes | Binding to CD4 receptor on lymphocytes | CHI | (Meiyu, G. et al. 2003) |
| Immune system | sulfated PMG (94)/alga | Polysaccharideg | Binding to lymphocytes | Interaction with CD4 receptor | CHI | (Miao, B. et al. 2004) |
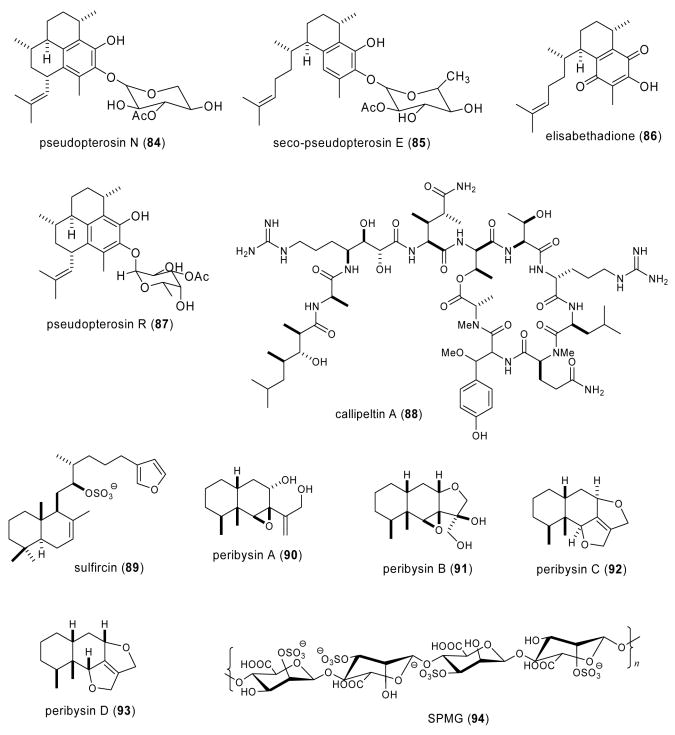
| Nervous system | petrosaspongiolide M (79)/sponge | Sesterterpenee | Reduction of morphine withdrawal in vitro | Undetermined | ITA | (Capasso, A. et al. 2003) |
| Nervous system | aplidine( 95)/tunicate | Depsipeptidef | Inhibition of aggregation of prion petide into β-sheet fibrils | Undetermined | SPA,USA | (Perez, M. et al. 2003) |
| Nervous system | aspermytin A (96)/fungus | Polyketided | Induction of neurite outgrowth | Undetermined | JAPN | (Tsukamoto, S. et al. 2004a) |
| Nervous system | labuanine A (97)/sponge | Pyridoacridine alkaloidf | Induction of neuite outgrowth | Undetermined | JAPN, INDO | (Aoki, S. et al. 2003) |
| Nervous system | linckosides C-E (98-100)/sea star | Steroidal glycosidee | Induction of neurite outgrowth | Undetermined | JAPN | (Qi, J. et al. 2004) |
| Nervous system | parguerolisoparguerol (101-102)/sea hare | Diterpenee | Induction of neurite outgrowth | Undetermined | JAPN | (Tsukamoto, S. et al. 2004b) |
| Nervous system | sargaquinoic acid (103)/alga | Meroditerpenee | Induction of neurite outgrowth | PI-3 kinase independent; TrKA-MAP kinase and adenylate cyclase-PKA dependent | JAPN | (Tsang, C. K. et al. 2004); (Kamei, Y. et al. 2003) |
| Nervous system | SJG-2 ganglioside (104)/sea cucumber | Ganglioside | Induction of neurite outgrowth | Undetermined | JAPN | (Kaneko, M. et al. 2003) |
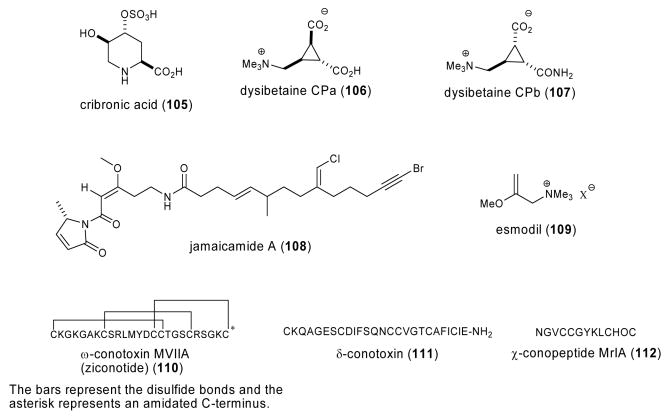
| Nervous system | cribronic acid (105)/sponge | Amino acidf | Convulsant activity in mice | Binding to NMDA-type receptor | JAPN | (Sakai, R. et al. 2003) |
| Nervous system | dysibetaine CPa-CPb 106,107)/sponge | Betaine | Binding to ionotropic glutamate receptors | Undetermined | JAPN | (Sakai, R. et al. 2004) |
| Nervous system | jamaicamide A (108)/cyanobacterium | Lipopeptide f | Sodium channel blocking | Undetermined | USA | (Edwards, D. J. et al. 2004) |
| Nervous system | esmodil (109)/sponge | Quaternary amine | Acetylcholine mimetic | Indetermined | AUS | (Capon, R. J. et al. 2004a) |
| Nervous system | ω-conopeptide MVIIA (110)/snail | Peptidef | Inhibition of refractory pain in patients with AIDS or Cancer | Binding to N-type Ca2+ channels | USA | (Staats, P. S. et al. 2004) |
| Nervous system | δ-conotoxin (111)/snail | Peptidef | Na+ current inhibition | Undetermined | IND | (Sudarslal, S. et al. 2003) |
| Nervous system | χ-conopeptide MrIA (112)/snail | Peptidef | Norepinephrine transporter inhibition | Non-competitive binding at the antidepressant binding site | AUS | (Sharpe, I. A. et al. 2003) |
| Nervous system | Acidic oligosaccharide/alga | Polysaccharideg | Inhibition of amyloid beta toxicity | Inhibition of apoptosis & intracellular Ca2+ | CHI | (Hu, J. et al. 2004) |
Organism: Kingdom Animalia: sea whip (Phylum Cnidaria); tunicate (Phylum Chordata), sea star and cucumber ( Phylum Echinodermata); sea hare and snail (Phylum Mollusca); sponge (Phylum Porifera); Kingdom Fungi: fungus; Kingdom Plantae: alga; Kingdom Monera: bacterium (Phylum Cyanobacteria).
MMOA: molecular mechanism of action, NO: nitric oxide.
Country: AUS: Australia; CHI: China; FRA: France; INDO: Indonesia; ITA: Italy; JAPN: Japan; N. ZEL: New Zealand; SPA: Spain; S.KOR: South Korea; UK: United Kingdom.
Polyketide.
Terpene.
Nitrogen-containing compound.
Polysaccharide.
Figure 2.
Table 3.
Marine pharmacology in 2003-4: Marine Compounds with Miscellaneous Mechanisms of Action.
| Compound/Organisma | Chemistry | Pharmacological Activity | IC50b | MMOAc | Countryd | References |
|---|---|---|---|---|---|---|
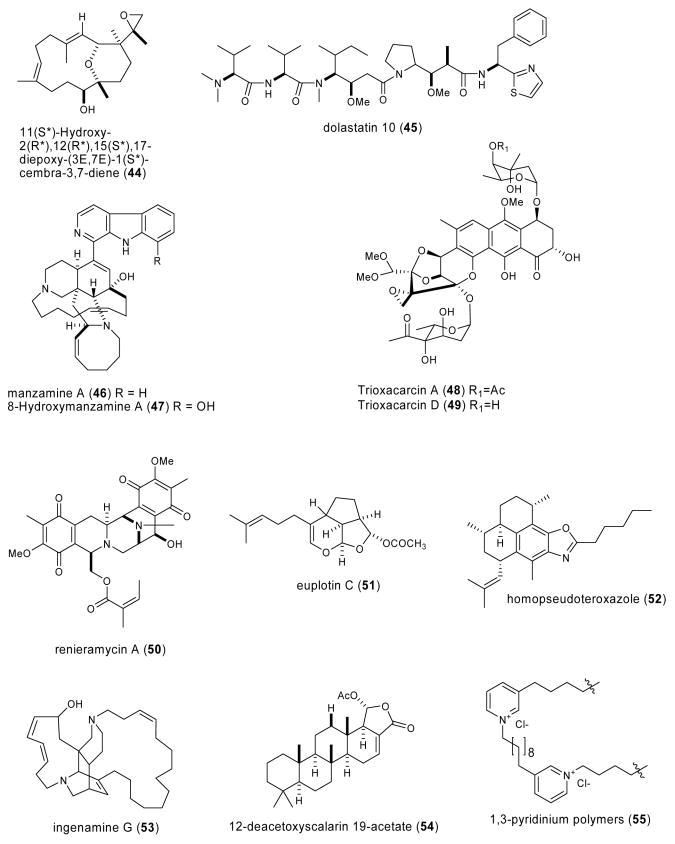
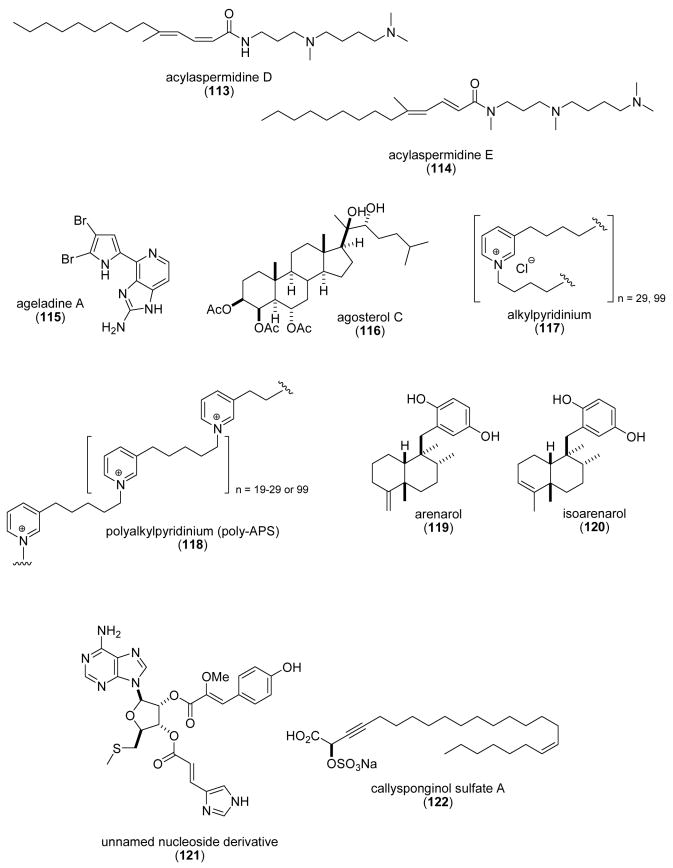
| acylspermidine D & E (113,114)/ soft coral | polyamineg | Vacuolar H+-pyrophosphatase inhibition | 0.98 μM | No inhibition of F-P- and V-type H+ ATPases and cytosolic pyrophosphatase | JAPN | (Hirono, M. et al. 2003) |
| ageladine A (115)/sponge | alkaloidg | Matrix metalloprotease (MMP) inhibition | 0.33-2 μg/mL | Noncompetitive inhibition of MMP-2 | NETH, JAPN | (Fujita, M. et al. 2003a) |
| agosterol C (116)/sponge | sterolf | Proteasome inhibition | 10 μg/mL | Undetermined | NETH, JAPN | (Tsukamoto, S. et al. 2003) |
| alkylpyridinium/(117)/sponge | pyridinium oligomerg | Pore-formation on neuronal membranes | ND | Reversible & irreversible increase [Ca2+]i ; membrane properties attenuated by zinc | TUR, SLO, UK | (McClelland, D. et al. 2003) |
| alkylpyridinium(poly-APS) (118)/sponge | pyridinium oligomerg | Stable DNA transfection | 0.5 μg/mL | Transient and reversible pore formation | SLO, UK | (Tucker, S. J. et al. 2003) |
| arenerol & isoarenarol (119, 120)/sponge | merosesquiter penef | Protein kinases inhibition | 4-7 μM | Undetermined | NETH, CAN | (Yoo, H. D. Leung D. et al. 2003) |
| Atriolum robustum nucleoside (121)/ascidian | Amino acid derivedg | Partial agonist at rat brain A1 adenosine receptors | 23±0.2 μM | Binding to A1 & A3 adenosine receptors | GER | (Kehraus, S. et al. 2004) |
| callysponginol sulfate A (122)/sponge | Polyketidee | MT1-matrix metalloproteinase inhibition | 15.0 μg/mL | Undetermined | JAPN, NETH | (Fujita, M. et al. 2003b) |
| calyculin A (123)/sponge | Polyketidee | Histone H1 kinase phosphorylation induction | ND | Type 1 phosphatase inhibition | JAPN | (Tosuji, H. et al. 2003) |
| CEL-III/sea cucumber | Proteing | Erythrocyte hemolysis | ND | Crystal structure suggests lectin domain 3 involved in pore formation | JAPN | (Uchida, T. et al. 2004) |
| clionasterol (124)/sponge | Sterolf | Complement component C1 inhibition | 4.1 μM | Undetermined | THAI, NETH, PORT | (Cerqueira, F. et al. 2003) |
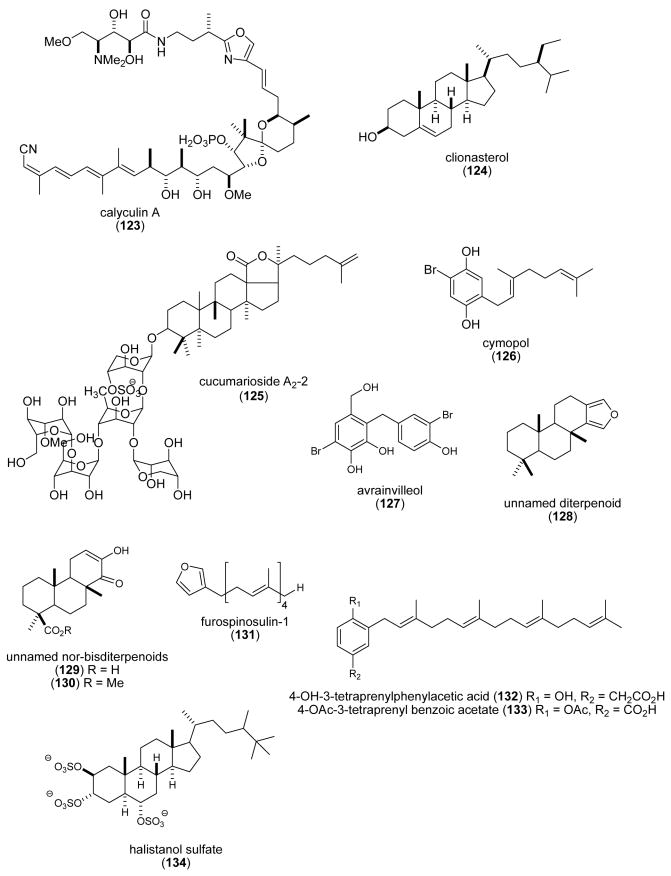
| cucumarioside A2-2 (125)/ sea cucumber | Triterpene glycosidef | Increased [Ca2+]i & lysosomal activity in mouse macrophages | ND | Undetermined | RUS | (Agafonova, I. G. et al. 2003) |
| cymopol (126) & avrainvilleol (127)/alga | Meromonoter penef/polykete | Antioxidant | 4.0-6.1 μM | Undetermined | USA | (Takamatsu, S. et al. 2003) |
| Dictyoceratida diterpenoids (128-130)/sponge | Diterpenef | DNA polymerase B lyase activity inhibition | 20.6-26 μg/mL | Undetermined | USA | (Chaturvedula, V. S. P. et al. 2004) |
| Furano- and aromatic terpenoids (131-133)/sponge | Terpenesf | CDC25 phosphatase inhibition | 0.4-4 μM | Undetermined | ITA, FRA, SPA, TUR, USA | (Erdogan-Orhan, I. et al. 2004) |
| halistanol sulfate (134)/sponge | Sterolf | P2Y12 purinergic receptor inhibition | 0.48 μM | Undetermined | USA | (Yang, S. W. et al. 2003a) |
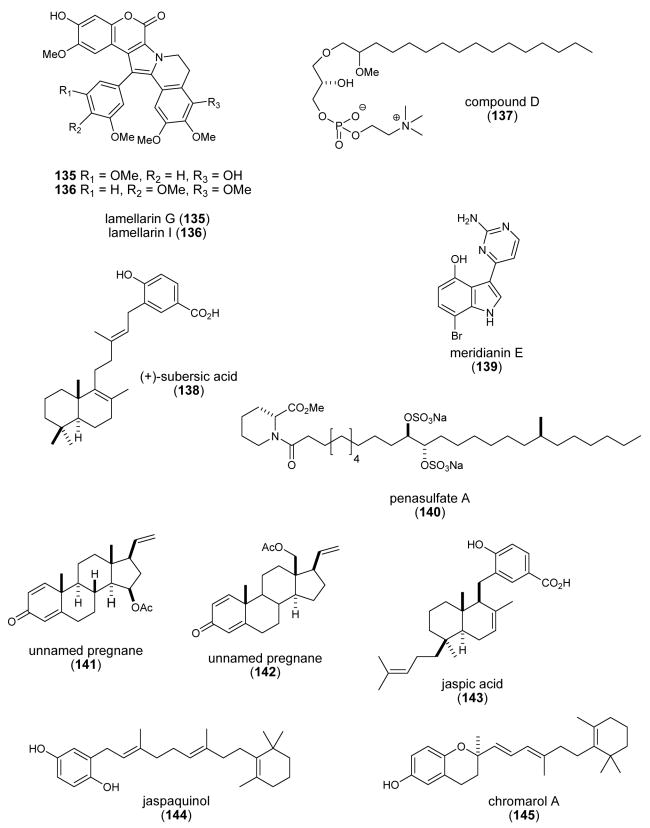
