Abstract
The cuticle fulfills multiple roles in the plant life cycle, including protection from environmental stresses and the regulation of organ fusion. It is largely composed of cutin, which consists of C16-18 fatty acids. While cutin composition and biosynthesis have been studied, the export of cutin monomers out of the epidermis has remained elusive. Here, we show that DESPERADO (AtWBC11) (abbreviated DSO), encoding a plasma membrane-localized ATP-binding cassette transporter, is required for cutin transport to the extracellular matrix. The dso mutant exhibits an array of surface defects suggesting an abnormally functioning cuticle. This was accompanied by dramatic alterations in the levels of cutin monomers. Moreover, electron microscopy revealed unusual lipidic cytoplasmatic inclusions in epidermal cells, disappearance of the cuticle in postgenital fusion areas, and altered morphology of trichomes and pavement cells. We also found that DSO is induced by salt, abscisic acid, and wounding stresses and its loss of function results in plants that are highly susceptible to salt and display reduced root branching. Thus, DSO is not only essential for developmental plasticity but also plays a vital role in stress responses.
One of the most critical adaptations of plants to a terrestrial environment 450 million years ago was the formation of their surface, the cuticle. The cuticular layer plays multiple roles in plants, including the regulation of epidermal permeability and nonstomatal water loss and protection against insects, pathogens, UV light, and frost (Sieber et al., 2000). It also functions in normal plant developmental processes, including the prevention of postgenital organ fusion and pollen-pistil interactions (Lolle et al., 1998).
The major component of the cuticle is cutin, which is a polyester insoluble in organic solvents consisting of oxygenated fatty acids with a chain length of 16 or 18 carbons. Embedded in the cutin matrix are cuticular waxes, which are complex mixtures of very-long-chain fatty acid (VLCFA; >C24) derivatives: aldehydes, ketones, primary and secondary alcohols, fatty acids, and wax esters (Kunst and Samuels, 2003). In many species, they also include triterpenoids and other secondary metabolites, such as sterols, alkaloids, phenylpropanoids, and flavonoids. The cuticular waxes are arranged into an intracuticular layer in close association with the cutin matrix, as well as an epicuticular film exterior to this, which may include epicuticular wax crystals (Jetter et al., 2000). Recently, 2-hydroxy- and α,ω-dicarboxylic fatty acids have been reported as the characteristic monomers of cutin in Arabidopsis (Arabidopsis thaliana; Bonaventure et al., 2004; Franke et al., 2005). This cutin monomer composition is similar to the aliphatic domain present in the Arabidopsis suberin polymer (Franke et al., 2005). Suberin is part of the plant apoplastic barrier that prevents uncontrolled nutritional and water loss, strengthens cell walls, and provides protection from pathogens. In Arabidopsis, suberin depositions were detected in the endodermis of primary roots and the periderm of mature roots.
Postgenital fusion is a unique phenomenon that occurs when alterations in cuticle properties cause augmentation of the contact responsiveness. During plant development, organ fusion is tightly regulated, and the cuticle plays a vital role in either preventing or permitting fusions. Postgenital organ fusion occurs most commonly during reproductive development (e.g. during carpel formation in angiosperms). One of the characteristic features of organ fusions is that adhesion of cell walls is often accompanied by disappearance of the cuticle in the contact area (Lolle et al., 1998).
To date, nearly 20 Arabidopsis mutants displaying postgenital fusions have been identified and for less than one-half of them has a corresponding gene product been associated (Lolle et al., 1998; Tanaka and Machida, 2006). One of the most known organ fusion mutant is the Arabidopsis fiddlehead (fdh). The FDH gene encodes a lipid biosynthetic enzyme that acts through the fatty acid elongation pathway and might be involved in cutin monomer biosynthesis. The fdh mutant leaves supported wild-type pollen germination on their surfaces and showed increased permeability of the cuticle to the toluidine blue dye. In addition, fdh mutants exhibited an enhanced rate of chlorophyll leaching from leaves submerged in alcoholic solution (Lolle and Cheung, 1993; Yephremov et al., 1999; Pruitt et al., 2000). Another mutant, abnormal leaf shape1 (ale1), showed defective cuticle in embryos and juvenile plants and, as a result, exhibited excessive water loss and organ fusion. The corresponding gene belongs to a large family of subtilisin-like Ser proteases in Arabidopsis that are typically involved in intercellular signaling, converting their substrates to active or inactive forms (Tanaka et al., 2001). The phenotypes of the ale1 mutants depend on the genetic background, and they could be observed in the Landsberg erecta background but not in the Columbia and Wassilewskija ecotype backgrounds (Watanabe et al., 2004). The double mutant of ale1 (in Wassilewskija) and the Arabidopsis homolog of crinkly4 (acr4) resulted in one-half of the seedlings showing deformed cotyledons and severely fused leaves. The authors suggested that ACR4 and ALE1 synergistically affected the epidermis and that ACR4 plays a major role in the differentiation of epidermal cells in both vegetative and reproductive tissues. The maize crinkly4 (cr4) mutation shows graft-like fusions between organs, and the CR4 gene encodes a putative receptor kinase that might generate a signal for epidermal cell differentiation (Jin et al., 2000; Becraft et al., 2001; Tanaka et al., 2002).
A Cyt P450 monooxygenase, CYP86A8, catalyzes the ω-hydroxylation of C12-18 fatty acids when assayed in vitro. The CYP86A8 loss-of-function mutant, lacerata (lcr), showed severe cuticle defects, as evidenced by epidermal ruptures and postgenital fusions (Wellesen et al., 2001). A different gene, HOTHEAD (HTH), putatively encoding an oxidoreductase, was suggested to be involved in the formation of α,ω-dicarboxylic fatty acids because the hth-12 mutant allele showed decreased load of these acids (Kurdyukov et al., 2006b). In the hth mutant, the majority of organ fusion events occur during floral development (Lolle et al., 1998; Krolikowski et al., 2003). Interestingly, the HTH gene is not epidermis specific, and its involvement in metabolism of additional compounds not essential for construction of the cuticle is not yet clear.
