Abstract
Plant salinity tolerance is a polygenic trait with contributions from genetic, developmental, and physiological interactions, in addition to interactions between the plant and its environment. In this study, we show that in salt-tolerant genotypes of barley (Hordeum vulgare), multiple mechanisms are well combined to withstand saline conditions. These mechanisms include: (1) better control of membrane voltage so retaining a more negative membrane potential; (2) intrinsically higher H+ pump activity; (3) better ability of root cells to pump Na+ from the cytosol to the external medium; and (4) higher sensitivity to supplemental Ca2+. At the same time, no significant difference was found between contrasting cultivars in their unidirectional 22Na+ influx or in the density and voltage dependence of depolarization-activated outward-rectifying K+ channels. Overall, our results are consistent with the idea of the cytosolic K+-to-Na+ ratio being a key determinant of plant salinity tolerance, and suggest multiple pathways of controlling that important feature in salt-tolerant plants.
Intracellular K+/Na+ homeostasis is crucial for cell metabolism and is considered to be a key component of salinity tolerance in plants (Niu et al., 1995; Maathuis and Amtmann, 1999; Hasegawa et al., 2000; Shabala, 2000; Tester and Davenport, 2003; Volkov et al., 2004; Chen et al., 2007). To maintain an optimal intracellular K+-to-Na+ ratio under saline conditions, accumulation of excessive amounts of Na+ in the cytosol should be prevented, along with retention of physiological concentrations of cytosolic K+. However, our understanding of how this is achieved is rather limited.
At the cellular level, maintenance of low cytosolic Na+ may be achieved through several major strategies. One is to restrict unidirectional Na+ uptake by roots (which is mediated mostly by nonselective cation channels [NSCC]; Demidchik and Tester, 2002; Essah et al., 2003; Demidchik and Maathuis, 2007). Another is active Na+ extrusion from the cytosol to the external medium (mediated by plasma membrane [PM]-located Na+/H+ antiporters; Blumwald et al., 2000; Shi et al., 2002; Shabala et al., 2005) and vacuolar compartmentation of Na+ (via tonoplast-located Na+/H+ antiporters; Apse et al., 1999; Blumwald, 2000; Zhang and Blumwald, 2001). The latter also contributes to cell osmotic adjustment thus providing a lower cellular osmotic potential under the hypertonic conditions of salt stress. At the whole-plant level, prevention of Na+ transport to the shoot (Tester and Davenport, 2003), and perhaps also recirculation of Na+ back to the roots through the phloem (Lohaus et al., 2000; Nublat et al., 2001; Berthomieu et al., 2003) appear to be crucial for salinity tolerance. Most glycophytes have a poor ability to exclude salt (Niu et al., 1995; Munns, 2002) and there is apparently a strong correlation between Na+ exclusion and salinity tolerance in many species (Tester and Davenport, 2003; Munns, 2005).
The high cytosolic K+-to-Na+ ratio may also be achieved by efficient cytosolic K+ homeostasis. Under saline conditions, the PM is strongly depolarized (by 60–80 mV; Shabala et al., 2003, 2005, 2006; Cuin and Shabala, 2006). Although this reduces the electrochemical driving force for Na+ uptake, the more important effect of this depolarization is to cause a drastic K+ efflux from both root (Chen et al., 2005; Cuin and Shabala, 2005) and mesophyll (Shabala, 2000; Shabala et al., 2006) cells, substantially reducing the cytosolic K+ pools (Carden et al., 2003; Cuin et al., 2003; Shabala et al., 2006) and compromising the metabolic competence of the cell. Increased uptake of K+ is difficult to attain under saline conditions (due to direct competition from Na+ for K+-binding sites on transport systems and also to a reduced electrochemical potential difference for passive K+ uptake). Hence, prevention of K+ loss from cells appears to be crucial for maintaining cytosolic K+ concentration.
We have previously reported a strong correlation between the ability of young barley (Hordeum vulgare) seedlings to restrict NaCl-induced K+ release and the salinity tolerance of mature plants, as measured by various physiological parameters (Chen et al., 2005). In a larger sample, the K+ release from 3-d-old seedlings of nearly 70 barley cultivars was also found to correlate significantly with major physiological and agronomical indices of adult plants (Chen et al., 2007). Genetic analysis has suggested that barley salinity tolerance, based on NaCl-induced K+ efflux, is under polygenic control—mainly by additive genes, with relatively smaller dominant and epistatic effects (Z.H. Chen, S. Shabala, N. Mendham, I.A. Newman, and G.P. Zhang, unpublished data). However, the specific ion transporters determining differential salt sensitivity among genotypes and the control modes of these transporters were not investigated.
This issue was addressed in this study. A range of biophysical measurements (membrane potential, noninvasive ion flux measurements, patch clamp, and radiotracers) and physiological and biochemical assays were applied to several barley cultivars contrasting in their salinity tolerance (Chen et al., 2005). Our results show that the superior ability of salt-tolerant cultivars to retain K+ is determined by several factors. They are consistent with the idea of the cytosolic K+-to-Na+ ratio being a key determinant of plant salinity tolerance, and suggest multiple pathways of controlling that important feature in salt-tolerant plants.
RESULTS
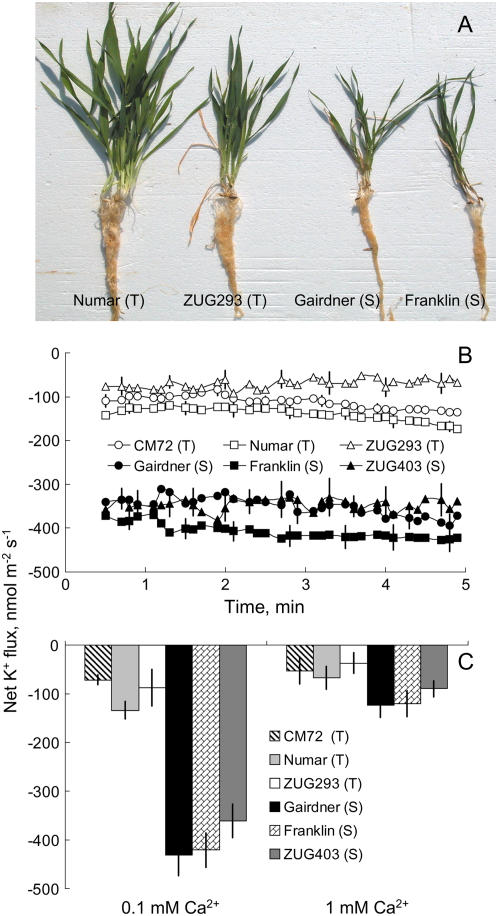
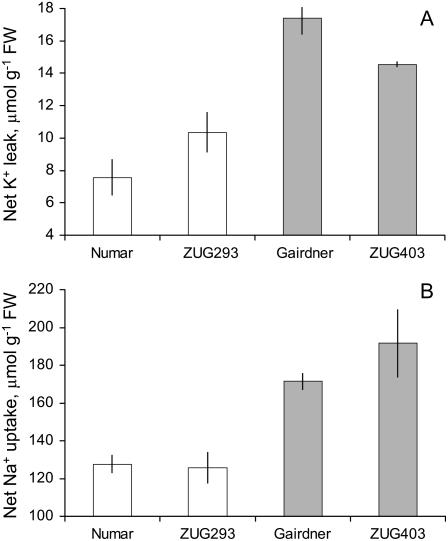
Six contrasting barley varieties were employed throughout this study. These clustered into two distinct groups: (1) salt tolerant: ‘CM72’, ‘Numar’, and ‘ZUG293’; and (2) salt sensitive: ‘Gairdner’, ‘Franklin’, and ‘ZUG403’ (as illustrated in Fig. 1A; see also Chen et al., 2007 for yield statistics). When measured at the 3-d-old stage, salt-tolerant genotypes showed a significant, 3-fold higher (P < 0.01) ability to retain K+ in the root by minimizing NaCl-induced K+ efflux from epidermal cells (Fig. 1B). The magnitude of NaCl-induced K+ loss showed a high correlation with salinity tolerance using conventional physiological and agronomical indices (Chen et al., 2007). Under severe (320 mm) salinity stress, salt-sensitive genotypes failed to produce any seed, while salt-tolerant ones attained approximately 15% the grain yield of the control (Chen et al., 2007). NaCl-induced K+ loss was significantly ameliorated by the addition of 1 mm Ca2+ (a concentration typically found in a soil solution; Tisdale et al., 1993) in both salt-tolerant and salt-sensitive genotypes (Fig. 1C). However, regardless of the Ca2+ concentration used, salt-tolerant varieties showed much a better K+ retention ability compared with salt-sensitive ones (Fig. 1C). Moreover, using low Ca2+ levels increased the resolution of the method, resulting in a larger K+ flux difference between contrasting varieties. This methodological advantage was kept in mind while conducting pharmacological and membrane potential measurements.