| lamellarin G & I (135, 136)/ ascidian | Alkaloidsg | Free radical scavenging activity | 2.96-3.28 mM | Undetermined | IND | (Krishnaiah, P. et al. 2004) |
| Lysophosphatidylcholine (137)/sponge | Polyketidee | Increased Ca2+ mobilization in HL-60 cells | ND | Undetermined | S.KOR | (Lee, E. H. et al. 2004) |
| (+)-subersic acid (138)/sponge | Meroterpenef | MAPKAP kinase 2 inhibition | 9.6-20μM | Undetermined | CAN, INDO, NETH, USA | (Williams, D. E. et al. 2004b) |
| meridianin E (139)/ascidian | Alkaloidsg | Cell proliferation and apoptosis inhibition | 0.18-0.6 μM | Cyclin B, p25, protein kinase A & G inhibition | ARG, FRA | (Gompel, M. et al. 2004) |
| penasulfate A (140)/sponge | Aminoacid/polyketidee | α-glucosidase inhibition | 3.5 μg/mL | Undetermined | JAPN, NETH | (Nakao, Y. et al. 2004) |
| pregnanes 1 & 2 (141-142)/ coral | Steroidsf | Mitochondrial respiratory chain inhibition | 1.1-1.9 μM | Undetermined | ITA, IND | (Ciavatta, M. L. et al. 2004) |
| Psammocinia spp. diterpenes (143-147)/sponge | Terpenef | Human 15-lipoxygenase inhibition | 0.3-0.8 μM | Reduction of lipoxygenase non-heme ferric center | USA | (Cichewicz, R. H. et al. 2004) |
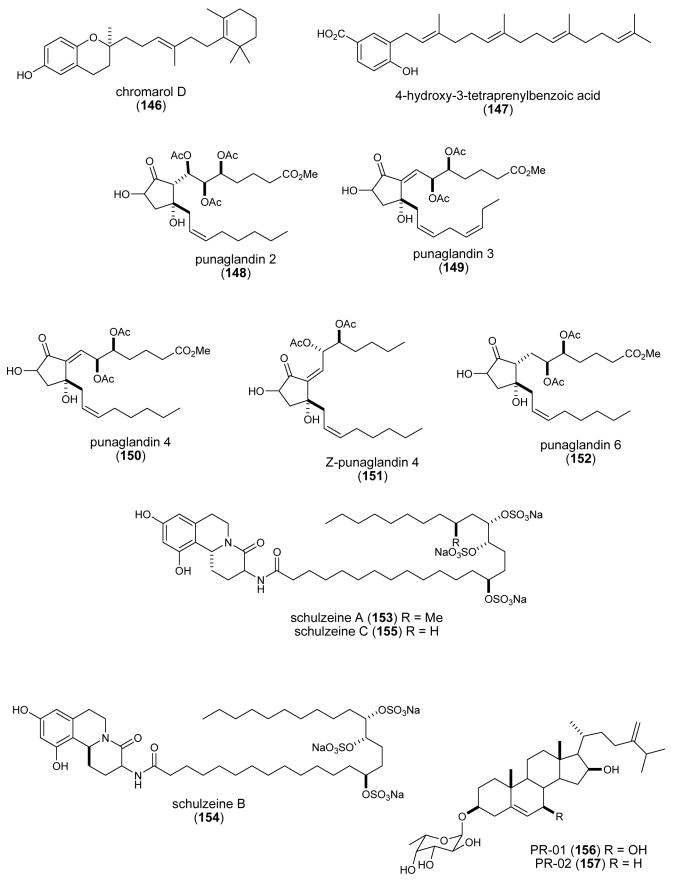
| punaglandins (148-152)/coral | Polyketidee | Cytotoxicity & apoptosis | 0.04-0.37μM | P53 accumulation & ubiquitin isopeptidase activity in vivo & in vitro | USA | (Verbitski, S. M. et al. 2004) |
| schulzeienes A-C (153-155)/ sponge | Alkaloidg/polyketide | α-glucosidase inhibition | 0.048-0.1 μM | Undetermined | JAPN, NETH | (Takada, K. et al. 2004) |
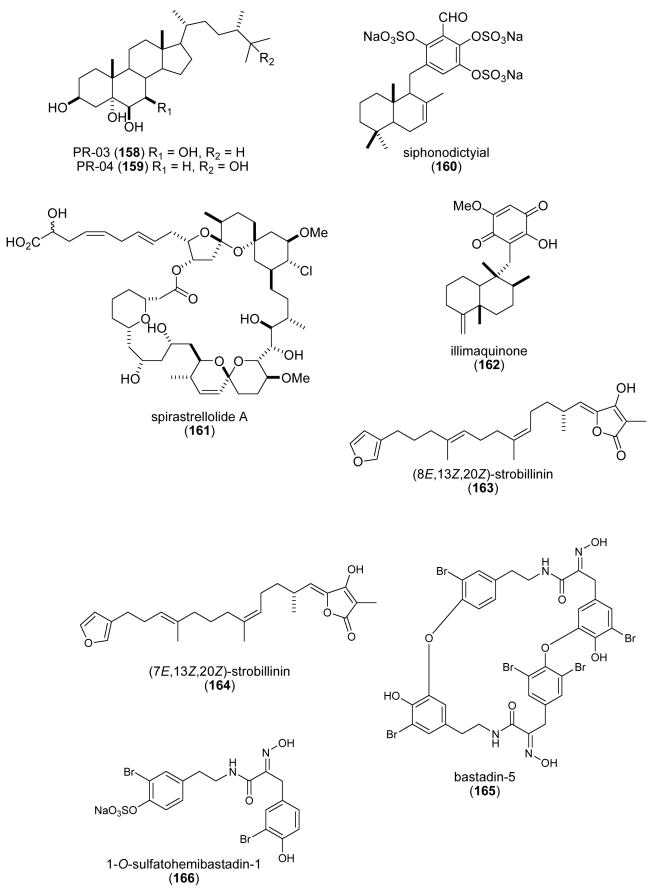
| Sinularia & Lobophytum sp. steroids (156-159)/soft coral | Glycosidic Steroidf | 5α-reductase inhibition | ND | Undetermined | IND, MEX | (Radhika, P. et al. 2004) |
| siphonodictyal C (160)/sponge | Merosesquiterpeneg | CDK4/cyclin D1 inhibition | 9 μg/mL | Undetermined | NZEL, SWI, USA | (Mukku, V. J. et al. 2003) |
| spirastrellolide A (161)/sponge | Polyketidee | Protein phosphatase 2A inhibition | 0.001 μM | Undetermined | CAN, USA | (Williams, D. E. et al. 2004a) |
| Spongia sesquiterpenoid (162)/sponge | Merosesquiterpenef | DNA polymerase B lyase activity inhibition | 16.2 μg/mL | Undetermined | USA | (Cao, S. et al. 2004) |
| strobilin-felixinin (163, 164)/ sponge | Sesterterpenef | Antioxidant and radical scavenger | ND | Superoxide scavenging inhibition & H2O2 induced DNA strand scission protection | CHI, S.KOR | (Jiang, Y. H. et al. 2004) |
| sulfatobastadins 1 & 2 (165, 166)/sponge | Bromotyrosine peptidesg | Inhibition of ryanodine binding | 13-29 μM | Undetermined | USA | (Masuno, M. N. et al. 2004) |
Organism, Kingdom Animalia: ascidians (Phylum Chordata), corals (Phylum Cnidaria), sea cucumber ( Phylum Echinodermata), sponge (Phylum Porifera); Kingdom Plantae: alga.
IC50, ND: not determined.
MMOA: molecular mechanism of action.
Country: ARG: Argentina; CAN: Canada; CHI: China; FRA: France; GER: Germany; IND: India; INDO: Indonesia; ITA: Italy; JAPN: Japan; MEX: Mexico; NETH: The Netherlands; NZEL: New Zealand; POR: Portugal; RUS: Russia; S. KOR: South Korea; SLO: Slovenia; SPA: Spain; THAI: Thailand; TUR: Turkey; UK: United Kingdom.
Polyketide.
Terpene.
Nitrogen-containing compound.
polysaccharide.
Figure 3.
2. Marine compounds with anthelmintic, antibacterial, anticoagulant, antifungal, antimalarial, antiplatelet, antiprotozoal, antituberculosis, and antiviral activities
Table 1 summarizes new pharmacological findings reported during 2003-4 on the preclinical anthelmintic, antibacterial, anticoagulant, antifungal, antimalarial, antiplatelet, antiprotozoal, antituberculosis, and antiviral pharmacology of the 67 marine natural products shown in Fig. 1.
2.1 Anthelmintic and antibacterial compounds
One study contributed to the search of novel anthelminthic marine natural products during 2003-4. The novel acyclic lipids thiocyanatins (1-4), were isolated from the Australian sponge Oceanapia sp. (Capon et al., 2004b) and were shown to be nematocidal (LD99= 3.1-8.3 μg/mL) to the commercial livestock parasite Haemonchus contortus. Although the mechanism of action of these compounds remains undetermined, the investigators noted that both the 2°-alcohol, SCN functionalities and chain length influenced the nematocidal activity.
In view of the fact that resistance to current antibiotics remains a significant challenge for pathogenic bacterial infections, during 2003-4, 19 studies contributed to the search for novel antibacterial marine natural products, an increase from 1998-2002 (Mayer and Lehmann, 2000; Mayer and Hamann 2002, 2004, 2005). Four studies reported on the mechanism of action of novel marine antibacterial agents (2-6; Fig. 1). Bugni et al., (Bugni et al., 2004) investigated a series of kalihinols, diterpenes isolated from the Philippine marine sponge Acanthella cavernosa, as potential bacterial folate biosynthesis inhibitors. The investigators reported that the pyranyl-type kalihinols Y (5) and X (6), although potent antibacterials (MIC=1.56 μg/mL), were however less selective inhibitors of bacterial folate biosynthesis than the furanyl type kalihinols, with the “C-10 position important for potency”. Isnansetyo and Kamei (Isnansetyo and Kamei, 2003) reported that a bactericidal compound named MC21-A (7), a 3,3′,5,5′-tetrabromo-2,2′-biphenyldiol, from the new marine bacterium Pseudoalteromonas phenolica sp. nov. MC21-A was bactericidal (MIC=1-2 μg/mL) against 10 clinical isolates of methicillin-resistant Staphylococcus aureus, and displayed comparable bioactivity to vancomycin (MIC=0.25-2 μg/mL). The mechanism of action of MC21-A involved permeabilizing bacterial cell membranes, and thus “might be a useful compound” because of a mode of action that differs from vancomycin. A new dimeric bromopyrrole alkaloid, nagelamide G (8) was isolated from the Okinawan marine sponge Agelas sp. (Endo et al., 2004). Nagelamide G exhibited antibacterial activity against M. luteus, B. subtilis and E. coli, but weakly inhibited protein phosphatase 2A (IC50=13 μM), thus suggesting that this enzyme may not be the main molecular target responsible for the antibacterial activity of this compound. Tincu et al. (Tincu et al., 2003) reported a new antimicrobial octapeptide plicatamide (9) from the hemocytes of the marine tunicate Styela plicata. In an extensive and detailed mechanistic study these investigators discovered that despite its small size, the octapeptide plicatamide proved to be a potent, rapidly acting and broad spectrum antimicrobial. The fact that both wild type and methicilin-resistant S. aureus responded to plicatamide with a massive and rapid potassium efflux is “consistent with an antimicrobial mechanism that targets their cell membrane”.
