Abstract
Human group II secretory phospholipase A2 (sPLA2) is an enzyme found in the α granules of platelets and at inflammatory sites. Although its physiological function is unclear, sPLA2 can inhibit blood coagulation reactions independent of its lipolytic action. To study the molecular basis of PLA2 activities, we developed a total chemical synthesis of sPLA2 by chemical ligation of large unprotected peptides. The synthetic segments PLA2-(1–58)-αCOSCH2COOH and PLA2-(59–124) were prepared by stepwise solid-phase peptide synthesis and ligated to yield a peptide bond between Gly58 and Cys59. The 124-residue polypeptide product (mass: 13,920 ± 2 Da) was folded to yield one major product (mass: 13,905 ± 1 Da), the loss of 15 ± 3 Da reflecting the formation of seven disulfide bonds. Circular dichroism studies of synthetic sPLA2 showed α-helix, β-structure, and random coil contents consistent with those found in the crystal structure of sPLA2. Synthetic sPLA2 had kcat and Km values identical to those of recombinant sPLA2 for hydrolysis of 1,2-bis(heptanoylthio)-phosphatidylcholine. Synthetic sPLA2, like recombinant sPLA2, inhibited thrombin generation from prothrombinase complex (factors Xa, V, II, Ca2+, and phospholipids). In the absence of phospholipids, both synthetic and recombinant sPLA2 inhibited by 70% prothrombin activation by factors Xa, Va, and Ca2+. Thus, synthetic sPLA2 is a phospholipid-independent anticoagulant like recombinant or natural sPLA2. This study demonstrates that chemical synthesis of sPLA2 yields a fully active native-like enzyme and offers a straightforward tool to provide sPLA2 analogs for structure–activity studies of anticoagulant, lipolytic, or inflammatory activities.
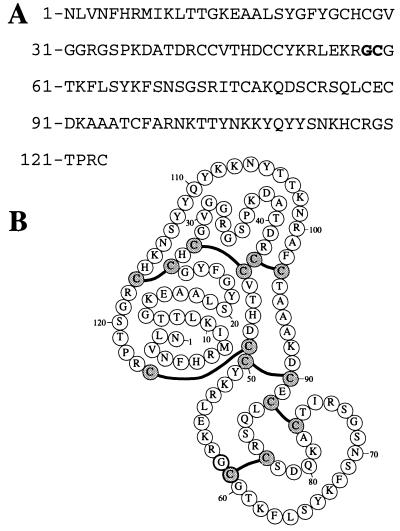
Secretory phospholipases A2 (sPLA2) catalyze the stereospecific hydrolylis of the fatty acid side chain ester bond at the 2 position of phospholipids in a Ca2+-dependent reaction (for a review see ref. 1). On the basis of similarities in polypeptide sequences, sPLA2 have been classified into three major groups, I, II, and III (2). Human sPLA2 purified from α granules of blood platelets and synovial fluid belongs to group II (3, 4) and is a 14-kDa enzyme consisting of a single polypeptide chain of 124 amino acids that contains 14 cysteine residues in seven disulfide bonds (4–6) (Fig. 1).
Figure 1.
(A) Amino acid sequence of the 124 residue human group II secretory phospholipase A2 (sPLA2). The Gly58-Cys59 ligation site is depicted in boldface type. (B) The covalent structure of the folded sPLA2 molecule. Disulfide-bonded cysteines are depicted in light gray, the boldface Gly58 residue is involved in the Gly58-Cys59 native chemical ligation site.
The physiological functions of sPLA2 remain unclear. sPLA2 has been proposed to be involved in inflammatory diseases, degradation of bacteria, and exocytosis/degranulation processes (1). Some snake-venom sPLA2 molecules express anticoagulant activity by inhibiting the prothrombinase complex activity and/or the extrinsic factor X-activating complex (7). It was proposed that in addition to the lipolytic activity due to the enzyme molecule, basic residues located in a variable surface loop (residues 51–77) were involved in the anticoagulant action of venom sPLA2 (8–10). Human sPLA2 exerts anticoagulant activity in plasma (11) and inhibits prothrombinase activity independent of its lipolytic activity (12). Thus, interesting questions arise concerning the structure–activity relationships of the sPLA2 molecule in the context of physiologically important activities.
The aim of the current study was to establish access to human sPLA2 through total chemical synthesis. This was achieved by native chemical ligation (13) of two large unprotected synthetic peptide segments (14). Extensive chemical, enzymatic, and biochemical characterizations of synthetic sPLA2 were performed. The results show that synthetic sPLA2 is indistinguishable from recombinant sPLA2. Total chemical synthesis of sPLA2 should allow the incorporation of noncoded elements such as unnatural amino acids and/or labeling with fluorescent, NMR, or x-ray crystallographic probes at specific sites and will establish the versatility of synthetic sPLA2 as a model to study the anticoagulant properties of sPLA2
EXPERIMENTAL PROCEDURES
Materials.