Figure 1.
A, Contrasting barley genotypes grown under 320 mm NaCl for 4 weeks in the glasshouse experiment. Salt-tolerant (T) and salt-sensitive (S) varieties are easily distinguished. B, Steady-state net K+ fluxes (inward positive). C, Effects of different external Ca2+ (0.1 and 1 mm) on NaCl-induced K+ flux measured from 3-d-old roots of barley genotypes contrasting in their salinity tolerance after 1 h of 80 mm NaCl treatment. Results in C are averaged over 15 min of K+ flux measurement. Means ± se (n = 7–10). [See online article for color version of this figure.]
Tetraethylammonium Chloride-Sensitive K+ Channels Determine the Difference in NaCl-Induced K+ Efflux between Contrasting Genotypes
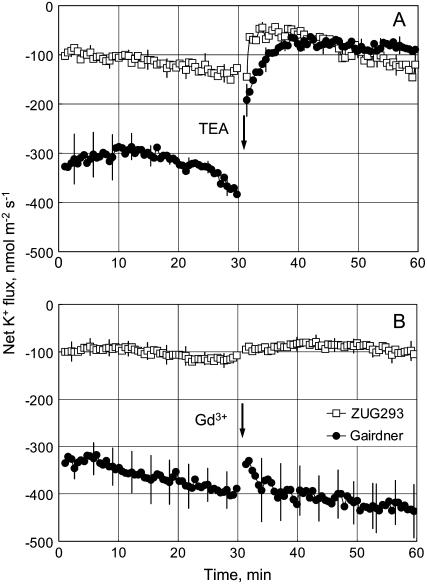
Two channel blockers, tetraethylammonium chloride (TEA+; a blocker of K+-selective channels) and GdCl3 (nonspecific cation channel blocker), were used in pharmacological experiments. Consistent with previous results, pretreatment with 80 mm NaCl for 1 h resulted in a significant difference in steady net K+ flux, with a 3-fold larger K+ loss from salt-sensitive ‘Gairdner’ compared with salt-tolerant ‘ZUG293’ (Fig. 2). Applying 20 mm TEA+ significantly (approximately 80%; P < 0.05) reduced the K+ loss from roots of ‘Gairdner’, but had a much smaller effect on the K+ loss from salt-treated ‘ZUG293’. As a result, no significant difference in the magnitude of K+ flux was observed between the contrasting varieties after TEA+ treatment for at least 30 min. This suggests that the TEA+-sensitive population of K+ efflux channels are the main contributors toward NaCl-induced K+ loss in salt-sensitive ‘Gairdner’, but this component has little contribution in salt-tolerant ‘ZUG293’. No significant effect of 50 μm Gd3+ treatment was observed for either cultivar after 1 h of NaCl treatment (Fig. 2B).
Figure 2.
Pharmacology of K+ flux responses. Net K+ fluxes were measured in response to 20 mm TEA+, applied at arrow, from roots of two contrasting barley genotypes (salt-tolerant ‘ZUG293’; salt-sensitive ‘Gairdner’) preincubated in 80 mm NaCl for 1 h. Means ± se (n = 7–8).
Salt-Tolerant Genotypes Have Intrinsically Higher H+ Pump Activity and Are Capable of Maintaining a More Negative Membrane Potential
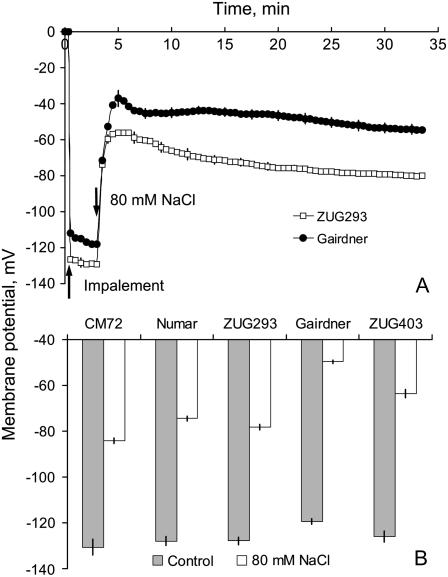
In plant cells, TEA+-sensitive K+ efflux channels are also voltage sensitive (Maathuis et al., 1997; Shabala et al., 2006). Thus, the different K+ retention ability of barley roots of contrasting genotypes might be related to a difference in their ability to maintain the PM potential (Em) after the imposition of salt stress. The Em of five genotypes was measured using conventional microelectrode impalement. Application of 80 mm NaCl caused an immediate and rapid depolarization by about 70 mV (Fig. 3A). Despite some recovery, the depolarized Em was maintained, allowing us to compare the magnitude of Em of all the cultivars before (2–4 min) and after (25–30 min) salt stress. This depolarization was significantly (P < 0.01) greater in salt-sensitive than in salt-tolerant genotypes (Fig. 3B) and strongly (r2 = 0.93, P < 0.01) correlated with net K+ flux. Notably, the Em of salt-sensitive cultivars was approximately 10 mV more positive than the Em of salt-tolerant varieties when measured under control conditions (Fig. 3B).
Figure 3.
A, Membrane potential of epidermal root cells of salt-tolerant ‘ZUG293’ and salt-sensitive ‘Gairdner’ measured in response to 80 mm NaCl treatment (at arrow). Means ± se (n = 6). B, Steady-state membrane potential (Em) values in control (prior to NaCl treatment) and after 20 min root exposure to 80 mm NaCl. Means ± se (n = 10).
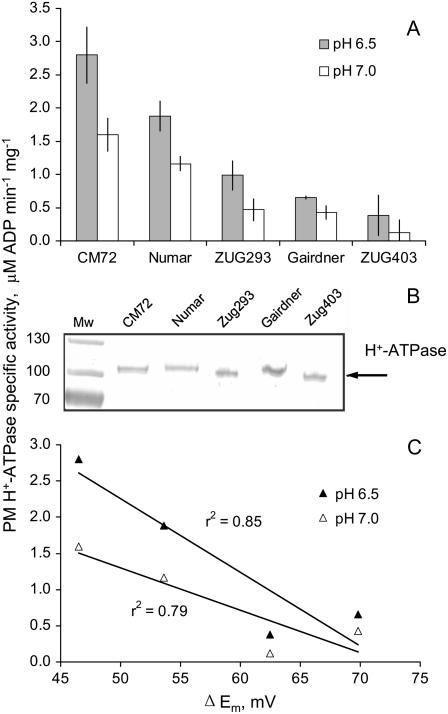
This issue was addressed directly by measuring ATP hydrolytic activity from PM vesicles isolated from the microsomal fraction of roots (Fig. 4A). The two salt-sensitive genotypes, ‘Gairdner’ and ‘ZUG403’, had the lowest level of PM H+-ATPase activity (5-fold lower than salt-tolerant ‘CM72’ and ‘Numar’). At pH 6.5 a strong correlation (r2 = 0.85) between PM H+-ATPase activity and NaCl-induced changes in Em was found (Fig. 4C). Western-blot analysis demonstrated that the observed difference in PM H+-ATPase activity could not be explained by a difference in enzyme level (Fig. 4B), implying that the observed differences in H+ pumping are the result of a posttranslational modification of activity. This suggests that a higher specific PM H+ pump activity is a characteristic of salt-tolerant varieties.
Figure 4.
A, ATP hydrolytic activity of PMs isolated from the microsomal fraction of roots of barley genotypes contrasting in salinity tolerance. Means ± se (n = 6). The statistics are based on two independent PM preparations and each of the preparations was tested three times with reproducible results. B, Western-blot results demonstrating that the observed difference in the H+-ATPase activity is not due to a difference in the enzyme level.