Chen et al. (2003) reported the isolation of the WAX2 gene and interpreted it to be required for both cutin and cuticular wax deposition. The cuticular membrane of wax2 weighed less and was thicker, disorganized, and less opaque. The total wax load on leaves and stems was decreased to nearly 80%, showing a reduction in the decarbonylase pathway products and an increase in the acyl reduction pathway products. The WAX2 protein contains certain regions with homology to sterol desaturases and short-chain dehydrogenases/reductases. It was suggested that it plays a metabolic role in both cutin and wax synthesis. The cloning and characterization of the same gene (termed YORE-YORE) was described by Kurata et al. (2003), and the yre mutant showed organ adhesion. The authors suggested that YRE might encode an enzyme catalyzing the formation of aldehydes in the wax decarbonylation pathway. Alterations to the fatty acid precursor pool could also result in plants showing organ fusion phenotypes. The enzyme acetyl-CoA carboxylase catalyses the ATP-dependant formation of malonyl-CoA. Acetyl-CoA carboxylase activity in the cytosol generates a malonyl-CoA pool that is required for a wide range of reactions, including VLCFA elongation, that are incorporated into cutin and waxes. Weak gurke and pasticcino3 mutant alleles that correspond to a defect in the ACC1 gene showed abnormal fused leaves that were often vitrified when plants were grown in vitro (Faure et al., 1998). A strong organ fusion phenotype was also seen in transgenic plants raised by Sieber et al. (2000) that expressed a fungal cutinase in Arabidopsis. Their results suggest that an intact cutin layer is crucial for preventing organ fusions.
The synthesis of cuticle constituents occurs in the epidermis layer from which they are transported out to the plant surface. Recently, the first clue to the export mechanism of cuticular lipids through the plasma membrane was provided by the characterization of the cer5 Arabidopsis mutant (Pighin et al., 2004). The CER5 gene encodes an ATP-binding cassette (ABC) transporter localized in the plasma membrane. Apart from the typical reduction in stem cuticular wax load (cer phenotype), the cer5 knockout mutant accumulated sheet-like inclusions in the wax-secreting cells. Epidermal peel staining and observation with light microscopy suggested that they are lipidic in nature. In addition, the overall levels of fatty acid were not altered, and this provided evidence that only wax transport was affected and not VLCFA biosynthesis. The CER5 gene expression was detected in all plant organs examined, including stems, leaves, siliques, flowers, and roots. This was unexpected because the cer5 phenotype is confined to leaves and stems. It was therefore suggested that additional transporters must be involved in delivering wax components. In Arabidopsis, there are over 120 putative ABC transporters; 29 of them, including CER5, belong to the white-brown complex (WBC) subfamily (Sanchez-Fernandez et al., 2001). In human and Drosophila, members of this family secrete cholesterol and plant sterols and play a role in the steroid hormone pathway, respectively (Berge et al., 2000; Hock et al., 2000). In Arabidopsis, ABC transporters are implicated in the transport of a wide range of substrates, including auxin, Suc, and mono- and divalent ions. They play a fundamental role in heavy metals transport, resistance to xenobiotics, and different aspects of plant development, including regulation of stomatal opening and closure (Schulz and Kolukisaoglu, 2006).
While the activity of CER5 could explain the transport of wax monomers out of the epidermal cells, the mechanism responsible for cutin monomer transport remained unknown. In this article, we describe the DESPERADO (DSO) gene putatively encoding a WBC-subtype ABC transporter. We provide several lines of evidence showing that DSO is vital for the export of both cutin and wax monomers to the surface of Arabidopsis plants. The various DSO phenotypes and the fact that its expression was not confined to vegetative organs but was also detected in the root suggest that it might also be involved in the transport of other types of lipids. Such a lipid molecule could be, for example, suberin that shows chemical analogy to cutin and is typically deposited in plant roots. The results obtained through this study also demonstrate that DSO is not only vital for proper plant development but also to plants' response to various stresses such as salinity and mechanical wounding.
RESULTS
Phenotypes of the DSO Loss-of-Function Lines
In human and Drosophila, members of the ABC transporters family play a role in the transport of lipid substrates (Pohl et al., 2005). To unravel the possible role of ABC transporters in plant lipid transport, we systematically generated, in Arabidopsis, RNA interference (RNAi) lines for more than 20 ABC transporters genes. All members investigated belonged to the WBC subfamily (Sanchez-Fernandez et al., 2001). One RNAi line (dso-1), targeted to silence the At1g17840 gene (Fig. 1A), showed an array of morphological phenotypes including inter-organ postgenital fusions (Fig. 1, B–D) that resembled those of mutant plants altered in their cuticle (Yephremov et al., 1999; Chen et al., 2003; Krolikowski et al., 2003; Kurata et al., 2003; Schnurr et al., 2004; Kurdyukov et al., 2006a). The DSO protein (AtWBC11; Sanchez-Fernandez et al., 2001) is a member of a small group of WBC transporters that includes the previously described CER5 wax transporter (AtWBC12; Pighin et al., 2004; Supplemental Fig. S2), AtWBC15/22, AtWBC13, and AtWBC3. DSO shows the highest identity to CER5 (52% at the amino acid level) and slightly less homology to AtWBC15/22 (51% identity) and AtWBC13 (48% identity). The closest homolog of the CER5 wax transporter is AtWBC15/22 (85% identity; Supplemental Table S3).
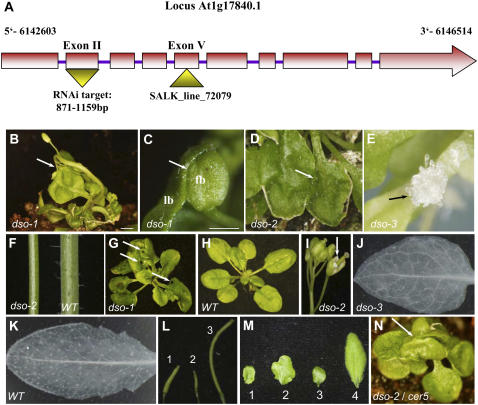
Figure 1.