Although additional novel marine antibacterials were reported in 2003-4, no mechanism of action studies were reported for compounds (10-29). Nevertheless, these studies highlight the fact that novel antibiotics are present in marine bacteria, tunicates, sea hares, soft corals, algae, sponges, worms, and fish. Two papers reported on antibacterial activity in compounds isolated from marine sponges: Namikoshi et al. (Namikoshi et al., 2004) reported the isolation of several manoalide derivatives (10) from a Luffariella sp. sponge collected in Palau, which were active against S. aureus at 5-10 μg/disk. The investigators noted that the presence of an “OH group at the C-25 position (hemiacetal moiety) is important for antibacterial activity.” Wang et al. (Wang et al., 2003) reported thirteen novel tetramic acids isolated from the marine sponge Melophlus sarassinorum. Interestingly, only melophlin C (11) displayed “pronounced antibacterial activity” against B. subtilis and S. aureus. One paper reported on new antimicrobial compounds isolated from marine tunicates: Schupp et al. (Schupp et al., 2003) discovered that the β-carboline eudistomin X (12), isolated from the Micronesian ascidian Eudistoma sp. was active against B. subtilis, S. aureus and E. coli. One paper reported on a new antimicrobial peptide isolated from sea hares: Iijima et al. (Iijima et al., 2003) reported a novel 33 amino acid antimicrobial peptide dolabellanin B2 (13) from the sea hare Dolabella auricularia. One hundred percent inhibition of growth of B. subtilis, H. influenza and Vibrio vulnificus was reported with 2.5-5 μg/mL dolabellanin B2. Two papers reported on new antimicrobial peptides isolated from marine soft corals: Ata et al. (Ata et al., 2004) reported two new diterpenes, pseudopterosin X and Y (14-15) from the soft coral Pseudopterogorgia elisabethae which showed antibacterial activity against Gram-positive bacteria Streptococcus pyogenes, S. aureus, and Enterococcus faecalis, while being inactive against Gram-negative bacteria. Dmitrenok et al. (Dmitrenok et al., 2003) reported several sphingolipids and glycolipids (16-18) from soft corals of the Andaman Islands (Indian Ocean). Although the MIC were not reported, “preliminary tests for antibacterial activity of lipids” demonstrated that these compounds inhibited the growth of E. coli, P. aeruginosa, B. subtilis and B. pumilus on solid agar. One paper reported on the presence of antibacterial compounds in marine algae: Xu et al. (Xu et al., 2003) reported that among 5 bromophenols isolated from the marine red alga Rhodomela confervoides, the known compound bis(2,3-dibromo-4,5-dihydroxybenzyl) ether (19) showed antibacterial activity against S. aureus (MIC=70 μg/mL), Staphylococcus epidermidis and Pseudomonas aeruginosa (MIC=70 μg/mL). Additional antibacterial marine natural products were isolated from sponges: Pettit et al. (Pettit et al., 2004) reported the antibacterial activity of a novel nitrogen heterocyclic compound cribrostatin 6 (20) isolated from the dark-blue marine Cribochalina sp. sponge. Cribrostatin 6 showed antibacterial activity against Gram-positive bacteria, and it was most active against S. pneumoniae (MIC= 0.5 μg/mL), a leading cause of infection and mortality worldwide. Goud et al. (Goud et al., 2003b) reported a novel purpuramine L (21) from the Indian marine sponge Psammaplysilla purpurea which was active against S. aureus, B. subtilis and C. violaceum. A new nitrogenous sesquiterpene germacrane (22) was isolated from an Axinyssa n. sp. sponge that demonstrated strong antimicrobial activity against S. aureus and B. subtilis (Satitpatipan and Suwanborirux, 2004). Yang et al. (Yang et al., 2003c) isolated a new bicyciclic guanidine alkaloid (23) from the marine sponge Ptilocaulis spiculifer, contributing a new member to the crambescin A class of compounds. Interestingly, 50 μg of the guanidine alkaloid was as potent as 10 μg gentamicin. Two diterpenes membranolides C and D (24, 25) derived from an Antarctic cactus sponge, displayed “modest yet broad spectrum” Gram-negative antibiotic activity (Ankisetty, S. et al. 2004). Two novel antibacterial peptides were isolated from marine worms: Ovchinnikova et al. (Ovchinnikova et al., 2004) purified and characterized two small 21-residue peptides arenicin-1 and -2 (26-27), from the coelomocytes of the marine lugworm Arenicola marina. Both arenicins were active against Gram-positive L. monocytogenes, Gram-negative E. coli and the fungus C. albicans. Pan et al. (Pan et al., 2004) isolated and characterized a 51-amino acid highly basic and hydrophobic peptide perinerin (28), from the marine clamworm Perinereis aibuhitensis, an organism that is extensively used as bait in fisheries and aquaculture. Perinerin, a peptide that is constitutively present in the marine worm and whose sequence appears to be novel among all know antimicrobial peptides, was active against Gram-negative and Gram-positive bacteria as well as fungi. Patrzykat et al. (Patrzykat et al., 2003b) reported active novel antimicrobials peptides by screening both genomic and mRNA transcripts from a number of different species of flatfish. The most active peptide coded as NRC-13 (29) which was derived from the American plaice Hippoglossoides platessoides Frabricius, “rapidly (5 to 10 min) and efficiently (95-100%)” killed antibiotic-resistant P. aeruginosa, methicillin-resistant S. aureus and C. albicans.
2.2 Anticoagulant compounds
During 2003-4 three articles reported on the anticoagulant properties of marine natural products, an increase from our previous reviews (Mayer and Lehmann, 2000; Mayer and Hamann, 2002, 2004, 2005). Carroll et al. (Carroll et al., 2004) reported three new peptides, dysinosins B, C (30) and D, isolated from the sponge Lamellodysidea chlorea, that inhibited the blood coagulation cascade serine proteases factor VIIa and thrombin. Furthermore, the study revealed that two structural motifs of the dysinosins contributed to the binding of these compounds to factor VIIa and thrombin proteases. Zancan et al. (Zancan and Mourao, 2004) extended the antithrombotic pharmacology of fucosylated chondroitin sulfate (31), a glycosaminoglycan isolated from the Brazilian sea cucumber Ludwigothurea grisea. The researchers noted that it was possible to dissociate the anticoagulant, bleeding and antithrombotic effect of this compound, i.e. the antithrombotic effect varied depending on the in vivo experimental model being used, and that it was “apparently unrelated to its effect on platelet aggregation”. Melo et al. (Melo et al., 2004) extended the pharmacology of anticoagulant sulfated galactans (32-33) isolated from the red alga Botryocladia occidentalis and the sea urchin Echinometra lucunter. The studies demonstrated that the antithrombin-activating conformational change appeared to be of minor significance for the sulfated galactans's anticoagulant activity, and that the antithrombin-sulfated galactan complex differed from the antithrombin-heparin complex, thus leading the researchers to propose that “the paradigm of the heparin-antithrombin interaction cannot necessarily be extended to other sulfated polysaccharides”.
2.3 Antifungal compounds
Six studies during 2003-4 reported on the antifungal properties of 6 novel marine natural products isolated from marine sponges and ascidians, a slight decrease from 1998-2002 (Mayer and Lehmann, 2000; Mayer and Hamann, 2002, 2004, 2005).
Several novel marine antifungals (34-38) were isolated from marine sponges. Yang et al. (Yang et al., 2003b) reported a new sterol sulfate (34) isolated from a deep-water marine sponge of the family Astroscleridae, which exhibited antifungal activity against “supersensitive” Saccharomyces cerevisiae (MIC=15 μg/ml). As part of an ongoing project to discover potential new drugs to treat resistant opportunistic fungal infections, Jacob et al. (Jacob et al., 2003) reinvestigated the antifungal properties of a previously described sterol (35) isolated from the marine sponge Dysidea arenaria. Interestingly, they observed a reversal of fluconazole resistance from 300 to 8.5 μM when combined with 3.8 μM of the Dysidea arenaria sterol, putatively as a result of inhibition of the MDR1-type efflux pump in multidrug-resistant C. albicans. With the purpose of finding more selective antifungal agents, Nishimura et al. (Nishimura et al., 2003) focused their research efforts in identifying inhibitors of the pathogenic fungus C. albicans geranylgeranyltransferase (GGTase), an enzyme that shares only 30% amino acid sequence homology with the human GGTase. Bioassay-guided fractionation resulted in the isolation of a novel alkaloid massadine (36) from the marine sponge Stylissa aff. massa, which inhibited fungal GGTase (IC50=3.9 μM). One novel imidazole alkaloid, naamine G (37) was reported from the Indonesian marine sponge Leucetta chagosensis that exhibited strong antifungal activity against the phytopathogenic fungus Cladosporium herbarum (Hassan et al., 2004). It remains to be determined if this compound will also be effective against fungi that infect mammalian hosts. Rifai et al. (Rifai et al., 2004) reported that untenospongin B (38), isolated from the Moroccan marine sponge Hippospongia communis, was more potent than amphotericin B, a clinically used antifungal agent, against Candida tropicalis (MIC=4-8 μg/mL) and Fusarium oxysporum (MIC=2-4 μg/mL). Further studies are required to determine the toxicity of untenospongin B in vivo as well as its molecular mechanism of action.
Kossuga et al. (Kossuga et al., 2004) reported a new antifungal agent polyketide, (2S, 3R)-2-aminododecan-3-ol (39), isolated from the Brazilian ascidian Clavelina oblonga, which was very active against C. albicans (MIC=0.7± 0.05 μg/mL). Although the mechanism of action of this compound remains undetermined its bioactivity was comparable to the clinically used antifungal agents nystatin (MIC=1-4 μg/mL) and ketoconazole (MIC=1.0-4.0 μg/ml).
2.4 Antimalarial, antiprotozoal, antituberculosis and antiplatelet compounds
During 2003-4, and as shown in Table 1, 16 studies were reported in the area of antimalarial, antiplatelet, antiprotozoal and antituberculosis pharmacology of structurally characterized marine natural products. Ten compounds (40-49; Fig. 1) were shown to possess antimalarial activity. Moderate antimalarial activity (IC50=10 μg/mL) against Plasmodium falciparum was observed with bielschowskysin (40), a new and highly oxygenated hexacyclic diterpene isolated from the Caribbean gorgonian octocoral Pseudopterogorgia kallos (Marrero et al., 2004), as well as the novel diterpenes of the eunicellin class, briarellins K hydroperoxide (41), D hydroperoxide (42) and L (43), isolated from the gorgonian Briareum polyanthes (IC50=9, 9 and 8 μg/mL, respectively) (Ospina et al., 2003). Wei et al. (Wei et al., 2004) reported moderate cytotoxic activity against the Plasmodium falciparum W2 (chloroquine-resistant) strain by the novel cembradiene (44) diterpenoids isolated from the Caribbean gorgonian read octocoral Eunicea sp., (IC50= 23, 15 and 16 μg/mL, respectively). Fennell et al. (Fennell et al., 2003) reported the antimalarial activity of dolastatin 10 (45), a peptide microtubule inhibitor isolated from the sea hare Dolabella auricularia which is a potent anticancer drug. Although an extensive structure-activity relationship study was described and dolastatin 10 showed potent inhibition of P. falciparum (IC50=0.1 nM) by affecting the schizont stage of intraerythrocytic development, which has the highest concentration of tubulin, the investigators concluded that dolastatin 10 was an “unpromising basis for further antimalarial evaluation” because of the lack of marked selectivity for parasite over mammalian cells. Rao et al. (Rao et al., 2003) reported structure-activity relationship studies with the manzamine alkaloids as potential antimalarial agents. Manzamine A (46) was observed to be particularly active against P. falciparum (D6 clone, IC50= 4.5 ng/mL) and P. falciparum (chlorine-resistant W2 clone, IC50=8.0 ng/mL), which compared well with artemisinin used as a control in these studies (IC50=10 & 6.3 ng/mL, respectively). As part of an ongoing screening program for novel bioactive compounds from marine Streptomycetes, Maskey et al. (Maskey et al., 2004) reported that the trioxacarcins A and D (48,49) isolated from the marine Streptomyces sp. isolate B8652 BCC 5149 possessed “extremely high antiplasmodial activity” against the parasite Plasmodium falciparum K1 & NF54 strains (IC50=1.5-1.6 & 2.3-1.7 ng/mL, respectively) which was much higher than the clinically used compound chloroquine (IC50=70 & 3.7 ng/mL, respectively).
Three compounds were shown to possess antiprotozoal activity. Nakao et al. (Nakao et al., 2004) reported the isolation of renieramycin A (50) a new compound from the Japanese sponge Neopetrosia sp. that dose-dependently inhibited recombinant Leishmania amazonensis proliferation (IC50=0.2 μg/mL) while showing cytotoxicity at “ten times higher concentration (IC50=2.2 μg/mL)”. Savoia et al. (Savoia et al., 2004) examined the activity of the sesquiterpene euplotin C (51), isolated from the marine ciliate Euplotes crassus on pathogenic protozoa Leishmania major and Leishmania infantum. Because a significant leishmanicidal activity was noted against both Leishmania species (LD50=4.6-8.1 μg/mL), the authors proposed evaluation of this natural product as “synergistic compound(s) for current antiprotozoon chemotherapeutics”. Roch et al. (Roch et al., 2004) reported that novel non-cytotoxic variant analogues of truncated defensins isolated from the Mediterranean mussel Mytilus galloprovincialis were antiprotozoal. Two defensin fragments, designated D (Sequence CGGWKRKRC) and P (Sequence CGGYCGGWKRKRCTSYRCG), killed both the African trypanosome Trypanosoma brucei (ID50=4-12 μM), which causes sleeping sickness, and the causative agent of cutaneous leishmaniasis, namely Leishmania major (ID50=12-45 μM), in a time- and concentration-dependent manner. The mechanism may involve binding of the defensin fragments “to parasite membranes”, perhaps affecting membrane fluidity.
Seven novel compounds (47, 52-57; Fig. 1) were contributed to the search for novel antituberculosis agents. Rodriguez et al. (Rodriguez and Rodriguez, 2003) reported that a novel diterpene alkaloid homopseudopteroxazole (52), isolated from the Caribbean sea plume Pseudopterogorgia elisabethae, inhibited growth of Mycobacterium tuberculosis H37Rv (MIC=12.5 μg/mL). As part of a manzamine alkaloid structure-activity relationship study, Rao et al. (Rao et al., 2003) noted that (+)-8-hydroxymanzamine A (47) was very potent against M. tuberculosis (H37Rv, MIC=0.91 μg/mL), comparing favorably with rifampin (MIC=0.5 μg/mL). De Oliveira et al. (de Oliveira et al., 2004) reported a new alkaloid ingenamine G (53) that demonstrated activity against Mycobacterium tuberculosis H37Rv at 8μg/ml. A new scalarane-type bioactive sesterterpene, 12-deacetoxyscalarin 19-acetate (54), which was purified from the Thai sponge Brachiaster sp (Wonganuchitmeta et al., 2004), inhibited growth of a nonvirulent strain of Mycobacterium tuberculosis by 50% at MIC= 4 μM, comparing favorably with kanamycin sulfate (MIC= 3.5-8.5 μM). As a result of a research program designed to identify marine natural products that inhibit the mycothiol-S-conjugate amidase, a mycobacterial detoxification enzyme, Nicholas et al. (Nicholas et al., 2003) reported several active compounds: a mixture of 1,3 pyridinium polymers (55) isolated from the marine sponge Amphimedon sp., IC50=0.1 μM; an Oceanapiside sp.-derived bromotyrosine compound (56), IC50=3 μM; and the glycosphingolipid oceanapiside (57), IC50=10 μM. Oceanapiside, was observed to be a “simple non-competitive inhibitor” of the mycothiol-S-conjugate amidase enzyme.
Two studies contributed to antiplatelet pharmacology of marine natural products during 2003-4. Pimentel et al. (Pimentel et al., 2003) using a new microplate assay for Ca2+-induced platelet aggregation, determined that xestospongin A (58), isolated from the marine sponge Xestospongia sp., inhibited both collagen-and epinephrine-induced platelet aggregation more potently than aspirin. Villar et al. (Villar et al., 2003) evaluated the effects of several zoanthamine-type alkaloids isolated from the zoanthids Zoanthus numphaeus and Zoanthus sp. on the aggregation of human platelets: 11-hydroxyzoanthamine (59) demonstrated strong inhibition of thrombin-, collagen- and arachidonic acid-induced platelet aggregation which appeared related to the hydroxyl group at C-11; in contrast, aromatization in ring A was probably responsible for the selectivity of zoanthenol (60) towards collagen- induced aggregation.