Salt-Tolerant Genotypes Accumulate Less Na+, But Do Not Differ in Unidirectional Na+ Uptake
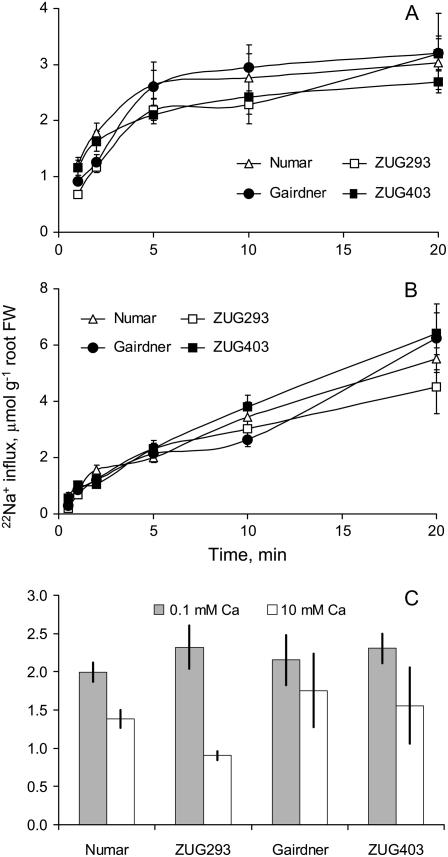
A reduced Na+ influx in salt-tolerant genotypes is another potential contributor to their better maintenance of membrane potential in saline conditions. We measured unidirectional 22Na+ influx in response to sudden salt shock (Fig. 5A) and after 24 h of salt treatment (Fig. 5B). Rapid accumulation of 22Na+ was measured in all genotypes upon addition of 80 mm NaCl, with 22Na+ influx gradually decreasing during the first 20 min (Fig. 5A), while a relatively steady 22Na+ influx was observed after 24 h of NaCl pretreatment (Fig. 5B). However, no clear difference between contrasting cultivars was evident either immediately upon NaCl treatment or after 24 h (Fig. 5, A and B).
Figure 5.
Unidirectional 22Na+ uptake, at times up to 20 min, into excised roots of four barley cultivars contrasting in their salinity tolerance. A, Immediately after 80 mm NaCl treatment. B, After 24 h incubation in 80 mm NaCl. C, For the immediate treatment, the 22Na+ uptake during 5 min with two levels of external Ca2+ (0.1 as in A and 10 mm in C). Means ± se (n = 8–13).
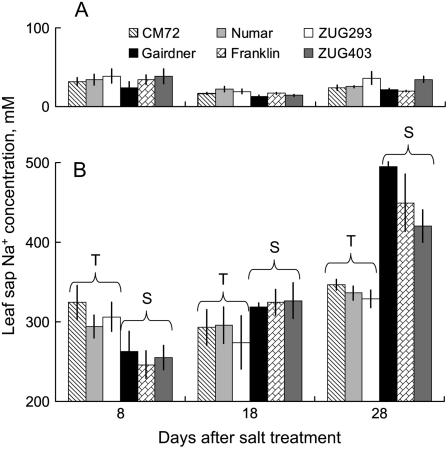
Solution depletion experiments showed that salt-tolerant genotypes were able not only to loose approximately 80% less K+ (Fig. 6A), but also to reduce significantly (by approximately 40%; P < 0.05) net root Na+ uptake compared with salt-sensitive genotypes (Fig. 6B). This implies that, given they have the same Na+ unidirectional influx as salt-sensitive varieties, salt-tolerant genotypes have a higher capacity to extrude the Na+ actively back to the external medium. This hypothesis was further tested by measuring Na+ concentration in the flag leaf sap of each of the six barley genotypes exposed to longer-term salt treatment (Fig. 7). As expected, salinity stress resulted in a substantial (10- to 14-fold) increase in the leaf sap Na+ content (Fig. 7). Interestingly, salt-tolerant varieties showed relatively constant sap Na+ levels, regardless of the duration of salt treatment (approximately 300 mm Na+; Fig. 7B). On the contrary, three salt-sensitive varieties showed a progressive accumulation of Na+ in the flag leaf (Fig. 7B). As a result, after 8 d of 320 mm NaCl treatment, salt-tolerant varieties had slightly larger quantities of Na+ in the leaf sap (308 ± 10 and 255 ± 10 mm [n = 12] for salt-tolerant and salt-sensitive group, respectively; significant at P < 0.05). Four weeks exposure to salt stress, however, resulted in salt-sensitive varieties accumulating 35% more leaf sap Na+ than salt-tolerant ones (455 ± 17 and 337 ± 5 mm [n = 12], respectively; significant at P < 0.05; Figure 7B).
Figure 6.
K+ loss (A) and Na+ uptake (B) by barley roots measured in depletion experiments. In each treatment, roots of ten 3-d-old seedlings were immersed in 10 mL saline solution (80 mm NaCl, 0.5 mm KCl, 0.1 mm CaCl2) in a plastic test tube and aerated for 24 h in the dark at 25°C. Two individual measurements were performed with three replicates for each genotype. Means ± se (n = 6).
Figure 7.
Leaf sap Na+ concentrations of six barley genotypes in both control (A) and 320 mm NaCl treatment (B). Flag leaf samples of all cultivars were collected 8, 18, and 28 d after the imposition of salinity. Means ± se (n = 4).
The above experiments were conducted under low (0.1 mm) external Ca2+ conditions. When 22Na+ influx was measured with high (10 mm) external Ca2+, the salt-tolerant genotypes ‘Numar’ and ‘ZUG293’ showed a significant reduction in unidirectional 22Na+ influx (on average approximately 46% of control values; P < 0.05; Fig. 5C), whereas unidirectional 22Na+ influx into roots of salt-sensitive genotypes (‘Gairdner’ and ‘ZUG403’) was much less affected by increased external Ca2+. This suggests that Na+ permeable transporters in the roots of tolerant genotypes have a higher sensitivity to supplemental Ca2+.
K+-Selective Outwardly Rectifying-Mediated Currents in Root Epidermal Protoplasts Do Not Differ in Salt-Tolerant and Salt-Sensitive Cultivars
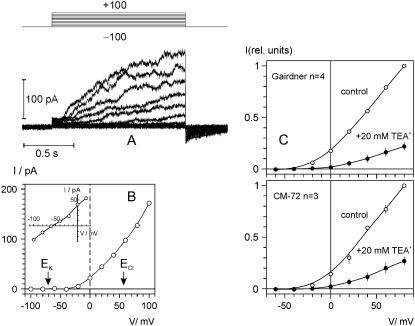
Further electrophysiological characterization of transport systems potentially involved in Na+ and K+ homeostasis in salinized barley roots was undertaken in a series of patch-clamp experiments. We found at least five cation currents, only one of which was sensitive to externally applied TEA+ (approximately 80% inhibition by 20 mm TEA+ at +80 mV; Fig. 8B) and was very similar to the K+-selective outwardly rectifying (KOR) channel current previously described in barley xylem parenchyma (Wegner and Raschke, 1994). In the ionic conditions used (5 mm external and 100 mm internal K+), this current was activated at potentials positive of −40 mV with a characteristic time delay. The time course could be explained by Hodgkin-Huxley kinetics/exponential power function, with n between 3 and 3.7 (results not shown). A typical recording of this KOR current is presented in Figure 8A. The reversal potential of the KOR current was approximately −60 mV, close to EK that was −70 mV under these conditions. This deviation from the ideal K+ selectivity probably reflects a limited Ca2+ permeability of this channel (compare with Roberts and Tester, 1997; Wegner and De Boer, 1997). External TEA+ tended to increase the outward rectification of KOR-mediated current (Fig. 8C), i.e. the blockage was more pronounced at less depolarizing voltages. In contrasting genotypes, the effective blocking charge of TEA was slightly higher in salt-sensitive ‘Gairdner’ compared to salt-tolerant ‘CM72’ (0.28 ± 0.07 and 0.22 ± 0.06, respectively). Higher voltage dependence of TEA+ blockage in ‘Gairdner’ as compared to ‘CM72’ resulted in a stronger suppression of KOR current at less depolarizing potentials (by 91% and 85% at 0 mV, respectively).
Figure 8.
Time- and voltage-dependent TEA-sensitive KOR currents in barley epidermal protoplasts. K+ concentration in bath/pipette was 5/100 mm (see “Materials and Methods” for detailed solution composition). A, Typical record of KOR currents in salt-sensitive ‘Gairdner’. Voltage was stepped from −100 mV (holding) in 20 mV increments up to +100 mV for 1.4 s and returned to −100 mV at the end of episode. B, I/V relation for the time-dependent component of the depolarization activated current; equilibrium potentials for K+ and Cl− are indicated by arrows. Inset shows amplitude of the tail currents (prepulse to +80 mV, subsequent test pulses to voltages between −100 and +20 mV) as a function of test voltage. C, KOR currents in two contrasting barley cultivars (salt-tolerant ‘CM72’ and salt-sensitive ‘Gairdner’) show similar sensitivity to external TEA+. Shown are normalized I/V relations of the time-dependent current component in control conditions and after external application of 20 mm TEACl. Means ± se (n = 3 and 4 protoplasts for ‘CM72’ and ‘Gairdner’, respectively). Note that in some cases error bar is smaller than the symbol size.