The dso loss-of-function mutant phenotypes. A, Scheme of the DSO locus (At1g17840) depicting the RNAi target sequence in the second exon and T-DNA insertion located in the fifth exon of the dso-3 line. B, Inter-organ postgenital fusions in the dso-1 mutant plant. A fusion area between inflorescence and leaf is indicated by an arrow. C, Fusion between a leaf blade (lb) and a floral bud (fb) in dso-1. D, Fusion between two leaves of dso-2 plant. E, Unusual protrusions in dso-3 plants grown in tissue culture. F, A cer phenotype in dso-2 plant stem (left) versus wild-type (WT) stem (right). G and H, dso-1 (G) and the wild type (H), 1-month-old plants after 2-min immersion in toluidine blue staining solution. Sites of the dye penetration are indicated by arrows. I, A dso-2 flower phenotype. Underdeveloped petal is indicated by an arrow. J, Vasculature phenotype detected in dso-3 rosette leaf. K, Wild-type rosette leaf vasculature. L, Siliques of dso-3 (1), dso-2 (2), and the wild type (3). M, Leaf phenotype of a severe dso-2 mutant line (1), mild dso-2 mutant line (2), dso-3 (3), and the wild type (4). N, The cer5-1/dso-2 (mild) double mutant phenotype. Fusion between leaves is indicated by an arrow. Bars = 2 mm in B and 1 mm in C.
The dso-1 plant phenotype was extremely severe as most of the plants were strongly retarded in growth and upon maturation produced multiple, thin, and short inflorescence stems (a bushy phenotype) probably due to the loss of apical dominance. The fusion of organs in dso-1 mutants often resulted in rosette leaves that were misshapen and torn. A toluidine dye uptake test (Tanaka et al., 2004) suggested malformation of the dso-1 mutant cuticle as they displayed strong coloration after 2 min of immersion in the dye, whereas no staining was observed in wild-type plants (Fig. 1, G and H). The leaf surfaces of several cuticular mutants were previously shown to support wild-type pollen germination (Lolle and Cheung, 1993; Lolle et al., 1998; Sieber et al., 2000; Wellesen et al., 2001; Kurdyukov et al., 2006b). Scanning electron microscopy (SEM) revealed that fully expanded rosette leaves of dso-1 plants do not support wild-type pollen germination (data not shown). In Arabidopsis rosette leaves, the vascular tissue is composed of the main vein in the middle of the leaf blade that is interconnected by secondary and higher order veins, forming a complex network. The vascular patterns in the dso-1 mutant appeared to be altered compared to the wild-type leaf patterns (Fig. 1, J and K). In the dso-1 leaf blade, fewer tertiary and quaternary veins were observed. Moreover, the veins along the edge of the leaf formed a discontinuous circle.
We also generated misexpression lines by expressing DSO under the control of the 35S cauliflower mosaic virus (CaMV) promoter (lines termed dso-2) and identified a T-DNA insertional line (SALK_072079; Fig. 1A) in the DSO gene (lines termed dso-3). Semiquantitative reverse transcription (RT)-PCR was performed for all three loss-of-function mutant genotypes to determine the levels of the DSO transcript. In the dso-1 and dso-2 lines, a significant reduction in transcript levels was evident, whereas in the dso-3 line no transcript was detected, indicating that dso-3 is a null mutant (Supplemental Fig. S1). The results with misexpression suggested that instead of overexpression we obtained cosuppression (detected in one-third of the primary transformants).
In Supplemental Table S2, we depicted the different phenotypes and their degree of penetration among the three DSO loss-of-function genotypes. Overall, the dso-2 and dso-3 plants showed similar phenotypes to the ones observed in the dso-1 RNAi line (Fig. 1) but with different levels of penetration. The cosuppression dso-2 lines had a relatively mild phenotype compared to dso-1 and dso-3, as they developed almost regular inflorescence stems that frequently had a glossy cer-type phenotype (Jenks et al., 1995; Fig. 1F). In some cases, cer-type phenotypes were observed only in certain stem parts but not others in the same plant. The dso-2 plants showed notched rosette leaves. The majority of dso-1 and dso-3 seedlings grown in tissue culture developed unusual, callus, or stigmatic-like protrusions from epidermal cells (Fig. 1E). A similar phenotype was observed previously in transgenic Arabidopsis plants expressing a fungal cutinase (Sieber et al., 2000). Leaf (Fig. 1M) and flower morphology was affected in all three dso genotypes, as petals were folded and twisted (Fig. 1I) and they produced short, almost seedless siliques (Fig. 1L). When seeds of dso-2 lines were immersed in toluidine blue dye solution, they showed increased staining compared to wild-type seeds (data not shown), suggesting impaired integrity of the seed surface. The stem cer-type phenotype detected by visual inspection was in agreement with the dramatic decrease in load of epicuticular wax crystals on dso-2 mutant stem surfaces observed by SEM (Fig. 2, A and B).
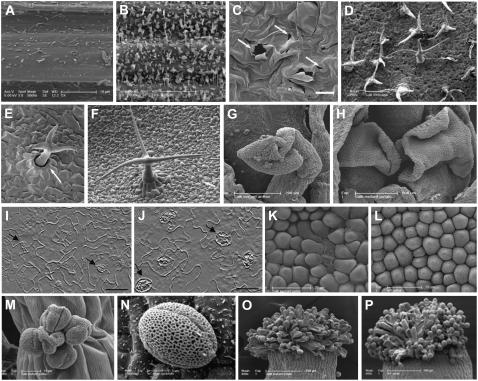
Figure 2.
SEM pictures. A and B, Stem wax load of dso-2 (A) and wild-type (B) plants. C, Occasional ruptures in the epidermis of dso-2. D, Distorted and underdeveloped trichomes of dso-3. E, Collapsed and underdeveloped trichome of dso-1. Misshapen support cells are indicated by an arrow. F, A wild-type trichome. G, Abnormal anther filament of a dso-3 flower. H, Curved petals of a dso-3 flower. I and J, Light microscopy images showing aberrant pavement cell pattern and abnormal stomatal cells (indicated by arrows) in dso-3 (I) and abaxial leaf epidermis of wild-type plants (J). K and L, SEM of abnormal conical cells in the abaxial epidermis of a dso-3 flower petal (K) and conical cells in the abaxial epidermis of a wild-type flower petal (L). M and N, SEM micrographs of shriveled pollen grains in dso-3 lines (M) and pollen grains in the wild type (N). O and P, Stigmata papillae of dso-3 (O) and those of the wild type (P); grains could not be detected in dso-3. Bars = 20 μm in C and 50 μm in I and J.