2.5 Antiviral Compounds
As shown in Table 1 interest in the antiviral pharmacology of marine natural products remained high during 2003-4. During this two-year period 7 novel marine compounds (61-67) (Fig. 1) were reported to possess antiviral properties against the human immunodeficiency (HIV) virus by targeting a number of diverse molecular targets. As a result of an effort to identify small molecules that disrupt protein-protein interactions involved in HIV-1 cellular entry, a new polycyclic guanidine alkaloid crambescidin 826 (61) was reported from the marine sponge Monanchora sp. (Chang et al., 2003). Crambescidin 826 inhibited HIV-1 envelope-mediated fusion in vitro (IC50=1-3 μM), thus suggesting that this class of compounds might ultimately aid in “the rational design…of small molecule HIV-1 fusion inhibitors”. Chill et al. (Chill et al., 2004) isolated a new C22 furanoterpene designated dehydrofurodendin (62) from a Madagascan Lendenfeldia sponge, that was active against HIV-1 reverse transcriptase- associated RNA- and DNA-directed DNA polymerase (IC50=3.2-5.6 μM). As a result of the National Cancer Institute's HIV-inhibitory natural product lead discovery program, a new HIV-inhibitory depsiundecapeptide neamphamide A (63) was isolated from the Papua New Guinea marine sponge Neamphius huxleyi (Oku et al., 2004). Neamphamide A potently inhibited the cytopathic effect of HIV-1 infection in a cell-based in vitro assay (EC50=28 nM). Pereira et al. (Pereira et al., 2004) reported an extensive study on the mechanism of action of two diterpenes, Da-1 and AcDa-1 (64, 65), isolated from the marine alga Dictyota menstrualis, that inhibited HIV-1 virus replication in the PM-1 cell line in vitro. Although both diterpenes did not affect viral attachment nor internalization of the virus into PM-1 cells, they inhibited the RNA-dependent DNA polymerase activity of the viral reverse transcriptase enzyme (IC50=10 & 35μM, for Da-1 and AcDa-1, respectively) in a cell-free in vitro assay. These results strongly suggested that “inhibition of synthesis of the proviral DNA by the diterpenes” was the probable mechanism involved in HIV replication inhibition in PM-1 cells. Goud et al. (Goud et al., 2003a) reported inhibition of HIV by two bis-quinolizidine alkaloids petrosins (66, 67) isolated from the Indian marine sponge Petrosia similis. The extensive investigation determined that both petrosins inhibited HIV-1 replication (IC50=41.3-86.8 μM), formation of giant cells (IC50=21.2-36.1 μM) and recombinant reverse transcriptase in vitro (IC50= 10.6-14.8 μM).
3. Marine compounds with anti-inflammatory effects and affecting the cardiovascular, immune and nervous system
Table 2 summarizes the preclinical pharmacological research completed during 2003-2004 with the 45 marine chemicals shown in Fig. 2.
3.1 Anti-inflammatory compounds
The anti-inflammatory pharmacology of the marine compounds astaxanthin, bolinaquinone, cacospongionolide B, clathriol B, conicamin, cycloamphilectene 2, elisabethadione, plakohypaphorine, pourewic acid A, methylpourewate B, cadlinolide C, petrocortyne A, petrosaspongiolides M-R, pseudopterosin N, pseudopterosin R, seco-pseudopterosin E was reported during 2003-4, a large increase from our previous reports (Mayer and Lehmann, 2000; Mayer and Hamann, 2002, 2004, 2005).
Ohgami et al. (Ohgami et al., 2003) reported the effect of astaxanthin (68), a carotenoid found in crustacean cells, salmon and sea stars on lipopolysaccharide-induced uveitis in rats both in vitro and in vivo. The investigators observed that astaxanthin, at 100 mg/kg, suppressed development of uveitis and was as potent as 10 mg/kg prednisolone. The mechanism of action determined for astaxanthin probably involved inhibition of nitric oxide, prostaglandin E2 and TNF-α generation. Lucas et al. (Lucas et al., 2003b) described a detailed mechanistic study on the modulatory effect of bolinaquinone (69), a sesquiterpenoid isolated form a Dysidea sp. sponge, in several models of acute and chronic inflammation. The observation that bolinaquinone significantly inhibited cytokine, iNOS expression and eicosanoid (LTB4, PGE2) generation in vitro and in vivo in several models of inflammation through secretory PLA2 inhibition led the authors to propose that bolinaquinone is a marine compound of “potential interest in the search for new anti-inflammatory agents”. Posadas et al. (Posadas et al., 2003a) extended the cellular and molecular pharmacology of the sesterterpene cacospongionolide B (70) isolated from the Mediterranean sponge Fasciospongia cavernosa, and previously shown to be an inhibitor of secretory phospholipase A2. In mouse peritoneal macrophages in vitro, as well as in an in vivo model of inflammation, cacospongionolide B decreased nitric oxide, prostaglandin E2 and TNF-α generation as well as the corresponding gene expression. At the molecular level, cacospongionolide B inhibited nuclear factor-κ-DNA binding activity and enhanced IκB-α expression. Keyzers et al. (Keyzers et al., 2003) contributed a novel anti-inflammatory sterol, clathriol B (71) from the New Zealand sponge Clathria lissosclera. Clathriol B was shown to moderately inhibit production of superoxide anion from agonist-stimulated human peripheral blood neutrophils. A novel indole alkaloid histamine antagonist, conicamin (72), was isolated from the Mediterranean tunicate Aplidium conicum (Aiello et al., 2003). Ex vivo studies with guinea pig ileum demonstrated a concentration-dependent reduction of histamine-induced contractions, probably by a non-competitive mechanism. Lucas et al. (Lucas et al., 2003a) examined the effects of a series of 6 new cycloamphilectenes isolated from Vanuatu sponge Axinella sp. on murine macrophage and human neutrophil functions. All compounds tested reduced nitric oxide (NO) production in the submicromolar range. Interestingly, cycloamphilectene 2 (73), which reduced NO production and elastase release without affecting TNF-α release, inhibited the nuclear factor-κB pathway and also exhibited in vivo activity. One novel iododinated plakohypaphorine (74) with antihistamine activity was isolated from the Caribbean sponge Plakortis simplex (Borrelli et al., 2004). The authors noted that the antihistamine activity appeared to be “connected to the number and nature of the halogen atoms”, because replacement of the iodine atom by chlorine resulted in a loss of the antihistamine property of this compound. Three novel diterpenes, namely pourewic acid A (75), methylpourewate B (76) and cadlinolide C (77), were purified from the New Zealand sponge Chelonaplysilla violacea (Keyzers et al., 2004). The three diterpenes moderately inhibited production of the inflammatory superoxide anion from human peripheral blood neutrophils stimulated with phorbol myristate acetate or N-formyl-methionine-leucine-phenylalanine. Hong et al. (Hong et al., 2003) investigated the anti-inflammatory properties of petrocortyne A (78), a C46 polyacetylenic alcohol isolated from the marine sponge Petrosia sp.. Petrocortyne A inhibited release of both TNF-α (IC50=2.35 μM) and nitric oxide from macrophages and induced homotypic aggregation of U937 human leukemic monocytes, a process that appears to involve phosphorylation of several intracellular signaling molecules. The molecular pharmacology of petrosaspongiolide M (79) was characterized by Posadas et al. (Posadas et al., 2003b), who determined that this marine sesterterpenoid inhibited production of nitrite, prostaglandin E2 and TNF-α both in vitro and in vivo while concomitantly inhibiting NF-κB signaling. The authors' results suggested that petrosaspongiolide M had “potentially (a) wide therapeutic spectrum” in inflammatory conditions. Monti et al. (Monti et al., 2004) extended the molecular pharmacology of the marine anti-inflammatory petrosaspongiolides M-R (79-83), bioactive sesterterpenes isolated from the marine sponge Petrosaspongia nigra. The irreversible inhibition of bee-venom phospholipase A2 (PLA2) was investigated by mass spectrometry and molecular modelling approaches and demonstrated that petrosapongiolides N, O, P and R shared the same inhibition mechanism and covalent binding site already reported for petrosaspongiolide M. Ata et al. (Ata et al., 2003) reported two new diterpenes pseudopterosin N (84) and seco-pseudopterosin E (85) and the hydroxyquinone elisabethadione (86) from the marine gorgonian Pseudopterogorgia elisabethae with in vivo anti-inflammatory activity. Interestingly all three compounds inhibited edema (inflammation) in a mouse ear anti-inflammatory assay more potently than “the well characterized pseudopterosins A and E”. With the purpose of contributing to the search of novel agents to treat neuroinflammation, several novel diterpene glycoside pseudopterosins and seco-pseudopterosins from the Caribbean sea whip Pseudopterogorgia elisabethae were evaluated in an in vitro anti-neuroinflammatory assay (Rodriguez et al., 2004). Pseudopterosin R (87) was observed to be the most promising compound because it significantly inhibited the generation of thromboxane B2 (IC50=4.7 μM) from activated rat brain macrophages. Although the molecular mechanism of action of pseudopterosin R remains currently undetermined, the authors concluded that it “could become an anti-inflammatory lead compound” for anti-neuroinflammatory drug design if its inhibitory effect on thromboxane B2 release was further enhanced.
3.2 Cardiovascular compounds
Trevisi et al. (2004) reported novel studies on the mechanism of action of callipeltin A (88), a previously described cyclic depsidecapeptide isolated from the marine sponges Callipelta sp. and Latrunculia sp.. In contrast to the lack of effect on guinea-pig aortic ring contractions, callipeltin A affected resting aorta contraction in a concentration-dependent manner (IC50=0.44 μM) by a mechanism that caused an increase in sodium influx, a phenomenon the authors describe as a “Na+-ionophore action”. Thus callipeltin A's cardiovascular effects are probably caused by “its capacity of mediating Na+ transport”.
3.3 Compounds affecting the immune system
With the purpose of contributing to the discovery of small molecule agonists and antagonists of chemokine receptors, Yang et al. (Yang et al., 2003d), reported a new sesterterpene sulfircin (89) purified from the marine sponge Ircinia sp. that inhibited the CCR7 chemokine receptor, a receptor involved in the regulation of T cells and dendritic cell mobilization into lymphoid organs. Anti-adhesive mucin-type glycoproteins were characterized from the mucus secretions of starfish Marthasterias glacialis and Porania pulvillus, and the brittlestar Ophiocomina nigra (Bavington et al., 2004). The investigators observed that partially purified mucus glycoproteins from all three species were not cytotoxic and inhibited neutrophil adhesion in a dose-dependent manner, thus suggesting that by blocking adhesion of leukocytes these mucins might be of therapeutic value to treat inflammatory disorders. Yamada et al. (Yamada et al., 2004) contributed a new series of eremophilane sesquiterpenoids, peribysins A-D (90-93), isolated from a strain of Periconia byssoides, a fungus previously isolated from the sea hare Aplysia kurodai. Although the molecular mechanism of action of the four peribysins currently remains undetermined, inhibition of the adhesion of HL-60 cells to HUVEC (IC50=0.1-2.7 μM), was significantly more potent than that observed with the control agent herbimycin A (IC50=38μM). A 1,3 β glucan, named phycarine, chemically indistinguishable from the known polysaccharide laminarin, was isolated from the alga Laminaria digitata (Vetvicka and Yvin, 2004). A detailed pharmacological investigation revealed that phycarine stimulated phagocytic activity in peritoneal macrophages and potentiated the synthesis and release of interleukin-1, 6 and tumor necrosis factor α. Interestingly, phycarine increased NK cell-mediated killing of tumor cells both in vitro and in vivo by interacting with the CD11b/CD18 receptor (the complement receptor type 3 receptor). Three studies were reported on the pharmacology of a sulfated polymannuroguluronate (SPMG) (94) a polysaccharide with an average molecular weight of 8.0 kDa isolated from brown algae, which recently entered Phase II clinical trial in China as the first anti-AIDS drug candidate. While SPMG was initially reported to bind to 28 amino acids located in the HIV viral glycoprotein gp120 V3 loop (Meiyu et al., 2003), more recently, SPMG was shown by flow cytometry and fluorescent microscopy analysis to bind to lymphocyte CD4 receptors, a receptor type known to interact with the HIV virus gp120 envelope glycoprotein, a finding that might contribute to additional mechanistic explanations for the clinical efficacy of SPMG in HIV-infected patients (Miao et al., 2004). Two previous studies by the same research group noted that SPMG upregulated endothelial intercellular adhesion molecule-1 expression in human umbilical vein endothelial cells (Meiyu et al., 2003; Wang et al., 2003).
3.4 Compounds affecting the nervous system
Reports on both central and autonomic nervous system pharmacology of marine natural products during 2003-4 studies involved aplidine, aspermytin A, cribronic acid, dysibetaines, esmodil, jamaicamides, labuanine A, linckosides C-E, acidic oligosaccharide sugar chain, parguerol and isoparguerol, petrosaspongiolide M, sargaquinoic acid, SJG-2 ganglioside, δ-conotoxin, ω-conopeptide MVIIA and χ-conopeptide MrIA.
Perez et al. (Perez et al., 2003) investigated the inhibitory effect of the proline-containing cyclic peptide aplidine (95), isolated from the tunicate Aplidium albican, on the in vitro aggregation of peptide PrP 106-126, a fraction of the prion protein which has been hypothesized to be involved in fatal neurodegenerative diseases and which can kill neuronal cells in culture. The fact that aplidine was observed to be a strong inhibitor of the aggregation of PrP 106-126 into β-sheet fibrils at a 1:1 molar ratio prompted these investigators to propose that it may be “a leading compound for drug development efforts”.
Induction of neurite outgrowth in vitro was reported for the marine natural compounds aspermytin A, labuanine A, linckosides C-E, parguerol and isoparguerol, ganglioside species SJG-2 and sargaquinoic acid. Tsukamoto et al., (Tsukamoto et al., 2004a) isolated a new polyketide, aspermytin A (96) from a cultured marine fungus, Aspergillus sp. that induced neurite outgrowth at 50 μM in more than 50% of rat pheochromocytoma (PC-12) cells after two days of in vitro treatment, an effect comparable to that of 50 ng/mL of nerve growth factor. Aoki et al. (Aoki et al., 2003) purified a novel pyridoacridine alkaloid labuanine A (97) from the Indonesian marine sponge Biemna fortis that induced multipolar type neurite outgrowth in Neuro 2A neuroblastoma cells (1-3 μM) by a putative mechanism that “may relate with inhibition of topoisomerase II”, a hypothesis currently under investigation. Qi et al. (Qi et al., 2004) contributed three new bioactive steroid glycosides linckosides C-E (98-100) from the Okinawan marine sea star Linckia laevigata, which potently induced neurite outgrowth in rat PC12 cells, as well as synergistically enhanced the neuritogenic activity of nerve growth factor, a chemical that has been shown to be essential for neuronal outgrowth, survival, function maintenance and prevention of aging in the central and peripheral nervous system. Bioassay-guided fractionation lead to the isolation of parguerol (101) and isoparguerol (102) from the sea hare Aplysia kurodai (Tsukamoto et al., 2004b). Both compounds, the first neurotrophic compounds reported from sea hare metabolites, stimulated neurite outgrowth in PC-12 cells treated with either 25 or 50 μg/mL of the compounds for two days. Further pharmacological research will be required to determine the molecular mechanism leading to morphological changes in PC-12 cells. Two studies were reported on the pharmacology of the low molecular weight quinonic compound sargaquinoic acid (103) isolated from the marine brown alga Sargassum macrocarpum, and previously noted to possess a novel nerve growth factor-dependent neurite outgrowth promoting activity at the nanogram range. Kamei and Tsang (Kamei and Tsang, 2003) investigated the signaling pathways involved using a pharmacological approach and concluded that sargaquinoic acid enhanced neurite outgrowth in PC12 neuronal cells by involving both TrkA-mitogen-activated protein kinase and adenylate cyclase-protein kinase A signal transduction pathways. In a subsequent study, the neuroprotective effect of sargaquinoic acid was shown to be independent of nerve growth factor and phosphatidylinositol 3-kinase, a key signaling molecule (Tsang and Kamei, 2004). Kaneko et al. (Kaneko et al., 2003) reported the structure of a novel ganglioside SJG-2 (104) isolated from the sea cucumber Stichopus japonicus which appears to be the “first ganglioside containing either the branched sugar chain moiety or the N-acetylgalactosamine residue”. While SJG-2 only displayed neuritogenic activity in the presence of nerve growth factor, it was more potent than the GM1 mammalian ganglioside.