2-(1H-Benzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HBTU) and tert-butoxycarbonyl (Boc)-amino acids were from Nova Biochem; Boc-Arg (p-toluenesulfonyl)-OH and Boc-Asn(xanthyl)-OH were from Bachem Bioscience (King of Prussia, PA). Boc-Gly-thioester resin was prepared as described (15). Boc-Cys-OCH2Pam resin and N,N-diisopropylethylamine (DIEA) were obtained from Applied Biosystems. N,N-Dimethylformamide (DMF) and HPLC-grade acetonitrile were purchased from Fischer. Trifluoroacetic acid (TFA) was obtained from Halocarbon (River Edge, NJ). HF was purchased from Matheson Gas (Cucamonga, CA). 1,2-bis(Heptanoylthio)-phosphatidylcholine (dithio-PC) and 5,5′-dithiobis(2-nitrobenzoic) acid were purchased from Cayman Chemical Company (Ann Arbor, MI). Factor Xa was obtained from Enzyme Research Laboratories (South Bend, IN), and factor V(a) (16) and prothrombin (17) were purified as described. Phosphatidylcholine (PC) and phosphatidylserine were purchased from Sigma.
Peptide Synthesis.
The N-terminal sPLA2 peptide (residues 1–58) and the C-terminal sPLA2 peptide (residues 59–124) were prepared by solid-phase peptide synthesis (SPPS) using the in situ neutralization/HBTU activation procedure for Boc chemistry as described (14). The N-terminal peptide was synthesized on Boc-Gly-S resin (15) (0.7 mmol/g of loaded resin), and the C-terminal peptide was synthesized on Boc-Cys-OCH2Pam resin (0.7 mmol/g of loaded resin) by using machine-assisted protocols on a custom-modified Applied Biosystems model 430A peptide synthesizer (14). Each synthetic cycle consisted of Nα-Boc removal by a 1- to 2-min treatment with TFA, a 1-min DMF flow wash, a 5- to 10-min coupling time with 2.0 mmol of activated Boc-amino acid in the presence of excess DIEA, and a second DMF flow wash. Nα-Boc amino acids (2.2 mmol) were preactivated for 3 min with 2.0 mmol of HBTU in the presence of excess DIEA (6 mmol). After each coupling step, yields were determined by measuring residual free amine with the quantitative ninhydrin assay (18). After coupling of Gln residues, a dichloromethane flow wash was used before and after deprotection using TFA, to prevent possible high-temperature (TFA/DMF)-catalyzed pyrrolidonecarboxylic acid formation (14). Side-chain-protected amino acids were Boc-Arg (p-toluenesulfonyl)-OH, Boc-Asn(xanthyl)-OH, Boc-Asp(O-cyclohexyl)-OH, Boc-Cys(4-methylbenzyl)-OH, Boc-Glu(O-cyclohexyl)-OH, Boc-His(dinitrophenyl)-OH, Boc-Lys(2-Cl-Z)-OH, Boc-Ser(benzyl)-OH, Boc-Thr(benzyl)-OH, and Boc-Tyr(2-Br-Z)-OH. Other amino acids were used without side-chain protection.
After chain assembly was completed, the peptides were deprotected and cleaved from the resin. For the C-terminal segment (residues 59–124), 2,4-dinitrophenyl (DNP) groups were removed from the His side chains by treatment with 20% 2-mercaptoethanol/10% DIEA in DMF (two 30-min treatments), then the Nα-Boc group was removed with TFA and the peptide resin was treated with 10% DIEA in DMF for two 1-min periods. The neutralized peptide resin was washed with DMF, dichloromethane, and dried. Remaining side-chain protecting groups were removed and the peptide was cleaved from the resin by treatment with anhydrous HF for 1 hr at 0°C with 4% p-cresol/p-thiocresol as a scavenger. The N-terminal peptide segment (residues 1–58) was treated as described above, although the His (DNP) groups were left intact and HF treatment was performed in the presence of 4% p-cresol. After cleavage, both peptides were precipitated, washed with ice-cold diethyl ether, dissolved in aqueous acetonitrile, and lyophilized. The crude N-terminal thioacid peptide (1–58-αCOSH) was dissolved at 10 mg/ml in 0.1 M sodium acetate/6 M guanidine hydrochloride (GuHCl) at pH 4.0 and treated with a 10-fold molar excess of bromoacetic acid. After 20 min, the mixture was applied to semipreparative HPLC for purification of the N-terminal peptide-αthioester, which was isolated and lyophilized.
High Pressure Liquid Chromatography (HPLC).
Analytical reversed-phase HPLC was performed on a Hewlett Packard HPLC 1050 system using Vydac C18 columns (5 μm, 0.46 × 15 cm). Semipreparative reversed-phase HPLC was performed on a Rainin HPLC system using a Vydac C18 column (10 μm, 1.0 × 25 cm). Linear gradients of acetonitrile in water/0.1% TFA were used to elute bound peptides. The flow rates used were 1 ml/min (analytical) and 3 ml/min (semipreparative).
Electrospray Mass Spectrometry (ESMS).
Mass spectra of all peptides were obtained with an API-III quadrupole electrospray mass spectrometer (PE-Sciex). Peptide masses were calculated from the experimental mass-to-charge (m/z) ratios from all the observed protonation states of a peptide with macspec software (Sciex, Thornhill, ON, Canada). Theoretical masses were calculated using macpromass software (Beckman).
Native Chemical Ligation.