SEM examination of leaf surfaces uncovered notable phenotypes in the dso lines. Apart from random ruptures in the epidermis (Fig. 2C), they developed dehydrated trichomes with shortened stalks and irregular branching patterns and they were often collapsed (Fig. 2, D–F). Misshapen, asymmetric stomata and abnormal leaf pavement cell patterns were regularly observed (Fig. 2, I and J).
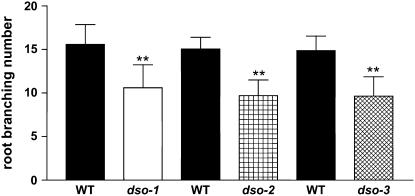
Alterations in reproductive organ morphology were also detected in dso plants examined by SEM. Flowers had curved petals and distorted anther filaments (Fig. 2, G and H). Moreover, the typical petal abaxial epidermis conical cells were variable in size and misshapen in the petal folding area (Fig. 2, K and L). Pollen grains were often absent from the stigmatic papillary cells, and they were often shriveled (Fig. 2, M–P). Alexander stain for a pollen viability test showed that 23% of dso-3 pollen is unviable and shriveled (data not shown). We also performed reciprocal crosses between dso-3 plants and wild-type plants. When dso-3 plants were used as male, short semi-sterile siliques were obtained. On the other hand, when dso-3 flowers were pollinated with wild-type pollen, no fertilization occurred. From these observations, we can conclude that the dso-3 sterility originates from both defective male and female reproductive organs. The descendants of the backcrossed dso-3 homozygous plants displayed the same phenotype, excluding the possibility that the phenotype originated from the background mutations. The effect of decreasing DSO transcript levels was also evidenced in below-ground tissues, as dso plants displayed reduced amounts of lateral roots compared to wild-type plants (Fig. 3; Supplemental Fig. S3).
Figure 3.
The dso mutants exhibit a root-branching phenotype. Root-branching number of 3-week-old dso and wild-type (WT) plants is shown. Error bars represent sd. **, P < 0.01.
DSO and CER5 Might Function in the Same Pathway
To evaluate the interaction between CER5 and DSO, we crossed the mild phenotype dso-2 plants (with glossy stems, curved petals, and without organ fusion phenotype) with the cer5-1 mutant. As mentioned above, the cer5-1 mutant does not display any additional visual phenotype apart from its glossy cer-type stem. The dso-2/cer5 double mutant showed severe fusion already at early developmental stages (Fig. 1N), suggesting an additive phenotype and providing evidence that these two genes could act in the same pathway.
Analysis of DSO and CER5 gene expression profiles using Genevestigator (https://www.genevestigator.ethz.ch/at/) revealed similar expression patterns for the two genes. Their expression is highest in seedlings, young leaves, and in the inflorescence. In the root organs, both genes display highest expression in lateral roots. Moreover, using the PRIME coexpression search tool, we found that expression of both genes is highly correlated (http://prime.psc.riken.jp/?action=coexpression_index).
DSO Loss-of-Function Lines Lack a Cuticular Layer in Organ Fusion Areas and Contain Unusual Lipidic Cytoplasmatic Inclusions in Epidermal Cells
We used transmission electron microscopy (TEM) to examine the changes in cuticle formation when two dso-1 rosette leaves are fused. These observations revealed that when complete fusion between the two leaf surfaces occurred, absolute disappearance of the cuticular layer was detected, suggesting copolymerization of adjacent cell walls (Fig. 4, A–C).
Figure 4.
TEM pictures. A, The fusion area between dso-1 leaves in which the cuticles (cut) of either leaf align next to each other. B and C, Areas of leaf fusions in dso-1; red arrowheads mark areas in which the cuticle is absent; cuticles are indicated by arrows. D, F, G, and H, Unusual inclusions (arrows) in the epidermal cell cytoplasm of a dso-3 leaf (D and F), in the epidermal cell of a dso-2 stem (G), and in an epidermal cell of a dso-3 stem (H). E, Epidermal cell cytoplasm of wild-type leaf. I, Epidermal cell cytoplasm of wild-type stem. Fluorescence images in J and K show Nile Red staining of the stem epidermis tissue isolated from dso-3 and the wild type, respectively. Arrows in J indicate the inclusions. Bars = 200 nm in A, 1 μm in B and E, 2 μm in D, 500 nm in G and H, 1 μm in F and I, and 100 μm in J and K. [See online article for color version of this figure.]
Unlike other cuticular mutants (Chen et al., 2003; Kurata et al., 2003; Xiao et al., 2004; Kurdyukov et al., 2006a, 2006b), dso mutant and transgenic line stems and leaves were not altered in the cuticle ultrastructure. On the other hand, detailed TEM inspection of both leaf and stems cells of dso-2 and dso-3 indicated that the transport of cuticular components might be compromised in the dso mutants. Unusual trilamellar cytoplasmatic inclusions were observed in epidermal cells of both leaves and stems of the mutants (Fig. 4, D–I). These structures could not be detected in epidermis cells of wild-type leaves and stems and not in other cell types of the mutants. To verify the nature of the cytoplasmatic inclusions, epidermal peels of dso-3 stem were stained with Nile Red and observed with fluorescence microscopy (Figs. 4, J and K). The results indicate that as in the case of the cer5 mutant, these inclusions are lipidic in nature (Pighin et al., 2004).
Expression of Reporter Genes Driven by the DSO 5′ Region
To study the tissue specificity of DSO, we examined the expression of GUS and GFP reporter genes under the control of the DSO 5′ upstream sequence. For GUS and GFP expression, 2,294 and 4,417 bp of the DSO 5′ upstream region were transcriptionally fused to either one of these reporters, respectively, and reporter activity was evaluated in different tissues of T2 plants. Reporter GUS and GFP expression indicated that DSO is expressed in the seed coat and the endosperm (data not shown) and later during embryo development in the radical tip and vasculature (Fig. 5A). DSO expression was detected in seedlings in the cotyledons, root tip, and young leaves (Fig. 5, B, C, F, and G). In both young and mature leaves, expression was detected in trichomes and stomatal cells (Fig. 5, J and K) and weaker in the rest of the blade. The strongest expression was detected in the main vein and the expanding basal portion of the leaf (Fig. 5G). In roots of mature plants, DSO expression was clearly observed in lateral root primordia and developing lateral roots but was also detected throughout the vasculature (Fig. 5, D–F). In the inflorescence, expression could be detected in all floral organs, predominantly in the anthers, styles, and in young siliques (Fig. 5H). In the developing siliques, the strongest expression was detected in young siliques (in the base and tip; Fig. 5I). Cross sections of the inflorescence stem DSO expression showed that DSO expression was not confined to epidermis, as it was detected in epidermal and mesophyll cells (Fig. 5L). Overall, developing rather than mature organs appear to express DSO.