Four marine compounds (105-108; Fig. 2) were shown to target receptors present in the nervous system. Sakai et al., (Sakai et al., 2003) contributed to the search for novel ionotropic glutamate receptor ligands by reporting the isolation of the novel amino acid cribronic acid (105) from the marine sponge Cribrochalina olemda. Cribronic acid induced potent convulsive behavior in mice upon intracerebroventricular injection (ED50= 29±3.0 pmol/mouse), as well as inhibited binding of an N-methyl-d-aspartic acid (NMDA) receptor ligand (IC50=83±15 nM). In 2004, Sakai et al. (Sakai et al., 2004) isolated two novel cyclopropane amino acids dysibetaine CPa (106) and dysibetaine CPb (107) from the marine sponge Dysidea herbacea collected in Micronesia, that showed weak affinity toward the NMDA-type and the kainic acid-type ionotropic glutamate receptors in a radioligand binding assay. The investigators concluded that the binding of these compounds to the glutamate receptor was of interest because they both lacked “a glutamate equivalent structure in the molecules”. A screening program for bioactive compounds from marine cyanobacteria led to the isolation of the novel lipopeptides jamaicamide A (108), B and C (Edwards et al., 2004), compounds that exhibited sodium channel blocking activity at 5 μM, producing about half the response of saxitoxin applied at 0.15 μM.
During 2003-4, additional marine compounds (109-112; Fig. 2) were reported to exhibit pharmacological effects on the nervous system. Bioassay-guided fractionation of the marine sponge Raspailia sp. yielded the known synthetic compound esmodil (109), reported in the patent literature in 1935 (Capon et al., 2004a). More than six decades ago, esmodil, an acetylcholine mimic, was shown to be potentially “useful in treating retention of urine in humans and as influencing the parasympathetic nervous system in cats, rabbits and the Indian buffalo”. Hu et al. (Hu et al., 2004) reported interactions of an acidic oligosaccharide sugar chain (AOSC) from the brown alga Echlonia kurome with the amyloid beta protein. AOSC was observed to inhibit the toxicity induced by amyloid beta protein in both rat cortical cells as well as human neuroblastoma cells by a mechanism that involved inhibition of apoptosis, reduction of intracellular Ca2+ and the generation of reactive oxygen species (ROS). Thus the authors concluded that AOSC “might be a potentially therapeutic compound for Alzheimer's disease”. Capasso et al. (Capasso et al., 2003) reported that petrosaspongiolide M (79) reduced morphine withdrawal in an in vitro model. Although the mechanism underlying petrosaspongiolide M-induced inhibition of morphine withdrawal remains undetermined, one possibility raised by the investigators was the putative inhibition of extracellular type II phospholipase A2 by this potent anti-inflammatory marine sesterterpene.
Extensive research on the preclinical and clinical pharmacology of conotoxin molecules resulted in the synthetic equivalent of ω-conopeptide MVIIA (110), a 25-amino-acid polybasic peptide derived from the marine snail Conus magus, receiving approval from the Food and Drug Administration on December 23, 2004. Currently, Ziconotide (Prialt®), which is marketed by Elan Biopharmaceuticals, Inc., constitutes the third drug in the U.S. Pharmacopeia which has been derived from marine chemicals. Staats et al. (Staats et al., 2004) reported a double-blind, placebo-controlled, randomized trial conducted from 1996-1998 in 32 study centers in the United States, Australia and the Netherlands which assessed the safety and efficacy of intrathecal ziconotide. Ziconotide selectively binds to the N-type voltage-sensitive calcium channels located in neurons thereby blocking neurotransmission and resulting in significant analgesia. In this clinical study, intrathecal ziconotide provided clinically and statistically significant analgesia in 111 patients with pain from cancer or AIDS. As part of a program to explore the diversity of conotoxins produced by Conus marine snails found off the Indian coast, Sudarslal et al. (Sudarslal et al., 2003) reported the isolation and characterization of a novel 26 peptide δ-conotoxin (111) isolated and purified from the venom of the marine snail Conus amadis, collected in the Bay of Bengal. The observation that this novel δ-conotoxin inhibited the inactivation of sodium current in cloned rat brain IIA α-subunit channels led the investigators to conclude that “conotoxins from some molluscivorous snails may also be active on mammalian Na+ channels”. Sharpe et al. (Sharpe et al., 2003) extended the molecular pharmacology of χ-conopeptide MrIA (112), a 13-residue peptide isolated form the venom of the marine snail Conus marmoreus. The investigators reported that the χ-conopeptide MrIA inhibited the norepinephrine transporter by a non-competitive mechanism that possibly involve a binding site “predicted to be distinct from the substrate binding site but to share some commonality with the antidepressant binding site”.
4. Marine Compounds with Miscellaneous Mechanisms of Action
Table 3 lists marine compounds with miscellaneous pharmacological mechanisms of action, with their respective structures presented in Fig. 3. Interestingly, and in contrast with the 109 chemicals included in Tables 1 and 2, this third group of 54 marine compounds which was isolated from a variety of marine organisms, includes not only nitrogen-containing compounds (i.e. proteins, peptides), but also terpenes and polyketides.
As shown in Table 3, for a limited number of these marine natural products, namely acylspermidine D & E (113,114), ageladine A (115), alkylpyridinium (117,118), Atriolum robustum nucleoside (121), calyculin A (123), Cell-III, meridianin E (139), Psammocinia spp. diterpenes (143-147), punaglandins (148-152) and strobilin-felixinin (163,164), both the pharmacological activity and a molecular mechanism of action have been investigated and reported. In contrast, for all the other compounds shown in Table 3, although a pharmacological activity was reported, no additional information was available on the molecular mechanism of action of these chemicals during 2003-2004.
5. Reviews on marine pharmacology
Specific areas of marine pharmacology research benefited from a number of excellent reviews published during 2003-4: (a) general marine pharmacology: natural products as sources of new drugs over the period 1981-2002 (Newman et al., 2003); marine natural products from marine invertebrates and sponge-associated fungi (Proksch et al. 2003b; Proksch et al., 2003a); the biopotential of marine sponges from China oceans (Zhang et al., 2003); marine natural products as therapeutic agents (patents): part 2 (Frenz et al., 2004); natural-product diversity of New Caledonia: a pharmacologically oriented view (Laurent and Pietra, 2004); (b) antimicrobial marine pharmacology: genomic screening to identify novel marine antimicrobial peptides (Patrzykat and Douglas, 2003a); marine natural products as anti-infective agents (Donia and Hamann, 2003); mining marine microorganisms as a source of new antimicrobials and antifungals (Bernan et al., 2004); antimicrobial peptides from marine invertebrates (Tincu and Taylor, 2004); bioactive peptides from marine sources: pharmacological properties and isolation procedures (Aneiros and Garateix, 2004); (c) anticoagulant marine pharmacology: recent advances in marine algal anticoagulants (Matsubara, 2004); the use of algae and invertebrate sulfated fucans as anticoagulant and antithrombotic agents (Mourao, 2004); (d) antituberculosis, antimalarial and antifungal marine pharmacology: antimycobacterial natural products (Copp, 2003); naturally occurring peroxides from marine sponges with antimalarial and antifungal activities (Jung al., 2003); antifungal compounds from marine organisms (Molinski, 2004); (e) antiviral marine pharmacology: algae as a potential source of antiviral agents (Luescher-Mattli, 2003); marine natural products as lead anti-HIV agents (Gochfeld et al., 2003); anti-HIV activity from marine organisms (Tziveleka et al., 2003); proteoglycans from sponges as tools to develop new agents for AIDS and Alzheimer's disease (Fernandez-Busquets and Burger, 2003); antiviral marine natural products (Gustafson et al., 2004); (f) anti-inflammatory marine pharmacology: anti-inflammatory metabolites from marine sponges (Keyzers and Davies-Coleman, 2005); (g) nervous system marine pharmacology: conotoxins as drug leads for neuropathic pain and other neurological conditions (Alonso et al., 2003); conotoxins and structural biology: a prospective paradigm for drug discovery (Grant et al., 2004); drugs from the sea: conopeptides as potential therapeutics (Livett et al., 2004); ziconotide: neuronal calcium channel blocker for treating severe chronic pain (Miljanich, 2004).
6. Conclusion
During 2003-4, and for the first time in many decades, a marine natural product, namely ziconotide (Prialt®) (110) was approved for patient care by the U.S. Food and Drug Administration for the management of severe chronic pain in patients for whom intrathecal therapy is necessary, and who appear to be intolerant of or refractory to other treatments, such as systemic analgesics, adjunctive therapies or intrathecal morphine. Although research into the non-antitumor and cytotoxic pharmacology of marine natural products remained concentrated in the specific areas we have highlighted in our present review, this contribution together with our previous ones (Mayer and Lehmann, 2000; Mayer and Hamann, 2002, 2004, 2005), demonstrates that marine pharmacology research continued to proceed at a very active pace in 2003-4, involving natural product chemists and pharmacologists from 28 foreign countries and the United States. Thus, if the rate of preclinical and clinical pharmacological research continues to be sustained over time, additional marine natural products will probably become available as novel therapeutic agents to treat multiple disease categories.
Acknowledgments
This publication was made possible by grant number 1R15 ES012654, from the National Institute of Environmental Health Sciences, NIH, to AMSM, and 1R01A136596, from the National Institute of Allergy and Infectious Diseases, NIH, and the Medicines for Malaria Venture (MMV), to MTH. Its content is solely the responsibility of the authors and does not necessarily represent the official views of the NIEHS, NIH. Additional financial support by Midwestern University is gratefully acknowledged. Jennifer Allman is acknowledged for her assistance with the preparation of Fig. 1 (MTH). The excellent support for literature searches in PubMed, Marinlit, Current Contents® and Chemical Abstracts®, as well as article retrieval by library staff members as well as medical and pharmacy students of Midwestern University is most gratefully acknowledged. The authors specially thank Ms. Mary Hall for carefully reviewing this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agafonova IG, Aminin DL, Avilov SA, Stonik VA. Influence of cucumariosides upon intracellular [Ca2+]i and lysosomal activity of macrophages. J Agric Food Chem. 2003;51:6982–6986. doi: 10.1021/jf034439x. [DOI] [PubMed] [Google Scholar]
- Aiello A, Borrelli F, Capasso R, Fattorusso E, Luciano P, Menna M. Conicamin, a novel histamine antagonist from the mediterranean tunicate Aplidium conicum. Bioorg Med Chem Lett. 2003;13:4481–4483. doi: 10.1016/j.bmcl.2003.08.081. [DOI] [PubMed] [Google Scholar]
- Alonso D, Khalil Z, Satkunanthan N, Livett BG. Drugs from the sea: conotoxins as drug leads for neuropathic pain and other neurological conditions. Mini Rev Med Chem. 2003;3:785–787. doi: 10.2174/1389557033487746. [DOI] [PubMed] [Google Scholar]
- Aneiros A, Garateix A. Bioactive peptides from marine sources: pharmacological properties and isolation procedures. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;803:41–53. doi: 10.1016/j.jchromb.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Ankisetty S, Amsler CD, McClintock JB, Baker BJ. Further membranolide diterpenes from the antarctic sponge Dendrilla membranosa. J Nat Prod. 2004;67:1172–1174. doi: 10.1021/np0340551. [DOI] [PubMed] [Google Scholar]
- Aoki S, Wei H, Matsui K, Rachmat R, Kobayashi M. Pyridoacridine alkaloids inducing neuronal differentiation in a neuroblastoma cell line, from marine sponge Biemna fortis. Bioorg Med Chem. 2003;11:1969–1973. doi: 10.1016/s0968-0896(03)00086-5. [DOI] [PubMed] [Google Scholar]
- Ata A, Kerr RG, Moya CE, Jacobs RS. Identification of anti-inflammatory diterpenes from the marine gorgonian Pseudopterogorgia elisabethae. Tetrahedron. 2003;59:4215–4222. [Google Scholar]