The ligation of the two unprotected synthetic peptide segments was performed as described (13). Briefly, benzylmercaptan (1%) and thiophenol (1%) were added to a solution of peptide 1–58-COSCH2COOH (9 mg/ml) and 59–124-COOH (9 mg/ml) in 0.1 M sodium phosphate/6 M GuHCl, pH 7.5. The ligation mixture was stirred for 22 hr at 37°C, and the reaction was monitored by HPLC and ESMS until completion. The mixture was subsequently treated with 10% 2-mercaptoethanol to remove remaining side-chain protecting (DNP) groups from histidine residues and with a 10-fold excess of Tris(2-carboxyethyl)phosphine to eliminate apparent 2-mercaptoethanol adducts of the cysteine-rich peptides. Semipreparative HPLC fractions containing the polypeptide chain of sPLA2 (residues 1–124) were identified by ESMS, pooled, and lyophilized.
Folding of sPLA2.
The purified reduced 124-amino acid polypeptide chain of sPLA2 was folded at a concentration of 0.2 mg/ml in 10 mM sodium borate buffer containing 0.9 M guanidine, 10 mM CaCl2, 8 mM l-cysteine, and 1 mM l-cystine, at pH 8.5, for 48 hr at 4°C (12). Formation of disulfides was monitored by analytical HPLC and ESMS. The folded disulfide-crosslinked product was subsequently purified by using semipreparative HPLC.
Recombinant sPLA2.
Recombinant Leu8-sPLA2 was expressed in E. coli as a fusion protein and purified as described (12, 19). The Leu8-sPLA2 variant retains full enzymatic activity (12, 19) and is herein called recombinant sPLA2.
Enzymatic sPLA2 Activity Assay.
The hydrolysis of dithio-PC substrate (20) by sPLA2 was analyzed on a HP-8452 diode array spectrophotometer at 40°C as described (21). Km and kcat values were determined with enzfitter software (Elsevier-Biosoft, Cambridge, U.K.).
Circular Dichroism (CD) Spectroscopy.
CD spectra were recorded on an AVIV 60DS spectropolarimeter at 25°C. Protein samples were dissolved in 25 mM boric acid at pH 7.5 and their concentrations were determined by quantitative amino acid analysis after acid hydrolysis. Secondary structure parameters were calculated with prosec software (Aviv Associates, Lakewood, NJ).
sPLA2 Anticoagulant Activity.
Factor Xa (20 pM) was preincubated with synthetic or recombinant sPLA2 (2.5 μM) in assay buffer [50 mM Tris⋅HCl/100 mM NaCl/0.02% azide, pH 7.4 (TBS) containing 5 mM CaCl2 and BSA at 0.1 mg/ml] at 37°C. After 15 min, a mixture of factor V (120 pM), prothrombin (1 μM), and phospholipid vesicles (5 μM phosphatidylserine/PC, 10:90) in the same buffer was added, and at different times, aliquots (10 μl) were transferred into microtiter wells containing 40 μl of amidolytic buffer (50 mM Tris⋅HCl/100 mM NaCl/0.02% azide/0.1% BSA, pH 8.35) and 50 mM EDTA to quench the prothrombinase reaction. Subsequently, 150 μl of 0.3 mM of the thrombin chromogenic substrate H-d-cyclohexylglycyl-l-α-aminobutyryl-l-arginine-p-nitroanilide was added to each well and the rate of hydrolysis was determined at 405 nm. Dilutions of purified α-thrombin were used to give a calibration curve. In other studies, factor Xa and Va (each at 2.5 nM) were preincubated with sPLA2 (2.5 μM) at 37°C in assay buffer for 10 min before prothrombin (1 μM) was added; thrombin generation was determined at different times (t = 10, 20, 30, 45, or 60 min) as described above.
RESULTS AND DISCUSSION
Human sPLA2 containing 124 amino acids and seven disulfide bonds (Fig. 1) was prepared by total chemical synthesis using native chemical ligation (13) of large unprotected peptide segments (14). Native chemical ligation is an extension of the chemoselective ligation approach (22), yielding an nonnative thioester bond at the ligation site (14, 22–24). In the native chemical ligation procedure, an initial thioester bond formed at the ligation site spontaneously rearranges to give a native backbone amide bond joining the two polypeptide segments at Xaa-Cys (13, 25).
Synthetic Strategy.