Figure 5.
DSO 5′ upstream region directed expression. A, GFP expression driven by the DSO 5′ upstream region in the embryo. Expression in radical tip is indicated by an arrow. B to I, GUS expression driven by the DSO 5′ upstream region detected (after 24-h incubation) in: 3-d-old seedling, expression in root cap is indicated by an arrow (B), a higher magnification image of a stained root cap (C); in the vasculature (v) of developing root and at the lateral root primordia (lrp; D); in the emerging lateral root (E); in a 7-d-old seedling (arrow marks lateral root emergence sites; F); in a 15-d-old seedling, expression in the lateral root (lr), main vein (mv), and basal segment (bs) of the leaf is indicated by arrows (G); in the inflorescence (H); and in the developing siliques (I). J, Confocal microscopy images of GFP expression driven by the DSO 5′ upstream region in the adaxial leaf epidermis (i, autofluorescence; ii, GFP signal; iii, merge of GFP with transmission; iv, merge between autofluorescence and GFP). The GFP signal is indicated by arrow. The blue signal marks autofluorescence in the cuticular ledges. K, Confocal microscopy images of GFP expression driven by the DSO 5′ upstream region in the adaxial leaf epidermis showing GFP signal in the trichome base (indicated by arrow) and support cells. L, Images of GFP expression driven by the DSO 5′ upstream region in a free-hand stem cross section. Arrows indicate GFP signal in epidermis. GFP signal was also detected in other stem tissues. Bar = 50 μm in A.
DSO Protein Subcellular Localization
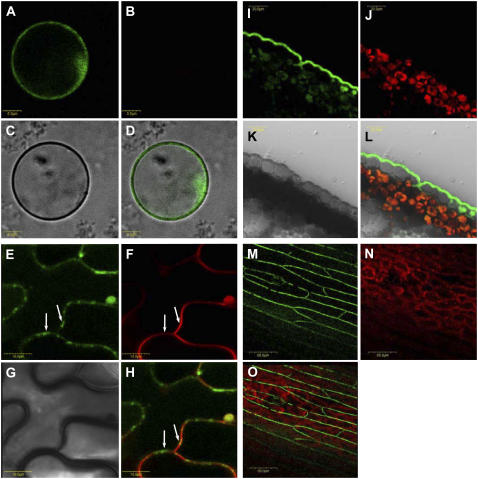
We also examined the subcellular localization of DSO by generating transgenic plants harboring a construct in which GFP was fused in frame to the N termini of the full-length DSO gene and expression was driven by the DSO 5′ upstream region. Two-thirds of the transformants displayed a cosuppression phenotype (data not shown). Five T2 GFP positive plants with no signs of cosuppression were used for whole-mount confocal microscopy of leaves (Fig. 6, E–H), protoplast preparation from stem epidermis-enriched tissues (Fig. 6, A–D), and preparation of free-hand stem cross sections (Fig. 6, I–L). In protoplasts, the GFP signal was detected in the periphery of the cells, suggesting plasma membrane localization. The use of protoplasts allowed us to exclude cell wall-specific expression. To determine whether the observed fluorescence was associated with the plasma membrane, we used a plasma membrane-specific marker (FM4-64). The results showed that DSO is colocalized with FM4-64 (Fig. 6, E–H). Analysis of the stem cross sections of the same plants indicated that, contrary to the promoter-directed expression, GFP was detected exclusively in the epidermis. Moreover, it was localized in a polar manner in the epidermis side facing the extracellular matrix (Fig. 6, I–L).
Figure 6.
Localization of DSO-GFP protein fusion to the plasma membrane of epidermal cells. A to D, Confocal microscopy of epidermal protoplasts derived from plants harboring the pDSO∷GFP-DSO construct (GFP fused in the N termini) are shown. Protoplasts were prepared from stem epidermis-enriched tissue and analyzed for DSO subcellular localization. Images were acquired through: GFP filter (A), chlorophyll filter (B), transmission (C), and a merge (D) between A and C. E to H, Whole-mount confocal microscopy of leaves derived from plants harboring the pDSO∷GFP-DSO construct (GFP fused in the N termini). Images were acquired through: GFP filter (E), chlorophyll filter (F), transmission (G), and a merge (H) between E and F. FM4-64 (red signal) is a plasma membrane marker and was used in F. FM4-64 was used in H for colocalization with the GFP signal. Arrows indicate GFP in E and H and FM4-64 in F and H. I to L, Confocal microscopy of stem cross sections of plants harboring the pDSO∷GFP-DSO construct. Images were acquired through: GFP filter (I), chlorophyll filter (J), transmission (K), and a merge (L) between I, J, and K. M to O, Whole-mount confocal microscopy of stems derived from plants harboring the pDSO∷GFP-DSO construct (GFP fused in the N termini). Images were acquired through: GFP filter (M), chlorophyll filter (N), and a merge (O) between M and N.
Changes in Cutin and Wax Monomer Composition in the dso Mutants
To gain more knowledge on the precise role of DSO in the transport of cuticle-associated lipids, we performed gas chromatography-mass spectrometry (GC-MS) analysis of epicuticular waxes on the surface of dso-3 and wild-type plants. Chemical analysis revealed a 3-fold decrease in total stem wax load in dso-3 compared to wild-type stems (6.77 ± 1.02 μg/cm2 versus 19.66 ± 2.22 μg/cm2; Table I). The C29 monomers, particularly, alkanes (11-fold decrease), ketone (2.6-fold decrease), and secondary alcohol (1.8-fold decrease), were largely responsible for this decrease (Fig. 7A).
Table I.