- Ata A, Win HY, Holt D, Holloway P, Segstro EP, Jayatilake GS. New antibacterial diterpenes from Pseudopterogorgia elisabethae. Helvetica Chimica Acta. 2004;87:1090–1098. [Google Scholar]
- Bavington CD, Lever R, Mulloy B, Grundy MM, Page CP, Richardson NV, McKenzie JD. Anti-adhesive glycoproteins in echinoderm mucus secretions. Comp Biochem Physiol B, Biochem & Molec Biol. 2004;139:607–617. doi: 10.1016/j.cbpc.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Bernan VS, Greenstein M, Carter GT. Mining marine microorganisms as a source of new antimicrobials and antifungals. Curr Med Chem -Anti-infective Agents. 2004;3:181–195. [Google Scholar]
- Borrelli F, Campagnuolo C, Capasso R, Fattorusso E, Taglialatela-Scafati O. Iodinated indole alkaloids from Plakortis simplex - New plakohypaphorines and an evaluation of their antihistamine activity. Eur J Org Chem. 2004;1994;(15):3227–3232. [Google Scholar]
- Bugni TS, Singh MP, Chen L, Arias DA, Harper MK, Greenstein M, Maiese WM, Concepcion GP, Mangalindan GC, Ireland CM. Kalihinols from two Acanthella cavernosa sponges: inhibitors of bacterial folate biosynthesis. Tetrahedron. 2004;60:6981–6988. [Google Scholar]
- Cao S, Gao Z, Thomas SJ, Hecht SM, Lazo JS, Kingston DG. Marine sesquiterpenoids that inhibit the lyase activity of DNA polymerase beta. J Nat Prod. 2004;67:1716–1718. doi: 10.1021/np049849+. [DOI] [PubMed] [Google Scholar]
- Capasso A, Casapullo A, Randazzo A, Gomez-Paloma L. Petrosaspongiolide M reduces morphine withdrawal in vitro. Life Sci. 2003;73:611–616. doi: 10.1016/s0024-3205(03)00319-9. [DOI] [PubMed] [Google Scholar]
- Capon RJ, Skene C, Liu EH, Lacey E, Gill JH, Heiland K, Friedel T. Esmodil: an acetylcholine mimetic resurfaces in a Southern Australian marine sponge Raspailia (Raspailia) sp. Nat Prod Res. 2004a;18:305–309. doi: 10.1080/14786410310001620619. [DOI] [PubMed] [Google Scholar]
- Capon RJ, Skene C, Liu EH, Lacey E, Gill JH, Heiland K, Friedel T. Nematocidal thiocyanatins from a southern Australian marine sponge Oceanapia sp. J Nat Prod. 2004b;67:1277–1282. doi: 10.1021/np049977y. [DOI] [PubMed] [Google Scholar]
- Carroll AR, Buchanan MS, Edser A, Hyde E, Simpson M, Quinn RJ. Dysinosins B-D, inhibitors of factor VIIa and thrombin from the Australian sponge Lamellodysidea chlorea. J Nat Prod. 2004;67:1291–1294. doi: 10.1021/np049968p. [DOI] [PubMed] [Google Scholar]
- Cerqueira F, Watanadilok R, Sonchaeng P, Kijjoa A, Pinto M, Quarles vU, Kroes B, Beukelman C, Nascimento MS. Clionasterol: a potent inhibitor of complement component C1. Planta Med. 2003;69:174–176. doi: 10.1055/s-2003-37719. [DOI] [PubMed] [Google Scholar]
- Chang L, Whittaker NF, Bewley CA. Crambescidin 826 and dehydrocrambine A: new polycyclic guanidine alkaloids from the marine sponge Monanchora sp. that inhibit HIV-1 fusion. J Nat Prod. 2003;66:1490–1494. doi: 10.1021/np030256t. [DOI] [PubMed] [Google Scholar]
- Chaturvedula VSP, Gao ZJ, Thomas SH, Hecht SA, Kingston DGI. New norditerpenoids and a diterpenoid from a sponge that inhibit the lyase activity of DNA polymerase beta. Tetrahedron. 2004;1994;60(44):9991–9995. [Google Scholar]
- Chill L, Rudi A, Aknin M, Loya S, Hizi A, Kashman Y. New sesterterpenes from Madagascan Lendenfeldia sponges. Tetrahedron. 2004;60(47):10619–10626. [Google Scholar]
- Ciavatta ML, Gresa MPL, Manzo E, Gavagnin M, Wahidulla S, Cimino G. New C-21 Delta(20) pregnanes, inhibitors of mitochondrial respiratory chain, from Indopacific octocoral Carijoa sp. Tetrahedron Lett. 2004;45:7745–7748. [Google Scholar]
- Cichewicz RH, Kenyon VA, Whitman S, Morales NM, Arguello JF, Holman TR, Crews P. Redox inactivation of human 15-lipoxygenase by marine-derived meroditerpenes and synthetic chromanes: Archetypes for a unique class of selective and recyclable inhibitors. J Amer Chem Soc. 2004;1994;126(45):14910–14920. doi: 10.1021/ja046082z. [DOI] [PubMed] [Google Scholar]
- Copp BR. Antimycobacterial natural products. Nat Prod Rep. 2003;20:535–557. doi: 10.1039/b212154a. [DOI] [PubMed] [Google Scholar]
- De Oliveira JH, Grube A, Kock M, Berlinck RG, Macedo ML, Ferreira AG, Hajdu E. Ingenamine G and cyclostellettamines G-I, K, and L from the new Brazilian species of marine sponge Pachychalina sp. J Nat Prod. 2004;67:1685–1689. doi: 10.1021/np0498713. [DOI] [PubMed] [Google Scholar]
- Dmitrenok AS, Radhika P, Anjaneyulu V, Subrahmanyam S, Rao PVS, Dmitrenok PS, Boguslavsky VM. New lipids from the soft corals of the Andaman Islands. Russ Chem Bull. 2003;52:1868–1872. [Google Scholar]
- Donia M, Hamann MT. Marine natural products and their potential applications as anti-infective agents. Lancet Infect Dis. 2003;3:338–348. doi: 10.1016/S1473-3099(03)00655-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards DJ, Marquez BL, Nogle LM, McPhail K, Goeger DE, Roberts MA, Gerwick WH. Structure and biosynthesis of the jamaicamides, new mixed polyketide-peptide neurotoxins from the marine cyanobacterium Lyngbya majuscula. Chem Biol. 2004;11:817–833. doi: 10.1016/j.chembiol.2004.03.030. [DOI] [PubMed] [Google Scholar]
- Endo T, Tsuda M, Okada T, Mitsuhashi S, Shima H, Kikuchi K, Mikami Y, Fromont J, Kobayashi J. Nagelamides A-H, new dimeric bromopyrrole alkaloids from marine sponge Agelas species. J Nat Prod. 2004;67:1262–1267. doi: 10.1021/np034077n. [DOI] [PubMed] [Google Scholar]
- Erdogan-Orhan I, Sener B, De Rosa S, Perez-Baz J, Lozach O, Leost M, Rakhilin S, Meijer L. Polyprenyl-hydroquinones and -furans from three marine sponges inhibit the cell cycle regulating phosphatase CDC25A. Nat Prod Res. 2004;18:1–9. doi: 10.1080/1478641031000111534. [DOI] [PubMed] [Google Scholar]
- Fennell BJ, Carolan S, Pettit GR, Bell A. Effects of the antimitotic natural product dolastatin 10, and related peptides, on the human malarial parasite Plasmodium falciparum. J Antimicrob Chemother. 2003;51:833–841. doi: 10.1093/jac/dkg151. [DOI] [PubMed] [Google Scholar]
- Fernandez-Busquets X, Burger MM. Circular proteoglycans from sponges: first members of the spongican family. Cell Mol Life Sci. 2003;60:88–112. doi: 10.1007/s000180300006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenz JL, Kohl AC, Kerr RG. Marine natural products as therapeutic agents: Part 2. Exp Opin Therap Patents. 2004;14:17–33. Review. [Google Scholar]
- Fujita M, Nakao Y, Matsunaga S, Seiki M, Itoh Y, Yamashita J, van Soest RW, Fusetani N. Ageladine A: an antiangiogenic matrixmetalloproteinase inhibitor from the marine sponge Agelas nakamurai. J Am Chem Soc. 2003a;125:15700–15701. doi: 10.1021/ja038025w. [DOI] [PubMed] [Google Scholar]
- Fujita M, Nakao Y, Matsunaga S, van Soest RW, Itoh Y, Seiki M, Fusetani N. Callysponginol sulfate A, an MT1-MMP inhibitor isolated from the marine sponge Callyspongia truncata. J Nat Prod. 2003b;66:569–571. doi: 10.1021/np020572s. [DOI] [PubMed] [Google Scholar]
- Gochfeld DJ, El Sayed KA, Yousaf M, Hu JF, Bartyzel P, Dunbar DC, Wilkins SP, Zjawiony JK, Schinazi RF, Schlueter WS, Tharnish PM, Hamann MT. Marine natural products as lead anti-HIV agents. Mini Rev Med Chem. 2003;3:401–424. doi: 10.2174/1389557033487962. [DOI] [PubMed] [Google Scholar]
- Gompel M, Leost M, Kier Joffe EB, Puricelli L, Franco LH, Palermo J, Meijer L. Meridianins, a new family of protein kinase inhibitors isolated from the ascidian Aplidium meridianum. Bioorg Med Chem Lett. 2004;14:1703–1707. doi: 10.1016/j.bmcl.2004.01.050. [DOI] [PubMed] [Google Scholar]
- Goud TV, Reddy NS, Swamy NR, Ram TS, Venkateswarlu Y. Anti-HIV active petrosins from the marine sponge Petrosia similis. Biol Pharm Bull. 2003a;26:1498–1501. doi: 10.1248/bpb.26.1498. [DOI] [PubMed] [Google Scholar]
- Goud TV, Srinivasulu M, Reddy VL, Reddy AV, Rao TP, Kumar DS, Murty US, Venkateswarlu Y. Two new bromotyrosine-derived metabolites from the sponge Psammaplysilla purpurea. Chem Pharm Bull (Tokyo) 2003b;51:990–993. doi: 10.1248/cpb.51.990. [DOI] [PubMed] [Google Scholar]
- Grant MA, Morelli XJ, Rigby AC. Conotoxins and structural biology: a prospective paradigm for drug discovery. Curr Protein Pept Sci. 2004;5:235–248. doi: 10.2174/1389203043379710. [DOI] [PubMed] [Google Scholar]
- Gustafson KR, Oku N, Milanowski DJ. Antiviral Marine Natural Products. Curr Med Chem. 2004;3:233–249. [Google Scholar]
- Hassan W, Edrada R, Ebel R, Wray V, Berg A, Van Soest R, Wiryowidagdo S, Proksch P. New imidazole alkaloids from the Indonesian sponge Leucetta chagosensis. J Nat Prod. 2004;67:817–822. doi: 10.1021/np0305223. [DOI] [PubMed] [Google Scholar]
- Hirono M, Ojika M, Mimura H, Nakanishi Y, Maeshima M. Acylspermidine derivatives isolated from a soft coral, Sinularia sp, inhibit plant vacuolar H(+)-pyrophosphatase. J Biochem (Tokyo) 2003;133:811–816. doi: 10.1093/jb/mvg103. [DOI] [PubMed] [Google Scholar]
- Hong S, Kim SH, Rhee MH, Kim AR, Jung JH, Chun T, Yoo ES, Cho JY. In vitro anti-inflammatory and pro-aggregative effects of a lipid compound, petrocortyne A, from marine sponges. Naunyn-Schmiedeberg's Arch. 2003;368:448–456. doi: 10.1007/s00210-003-0848-7. [DOI] [PubMed] [Google Scholar]
- Hu J, Geng M, Li J, Xin X, Wang J, Tang M, Zhang J, Zhang X, Ding J. Acidic oligosaccharide sugar chain, a marine-derived acidic oligosaccharide, inhibits the cytotoxicity and aggregation of amyloid beta protein. J Pharmacol Sci. 2004;95:248–255. doi: 10.1254/jphs.fpj04004x. [DOI] [PubMed] [Google Scholar]
- Iijima R, Kisugi J, Yamazaki M. A novel antimicrobial peptide from the sea hare Dolabella auricularia. Dev Comp Immunol. 2003;27:305–311. doi: 10.1016/s0145-305x(02)00105-2. [DOI] [PubMed] [Google Scholar]
- Isnansetyo A, Kamei Y. MC21-A, a bactericidal antibiotic produced by a new marine bacterium, Pseudoalteromonas phenolica sp. nov. O-BC30(T), against methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2003;47:480–488. doi: 10.1128/AAC.47.2.480-488.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob MR, Hossain CF, Mohammed KA, Smillie TJ, Clark AM, Walker LA, Nagle DG. Reversal of fluconazole resistance in multidrug efflux-resistant fungi by the Dysidea arenaria sponge sterol 9alpha,11alpha-epoxycholest-7-ene-3beta,5alpha,6alpha,19-tetrol 6-acetate. J Nat Prod. 2003;66:1618–1622. doi: 10.1021/np030317n. [DOI] [PubMed] [Google Scholar]
- Jiang YH, Ryu SH, Ahn EY, You S, Lee BJ, Jung JH, Kim DK. Antioxidant activity of (8E,13Z, 20Z)-strobilinin/(7E,13Z, 20Z)-felixinin from a marine sponge Psammocinia sp. Nat Prod Sci. 2004;10:272–276. [Google Scholar]
- Jung M, Kim H, Lee K, Park M. Naturally occurring peroxides with biological activities. Mini Rev Med Chem. 2003;3:159–165. doi: 10.2174/1389557033405313. [DOI] [PubMed] [Google Scholar]
- Kamei Y, Tsang CK. Sargaquinoic acid promotes neurite outgrowth via protein kinase A and MAP kinases-mediated signaling pathways in PC12D cells. Int J Dev Neurosci. 2003;21:255–262. doi: 10.1016/s0736-5748(03)00068-6. [DOI] [PubMed] [Google Scholar]
- Kaneko M, Kisa F, Yamada K, Miyamoto T, Higuchi R. Structure of a new neuritogenic-active ganglioside from the sea cucumber Stichopus japonicus. Eur J Org Chem. 2003;(6):1004–1008. [Google Scholar]
- Kehraus S, Gorzalka S, Hallmen C, Iqbal J, Muller CE, Wright AD, Wiese M, Konig GM. Novel amino acid derived natural products from the ascidian Atriolum robustum: identification and pharmacological characterization of a unique adenosine derivative. J Med Chem. 2004;47:2243–2255. doi: 10.1021/jm031092g. [DOI] [PubMed] [Google Scholar]
- Keyzers RA, Davies-Coleman MT. Anti-inflammatory metabolites from marine sponges. Chem Soc Rev. 2005;34:355–365. doi: 10.1039/b408600g. [DOI] [PubMed] [Google Scholar]
- Keyzers RA, Northcote PT, Berridge MV. Clathriol B, a new 14 beta marine sterol from the New Zealand sponge Clathria lissosclera. Australian Journal of Chemistry. 2003;56:279–282. [Google Scholar]
- Keyzers RA, Northcote PT, Zubkov OA. Novel anti-inflammatory spongian diterpenes from the New Zealand marine sponge Chelonaplysilla violacea. Eur J Org Chem. 20041994:419–425. [Google Scholar]
- Kossuga MH, MacMillan JB, Rogers EW, Molinski TF, Nascimento GG, Rocha RM, Berlinck RG. (2S,3R)-2-aminododecan-3-ol, a new antifungal agent from the ascidian Clavelina oblonga. J Nat Prod. 2004;67:1879–1881. doi: 10.1021/np049782q. [DOI] [PubMed] [Google Scholar]
- Krishnaiah P, Reddy VL, Venkataramana G, Ravinder K, Srinivasulu M, Raju TV, Ravikumar K, Chandrasekar D, Ramakrishna S, Venkateswarlu Y. New lamellarin alkaloids from the Indian ascidian Didemnum obscurum and their antioxidant properties. J Nat Prod. 2004;67:1168–1171. doi: 10.1021/np030503t. [DOI] [PubMed] [Google Scholar]
- Lakshmi V, Kumar R, Gupta P, Varshney V, Srivastava MN, Dikshit M, Murthy PK, Misra-Bhattacharya S. The antifilarial activity of a marine red alga, Botryocladia leptopoda, against experimental infections with animal and human filariae. Parasitol Res. 2004;93:468–474. doi: 10.1007/s00436-004-1159-8. [DOI] [PubMed] [Google Scholar]