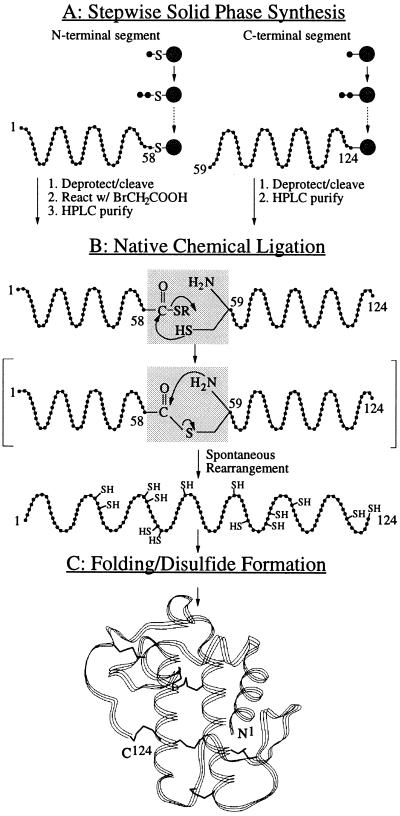
sPLA2 contained three Xaa-Cys ligation sites approximately in the middle of the polypeptide chain (Asp48-Cys49, Gly58-Cys59, and Thr76-Cys77; Fig. 1). The choice of the Gly-Cys ligation site has the advantage of a sterically unhindered Gly residue that is most centrally located within the sPLA2 polypeptide chain. Moreover, the use of a Gly-Cys ligation site obviates any possible racemization at the C-terminal residue of the N-terminal segment (26). For these reasons, and because it was previously shown that the use of a Gly-Cys ligation site allows rapid native chemical ligation (13, 27), the Gly58-Cys59 site was the preferred target of the chemical ligation strategy. Completion of the ligation reaction to form an amide bond at the ligation site could be demonstrated by the ability of the Cys59 to form a disulfide bond with its partner, possible only after the spontaneous rearrangement of the initial thioester yielding the free Cys59 (Fig. 2).
Figure 2.
Strategy for the total chemical synthesis of human sPLA2. (A) The 66-residue C-terminal segment of sPLA2 and the 58-residue N-terminal peptide-αCOSH segment were synthesized by stepwise SPPS techniques using Boc chemistry protocols, and the N-terminal thioacid peptide was activated by reaction with bromoacetic acid to form the peptide-αthioester. (B) Native chemical ligation of the two unprotected peptide segments results in formation of an amide bond at Gly58-Cys59. The two segments are initially joined by thioester formation (not observed as a discrete intermediate); subsequent spontaneous rapid rearrangement forms a native peptide bond at the ligation site (13). (C) Folding of the synthetic 124-residue polypeptide chain results in the formation of seven disulfide bonds and the generation of the active sPLA2 enzyme molecule.
A special note about the choice of the protecting group of the His side chain is appropriate. When benzyloxymethyl-protected His was used for peptide synthesis, deprotection of the His residues occurred during anhydrous HF treatment at 0°C, accompanied by the generation of one equivalent of formaldehyde for every His residue. This contaminating formaldehyde could not be quantitatively removed by HPLC purification and lyophilization and lead to an instantaneous formyl cyclization of more than 75% of the N-terminal Cys under the ligation conditions. This reaction product, observed as a +12-Da derivative of the C-terminal segment, was unreactive for ligation. To avoid this side reaction, His(DNP) was used in the synthesis of both peptide segments.
Synthesis of the Peptide Segment sPLA2-(59–124).
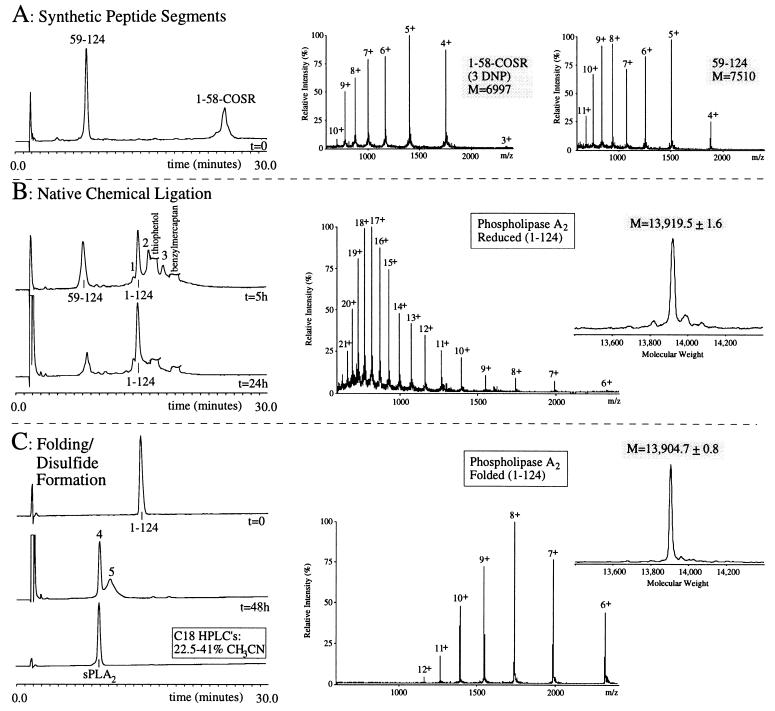
The C-terminal segment of human sPLA2 was synthesized by optimized SPPS (14) on Boc-Cys-(4-CH3Bzl)OCH2Pam resin on a 0.15-mmol scale. Subsequent machine-assisted coupling of residues 123 through 59 was achieved as described (Fig. 2A). After the deprotection and cleavage from the solid support, the crude 59–124 peptide with a C-terminal α-carboxyl group was obtained. ESMS revealed a mass of 7,510.1 ± 2.6 Da, corresponding to the calculated mass for the reduced peptide (7,510.6 Da, average isotope composition). The peptide was purified by semipreparative HPLC (C18; 20.7–29.7% CH3CN; 0.15% per min), lyophilized, and stored at −20°C after lyophilization (Fig. 3A, 59–124). The yield for cleavage of 16 μmol of 59–124 peptide resin was 13 μmol of crude and 1 μmol of purified 59–124 polypeptide.