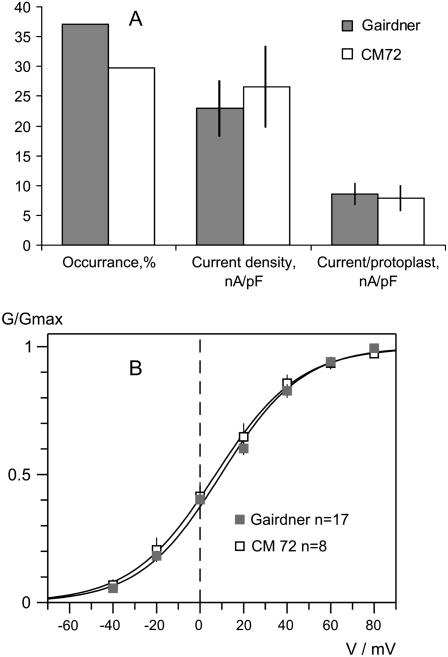
Analysis of the occurrence (percentage of successful recordings in the total number of protoplasts recorded) and current densities of KOR channels for two contrasting genotypes, salt-sensitive ‘Gairdner’ and salt-tolerant ‘CM72’, suggest that, despite having a slightly higher percentage of KOR channels in the salt-sensitive genotype (37% versus 30% of the total protoplast population studied, n = 70, 37, respectively; Fig. 9A), the actual current density through KOR channels was slightly higher in the protoplasts from the salt-tolerant variety ‘CM72’ (Fig. 9A). As a result, the overall K+ current through KOR channels per protoplast was not significantly different between the contrasting varieties (Fig. 9A). Also, no significant difference in KOR voltage gating was found between contrasting genotypes (Fig. 9B).
Figure 9.
Comparison of KOR-mediated currents in two contrasting barley cultivars: salt-tolerant ‘CM72’ and salt-sensitive ‘Gairdner’. A, Frequency of detection (successful/total records) of KOR channels, average current densities at +60 mV, and average KOR current per protoplast. Means ± se (n = 36 for ‘CM72’; 70 for ‘Gairdner’). B, Voltage dependence of KOR-mediated conductance. Solid lines are best fits to Boltzmann equation, with midpoint potential values of 6.4 ± 1.4 mV and 9.6 ± 2.4 mV, and the slope factor (membrane depolarization that increases open/closed states ratio e times) of 18.6 ± 0.8 mV and 18.8 ± 1.2 mV for ‘CM72’ and ‘Gairdner’, respectively.
DISCUSSION
Most authors agree that K+/Na+ homeostasis is a key feature of plant salinity tolerance (Gorham et al., 1990; Rubio et al., 1995; Dubcovsky et al., 1996; Maathuis and Amtmann, 1999; Cuin and Shabala, 2006; Volkov and Amtmann, 2006). Carden et al. (2003) found that a salt-tolerant barley cultivar was better at maintaining root cytosolic K+ under saline conditions compared with a salt-sensitive variety. However, most work has only considered whole cell or whole tissue cation contents.
Increased salinity tolerance has been reported in transgenic plants expressing the yeast (Saccharomyces cerevisiae) HAL1 gene (Bordas et al., 1997; Gisbert et al., 2000; Rus et al., 2001), and transcription of HAL1 favors Na+ extrusion and restricts K+ efflux through an unknown pathway (Bordas et al., 1997), the combined effect effectively increasing the intracellular K+-to-Na+ ratio (Gaxiola et al., 1992). Overexpression of the yeast homolog of the HAL3 gene in transgenic Arabidopsis (Arabidopsis thaliana) plants improves salinity tolerance by increasing the cytoplasmic K+ concentration and decreasing Na+ concentrations (Espinosa-Ruiz et al., 1999). Thellungiella halophila, a salt-tolerant relative of Arabidopsis, possesses a greater ability to retain, or even to increase, shoot K+ content compared with Arabidopsis under salt stress (Volkov et al., 2004). A strong correlation between a plant's ability to retain K+ in root epidermal cells and salinity tolerance was reported in our previous work screening nearly 70 barley cultivars covering a wide range of salinity tolerance (Chen et al., 2005, 2007). Thus, targeting mechanistic components responsible for intracellular K+/Na+ homeostasis may be an effective way of improving salinity tolerance in crops. This leads to the question: What are these components, and what genes encode their function?
Activity of a PM Na+/H+ Exchanger, But Not Na+ Uptake Systems, Contributes to Differential Salinity Tolerance in Barley
Several pathways for Na+ uptake across the PM have been identified recently using electrophysiological (patch-clamp) and molecular genetic approaches. The major route for Na+ uptake into the root is believed to be through NSCCs, either voltage independent (so-called VIC channels; Roberts and Tester, 1997; White and Davenport, 2002), or weakly voltage dependent (Davenport and Tester, 2000; Demidchik et al., 2002). No significant difference in unidirectional 22Na+ influx was found between salt-tolerant and salt-sensitive barley genotypes under the low Ca2+ conditions in this study (Fig. 5, A and B). Conversely, net Na+ uptake by the root (Fig. 6B) and Na+ accumulation in the leaf (Fig. 7B) was found to be significantly (by approximately 40% and 35%, respectively) lower in salt-tolerant cultivars. Thus, it appears that salt-tolerant cultivars have a superior ability to pump Na+ from the cytosol back to the external medium. To date, the only known candidate for such active Na+ extrusion in higher plants is a PM-bound Na+/H+ antiporter (Blumwald et al., 2000), possibly SOS1 (Shabala et al., 2005). Thus, we suggest that the differential salt sensitivity between contrasting barley cultivars is in part conferred by higher Na+/H+ antiporter activity in salt-tolerant varieties.
Consistent with our results, no significant differences in Na+ influx were found between the wild type and any of the Arabidopsis sos and hkt mutants with altered salinity tolerance (Essah et al., 2003), or between salt-tolerant and -sensitive wheat (Triticum aestivum) cultivars under 0.5 or 2 mm external Ca2+ (Davenport et al., 1997, 2005).
Na+ Influx Transporters in Salt-Tolerant Genotypes Have a Higher Sensitivity to Supplementary Ca2+
Calcium can ameliorate Na+ toxicity in plants by decreasing Na+ influx through NSCCs (Schachtman and Liu, 1999; Davenport and Tester, 2000; Demidchik and Maathuis, 2007). For instance, a 50% inhibition of NSCC current was observed at 0.1 mm Ca2+ activity (Demidchik and Tester, 2002). Recently we have shown that elevated external [Ca2+] also inhibits Na+-induced K+ efflux through outwardly directed K+-permeable channels (Shabala et al., 2006). These experiments have demonstrated that two populations of Ca2+-sensitive K+ efflux channels exist in protoplasts isolated from the mature epidermis of Arabidopsis root and leaf mesophyll cells. The instantaneously activating K+ efflux channels showed weak voltage dependence and insensitivity to external and internal Na+. Another population of K+ efflux channels was slowly activating, steeply rectifying, and highly sensitive to Na+ (Shabala et al., 2006).
In this work, most experiments were conducted at low (0.1 mm) Ca2+ to avoid Ca2+ inhibition of either NSCC or K+ efflux channels. Under these conditions, neither immediately upon, nor after 24 h of NaCl treatment, was a clear difference between contrasting cultivars evident in unidirectional 22Na+ influx (Fig. 5, A and B). At high Ca2+ levels (10 mm), however, unidirectional 22Na+ influx in salt-tolerant genotypes was reduced (Fig. 5C). During the first 5 min of salt supply, salt-sensitive genotypes accumulated on average 31% more Na+ than salt-tolerant ones (Fig. 5C; P < 0.05). This suggests that supplemental external Ca2+ is better able to regulate NSCC in salt-tolerant genotypes. This is consistent with Davenport et al. (1997) who showed that in wheat, Na+ influx into roots was more sensitive to 10 mm Ca2+ in salt-tolerant cultivars compared with salt-sensitive ones.