GC-MS analysis of wax monomers load of dso-3 mutants and wild-type plants
Data presented here are the average of three replicates.
| Compound Class | Wild Type | dso-3 | ||
|---|---|---|---|---|
| mean μg/cm2 | sd | mean μg/cm2 | sd | |
| Alkanes | 11.46 | 1.02 | 2.05 | 0.19 |
| Secondary alcohols | 1.87 | 0.17 | 1.12 | 0.20 |
| Ketones | 3.79 | 0.90 | 1.45 | 0.43 |
| Primary alcohols | 1.56 | 0.07 | 1.43 | 0.07 |
| Fatty acids | 0.03 | 0.01 | 0.00 | 0.00 |
| Aldehydes | 0.25 | 0.02 | 0.10 | 0.02 |
| Wax esters | 0.30 | 0.02 | 0.31 | 0.05 |
| Unknown | 0.41 | 0.01 | 0.31 | 0.06 |
| Total wax load | 19.66 | 2.22 | 6.77 | 1.02 |
Figure 7.
Reduced epicuticular wax and cutin monomers load in dso-3 plants. A, Stem wax load of dso-3 plants versus the wild type. *, P < 0.05; **, P < 0.01. B, Cutin monomer load of dso-3 plants versus the wild type. The differences were significant between all bars with P < 0.01. Error bars are sd in both A and B. For identities of major cutin monomers identified in leaf cuticles of dso-3 and wild-type plants, see Table II.
The data gathered to this point suggested that DSO might not only be required for wax but also for cutin monomer transport. Consequently, we conducted GC-MS analysis of the previously reported Arabidopsis cutin constituents, including regular fatty acids, 2-hydroxy fatty acids, ω-hydroxy acids, and α,ω-dicarboxylic acids (Bonaventure et al., 2004; Xiao et al., 2004; Franke et al., 2005). The results showed that total cutin monomer load per leaf area in dso-3 was reduced 3.3-fold compared to the wild type (63.96 ± 4.38 ng/cm2 versus 211.58 ± 11.63 ng/cm2; Table II; Supplemental Table S1). Moreover, levels of all 22 detected cutin monomers were dramatically decreased in dso-3 plants (Fig. 7B; Supplemental Table S1). Interestingly, chemical analysis of dso-2 leaf cuticular lipids showed reduction only in wax load, while no significant difference in cutin monomer load was noted. However, cutin composition in dso-2 was different from the wild type. Levels of 2-hydroxy and ω-hydroxy fatty acid levels were up-regulated, whereas the levels of α,ω-dicarboxylic acids (Bonaventure et al., 2004; Franke et al., 2005) were significantly reduced (Supplemental Fig. S4). Thus, DSO loss of function results in altered level and composition of both cutin and wax monomers in the cuticle.
Table II.
List of cutin monomers identified after GC-MS analysis with their respective concentrations in dso-3 mutants and wild-type plants
Data presented here are the average of three replicates.
| n | Compound Class | Wild Type | sd | dso-3 | sd | P Value | Change versus Wild Type | |
|---|---|---|---|---|---|---|---|---|
| mean ng/cm2 | mean ng/cm2 | |||||||
| Alkan-1-oic acids | ||||||||
| 1 | C18 octadecenoic acid(1) | 1.48 | 0.10 | 0.76 | 0.11 | P < 0.01 | ↓ | |
| 2 | C18 octadeca(dien+trien)oic acid(2,3) | 17.37 | 1.54 | 6.78 | 1.01 | P < 0.01 | ↓ | |
| 3 | C22 docosanoic acid | 3.53 | 0.43 | 1.25 | 0.07 | P < 0.01 | ↓ | |
| 4 | C24 tetracosanoic acid | 4.20 | 0.36 | 1.94 | 0.03 | P < 0.01 | ↓ | |
| Total | 26.58 | 2.43 | 10.73 | 1.21 | ||||
| 2-Hydroxy acids | ||||||||
| 5 | C16 2-hydroxy-hexadecanoic acid | 4.57 | 0.06 | 1.75 | 0.08 | P < 0.01 | ↓ | |
| 6 | C20 2-hydroxy-eicosanoic acid | 1.51 | 0.12 | 0.53 | 0.06 | P < 0.01 | ↓ | |
| 7 | C22 2-hydroxy-docosanoic acid | 7.92 | 0.08 | 3.49 | 0.15 | P < 0.01 | ↓ | |
| 8 | C23 2-hydroxy-tricosanoic acid | 1.20 | 0.11 | 0.56 | 0.06 | P < 0.01 | ↓ | |
| 9 | C24 2-hydroxy-tetracosenoic acid(1) | 23.77 | 0.56 | 5.98 | 0.38 | P < 0.01 | ↓ | |
| 10 | C24 2-hydroxy-tetracosanoic acid | 28.79 | 0.64 | 10.17 | 0.09 | P < 0.01 | ↓ | |
| 11 | C25 2-hydroxy-pentacosenoic acid(1) | 0.94 | 0.12 | 0.27 | 0.01 | P < 0.01 | ↓ | |
| 12 | C25 2-hydroxy-pentacosanoic acid | 1.40 | 0.08 | 0.66 | 0.03 | P < 0.01 | ↓ | |
| 13 | C26 2-hydroxy-hexacosenoic acid(1) | 2.30 | 0.11 | 0.74 | 0.02 | P < 0.01 | ↓ | |
| 14 | C26 2-hydroxy-hexacosanoic acid | 9.45 | 0.04 | 4.31 | 0.13 | P < 0.01 | ↓ | |
| Total | 81.85 | 1.94 | 28.46 | 1.01 | ||||
| ω-Hydroxy acids | ||||||||
| 15 | C16 16-hydroxy-hexadecanoic acid | 1.95 | 0.09 | 0.38 | 0.04 | P < 0.01 | ↓ | |
| 16 | C17 17-hydroxy-heptadecanoic acid | 1.90 | 0.19 | 0.31 | 0.01 | P < 0.01 | ↓ | |
| 17 | C18 18-hydroxy-octadecadienoic acid(2) | 1.80 | 0.11 | 0.60 | 0.10 | P < 0.01 | ↓ | |
| 18 | C18 18-hydroxy-octadecatrienoic acid(3) | 1.95 | 0.16 | 0.42 | 0.09 | P < 0.01 | ↓ | |
| Total | 7.59 | 0.55 | 1.71 | 0.25 | ||||
| α,ω-Dicarboxylic acids | ||||||||
| 19 | C16 hexadecane-(1,16)-dioic acid | 13.89 | 1.41 | 1.91 | 0.12 | P < 0.01 | ↓ | |
| 20 | C18 octadecane-(1,18)-dioic acid | 2.98 | 0.26 | 0.59 | 0.02 | P < 0.01 | ↓ | |
| 21 | C18 octadecen-(1,18)-dioic acid(1) | 9.16 | 0.89 | 1.69 | 0.06 | P < 0.01 | ↓ | |
| 22 | C18 octadecadien-(1,18)-dioic acid(2) | 29.94 | 1.88 | 6.69 | 0.73 | P < 0.01 | ↓ | |
| Total | 55.96 | 4.43 | 10.88 | 0.94 | ||||
| Midchain oxygenated fatty acids | 21.03 | 1.35 | 5.57 | 0.31 | ||||
| Unknown aliphatics | 10.42 | 0.46 | 3.67 | 0.28 | ||||
| Unidentified compounds | 8.16 | 0.46 | 2.94 | 0.38 | ||||
| Sum total | 211.58 | 11.63 | 63.96 | 4.38 | ||||
DSO Is Induced under Salt, Abscisic Acid, and Wound Stresses
Salinity is a polymorphous stress that impedes plant development and viability through two shared effects: osmotic and nutritional. High salinity affects plants through ion toxicity as well. To assess how DSO is implicated in these environmental stresses, we subjected dso-1 seedlings to salinity stress (200 mm NaCl). We found that dso-1 seedlings are more susceptible to salinity stress than the wild type (Fig. 8, C and D). Using semiquantitative RT-PCR, we evaluated DSO transcript levels in wild-type plants under the same conditions and found up-regulation in DSO expression upon salt stress (24-h exposure; Fig. 8A).