Figure 3.
Total synthesis of sPLA2 by native chemical ligation. (A) Reaction at t = 0. HPLC chromatogram of the starting synthetic peptide segments (Cys59-124) and (1-Gly58)-αCOSR, dissolved at 9 mg/ml in phosphate buffer (pH 7.5) containing 6 M GuHCl. ESMS spectra show the m/z ratios of the (1–58)-αCOSR peptide (3rd–10th ionized states) observed mass 6,996.5 ± 0.7 Da (calculated 6,997.7 Da, average isotope composition of the reduced peptide), and the (59–124)-peptide (4th–11th ionized states) observed mass of 7,510.1 ± 2.6 Da (calculated 7,510.6 Da, average isotope composition of the reduced peptide. (B) HPLC chromatograms of the ligation reaction, started by the addition of 1% thiophenol and 1% benzylmercaptan. At t = 5 hr (Upper), ligated product (1–124) is shown, as well as the C-terminal segment starting material. The unreacted N-terminal material can be accounted for as multiple intermediates eluting between 15 and 20 min (see text). Peak 1 represents a N-terminal peptide derivative; peaks 2 and 3 represent the ligated material (1–124) with, respectively, one and two DNP groups still attached to His side chains (see text). At t = 24 h (Lower), an almost quantitative ligation has occurred. The m/z pattern of the ligated material is shown (6th–21th ionized states) observed mass of 13,919.5 ± 1.6 Da (calculated 13,918.0 Da, average isotopic mass of the reduced peptide). (C) Folding and disulfide formation of the sPLA2 purified 124 residue polypeptide. HPLC chromatograms are shown of the purified reduced sPLA2 polypeptide (t = 0), the crude folded material (t = 48 hr), and the purified final product (sPLA2). The m/z pattern from the material in peak 4 is shown (6th–12th ionized state), observed molecular mass of 13,904.7 ± 0.8 Da (calculated 13,904.0 Da, average isotopic mass of the sPLA2 containing seven disulfides). Note the shift in m/z intensities between the reduced and folded sPLA2 (see text).
Synthesis of the Peptide Segment (1–58)-αCOSCH2COOH.
To obtain a C-terminal thioacid group on Gly58 of peptide 1–58 of sPLA2, peptide synthesis was started on a 0.15-mmol scale on aminomethyl resin to which a preformed HF-labile Gly-thioester linker was coupled (15). The usual procedure for His side-chain deprotection (DNP removal) by 2-mercaptoethanol before HF treatment of the peptide resin could not be used because of the vulnerability of the thioester-resin linker. Therefore, HF cleavage of the peptide resin yielded a crude N-terminal peptide with a C-terminal thioacid group and three DNP groups attached to the three His residues. ESMS analysis yielded a mass of 6,939.1 ± 1.1 Da, consistent with the calculated mass for the reduced peptide with three DNP groups attached (6,939.7 Da, average isotope composition).
The crude thioacid peptide was subsequently treated at pH 4.0 with a 10-fold excess of bromoacetic acid to obtain the peptide-αthioester. ESMS revealed a mass of 6,996.5 ± 0.7 Da, corresponding to the calculated mass for the reduced peptide (6,997.7 Da, average isotope composition) with three DNP groups attached (Fig. 3A, 1–58-COSR). The N-terminal peptide appeared as a broad peak by HPLC after bromoacetic acid treatment. The apparent heterogeneity is not understood, but throughout the range of the peak, the ESMS m/z patterns were identical, as were the obtained masses. The complete peptide peak was isolated by semipreparative HPLC (C18; 24.3–30.6% CH3CN; 0.11% per min) and stored at −20°C after lyophilization. The yield for cleavage of 25 μmol of 1–58 peptide resin was 5 μmol of crude (1–58)peptide-αthioacid and 0.9 μmol of purified (1–58)peptide-αthioester.
Native Chemical Ligation.