The Ca2+ block of NSCC is not complete (even at saturating Ca2+ concentrations), with both Ca2+-sensitive and Ca2+-insensitive components of Na+ influx being reported for wheat (Demidchik and Tester, 2002) and Arabidopsis (Essah et al., 2003). Based on our results, we propose that there are two populations of NSCC: Ca2+ sensitive and Ca2+ insensitive, in barley root epidermal cells. The relative size of these two pools differs between salt-tolerant and -sensitive cultivars, with an apparently larger Ca2+-sensitive pool of NSCC in salt-tolerant genotypes.
Barley Salinity Tolerance Correlates with Higher H+-ATPase Activity in Root Cells
Being an electroneutral exchanger (Serrano and Rodriguez-Navarro, 2001), the Na+/H+ antiporter cannot be directly responsible for the less pronounced membrane depolarization found in salt-tolerant barley genotypes under saline conditions (Fig. 3). This difference in the magnitude of membrane depolarization can be explained by the intrinsically higher activity of H+-ATPase in the salt-tolerant cultivars (Fig. 4A). This higher activity significantly correlated with the smaller ΔEm and the lower NaCl-induced K+ efflux (Fig. 4C). With PM H+-ATPase being a major determinant of Em (Michelet and Boutry, 1995; Palmgren, 2001), more negative Em values in salt-tolerant genotypes under steady-state conditions could be a direct consequence of a more active H+ pump. However, in contrast to some other species (Elkahoui et al., 2005; Yang et al., 2006), western-blot analysis revealed no difference in the amount of protein present between different cultivars (Fig. 4B). This suggests that the 5-fold difference in H+-ATPase activity observed between contrasting cultivars (Fig. 4A) is due to posttranslational modulation of the ATPase. The specific nature of such a posttranslational modification requires separate investigation.
Different Membrane Depolarization But Not the Difference in KOR Properties Underlies Different K+ Efflux Responses in Contrasting Cultivars
Consistent with the general view that salinity is a polygenic trait (Flowers, 2004; Munns, 2005), genetic analysis in our laboratory has indicated that NaCl-induced K+ efflux is under polygenic control, mainly by additive genes with relatively smaller dominant and epistatic effects (Z.H. Chen, S. Shabala, N. Mendham, I.A. Newman, and G.P. Zhang, unpublished data). Nonetheless, plant breeders are still in search of a primary gene to improve salinity tolerance in crops. Based on our findings that (1) NaCl-induced K+ efflux was TEA+ sensitive and (2) TEA+ application eliminated the difference in magnitude of K+ efflux between salt-sensitive and -tolerant genotypes after 1 h of NaCl treatment (Fig. 2A), it would be logical to propose that contrasting salinity tolerance between the genotypes is determined (at the genetic level) by the different occurrences of PM KOR channels. Surprisingly, patch-clamp experiments on two contrasting genotypes revealed no major difference in KOR channel occurrence. Moreover, salt-tolerant genotypes showed slightly higher KOR-mediated K+ current density (Fig. 9A). As a result, at a given membrane potential, the KOR-mediated K+ current per protoplast was not significantly different between the contrasting varieties. In addition, no significant difference in KOR voltage dependence was found in the whole cell mode between salt-sensitive (‘Gairdner’) and salt-tolerant (‘CM72’) cultivars (Fig. 9B).
Based on the slope of the voltage dependence, a 20 mV depolarization difference, as found between the contrasting cultivars (Fig. 3B), will cause up to a 3-fold difference between their KOR channel open probability. Combining this with the 20 mV difference in driving force for K+, the difference in K+ outward current through KOR channels may indeed equal the difference in the NaCl-induced K+ efflux observed between ‘Gairdner’ and ‘CM72’ cultivars (Fig. 1B). Similar conclusions have been drawn by Murthy and Tester (2006) in a patch-clamp study of Na+-including and -excluding genotypes of capsicum.
For the quantitative comparison of the K+ outward current measured by the patch-clamp technique with K+ loss measured by MIFE, one must also take into the account the differences in external K+ concentration, 5 and 0.5 mm, respectively. A correction can be made based on the biophysical properties of KOR-mediated currents. The properties (selectivity, inhibition by external TEA+, activation kinetics, voltage dependence) of KOR-mediated currents in epidermal protoplasts are very similar to those of KOR currents described for barley xylem parenchyma (Wegner and Raschke, 1994; Wegner and De Boer, 1999). Increase of external K+ causes a shift of KOR activation threshold and the concomitant shift of the reversal potential so that the entire I/V curve is shifted roughly in parallel to the right by approximately 50 mV per 10-fold increase of external K+ concentration (figure 6 in Wegner and De Boer, 1997). Therefore, for ‘CM72’ plants, a specific current at −65 mV (the value of the free-running membrane potential after NaCl application during MIFE K+ flux measurements) in 0.5 mm K+ bath concentration, will be roughly equivalent to the current at −15 mV in a 5 mm K+ bath (patch-clamp conditions, Fig. 8). Respective values for ‘Gairdner’ will be −45 and +5 mV. Now net K+ fluxes measured by MIFE can be compared with currents measured by patch clamp. Assuming a specific membrane capacitance of 1 μF cm−2, 120 and 350 nmol m−2 s−1 net K+ flux (Fig. 1B) are (if all flux is efflux) equivalent to specific currents of 1.16 and 3.36 pA/pF, for salt-tolerant and -sensitive cultivars, respectively. At the same time, from Fig. 8, the K+ outward current at −15 and +5 mV will be 0.75 and 2.4 pA/pF, respectively. Therefore, MIFE and patch results are in a good agreement. Two conclusions can be made from these observations: (1) the major portion of NaCl-induced K+ efflux is mediated by TEA+-sensitive KOR channels, although contributions from other K+ permeable channels cannot be excluded (given the fact that 20 mm TEA suppressed KOR-mediated currents by 91% and 85% in salt-sensitive and salt-tolerant cultivars [Fig. 8C] and nonetheless net K+ fluxes were suppressed by approximately 20% and 50% of control fluxes [Fig. 2], respectively), and (2) the difference in NaCl-induced K+ efflux between salt-sensitive and salt-tolerant barley cultivars mainly reflects the difference in NaCl-induced membrane depolarization, which may, in turn, be primarily determined by the activity of PM H+-transporting ATPases.
Physiological Implications and Prospects for Breeding
Plant salinity tolerance is a polygenic trait with contributions from genetic, developmental, and physiological interactions, in addition to interactions between the plant and its environment. In this study we show that multiple mechanisms are well combined in salt-tolerant barley genotypes, enabling them to withstand saline conditions. In addition to efficient Na+ extrusion (most likely, through a PM Na+/H+ exchanger), better retention of K+ makes a crucial contribution to salinity tolerance in barley. K+ retention is achieved primarily through the 5-fold higher PM H+-ATPase activity in salt-tolerant genotypes, leading to smaller membrane depolarization and, consequently, less K+ efflux through PM K+-permeable channels (primarily KORs). Taken together, this leads both to superior K+ retention in the cell and to a reduced concentration of Na+ in the cytosol. This enables optimal cytosolic K+/Na+ homeostasis, hence, normal cell metabolism even under saline conditions.
Suitable manipulation of the PM Na+ and K+ transporters either to decrease K+ loss via KORs, to enhance H+-pump-fueled Na+ extrusion, or to increase efficiency of inhibition of Na+-sensitive nonselective channels by external Ca2+, could all contribute to improving salinity tolerance in barley and other crops as well. These characters could be introgressed into commercial varieties by marker-assisted selection or by using transgenic methods.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Six barley (Hordeum vulgare) genotypes (three salt tolerant: ‘CM72’, ‘Numar’, and ‘ZUG293’; and three salt sensitive: ‘Franklin’, ‘Gairdner’, and ‘ZUG403’) were obtained from the Australian Winter Cereals Collection and the Barley Genotypic Collection (Zhejiang University, China). General growth conditions and salt treatments were as previously described (Chen et al., 2005; Cuin and Shabala, 2005). Hydroponically grown 3-d-old barley seedlings were used for all electrophysiological and ion depletion experiments. For the H+-ATPase assay, seeds were germinated on wet filter paper in petri dishes, then grown for 7 d in the dark in vermiculite. In both cases, 0.5 mm KCl and 0.1 mm CaCl2 were used as the bath or hydroponic solution. For leaf sap Na+ analysis, barley plants were grown semihydroponically in a glasshouse. Growth medium and condition were previously described in Chen et al. (2005). A total of 320 mm NaCl treatment, added to 3-week-old plants, was reached by starting at 80 mm NaCl with a 40 mm daily increment. Salinity treatment lasted for 4 weeks.