Figure 8.
Induction of DSO and related gene expression by different stresses and sensitivity of the dso-1 lines to salinity. A, Semiquantitative RT-PCR experiments showing DSO (WBC11), CER5 (WBC12), and WBC13 expression under 200 mm NaCL and 50 μm ABA treatments. The β-actin gene served as a control for equal loading of cDNA. B, Wound induction detected in plants expressing GUS driven by the DSO 5′ upstream region. C and D, The decrease in DSO expression results in salt susceptibility as detected in 2-week-old dso-1 seedlings exposed to 200 mm NaCl for 4 d (C) and a wild-type seedling exposed to the same treatment (D).
Abscisic acid (ABA) is a universal plant hormone widely implicated in adaptation to stress. It regulates stomatal closure, and increasing evidence suggests that it is involved in root branching (De Smet et al., 2006). Semiquantitative RT-PCR showed up-regulation of DSO transcripts in RNA derived from seedlings treated for 24 h with 50 μm ABA (Fig. 8A). DSO expression was not only induced by salinity and increased ABA levels but also upon mechanical wounding, as detected in leaves expressing GUS driven by the DSO 5′ upstream region (Fig. 8B). We also examined the expression of CER5 (AtWBC12) and AtWBC13 genes under the same salt and ABA treatments (Fig. 8A). The results show that while CER5 expression is induced in salt and ABA treatments (similar to DSO), expression of WBC13 is induced by salt but not by ABA.
DISCUSSION
Cutin is the third most abundant biopolymer on earth after lignin and cellulose. It is a major component of the cuticle that covers all plant surfaces exposed to air. Despite its significance, little is known about the transport of cutin monomers from their synthesis site in the epidermal cells to the extracellular domain where the cuticle is assembled. This study demonstrates that in Arabidopsis, DSO, a plasma membrane-localized ABC transporter, is required for proper export of cutin monomers through the plasma membrane to the cuticle. It is also required for the transport of wax monomers, a different set of cuticular components, as reported earlier for the Arabidopsis CER5 transporter (Pighin et al., 2004). The transport of these two compound classes (i.e. cutin and wax) by the same transporter is likely because a large number of the ABC transporters characterized to date were able to handle several structurally different compounds (Yazaki, 2006). Moreover, the information regarding DSO gene expression and the array of loss-of-function phenotypes suggest that the DSO protein might also be associated with transport of other wax- or cutin-like molecules.
The DSO protein sequence shares 52% identity with CER5 but, interestingly, an even higher level of similarity with the cotton (Gossypum hirsutum) GhWBC1 protein (84% identity). GhWBC11 is highly expressed in developing cotton fiber cells, and its overexpression in Arabidopsis resulted in plants with short siliques containing severely shriveled embryos and with only several seeds per silique (Zhu et al., 2003). A variable amount of suberin could be found at the cotton fiber base that is typically deposited in concentric layers, alternating with polysaccharides (Ryser, 1992). The relatively high similarity in sequence between DSO and GhWBC1, the overexpression phenotype, and the presence of suberin and a thin cuticle with wax and cutin components in cotton fibers (Schmutz et al., 1996) suggest a similar role to the two transporter proteins in Arabidopsis and cotton.
DSO expression was not confined to the epidermis of aerial parts as anticipated for a transporter of cuticular components. Its expression was also detected in other aerial plant organs and cell types as, for example, in the stem mesophyll cells. Interestingly, relatively strong DSO expression was detected in lateral root primordia, the developing lateral root, and in the root vasculature. Moreover, we also detected reduced root branching in the dso lines. In a different cuticular mutant, bodyguard (bdg), an increase in root branching was observed (Kurdyukov et al., 2006a). This is not the first study in which root expression of genes associated with cutin and wax metabolism is reported. Root expression was also detected for the β-KETO ACYL-COA SYNTHASE1 (KCS1; Todd et al., 1999), YRE (Kurata et al., 2003), BDG (Kurdyukov et al., 2006a), SHINE3 (SHN3; Aharoni et al., 2004), HTH (Kurdyukov et al., 2006b), and CER5 (Pighin et al., 2004) genes. Fatty acid analysis of the kcs1-1 mutant roots revealed a 2-fold increase in α,ω-dicarboxylic acids, and this result led the authors to suggest that KCS1 is not only implicated in wax metabolism but also in the suberin biosynthesis pathway (Todd et al., 1999). Apart from expression of DSO in roots, two more lines of evidence suggest that DSO is involved in transport of other lipid-derived chemicals (such as suberin) that are similar in structure to cuticular lipids. The first supporting evidence is the altered leaf vascular patterns in the dso-3 mutant that might be a result of changes in suberin deposition during secondary growth in the vascular tissue.