The ligation of the (1–58)-αCOSCH2COOH and Cys59(59–124)-COOH peptides was performed at pH 7.5 in 6 M GuHCl. The concentration of each reactant was 9 mg/ml (1.2 mM in each segment; scale, 0.4 μmol for each segment), and 1% benzylmercaptan and 1% thiophenol were added to create a reducing environment and facilitate the ligation reaction (27). After the first 40 min of the reaction, all of the (1–58)-COSCH2COOH starting material with an average retention time of 25 min (Fig. 3A, 1–58-COSR) had disappeared to form a collection of derivatives from the N-terminal peptide (residues 1–58) and the ligated material (residues 1–124), eluting on analytical HPLC between 14 and 21 min. At this point in the reaction, more than eight ligation reaction intermediates were detected caused by the following reactions (I–III) or combinations thereof. Reaction I: Due to the presence of benzylmercaptan and thiophenol, DNP groups were gradually removed during the ligation reaction, giving the N-terminal peptide or the ligated product containing 1, 2, or 3 DNP groups attached to His residues, observed as changes in mass of +166.1, +332.2, or +498.3 Da. Reaction II: N-terminal peptide that had undergone thiol exchange with benzylmercaptan at the C-terminal COSR group to yield the benzyl thioester (+32 Da). Reaction III: N-terminal peptide that had undergone cyclization at the C terminus (−92 Da) (Fig. 3B). Remarkably, all these intermediates eventually converged into one final ligation product, the 1–124 polypeptide chain of sPLA2. After 22 hr of ligation reaction, some DNP groups were still attached to the sPLA2 polypeptide chains in the ligation mixture. To yield the maximal amount of the target polypeptide (residues 1–124), the reaction mixture was treated with 10% 2-mercaptoethanol for 1 hr at 37°C. The removal of DNP groups was complete after this treatment. However, as a result, 2-mercaptoethanol adducts were formed as indicated by +76, +156, and +228 Da changes in molecular mass of the cysteine-rich 1–124 polypeptide. To remove the 2-mercaptoethanol adducts, the reaction mixture was subsequently treated with a 10-fold excess of Tris(2-carboxyethyl)phosphine for 1 hr at 37°C. Overall, an almost quantitative ligation reaction was observed after 24 hr (Fig. 3B, t = 24 h). ESMS confirmed the molecular mass for the 1–124 polypeptide chain of 13,919.5 ± 1.6 Da, in good agreement with the calculated average isotopic mass of 13,918.0 Da for the reduced sPLA2 (Fig. 3B, Middle and Left). The sPLA2 polypeptide chain was subsequently purified using semipreparative HPLC (C18; 22.5–40.5% CH3CN; 0.3% per min), lyophilized, and stored at −20°C (yield, 0.1 μmol).
Folding of sPLA2 and Disulfide Formation.
The purified sPLA2 124-residue polypeptide chain (65 nmol) was folded as described. After 48 hr, the formation of disulfide bonds in the folded sPLA2 was revealed as a sharp peak at a earlier retention time (Fig. 3C, peak 4), with additional material in a secondary slower-eluting peak (peak 5). According to ESMS, the materials in both peaks had the same molecular mass of 13,904.7 ± 0.8 Da, similar to the calculated mass (13,904.0 Da, average isotope composition) for sPLA2 containing seven disulfide bonds. Therefore, the differences in retention times are most likely caused by different disulfide bond patterns of the folded sPLA2. The sharp peak eluting early is consistent with the behavior of correctly folded disulfide-crosslinked globular proteins in numerous previous cases since, typically, formation of the hydrophobic core of the folded protein buries the maximum proportion of the hydrophobic side chains and leads to early elution under reverse-phase HPLC conditions. Interestingly, ESMS observations on this material were also consistent with this notion. When compared with the m/z pattern of the reduced sPLA2 (Fig. 3B Middle), the m/z pattern of the oxidized sPLA2 shows a striking difference in the intensity of the various charged states. The shift of maximal intensity from the 17th or 18th charged state of the reduced polypeptide to the 8th charged state of the folded polypeptide is characteristic of the ESMS ionization behavior of folded globular proteins, compared with their denatured counterparts (28, 29).
The folded disulfide-crosslinked sPLA2 was HPLC-purified (18–31.5% acetonitrile, 0.23% per min), lyophilized, and stored at −20°C (yield, 11 nmol). The amino acid composition of the protein determined after acid hydrolysis agreed with that expected for sPLA2.
CD Spectroscopy.
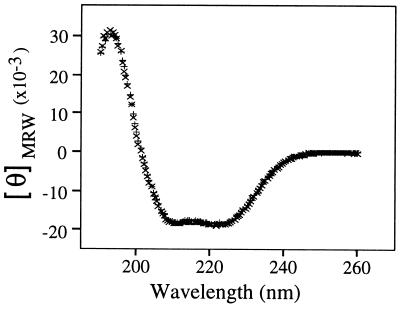
CD studies were performed to quantify the secondary structural elements present in the chemically synthesized sPLA2. The CD spectra of chemically synthesized and recombinant sPLA2 showed identical curves (Fig. 4). The secondary structures calculated from these CD data were compared with that calculated from the crystal structure of sPLA2 (5, 6) and were found to be essentially identical (Table 1). The minor differences in β-structure content may not be significant and are probably caused by the difficulties to quantify β-structure underneath the predominant α-helix structure in the CD spectra. It should be noted that the total amount of β-structure is the same in all three cases (Table 1).
Figure 4.
CD spectra of synthetic (×) and recombinant (+) sPLA2. For each sPLA2, the average of three CD spectra are shown.
Table 1.
Secondary structural elements of sPLA2
| %
|
||||
|---|---|---|---|---|
| α-helix | β-strand | β-turn | Random coil | |
| Synthetic sPLA2 | 43.8 | — | 18.8 | 37.5 |
| Recombinant sPLA2 | 44.7 | — | 20.1 | 35.2 |
| sPLA2 [x-ray structure (5, 6)] | 44.4 | 6.5 | 12.9 | 36.3 |
Secondary structural content was determined by using an average of three CD spectra of synthetic and recombinant sPLA2.
Enzymatic sPLA2 Activity Assay.