Ion Depletion Experiments
Net Na+ uptake and K+ loss from barley roots were studied in depletion experiments. Roots of 10 intact 3-d-old seedlings were immersed in a plastic vial with 10 mL saline solution (80 mm NaCl, 0.5 mm KCl, and 0.1 mm CaCl2) and aerated with an aquarium air pump. Seedlings were kept at 25°C in the dark for 24 h, then roots were blotted dry, cut, and weighed. Na+ and K+ concentrations in the remaining solution were determined using flame photometry, and net Na+ uptake and K+ loss were calculated on a fresh weight basis. Two independent experiments were conducted with three replicates per cultivar in each experiment.
Leaf Sap Na+ Concentration
Measurement of tissue Na+ concentration was described by Cuin and Shabala (2005). Flag leaves were collected into 1.5-mL microcentrifuge tubes and immediately frozen by liquid nitrogen. A basal opening in the tube allows cell sap but not tissue fragments to pass through to a collection tube. The sample was then thawed and spun for 3 min at 11,000g in a microcentrifuge. The collected sample was measured for its Na+ concentration (in mm) using a flame photometer.
K+ Flux Measurements
Net K+ fluxes were measured at the mature root zone, about 10 mm from the root tip, using the noninvasive ion-selective microelectrode MIFE technique (University of Tasmania, Hobart, Australia) essentially as described by Shabala et al. (1997, 2003). In brief, glass microelectrodes with an external tip diameter of 3 μm were filled with a potassium-selective cocktail (K+ 60031, Fluka). Electrodes were moved in a slow (10-s cycle, 40 μm amplitude) square wave by a computer-driven micromanipulator (Patchman NP2, Eppendorf). From the electrical recordings, net K+ fluxes were calculated as described by Newman (2001).
One hour prior to measurement, a 3-d-old seedling was taken from the growth cabinet and placed in a Perspex measuring chamber containing 10 mL of a saline solution (80 mm NaCl, 0.5 mm KCl, and either 0.1 mm or 1 mm CaCl2). For pharmacological experiments, seedlings were pretreated in the low Ca2+ (0.1 mm) saline solution for 1 h as above. After K+ fluxes had been measured for 30 min, channel blockers (either 20 mm TEA+ or 50 μm GdCl3) were added to the solution and K+ fluxes recorded for another 30 min.
Na+ Influx Measurements
Na+ influx was measured using 22Na+ radiotracer essentially as described by Essah et al. (2003). Entire root systems of 3-d-old seedlings were excised from the shoot (to eliminate potential complications arising from transpiration) and used for experiments immediately after blotting with tissue paper. To determine the steady-state Na+ influx (avoiding the effects of sudden salt shock), seedlings were pretreated for 24 h in the saline solution. Tracer influx was measured from 10 mL of unbuffered saline solution containing approximately 40 kBq mL−1 of 22Na+, at two Ca2+ concentrations (0.1 mm and 10 mm) and over five time durations (1, 2, 5, 10, and 20 min). On average, 10 roots per treatment were measured for each duration. At the end of the influx period, roots were blotted dry and transferred into 500 mL of ice-cold 80 mm NaCl plus 10 mm CaCl2 for two successive rinses of 2 min and then 3 min to displace any apoplastic 22Na+. All solutions were stirred on gently moving shakers at 45 rpm. Roots were blotted gently, rapidly weighed, and transferred to plastic vials containing 2.5 mL scintillation cocktail (Optiphase Hisafe, Fisher Chemicals). Samples were counted with a liquid scintillation counter (Beckman coulter LS6500).
Membrane Potential Measurements
Conventional KCl-filled Ag/AgCl microelectrodes (Shabala and Lew, 2002; Cuin and Shabala, 2005) with a tip diameter of 0.5 μm were used with the MIFE electrometer to measure Em from epidermal cells in the root mature zone. Following cell penetration, Em was recorded for 2 min, then an equal quantity of the standard solution having 160 mm NaCl was added, giving the required 80 mm NaCl concentration after mixing. Measurements continued for at least 20 min after addition of the saline solution. In steady-state experiments, measurements were taken in control roots and in roots 20 min after 80 mm NaCl treatment. At least five individual plants for both control and treated roots were determined, with up to three measurements from each individual root.
Protoplast Isolation for Patch Clamping
An effective protocol for the quick isolation of root epidermal protoplasts was developed by modifying the previously described protocols used for mesophyll protoplasts (Demidchik and Tester, 2002; Shabala et al., 2006). The advantages of the method are: (1) short preparation time (approximately 30 min altogether); (2) minimum contamination of the measuring chamber by cell debris; (3) direct release of the fresh protoplasts into the measuring chamber, without any centrifugation step (hence with minimal disturbance to protoplasts); and (4) a fresh isolation for each patch-clamp measurement (hence, higher success rate of gigaohm seal formation).
According to the protocol developed, a 3-d-old barley seedling was removed from the growing container. Seminal roots were cut at about 5 mm below the seed and their apical 7 to 10 mm were also cut and discarded. The remaining segments were cut into approximately 10 mm lengths and split longitudinally under a dissecting microscope. Split root segments were placed into 3 mL of the enzyme solution containing 2% (w/v) cellulose (Yakult Honsha), 1.2% (w/v) cellulysin (Biosciences Inc.), 0.1% (w/v) pectolyase, 0.1% (w/v) bovine serum albumin, 10 mm KCl, 10 mm CaCl2, and 2 mm MgCl2, pH 5.7 adjusted with 2 mm MES. All chemicals and reagents were purchased from Sigma unless specified otherwise. The osmolality of the enzyme solution was adjusted to 760 to 800 mOsM with mannitol. After 20 to 25 min of incubation in the enzyme solution (in the dark at 30°C; agitated on a 90 rpm rotary shaker), root segments were transferred to the so-called wash solution (as above, minus enzymes) and thoroughly washed for another 2 min. Segments were then transferred into the measuring chamber filled with release solution (10 mm KCl, 2 mm CaCl2, 1 mm MgCl2; 2 mm MES, pH 5.7; osmolality 380 mOsM). By gently shaking the plasmolyzed and digested root tissue, protoplasts were released into the measuring chamber. Root tissues were removed from the solution and the chamber was then perfused with the bath solution used for patch-clamp experiments (see next section), removing all protoplasts that were not attached to the bottom of the measuring chamber.
The above protocol provides protoplasts from entire roots. We wished to use epidermal protoplasts for optimal match with the Em and flux studies. To the best of our knowledge, no suitable tissue-specific staining technique is available for barley to provide specific tissue identification. As a result, protoplast selection for patch-clamp experiments was based on the protoplast diameter (approximately 20 μm), which is indicative of epidermal origin. To justify this choice, separate experiments were undertaken. Protoplasts were isolated from (1) the whole root; (2) isolated root epidermis; (3) stele; and (4) cortex. Overall, more than 5,000 protoplasts were measured (Supplemental Table S1). Our results showed that cortical protoplasts were twice as large as those isolated from epidermal or stele tissues, thus they could be easily distinguished and avoided in patch experiments. Accordingly, protoplasts isolated from the whole root showed a clear bimodal distribution in diameter (Supplemental Fig. S1). The average diameter of stele and epidermal protoplasts, however, was somewhat similar (Supplemental Table S1) and close to the 20 μm size chosen (Supplemental Fig. S1, arrow) for patch experiments. However, the yield of stellar protoplasts was much lower (only 6% compared with epidermal ones), perhaps due to the lignification pattern of the stele making it almost inaccessible to enzymes during the digestion. Therefore, although we cannot exclude the possibility that some protoplasts measured in our study originated from the xylem parenchyma, the proportion of them is low (approximately 6%; Supplemental Fig. S1; Supplemental Table S1).
Patch-Clamp Experiments
Barley root protoplasts of 14 to 22 μm diameter were patch clamped in the whole-cell mode. GΩ resistance seals were obtained in the bath solution containing (in mm): 1 to 2 CaCl2, 5 KCl, 2 MES, pH 5.7, and 500 mOsM adjusted with d-sorbitol. The basic pipette solution (PS) contained (in mm): 100 KCl, 2 MgCl2, 0.8 CaCl2 (100 nm free Ca2+), 2 EGTA, and 10 HEPES, pH 7.4 adjusted with Tris base. Osmolality of the PS was 560 mOsM.