- Laurent D, Pietra F. Natural-product diversity of the New Caledonian marine ecosystem compared to other ecosystems: A pharmacologically oriented view. Chem Biodiv. 2004;1:539–594. doi: 10.1002/cbdv.200490048. Review. [DOI] [PubMed] [Google Scholar]
- Lee EH, Yun MR, Wang WH, Jung JH, Im DS. Structure-activity relationship of lysophosphatidylcholines in HL-60 human leukemia cells. Acta Pharmacol Sin. 2004;25:1521–1524. [PubMed] [Google Scholar]
- Lippert H, Brinkmeyer R, Mulhaupt T, Iken K. Antimicrobial activity in sub-Arctic marine invertebrates. Polar Biol. 2003;26:591–600. [Google Scholar]
- Livett BG, Gayler KR, Khalil Z. Drugs from the sea: conopeptides as potential therapeutics. Curr Med Chem. 2004;11:1715–1723. doi: 10.2174/0929867043364928. [DOI] [PubMed] [Google Scholar]
- Lucas R, Casapullo A, Ciasullo L, Gomez-Paloma L, Paya M. Cycloamphilectenes, a new type of potent marine diterpenes: inhibition of nitric oxide production in murine macrophages. Life Sci. 2003a;72:2543–2552. doi: 10.1016/s0024-3205(03)00167-x. [DOI] [PubMed] [Google Scholar]
- Lucas R, Giannini C, D'Auria MV, Paya M. Modulatory effect of bolinaquinone, a marine sesquiterpenoid, on acute and chronic inflammatory processes. J Pharmacol Exp Ther. 2003b;304:1172–1180. doi: 10.1124/jpet.102.045278. [DOI] [PubMed] [Google Scholar]
- Luescher-Mattli M. Algae, a possible source for new drugs in the treatment of HIV and other viral diseases. Curr Med Chem. 2003;2:219–225. [Google Scholar]
- Marrero J, Rodriguez AD, Baran P, Raptis RG, Sanchez JA, Ortega-Barria E, Capson TL. Bielschowskysin, a gorgonian-derived biologically active diterpene with an unprecedented carbon skeleton. Org Lett. 2004;6:1661–1664. doi: 10.1021/ol049495d. [DOI] [PubMed] [Google Scholar]
- Maskey RP, Helmke E, Kayser O, Fiebig HH, Maier A, Busche A, Laatsch H. anti-cancer and antibacterial trioxacarcins with high anti-malaria activity from a marine streptomycete and their absolute stereochemistry. J Antibiot. 2004;57:771–779. doi: 10.7164/antibiotics.57.771. [DOI] [PubMed] [Google Scholar]
- Masuno MN, Hoepker AC, Pessah IN, Molinski TF. 1-O-Sulfatobastadins-1 and -2 from Ianthella basta (Pallas). Antagonists of the RyR1-FKBP23 Ca+2 Channel. Marine Drugs. 2004;2:176–184. [Google Scholar]
- Matsubara K. Recent advances in marine algal anticoagulants. Curr Med Chem. 2004;2:13–19. doi: 10.2174/1568016043477314. [DOI] [PubMed] [Google Scholar]
- Mayer AMS, Hamann MT. Marine pharmacology in 1999: compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, anthelmintic, anti-inflammatory, antiplatelet, antiprotozoal and antiviral activities;affecting the cardiovascular, endocrine, immune, and nervous systems; and other miscellaneous mechanisms of action. Comp Biochem Physiol C, Pharmacol Toxicol. 2002;132:315–339. doi: 10.1016/s1532-0456(02)00094-7. [DOI] [PubMed] [Google Scholar]
- Mayer AMS, Hamann MT. Marine pharmacology in 2000: marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiplatelet, antituberculosis, and antiviral activities; affecting the cardiovascular, immune, and nervous systems and other miscellaneous mechanisms of action. Mar Biotechnol (NY) 2004;6:37–52. doi: 10.1007/s10126-003-0007-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AMS, Hamann MT. Marine pharmacology in 2001-2002: marine compounds with anthelmintic, antibacterial, anticoagulant, antidiabetic, antifungal, anti-inflammatory, antimalarial, antiplatelet, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems and other miscellaneous mechanisms of action. Comp Biochem Physiol C Toxicol Pharmacol. 2005;140:265–286. doi: 10.1016/j.cca.2005.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AMS, Lehmann VKB. Marine pharmacology in 1998: marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, anthelmintic, antiplatelet, antiprotozoal, and antiviral activities; with actions on the cardiovascular, endocrine, immune, and nervous systems; and other miscellaneous mechanisms of action. Pharmacologist. 2000;42:62–69. [Google Scholar]
- McClelland D, Evans RM, Abidin I, Sharma S, Choudhry FZ, Jaspars M, Sepcic K, Scott RH. Irreversible and reversible pore formation by polymeric alkylpyridinium salts (poly-APS) from the sponge Reniera sarai. Br J Pharmacol. 2003;139:1399–1408. doi: 10.1038/sj.bjp.0705374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiyu G, Fuchuan L, Xianliang X, Jing L, Zuowei Y, Huashi G. The potential molecular targets of marine sulfated polymannuroguluronate interfering with HIV-1 entry. Interaction between SPMG and HIV-1 rgp120 and CD4 molecule. Antiviral Res. 2003;59:127–135. doi: 10.1016/s0166-3542(03)00068-8. [DOI] [PubMed] [Google Scholar]
- Melo FR, Pereira MS, Foguel D, Mourao PA. Antithrombin-mediated anticoagulant activity of sulfated polysaccharides: different mechanisms for heparin and sulfated galactans. J Biol Chem. 2004;279:20824–20835. doi: 10.1074/jbc.M308688200. [DOI] [PubMed] [Google Scholar]
- Miao B, Geng M, Li J, Li F, Chen H, Guan H, Ding J. Sulfated polymannuroguluronate, a novel anti-acquired immune deficiency syndrome (AIDS) drug candidate, targeting CD4 in lymphocytes. Biochem Pharmacol. 2004;68:641–649. doi: 10.1016/j.bcp.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Miljanich GP. Ziconotide: neuronal calcium channel blocker for treating severe chronic pain. Curr Med Chem. 2004;11:3029–3040. doi: 10.2174/0929867043363884. [DOI] [PubMed] [Google Scholar]
- Molinski TF. Antifungal Compounds from Marine Organisms. Curr Med Chem. 2004;3:197–220. [Google Scholar]
- Monti MC, Casapullo A, Riccio R, Gomez-Paloma L. Further insights on the structural aspects of PLA(2) inhibition by gamma-hydroxybutenolide-containing natural products: a comparative study on petrosaspongiolides M-R. Bioorg. Med Chem. 2004;12:1467–1474. doi: 10.1016/j.bmc.2003.12.038. [DOI] [PubMed] [Google Scholar]
- Mourao PA. Use of sulfated fucans as anticoagulant and antithrombotic agents: future perspectives. Curr Pharm Des. 2004;10:967–981. doi: 10.2174/1381612043452730. [DOI] [PubMed] [Google Scholar]
- Mukku VJ, Edrada RA, Schmitz FJ, Shanks MK, Chaudhuri B, Fabbro D. New sesquiterpene quinols from a Micronesian sponge Aka sp. J Nat Prod. 2003;66:686–689. doi: 10.1021/np0205506. [DOI] [PubMed] [Google Scholar]
- Nakao Y, Shiroiwa T, Murayama S, Matsunaga S, Goto Y, Matsumoto Y, Fusetani N. Identification of Renieramycin A as an Antileishmanial substance in a marine sponge Neopetrosia sp. Mar Drugs. 2004;2:55–62. [Google Scholar]
- Nakao Y, Maki T, Matsunaga S, van Soest RW, Fusetani N. Penasulfate A, a new alpha-glucosidase inhibitor from a marine sponge Penares sp. J Nat Prod. 2004;67:1346–1350. doi: 10.1021/np049939e. [DOI] [PubMed] [Google Scholar]
- Namikoshi M, Suzuki S, Meguro S, Nagai H, Koike Y, Kitazawa A, Kobayashi H, Oda T, Yamada J. Manoalide derivatives from a marine sponge Luffariella sp collected in Palau. Fisheries Sci. 2004;70:152–158. [Google Scholar]
- Newman DJ, Cragg GM, Snader KM. Natural products as sources of new drugs over the period 1981-2002. J Nat Prod. 2003;66:1022–1037. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
- Nicholas GM, Eckman LL, Newton GL, Fahey RC, Ray S, Bewley CA. Inhibition and kinetics of mycobacterium tuberculosis and mycobacterium smegmatis mycothiol-S-conjugate amidase by natural product inhibitors. Bioorg Med Chem. 2003;11:601–608. doi: 10.1016/s0968-0896(02)00345-0. [DOI] [PubMed] [Google Scholar]
- Nika K, Mulloy B, Carpenter B, Gibbs R. Specific recognition of immune cytokines by sulphated polysaccharides from marine algae. Eur J Phycol. 2003;38:257–264. [Google Scholar]
- Nishimura S, Matsunaga S, Shibazaki M, Suzuki K, Furihata K, van Soest RW, Fusetani N. Massadine, a novel geranylgeranyltransferase type I inhibitor from the marine sponge Stylissa aff. massa. Org Lett. 2003;5:2255–2257. doi: 10.1021/ol034564u. [DOI] [PubMed] [Google Scholar]
- Ohgami K, Shiratori K, Kotake S, Nishida T, Mizuki N, Yazawa K, Ohno S. Effects of astaxanthin on lipopolysaccharide-induced inflammation in vitro and in vivo. Invest Ophthalmol Vis Sci. 2003;44:2694–2701. doi: 10.1167/iovs.02-0822. [DOI] [PubMed] [Google Scholar]
- Oku N, Gustafson KR, Cartner LK, Wilson JA, Shigematsu N, Hess S, Pannell LK, Boyd MR, McMahon JB. Neamphamide A, a new HIV-inhibitory depsipeptide from the Papua New Guinea marine sponge Neamphius huxleyi. J Nat Prod. 2004;67:1407–1411. doi: 10.1021/np040003f. [DOI] [PubMed] [Google Scholar]
- Ospina CA, Rodriguez AD, Ortega-Barria E, Capson TL. Briarellins J-P and polyanthellin A: new eunicellin-based diterpenes from the gorgonian coral Briareum polyanthes and their antimalarial activity. J Nat Prod. 2003;66:357–363. doi: 10.1021/np0204500. [DOI] [PubMed] [Google Scholar]
- Ovchinnikova TV, Aleshina GM, Balandin SV, Krasnosdembskaya AD, Markelov ML, Frolova EI, Leonova YF, Tagaev AA, Krasnodembsky EG, Kokryakov VN. Purification and primary structure of two isoforms of arenicin, a novel antimicrobial peptide from marine polychaeta Arenicola marina. FEBS Lett. 2004;577:209–214. doi: 10.1016/j.febslet.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Pan W, Liu X, Ge F, Han J, Zheng T. Perinerin, a novel antimicrobial peptide purified from the clamworm Perinereis aibuhitensis grube and its partial characterization. J Biochem (Tokyo) 2004;135:297–304. doi: 10.1093/jb/mvh036. [DOI] [PubMed] [Google Scholar]
- Pascual I, Gil-Parrado S, Cisneros M, Joseph-Bravo P, Diaz J, Possani LD, Charli JL, Chavez M. Purification of a specific inhibitor of pyroglutamyl aminopeptidase II from the marine annelide Hermodice carunculata. in vivo effects in rodent brain. Int J Biochem Cell Biol. 2004;36:138–152. doi: 10.1016/s1357-2725(03)00175-4. [DOI] [PubMed] [Google Scholar]
- Patrzykat A, Douglas SE. Gone gene fishing: how to catch novel marine antimicrobials. Trends Biotechnol. 2003a;21:362–369. doi: 10.1016/S0167-7799(03)00145-8. [DOI] [PubMed] [Google Scholar]
- Patrzykat A, Gallant JW, Seo JK, Pytyck J, Douglas SE. Novel antimicrobial peptides derived from flatfish genes. Antimicrob Agents Chemother. 2003b;47:2464–2470. doi: 10.1128/AAC.47.8.2464-2470.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira HS, Leao-Ferreira LR, Moussatche N, Teixeira VL, Cavalcanti DN, Costa LJ, Diaz R, Frugulhetti IC. Antiviral activity of diterpenes isolated from the Brazilian marine alga Dictyota menstrualis against human immunodeficiency virus type 1 (HIV-1) Antiviral Res. 2004;64:69–76. doi: 10.1016/j.antiviral.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Perez M, Sadqi M, Munoz V, Avila J. Inhibition by Aplidine of the aggregation of the prion peptide PrP 106-126 into beta-sheet fibrils. Biochim Biophys Acta. 2003;1639:133–139. doi: 10.1016/j.bbadis.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Pettit RK, Fakoury BR, Knight JC, Weber CA, Pettit GR, Cage GD, Pon S. Antibacterial activity of the marine sponge constituent cribrostatin 6. J Med Microbiol. 2004;53:61–65. doi: 10.1099/jmm.0.05250-0. [DOI] [PubMed] [Google Scholar]
- Pimentel SM, Bojo ZP, Roberto AV, Lazaro JE, Mangalindan GC, Florentino LM, Lim-Navarro P, Tasdemir D, Ireland CM, Concepcion GP. Platelet aggregation inhibitors from philippine marine invertebrate samples screened in a new microplate assay. Mar Biotechnol (NY) 2003;5:395–400. doi: 10.1007/s10126-002-0080-3. [DOI] [PubMed] [Google Scholar]
- Posadas I, De Rosa S, Terencio MC, Paya M, Alcaraz MJ. Cacospongionolide B suppresses the expression of inflammatory enzymes and tumour necrosis factor-alpha by inhibiting nuclear factor-kappa B activation. Br J Pharmacol. 2003a;138:1571–1579. doi: 10.1038/sj.bjp.0705189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posadas I, Terencio MC, Randazzo A, Gomez-Paloma L, Paya M, Alcaraz MJ. Inhibition of the NF-kappaB signaling pathway mediates the anti-inflammatory effects of petrosaspongiolide. M Biochem Pharmacol. 2003b;65:887–895. doi: 10.1016/s0006-2952(02)01659-3. [DOI] [PubMed] [Google Scholar]
- Proksch P, Ebel R, Edrada RA, Schupp P, Lin HW, Sudarsono, Wray V, Steube K. Detection of pharmacologically active natural products using ecology. Selected examples from Indopacific marine invertebrates and sponge-derived fungi. Pure Appl Chem. 2003a;75:343–352. [Google Scholar]
- Proksch P, Ebel R, Edrada RA, Wray V, Steube K. SPONGES: (PORIFERA) 2003b. Bioactive natural products from marine invertebrates and associated fungi; pp. 117–142. [DOI] [PubMed] [Google Scholar]
- Qi J, Ojika M, Sakagami Y. Linckosides C-E, three new neuritogenic steroid glycosides from the Okinawan starfish Linckia laevigata. Bioorg Med Chem. 2004;12:4259–4265. doi: 10.1016/j.bmc.2004.04.049. [DOI] [PubMed] [Google Scholar]