Hydrolysis of the synthetic dithio-PC substrate was used to study the kinetic parameters of sPLA2 (20, 21). Table 2 shows the enzymatic properties of the material from peak 4 (Fig. 3C), compared with recombinant sPLA2. The Km and kcat values for the synthetic and recombinant enzymes were very similar (Table 2). No enzymatic activity could be detected in the material from peak 5 (Fig. 3C), supporting the hypothesis that this peak reflects formation of incorrect disulfide bonds resulting in nonnative conformations and inactivity of the enzyme.
Table 2.
Enzyme kinetics of sPLA2
| Km, mM | kcat, sec−1 | |
|---|---|---|
| Chemically synthesized sPLA2 | 0.96 ± 0.15 | 3.5 ± 0.3 |
| Recombinant sPLA2 | 0.94 ± 0.19 | 3.1 ± 0.2 |
Dithio-PC hydrolysis was used to study the kinetic parameters of sPLA2. The data represent the average ± SD of three experiments.
sPLA2 Anticoagulant Activity.
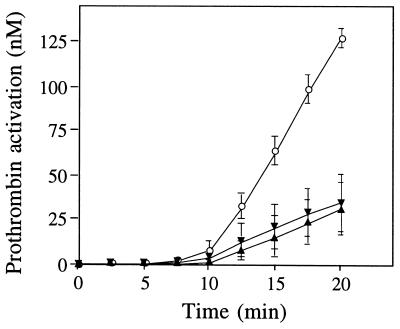
PLA2 is an anticoagulant and inhibits the prothrombinase complex activity of blood coagulation (8–12). To compare synthetic sPLA2 with recombinant sPLA2, each protein was preincubated with factor Xa at 37°C for 15 min before adding the other components of prothrombinase, i.e., factor V, prothrombin, Ca2+, and phospholipid vesicles. After a lag time during which factor V was activated to the active cofactor factor Va, synthetic sPLA2 inhibited thrombin generation by approximately 70% throughout the course of the experiment, in a manner indistinguishable from recombinant sPLA2 (Fig. 5). To verify the phospholipid-independent inhibitory anticoagulant activity of sPLA2, we used a prothrombinase assay performed in the absence of phospholipids. The prothrombin activation rate due to factor Xa and Va (each at 2.5 nM) in the presence of Ca2+ ions but in the absence of phospholipids was 132 ± 8 pM/min (average ± SD, n = 3). This prothrombin activation rate was equally well inhibited by either chemically synthesized sPLA2 (35 ± 5 pM/min; 70% inhibition) or recombinant sPLA2 (25 ± 5 pM/min; 80% inhibition). Thus, the anticoagulant effect of synthetic sPLA2, like recombinant sPLA2, cannot be solely due to phospholipid hydrolysis, and sPLA2 is a phospholipid-independent anticoagulant.
Figure 5.
Inhibition of prothrombinase activity by sPLA2. Factor Xa was preincubated with synthetic (▾) or recombinant (▴) sPLA2 (2.5 μM, final concentration). After 15 min, a mixture of factor V, prothrombin, and phospholipid vesicles was added, and prothrombin activation was measured. At different times, thrombin amidolytic activity was determined and quantitated by using an appropriate standard curve. The data represent the average ± SD of three experiments.
From the chemist’s viewpoint, the total chemical synthesis of fully active human sPLA2 shows the facility and robust nature of the native chemical ligation method for the total chemical synthesis of proteins and illustrates the extraordinary regioselecivity of the reaction, i.e., specific ligation at a single Cys in the presence of a total of 14 Cys residues. From the biochemist’s viewpoint, the modular chemical synthesis approach to the preparation of sPLA2 gives versatile access to this enzyme and its analogs for broad studies of structure–activity relationships and of the anticoagulant, lipolytic, or inflammatory activities of sPLA2.
Acknowledgments
This work was in part supported by National Institutes of Health Grants RO1-HL21544, PO1-HL31950, and M01-RR00833.