Measurements were made using an Axopatch 200 patch-clamp amplifier (Axon Instruments) in the conventional whole cell configuration as described by Shabala et al. (2006). Membrane potentials were clamped at −100 mV throughout the experiments, and voltage pulses were applied in 20 mV steps, from −160 mV to +80 or + 100 mV. Typical access resistance was 11 to 32 MΩ, and mean whole cell capacitance, 11.9 ± 0.8 and 11.0 ± 1.0 pF for ‘Gairdner’ and ‘CM72’ protoplasts, respectively.
Isolation of PMs for PM H+-ATPase Assay
Barley roots (5–12 g fresh weight) were rinsed with bathing solution or water to remove the vermiculite. Roots were then homogenized in 200 mL buffer (250 mm Tris-HCl pH 8.0, 300 mm Suc, 25 mm EDTA, 5 mm dithiothreitol, 5 mm ascorbate, 0.6% polyvinylpyrrolidone, and 1 mm phenylmethylsulfonyl fluoride) containing phosphatase inhibitors (25 mm NaF, 1 mm NaMo, 50 mm Na pyrophosphate). PMs were isolated from the microsomal fraction (30,000 g) by partitioning at 4°C in an aqueous polymer two-phase system (9 g + 3 g) composed of 6.3% (w/w) dextran T500 (Amersham Biosciences, GE Healthcare), 6.3% (w/w) polyethylene glycol PEG 1500 (Sigma), 330 mm Suc, 5 mm potassium phosphate pH 7.8, 3 mm KCl, 0.1 mm EDTA, and 1 mm dithiothreitol (Palmgren, 1990; Larsson et al., 1994). The final PM pellet was suspended in 330 mm Suc, 5 mm potassium phosphate pH 7.8, 50 mm KCl, and 5 mm EDTA.
Western Blotting
Protein concentration was determined by Bradford assay using γ-globulin as a reference. Proteins (20 μg/lane) were solubilized in SDS cocktail and subjected to SDS-PAGE (10%). Proteins were transferred to nitrocellulose paper for immunostaining. Antiserum number 759 against the C-terminal domain of the PM H+-ATPase was employed (dilution 1:4,000; Pardo and Serrano, 1989).
ATPase Assay
ATP hydrolytic activity was measured essentially as described by Regenberg et al. (1995). The assay medium (20 mm MOPS, 8 mm MgSO4, 50 mm KNO3, 5 mm NaNO3, 250 μm NaMo; pH adjusted to 6.5 or 7.0 with KOH) included 3 mm ATP and 0.02% Brij-58. The PMs were preincubated for 10 min with 0.02% Brij-58 to obtain inside-out vesicles. The reaction was initiated by the addition of 2 μg of barley PMs to the assay medium.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Protoplast size distributions for the whole root and the three tissue types isolated. Arrow shows the protoplast size chosen for patch experiments.
Supplemental Table S1. Basic characteristics of protoplasts isolated from different parts of the mature region of barley roots.
Supplementary Material
This work was supported by Australian Research Council (ARC) Discovery (grant no. DP0449856) and Department of Education, Science and Training (DEST) grants (to S.S.), Grains Research and Development Corporation (GRDC; UT8) and DEST grants (to M.Z.), and ARC Discovery (grant no. A00105708 to I.A.N.). M.T. was supported by the ARC and GRDC.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Sergey Shabala (sergey.shabala@utas.edu.au).
Some figures in this article are displayed in color online but in black and white in the print edition.
The online version of this article contains Web-only data.
References
- Apse MP, Aharon GS, Sneddon WA, Blumwald E (1999) Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science 285 1256–1258 [DOI] [PubMed] [Google Scholar]
- Berthomieu P, Conéjéro G, Nublat A, Brackenbury WJ, Lambert C, Savio C, Uozumi N, Oiki S, Yamada K, Cellier F, et al (2003) Functional analysis of AtHKT1 in Arabidopsis shows that Na+ recirculation by the phloem is crucial for salt tolerance. EMBO J 22 2004–2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumwald E (2000) Sodium transport and salt tolerance in plants. Curr Opin Cell Biol 12 431–434 [DOI] [PubMed] [Google Scholar]
- Blumwald E, Aharon GS, Apse MP (2000) Sodium transport in plant cells. Biochim Biophys Acta 1465 140–151 [DOI] [PubMed] [Google Scholar]
- Bordas M, Montesinos C, Dabauza M, Salvador A, Roig LA, Serrano R, Moreno V (1997) Transfer of the yeast salt tolerance gene HAL1 to Cucumis melo L. cultivars and in vitro evaluation of salt tolerance. Transgenic Res 5 1–10 [DOI] [PubMed] [Google Scholar]
- Carden DE, Walker DJ, Flowers TJ, Miller AJ (2003) Single-cell measurements of the contributions of cytosolic Na+ and K+ to salt tolerance. Plant Physiol 131 676–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Newman I, Zhou M, Mendham N, Zhang G, Shabala S (2005) Screening plants for salt tolerance by measuring K+ flux: a case study for barley. Plant Cell Environ 28 1230–1246 [Google Scholar]
- Chen Z, Zhou M, Newman I, Mendham N, Zhang G, Shabala S (2007) Potassium and sodium relations in salinised barley tissues as a basis of differential salt tolerance. Funct Plant Biol 34 150–162 [DOI] [PubMed] [Google Scholar]
- Cuin TA, Miller AJ, Laurie SA, Leigh R (2003) Potassium activities in cell compartments of salt-grown barley leaves. J Exp Bot 54 657–661 [DOI] [PubMed] [Google Scholar]
- Cuin TA, Shabala S (2005) Exogenously supplied compatible solutes rapidly ameliorate NaCl-induced potassium efflux from barley roots. Plant Cell Physiol 46 1924–1933 [DOI] [PubMed] [Google Scholar]
- Cuin TA, Shabala S (2006) Potassium homeostasis in salinised plant tissues. In A Volkov, ed, Plant Electrophysiology—Theory and Methods. Springer, Heidelberg, pp 287–317
- Davenport RJ, James RA, Zakrisson-Plogander A, Tester M, Munns R (2005) Control of sodium transport in durum wheat. Plant Physiol 137 807–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport RJ, Reid RJ, Smith FA (1997) Sodium-calcium interactions in two wheat species differing in salinity tolerance. Physiol Plant 99 323–327 [Google Scholar]
- Davenport RJ, Tester M (2000) A weakly voltage-dependent, non-selective cation channel mediates toxic sodium influx in wheat. Plant Physiol 122 823–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demidchik V, Bowen HC, Maathuis FJM, Shabala SN, Tester MA, White PJ, Davies JM (2002) Arabidopsis thaliana root nonselective cation channels mediate calcium uptake and are involved in growth. Plant J 32 799–808 [DOI] [PubMed] [Google Scholar]
- Demidchik V, Maathuis FJM (2007) Physiological roles of nonselective cation channels in plants: from salt stress to signalling and development. New Phytol 175 387–404 [DOI] [PubMed] [Google Scholar]
- Demidchik V, Tester MA (2002) Sodium fluxes through nonselective cation channels in the plant plasma membrane of protoplasts from Arabidopsis roots. Plant Physiol 128 379–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubcovsky J, Luo MC, Zhong GY, Bransteiter R, Desai A, Kilian A, Kleinhofs A, Dvorak J (1996) Genetic map of diploid wheat, Triticum monococcum L., and its comparison with maps of Hordeum vulgare L. Genetics 143 983–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkahoui S, Carvajal M, Ghrir R, Limam F (2005) Study of the involvement of osmotic adjustment and H+-ATPase activity in the resistance of Catharanthus roseus suspension cells to salt stress. Plant Cell Tissue Organ Cult 80 287–294 [Google Scholar]
- Espinosa-Ruiz A, Belles JM, Serrano R, Culianez-Macia FA (1999) Arabidopsis thaliana AtHAL3: a flavoprotein related to salt and osmotic tolerance and plant growth. Plant J 20 529–539 [DOI] [PubMed] [Google Scholar]
- Essah PA, Davenport R, Tester M (2003) Sodium influx and accumulation in Arabidopsis. Plant Physiol 133 307–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flowers TJ (2004) Improving crop salt tolerance. J Exp Bot 55 307–319 [DOI] [PubMed] [Google Scholar]
- Gaxiola R, De Larrinoa IF, Villalba JM, Serrano R (1992) A novel and conserved salt-induced protein is an important determinant of salt tolerance in yeast. EMBO J 11 3157–3164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisbert C, Rus AM, Bolarín MC, Coronado JM, Arrillaga I, Montesinos C, Caro M, Serrano R, Moreno V (2000) The yeast HAL1 gene improves salt tolerance of transgenic tomato. Plant Physiol 123 393–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorham J, Bristol A, Young EM, Wyn Jones RG, Kashour G (1990) Salt tolerance in the Triticeae: K+/Na+ discrimination in barley. J Exp Bot 41 1095–1101 [Google Scholar]
- Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ (2000) Plant cellular and molecular responses to high salinity. Annu Rev Plant Biol 51 463–499 [DOI] [PubMed] [Google Scholar]
- Larsson C, Sommarin M, Widell S (1994) Isolation of highly purified plasma membranes and the separation of inside-out and right-side-out vesicles. Methods Enzymol 228 451–469 [Google Scholar]
- Lohaus G, Hussmann M, Pennewiss K, Schneider H, Zhu JJ, Sattelmacher B (2000) Solute balance of a maize (Zea mays L.) source leaf as affected by salt treatment with special emphasis on phloem retranslocation and ion leaching. J Exp Bot 51 1721–1732 [DOI] [PubMed] [Google Scholar]
- Maathuis FJM, Amtmann A (1999) K+ nutrition and Na+ toxicity: the basis of cellular K+/Na+ ratios. Ann Bot (Lond) 84 123–133 [Google Scholar]
- Maathuis FJM, Ichida AM, Sanders D, Schroeder JI (1997) Roles of higher plant K+ channels. Plant Physiol 114 1141–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelet B, Boutry M (1995) The plasma membrane H+-ATPase: a highly regulated enzyme with multiple physiological functions. Plant Physiol 108 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munns R (2002) Comparative physiology of salt and water stress. Plant Cell Environ 25 239–250 [DOI] [PubMed] [Google Scholar]
- Munns R (2005) Genes and salt tolerance: bringing them together. New Phytol 167 645–663 [DOI] [PubMed] [Google Scholar]
- Murthy M, Tester M (2006) Cation currents in protoplasts from the roots of a Na+ hyperaccumulating mutant of Capsicum annuum. J Exp Bot 57 1171–1180 [DOI] [PubMed] [Google Scholar]
- Newman IA (2001) Ion transport in roots: measurement of fluxes using ion-selective microelectrodes to characterize transporter function. Plant Cell Environ 24 1–14 [DOI] [PubMed] [Google Scholar]
- Niu X, Bressan RA, Hasegawa PM, Pardo JM (1995) Ion homeostasis in NaCl stress environments. Plant Physiol 109 735–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nublat A, Desplans J, Casse F, Berthomieu P (2001) sas1, an Arabidopsis mutant overaccumulating sodium in the shoot, shows deficiency in the control of the root radial transport of sodium. Plant Cell 13 125–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmgren MG (1990) An H+-ATPase assay: proton pumping and ATPase activity determined simultaneously in the same sample. Plant Physiol 94 882–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmgren MG (2001) Plant plasma membrane H+-ATPases: powerhouses for nutrient uptake. Annu Rev Plant Physiol Plant Mol Biol 52 817–845 [DOI] [PubMed] [Google Scholar]
- Pardo JM, Serrano R (1989) Structure of a plasma membrane H+-ATPase gene from the plant Arabidopsis thaliana. J Biol Chem 264 8557–8562 [PubMed] [Google Scholar]
- Regenberg B, Villalba JM, Lanfermeijer FC, Palmgren MG (1995) C-terminal deletion analysis of plant plasma membrane H+-ATPase: yeast as a model system for solute transport across the plant plasma membrane. Plant Cell 7 1655–1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts SK, Tester M (1997) Patch clamp study of Na+ transport in maize roots. J Exp Bot 48 431–440 [DOI] [PubMed] [Google Scholar]
- Rubio F, Gassmann W, Schroeder JI (1995) Sodium-driven potassium uptake by the plant potassium transporter HKT1 and mutations conferring salt tolerance. Science 270 1660–1663 [DOI] [PubMed] [Google Scholar]
- Rus A, Yokoi S, Sharkhuu A, Reddy M, Lee BH, Matsumoto TK, Koiwa H, Zhu JK, Bressan RA, Hasegawa PM (2001) AtHKT1 is a salt tolerance determinant that controls Na+ entry into plant roots. Proc Natl Acad Sci USA 98 14150–14155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtman DP, Liu W (1999) Molecular pieces to the puzzle of the interaction between potassium and sodium uptake in plants. Trends Plant Sci 4 281–287 [DOI] [PubMed] [Google Scholar]
- Serrano R, Rodriguez-Navarro A (2001) Ion homeostasis during salt stress in plants. Curr Opin Cell Biol 13 399–404 [DOI] [PubMed] [Google Scholar]
- Shabala L, Cuin TA, Newman I, Shabala S (2005) Salinity-induced ion flux patterns from the excised roots of Arabidopsis sos mutants. Planta 222 1041–1050 [DOI] [PubMed] [Google Scholar]
- Shabala S (2000) Ionic and osmotic components of salt stress specifically modulate net ion fluxes from bean leaf mesophyll. Plant Cell Environ 23 825–837 [Google Scholar]
- Shabala S, Demidchik V, Shabala L, Cuin TA, Smith SJ, Miller AJ, Davies JM, Newman IA (2006) Extracellular Ca2+ ameliorates NaCl-induced K+ loss from Arabidopsis root and leaf cells by controlling plasma membrane K+-permeable channels. Plant Physiol 141 1653–1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabala S, Lew RR (2002) Turgor regulation in osmotically stressed Arabidopsis thaliana epidermal root cells: direct support for the role of inorganic ion uptake as revealed by concurrent flux and cell turgor measurements. Plant Physiol 129 290–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabala S, Newman IA, Morris J (1997) Oscillations in H+ and Ca2+ ion fluxes around the elongation region of corn roots and effects of external pH. Plant Physiol 113 111–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabala S, Shabala L, Volkenburgh E (2003) Effect of calcium on root development and root ion fluxes in salinized barley seedlings. Funct Plant Biol 30 507–514 [DOI] [PubMed] [Google Scholar]
- Shi H, Quintero FJ, Pardo JM, Zhu JK (2002) The putative plasma membrane Na+/H+ antiporter SOS1 controls long distance Na+ transport in plants. Plant Cell 14 465–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tester M, Davenport R (2003) Na+ tolerance and Na+ transport in higher plants. Ann Bot (Lond) 91 503–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisdale SL, Nelson WL, Beaton JD, Havlin JL (1993) Soil Fertility and Fertilisers, Ed 5. Prentice-Hall, Upper Saddle River, NJ
- Volkov V, Amtmann A (2006) Thellungiella halophila, a salt-tolerant relative of Arabidopsis thaliana, has specific root ion-channel features supporting K+/Na+ homeostasis under salinity stress. Plant J 48 342–353 [DOI] [PubMed] [Google Scholar]
- Volkov V, Wang B, Dominy PJ, Fricke W, Amtmann A (2004) Thellungiella halophila, a salt-tolerant relative of Arabidopsis thaliana, possesses effective mechanisms to discriminate between potassium and sodium. Plant Cell Environ 27 1–14 [Google Scholar]
- Wegner LH, De Boer AH (1997) Properties of two outward-rectifying channels in root xylem parenchyma cells suggest a role in K+ homeostasis and long-distance signaling. Plant Physiol 115 1707–1719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner LH, De Boer AH (1999) Activation kinetics of the K+ outward rectifying conductance (KORC) in xylem parenchyma cells from barley root. J Membr Biol 170 103–119 [DOI] [PubMed] [Google Scholar]
- Wegner LH, Raschke K (1994) Ion channels in the xylem parenchyma of barley roots. Plant Physiol 105 799–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White PJ, Davenport RJ (2002) The voltage independent cation channel in the plasma membrane of wheat roots is permeable to divalent cations and may be involved in cytosolic Ca2+ homeostasis. Plant Physiol 130 1386–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Zhang F, Zhao M, An L, Zhang L, Chen N (2006) Properties of plasma membrane H+-ATPase in salt-treated Populus euphratica callus. Plant Cell Rep 26 229–235 [DOI] [PubMed] [Google Scholar]
- Zhang HX, Blumwald E (2001) Transgenic salt-tolerant tomato plants accumulate salt in foliage but not in fruit. Nat Biotechnol 19 765–768 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.