- Radhika P, Cabeza M, Bratoeff E, Garcia G. 5 alpha-Reductase inhibition activity of steroids isolated from marine soft corals. Steroids. 2004;69:439–444. doi: 10.1016/j.steroids.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Rao KV, Santarsiero BD, Mesecar AD, Schinazi RF, Tekwani BL, Hamann MT. New manzamine alkaloids with activity against infectious and tropical parasitic diseases from an Indonesian sponge. J Nat Prod. 2003;66:823–828. doi: 10.1021/np020592u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifai S, Fassouane A, Kijjoa A, Van Soest R. Antimicrobial activity of untenospongin B, a metabolite from the marine sponge Hippospongia communis collected from the Atlantic coast of Morocco. Mar Drugs. 2004;2:147–153. [Google Scholar]
- Roch P, Beschin A, Bernard E. Antiprotozoan and Antiviral Activities of Non-cytotoxic Truncated and Variant Analogues of Mussel Defensin. Evid Based Complement Alternat Med. 2004;1:167–174. doi: 10.1093/ecam/neh033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez II, Rodriguez AD. Homopseudopteroxazole, a new antimycobacterial diterpene alkaloid from Pseudopterogorgia elisabethae. J Nat Prod. 2003;66:855–857. doi: 10.1021/np030052c. [DOI] [PubMed] [Google Scholar]
- Rodriguez II, Shi YP, Garcia OJ, Rodriguez AD, Mayer AM, Sanchez JA, Ortega-Barria E, Gonzalez J. New pseudopterosin and seco-pseudopterosin diterpene glycosides from two Colombian isolates of Pseudopterogorgia elisabethae and their diverse biological activities. J Nat Prod. 2004;67:1672–1680. doi: 10.1021/np049802o. [DOI] [PubMed] [Google Scholar]
- Sakai R, Matsubara H, Shimamoto K, Jimbo M, Kamiya H, Namikoshi M. Isolations of N-methyl-D-aspartic acid-type glutamate receptor ligands from Micronesian sponges. J Nat Prod. 2003;66:784–787. doi: 10.1021/np020590+. [DOI] [PubMed] [Google Scholar]
- Sakai R, Suzuki K, Shimamoto K, Kamiya H. Novel betaines from a micronesian sponge Dysidea herbacea. J Org Chem. 2004;69:1180–1185. doi: 10.1021/jo0355045. [DOI] [PubMed] [Google Scholar]
- Sandsdalen E, Haug T, Stensvag K, Styrvold OB. The antibacterial effect of a polyhydroxylated fucophlorethol from the marine brown alga, Fucus vesiculosus. World J Microbiol Biotechnol. 2003;19:777–782. [Google Scholar]
- Satitpatipan V, Suwanborirux K. New nitrogenous Germacranes from a Thai marine sponge, Axinyssa n. sp. J Nat Prod. 2004;67:503–505. doi: 10.1021/np030349a. [DOI] [PubMed] [Google Scholar]
- Savoia D, Avanzini C, Allice T, Callone E, Guella G, Dini F. Antimicrobial activity of euplotin C, the sesquiterpene taxonomic marker from the marine ciliate Euplotes crassus. Antimicrob Agents Chemother. 2004;48:3828–3833. doi: 10.1128/AAC.48.10.3828-3833.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz FJ, Bowden BF, Toth SI. Antitumor and cytotoxic compounds from marine organisms. In: Attaway DH, Zaborsky OR, editors. Marine Biotechnology, Pharmaceutical and Bioactive Natural Products, vol 1. Plenum Press; New York: pp. 197–308. [Google Scholar]
- Schupp P, Poehner T, Edrada R, Ebel R, Berg A, Wray V, Proksch P. Eudistomins W and X, two new beta-carbolines from the micronesian tunicate Eudistoma sp. J Nat Prod. 2003;66:272–275. doi: 10.1021/np020315n. [DOI] [PubMed] [Google Scholar]
- Sharpe IA, Palant E, Schroeder CI, Kaye DM, Adams DJ, Alewood PF, Lewis RJ. Inhibition of the norepinephrine transporter by the venom peptide chi-MrIA. Site of action, Na+ dependence, and structure-activity relationship. J Biol Chem. 2003;278:40317–40323. doi: 10.1074/jbc.M213030200. [DOI] [PubMed] [Google Scholar]
- Staats PS, Yearwood T, Charapata SG, Presley RW, Wallace MS, Byas-Smith M, Fisher R, Bryce DA, Mangieri EA, Luther RR, Mayo M, McGuire D, Ellis D. Intrathecal ziconotide in the treatment of refractory pain in patients with cancer or AIDS - A randomized controlled trial. J Amer Med Ass. 2004;291:63–70. doi: 10.1001/jama.291.1.63. [DOI] [PubMed] [Google Scholar]
- Sudarslal S, Majumdar S, Ramasamy P, Dhawan R, Pal PP, Ramaswami M, Lala AK, Sikdar SK, Sarma SP, Krishnan KS, Balaram P. Sodium channel modulating activity in a delta-conotoxin from an Indian marine snail. FEBS Lett. 2003;553:209–212. doi: 10.1016/s0014-5793(03)01016-0. [DOI] [PubMed] [Google Scholar]
- Takada K, Uehara T, Nakao Y, Matsunaga S, van Soest RW, Fusetani N. Schulzeines A-C, new alpha-glucosidase inhibitors from the marine sponge Penares schulzei. J Am Chem Soc. 2004;126:187–193. doi: 10.1021/ja037368r. [DOI] [PubMed] [Google Scholar]
- Takamatsu S, Hodges TW, Rajbhandari I, Gerwick WH, Hamann MT, Nagle DG. Marine natural products as novel antioxidant prototypes. J Nat Prod. 2003;66:605–608. doi: 10.1021/np0204038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tincu JA, Menzel LP, Azimov R, Sands J, Hong T, Waring AJ, Taylor SW, Lehrer RI. Plicatamide, an antimicrobial octapeptide from Styela plicata hemocytes. J Biol Chem. 2003;278:13546–13553. doi: 10.1074/jbc.M211332200. [DOI] [PubMed] [Google Scholar]
- Tincu JA, Taylor SW. Antimicrobial peptides from marine invertebrates. Antimicrob Agents Chemother. 2004;48:3645–3654. doi: 10.1128/AAC.48.10.3645-3654.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosuji H, Fusetani N, Seki Y. Calyculin A causes the activation of histone H1 kinase and condensation of chromosomes in unfertilized sea urchin eggs independently of the maturation-promoting factor. Comp Biochem Physiol C, Toxicol Pharmacol. 2003;135:415–424. doi: 10.1016/s1532-0456(03)00143-1. [DOI] [PubMed] [Google Scholar]
- Trevisi L, Cargnelli G, Ceolotto G, Papparella I, Semplicini A, Zampella A, D'Auria MV, Luciani S. Callipeltin A: sodium ionophore effect and tension development in vascular smooth muscle. Biochem Pharmacol. 2004;68:1331–1338. doi: 10.1016/j.bcp.2004.04.032. [DOI] [PubMed] [Google Scholar]
- Tsang CK, Kamei Y. Sargaquinoic acid supports the survival of neuronal PC12D cells in a nerve growth factor-independent manner. Eur J Pharmacol. 2004;488:11–18. doi: 10.1016/j.ejphar.2004.01.033. [DOI] [PubMed] [Google Scholar]
- Tsukamoto S, Miura S, Yamashita Y, Ohta T. Aspermytin A: a new neurotrophic polyketide isolated from a marine-derived fungus of the genus Aspergillus. Bioorg Med Chem Lett. 2004a;14:417–420. doi: 10.1016/j.bmcl.2003.10.053. [DOI] [PubMed] [Google Scholar]
- Tsukamoto S, Tatsuno M, van Soest RW, Yokosawa H, Ohta T. New polyhydroxy sterols: proteasome inhibitors from a marine sponge Acanthodendrilla sp. J Nat Prod. 2003;66:1181–1185. doi: 10.1021/np030120v. [DOI] [PubMed] [Google Scholar]
- Tsukamoto S, Yamashita Y, Yoshida T, Ohta T. Parguerol and Isoparguerol Isolated from the Sea Hare, Aplysia kurodai, Induce Neurite Outgrowth in PC-12 Cells. Mar Drugs. 2004b;2:170–175. [Google Scholar]
- Tucker SJ, McClelland D, Jaspars M, Sepcic K, MacEwan DJ, Scott RH. The influence of alkyl pyridinium sponge toxins on membrane properties, cytotoxicity, transfection and protein expression in mammalian cells. Biochim Biophys Acta. 2003;1614:171–181. doi: 10.1016/s0005-2736(03)00175-5. [DOI] [PubMed] [Google Scholar]
- Tziveleka LA, Vagias C, Roussis V. Natural products with anti-HIV activity from marine organisms. Curr Top Med Chem. 2003;3:1512–1535. doi: 10.2174/1568026033451790. [DOI] [PubMed] [Google Scholar]
- Uchida T, Yamasaki T, Eto S, Sugawara H, Kurisu G, Nakagawa A, Kusunoki M, Hatakeyama T. Crystal structure of the hemolytic lectin CEL-III isolated from the marine invertebrate Cucumaria echinata: implications of domain structure for its membrane pore-formation mechanism. J Biol Chem. 2004;279:37133–37141. doi: 10.1074/jbc.M404065200. [DOI] [PubMed] [Google Scholar]
- Verbitski SM, Mullally JE, Fitzpatrick FA, Ireland CM. Punaglandins, chlorinated prostaglandins, function as potent Michael receptors to inhibit ubiquitin isopeptidase activity. J Med Chem. 2004;47:2062–2070. doi: 10.1021/jm030448l. [DOI] [PubMed] [Google Scholar]
- Vetvicka V, Yvin JC. Effects of marine beta-1,3 glucan on immune reactions. Int Immunopharmacol. 2004;4:721–730. doi: 10.1016/j.intimp.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Villar RM, Gil-Longo J, Daranas AH, Souto ML, Fernandez JJ, Peixinho S, Barral MA, Santafe G, Rodriguez J, Jimenez C. Evaluation of the effects of several zoanthamine-type alkaloids on the aggregation of human platelets. Bioorg Med Chem. 2003;11:2301–2306. doi: 10.1016/s0968-0896(03)00107-x. [DOI] [PubMed] [Google Scholar]
- Wang CY, Wang BG, Wiryowidagdo S, Wray V, Van Soest R, Steube KG, Guan HS, Proksch P, Ebel R. Melophlins C-O, thirteen novel tetramic acids from the marine sponge Melophlus sarassinorum. J Nat Prod. 2003;66:51–56. doi: 10.1021/np0202778. [DOI] [PubMed] [Google Scholar]
- Wang L, Geng M, Li J, Guan H, Ding J. Studies of marine sulfated polymannuroguluronate on endothelial cell proliferation and endothelial immunity and related mechanisms. J Pharmacol Sci. 2003;92:367–373. doi: 10.1254/jphs.92.367. [DOI] [PubMed] [Google Scholar]
- Wei XM, Rodriguez AD, Baran P, Raptis RG, Sanchez JA, Ortega-Barria E, Gonzalez J. Antiplasmodial cembradiene diterpenoids from a Southwestern Caribbean gorgonian octocoral of the genus Eunicea. Tetrahedron. 2004;60:11813–11819. [Google Scholar]
- Williams DE, Lapawa M, Feng X, Tarling T, Roberge M, Andersen RJ. Spirastrellolide A: revised structure, progress toward the relative configuration, and inhibition of protein phosphatase 2A. Org Lett. 2004a;6:2607–2610. doi: 10.1021/ol0490983. [DOI] [PubMed] [Google Scholar]
- Williams DE, Telliez JB, Liu J, Tahir A, Van Soest R, Andersen RJ. Meroterpenoid MAPKAP (MK2) inhibitors isolated from the indonesian marine sponge Acanthodendrilla sp. J Nat Prod. 2004b;67:2127–2129. doi: 10.1021/np049808d. [DOI] [PubMed] [Google Scholar]
- Wonganuchitmeta SN, Yuenyongsawad S, Keawpradub N, Plubrukarn A. Antitubercular sesterterpenes from the Thai sponge Brachiaster sp. J Nat Prod. 2004;67:1767–1770. doi: 10.1021/np0498354. [DOI] [PubMed] [Google Scholar]
- Xu N, Fan X, Yan X, Li X, Niu R, Tseng CK. Antibacterial bromophenols from the marine red alga Rhodomela confervoides. Phytochemistry. 2003;62:1221–1224. doi: 10.1016/s0031-9422(03)00004-9. [DOI] [PubMed] [Google Scholar]
- Yamada T, Iritani M, Minoura K, Kawai K, Numata A. Peribysins A-D, potent cell-adhesion inhibitors from a sea hare-derived culture of Periconia species. Org Biomolec Chem. 2004;2:2131–2135. doi: 10.1039/b404459b. [DOI] [PubMed] [Google Scholar]
- Yang SW, Buivich A, Chan TM, Smith M, Lachowicz J, Pomponi SA, Wright AE, Mierzwa R, Patel M, Gullo V, Chu M. A new sterol sulfate, Sch 572423, from a marine sponge, Topsentia sp. Bioorg Med Chem Lett. 2003a;13:1791–1794. doi: 10.1016/s0960-894x(03)00260-9. [DOI] [PubMed] [Google Scholar]
- Yang SW, Chan TM, Pomponi SA, Chen G, Loebenberg D, Wright A, Patel M, Gullo V, Pramanik B, Chu M. Structure elucidation of a new antifungal sterol sulfate, Sch 575867, from a deep-water marine sponge (Family: Astroscleridae) J Antibiot (Tokyo) 2003b;56:186–189. doi: 10.7164/antibiotics.56.186. [DOI] [PubMed] [Google Scholar]
- Yang SW, Chan TM, Pomponi SA, Chen G, Wright AE, Patel M, Gullo V, Pramanik B, Chu M. A new bicyclic guanidine alkaloid, Sch 575948, from a marine sponge, Ptilocaulis spiculifer. J Antibiot (Tokyo) 2003c;56:970–972. doi: 10.7164/antibiotics.56.970. [DOI] [PubMed] [Google Scholar]
- Yang SW, Chan TM, Pomponi SA, Gonsiorek W, Chen G, Wright AE, Hipkin W, Patel M, Gullo V, Pramanik B, Zavodny P, Chu M. A new sesterterpene, Sch 599473, from a marine sponge, Ircinia sp. J Antibiot (Tokyo) 2003d;56:783–786. doi: 10.7164/antibiotics.56.783. [DOI] [PubMed] [Google Scholar]
- Yim JH, Kim SJ, Ahn SH, Lee CK, Rhie KT, Lee HK. Antiviral effects of sulfated exopolysaccharide from the marine microalga Gyrodinium impudicum strain KG03. Mar Biotechnol (NY) 2004;6:17–25. doi: 10.1007/s10126-003-0002-z. [DOI] [PubMed] [Google Scholar]
- Yoo HD, L D, Sanghara J, Daley D, Van Soest R, Andersen RJ. Isoarenarol, A New Protein Kinase Inhibitor from the Marine Sponge Dysidea arenaria. Pharm Biol. 2003;41:223–225. [Google Scholar]
- Zancan P, Mourao PA. Venous and arterial thrombosis in rat models: dissociation of the antithrombotic effects of glycosaminoglycans. Blood Coagul Fibrinol. 2004;15:45–54. doi: 10.1097/00001721-200401000-00008. [DOI] [PubMed] [Google Scholar]
- Zhang W, Xue S, Zhao Q, Zhang X, Li J, Jin M, Yu X, Yuan Q. Biopotentials of marine sponges from China oceans: past and future. Biomol Eng. 2003;20:413–419. doi: 10.1016/s1389-0344(03)00066-2. [DOI] [PubMed] [Google Scholar]
- Zhu W, Chiu LC, Ooi VE, Chan PK, Ang PO., Jr Antiviral property and mode of action of a sulphated polysaccharide from Sargassum patens against herpes simplex virus type 2. Int J Antimicrob Agents. 2004;24:279–283. doi: 10.1016/j.ijantimicag.2004.02.022. [DOI] [PubMed] [Google Scholar]