ABBREVIATIONS
- Boc
tert-butoxycarbonyl
- DIEA
N,N-diisopropylethylamine
- DMF
N,N-dimethylformamide
- DNP
2,4-dinitrophenyl
- ESMS
electrospray mass spectrometry
- sPLA2
secretory phospholipase A2
- SPPS
solid phase peptide synthesis
- TFA
trifluoroacetic acid
- HBTU
2-(1H-benzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate
- PC
phosphatidylcholine
- dithio-PC
1,2-bis(heptanoylthio)-phosphatidylcholine
- GuHCl
guanidine hydrochloride
References
- 1.Dennis E A. J Biol Chem. 1994;269:13057–13060. [PubMed] [Google Scholar]
- 2.Davidson F F, Dennis E A. J Mol Evol. 1990;31:228–238. doi: 10.1007/BF02109500. [DOI] [PubMed] [Google Scholar]
- 3.Seilhamer J J, Pruzanski W, Vadas P, Plant S, Miller J A, Kloss J, Johnson L K. J Biol Chem. 1989;264:5335–5338. [PubMed] [Google Scholar]
- 4.Kramer R M, Hession C, Johansen B, Hayes G, McGray P, Pingchang Chow E, Tizard R, Blake Pepinsky R. J Biol Chem. 1989;264:5768–5775. [PubMed] [Google Scholar]
- 5.Wery J-P, Schevitz R W, Clawson D K, Bobbitt J L, Dow E R, Gamboa G, Goodson T, Jr, Hermann R B, Kramer R M, McClure D B, Mihelich E D, Putnam J E, Sharp J D, Stark D H, Teater C, Warrick M W, Jones N D. Nature (London) 1991;352:79–82. doi: 10.1038/352079a0. [DOI] [PubMed] [Google Scholar]
- 6.Scott D L, White S P, Browning J L, Rosa J J, Gelb M H, Sigler P B. Science. 1991;254:1007–1010. doi: 10.1126/science.1948070. [DOI] [PubMed] [Google Scholar]
- 7.Ouyang C, Teng C M, Huang T F. Toxicon. 1992;30:945–966. doi: 10.1016/0041-0101(92)90040-c. [DOI] [PubMed] [Google Scholar]
- 8.Kini R M, Evans H J. J Biol Chem. 1987;262:14402–14407. [PubMed] [Google Scholar]
- 9.Stefansson S, Kini R M, Evans H J. Biochemistry. 1990;29:7742–7746. doi: 10.1021/bi00485a024. [DOI] [PubMed] [Google Scholar]
- 10.Inada M, Crowl R M, Bekkers A C A P A, Verheij H M, Weiss J. J Biol Chem. 1994;269:26338–26343. [PubMed] [Google Scholar]
- 11.Cirino G, Cicala C, Sorrentino L, Browning J L. Thromb Res. 1993;70:337–342. doi: 10.1016/0049-3848(93)90106-x. [DOI] [PubMed] [Google Scholar]
- 12.Mounier C, Franken P A, Verheij H M, Bon C. Eur J Biochem. 1996;237:778–785. doi: 10.1111/j.1432-1033.1996.0778p.x. [DOI] [PubMed] [Google Scholar]
- 13.Dawson P E, Muir T W, Clark-Lewis I, Kent S B H. Science. 1994;266:776–779. doi: 10.1126/science.7973629. [DOI] [PubMed] [Google Scholar]
- 14.Schnölzer M, Alewood P, Jones A, Alewood D, Kent S B H. Int J Pept Protein Res. 1992;40:180–193. doi: 10.1111/j.1399-3011.1992.tb00291.x. [DOI] [PubMed] [Google Scholar]
- 15.Canne L E, Walker S M, Kent S B H. Tetrahedon Lett. 1995;36:1217–1220. [Google Scholar]
- 16.Hackeng T M, van’t Veer C, Meijers J C M, Bouma B N. J Biol Chem. 1994;269:21051–21058. [PubMed] [Google Scholar]
- 17.DiScipio R G, Davie E W. Biochemistry. 1979;18:899–904. doi: 10.1021/bi00572a026. [DOI] [PubMed] [Google Scholar]
- 18.Sarin V K, Kent S B H, Tam J P, Merrifield R B. Anal Biochem. 1981;117:147–157. doi: 10.1016/0003-2697(81)90704-1. [DOI] [PubMed] [Google Scholar]
- 19.Franken P A, van den Berg L, Huang J, Gunyuzlu P, Lugtigheid R B, Verheij H M, De Haas G H. Eur J Biochem. 1992;203:89–98. doi: 10.1111/j.1432-1033.1992.tb19832.x. [DOI] [PubMed] [Google Scholar]
- 20.Hendrickson H S, Hendrickson E K, Dybvig R H. J Lipid Res. 1982;24:1532–1537. [PubMed] [Google Scholar]
- 21.Reynolds L R, Hughes L L, Dennis E A. Anal Biochem. 1992;204:190–197. doi: 10.1016/0003-2697(92)90160-9. [DOI] [PubMed] [Google Scholar]
- 22.Schnölzer M, Kent S B H. Science. 1992;256:221–225. doi: 10.1126/science.1566069. [DOI] [PubMed] [Google Scholar]
- 23.Muir T W, Williams M J, Ginsberg M H, Kent S B H. Biochemistry. 1994;33:7701–7708. doi: 10.1021/bi00190a025. [DOI] [PubMed] [Google Scholar]
- 24.Canne L E, Ferré-D’Amaré A R, Burley S K, Kent S B H. J Am Chem Soc. 1995;117:2998–3007. [Google Scholar]
- 25.Tam J P, Lu Y-A, Liu C-F, Shao J. Proc Natl Acad Sci USA. 1995;92:12485–12489. doi: 10.1073/pnas.92.26.12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu W, Quasim M A, Kent S B H. J Am Chem Soc. 1996;118:8518–8523. [Google Scholar]
- 27.Dawson P E, Churchill M, Ghadiri M R, Kent S B H. J Am Chem Soc. 1997;119:4325–4329. [Google Scholar]
- 28.Chowdhury S K, Katta V, Chait B T. J Am Chem Soc. 1990;112:9012–9013. [Google Scholar]
- 29.Loo J A, Edmonds C G, Udseth H R, Smith R D. Anal Chem. 1990;62:693–698. doi: 10.1021/ac00206a009. [DOI] [PubMed] [Google Scholar]