A second point supporting this argument is the striking similarity between the aliphatic monomer composition of Arabidopsis cutin and suberin (Bonaventure et al., 2004; Franke et al., 2005). Indeed, a few recent studies suggested that the long-chain α,ω-dicarboxylic fatty acids are not only constituents of the cutin polyester in Arabidopsis (Bonaventure et al., 2004; Kurdyukov et al., 2006b). They might play additional roles as a “suberin-like” network in the secondary cell wall or be required for the cross linking that ensures the integrity of the primary epidermis cell wall. Evolutionarily, it is feasible that during the course of adaptation to terrestrial environments, plants modified the substrate specificity of a lipid transporter to a protein that could fulfill the requirements for constructing interface layers using three different building blocks, namely, cutin, wax, and suberin. We therefore anticipate that in the near future, as suggested here for DSO and previously for KCS1 (Todd et al., 1999), more genes associated with wax and cutin metabolism will also be recognized as playing a role in the biosynthesis and transport of other plant interface components (e.g. suberin).
Full-size ABC transporters contain two ABC and two transmembrane domains in a single polypeptide chain (Schulz and Kolukisaoglu, 2006). On the other hand, half-size transporters, such as the one encoded by DSO, achieve their functionality by combining two ABC-transmembrane domain units as homo- or heterodimers in a membrane-bound transporter complex. One possible dimerization candidate for wax transport is CER5, although two other proteins in the same phylogenetic clade (At3g21090, WBC15; and At1g51460, WBC13; see Sanchez-Fernandez et al., 2001) might also act as partners for the transport of cutin and other molecules. For example, in Drosophila, dimerization of three half-transporter ABC proteins related in sequence, White with Brown and White with Scarlet, is required for the transport of different eye pigment precursors into pigment cells (Mackenzie et al., 2000). Further experiments should identify the interacting partners between the transporters, their substrate specificity, and their mode of action.
With respect to their mode of action, ABC transporters might actively expel the substrates into the extramembrane space or through a side port of the transporter into the upper leaflet of the plasma membrane bilayer in an ATP-dependant process. Alternatively, they might act by turning over the substrates from the inner to the outer leaflet of the plasma membrane acting as an “hydrophobic vacuum cleaner” (flippase activity; Chang and Roth, 2001). Another important question is: How do cuticular lipids reach the ABC transporter localized in the plasma membrane? The possible routes include: (1) they are picked up by fatty acid-binding proteins and relocated to the transporter; or (2) relocation through a vesicular pathway either by the formation of oleosome bodies coated by oleosin-like proteins or the formation of uncoated vesicles that contain the cuticular lipids in lipid rafts (Schulz and Frommer, 2004).
Wax load in, particularly, the C29 alkanes was dramatically reduced in stems of both the dso and the cer5 mutants. It should be noted that in the case of dso, levels of several stem wax components were significantly increased in the mutant compared to the wild type (i.e. C27 alkanes, C31 secondary alcohols, and C24 primary alcohols), suggesting compensation for the loss of other cuticle components. Leaves and fruit of a transposon insertion mutant in the tomato LeCER6 encoding a VLCFA elongase (KCS) were deficient in n-alkanes and aldehydes with chain lengths beyond C30. In the same plants, much higher levels of pentacyclic triterpenoids (α-, β-, and δ-amyrin) were detected, suggesting compensation for the reduction in aliphatics (Vogg et al., 2004). In contrast to the cer5 knockout mutant showing the typical glossy/cer-like stem phenotype with no effect on plant architecture, the dso mutants exhibited a range of dramatic phenotypes in nearly every plant organ examined. The lesion in DSO had a dramatic effect on epidermal cell development, including alterations to trichomes, stomata, and pavement cells. In the case of trichomes, they were collapsed and underdeveloped. Similar phenotypes were observed in trichomes of several mutants and transgenic plants involved in wax and cutin metabolism, including SHN1 overexpression lines (Aharoni et al., 2004), fdh (Yephremov et al., 1999), lcr (Wellesen et al., 2001), cer10 (Zheng et al., 2005), and bdg (Kurdyukov et al., 2006a). It is intriguing that SHN3, one of three AP2 domain transcription factors suggested to act as activators of the wax biosynthetic pathway in Arabidopsis, showed strong and specific expression in the trichome support cells that surround the base of the trichome (Aharoni et al., 2004). The collapse of trichome phenotype detected in dso and bdg might be a result of altered development of the support cells and suggests that these cells might contain a unique component such as suberin that provides them with the strength to hold the trichomes. This hypothesis is further supported by an earlier report on the presence of suberized cell walls in the boundary between plants and secretory organs such as trichomes (Kolattukudy, 2001).
Basal or support cells of trichomes, cuticular ledges, and cuticles over anticlinal cell walls together provide aqueous pores for plants cuticles (Schonherr, 2006). The exact biological impact of the collapsed trichomes supporting cells of the dso mutants with regard to the aqueous solutes movement requires further investigation. Because aqueous pores serve as a main gate for foliar penetration of exogenously applied compounds including agricultural chemicals, deciphering the exact role of cutin metabolism genes on cuticular aqueous pores assembly in plants will have paramount significance for agriculture and ecophysiology.
A major phenotype of the dso loss-of-function genotypes was the occurrence of postgenital fusions that involve surface contact between organs that have already developed as individual entities (Verbeke, 1992). In dso, fusions could be noticed between different types of vegetative and reproductive organs, including between distal parts (even tips) of rosette leaves. Although multiple mutants have been described that posses postgenital organ fusion (see introduction), one cannot identify the factor(s) mediating this phenomenon. The assortment of mutants exhibiting postgenital fusions described up to date differ in most of the parameters used to phenotype cuticular mutants, including in: permeability to a cationic dye, rate of chlorophyll leaching from leaves in alcoholic solution, rate of water loss, defects in pollen hydration, male sterility recovery under high humidity, glossy appearance, alterations to the cuticle ultrastructure, stomatal index, trichome number and branching, and changes in the cuticle chemical composition. This difference in phenotypes in the class of postgenital organ fusion mutants might be simply due to the nature of mutations, gene redundancy, and the degree of phenotype penetration. However, it may also be that a single factor, either a not-yet-identified signaling lipid-based molecule or a specific structural change in the cuticle or the epidermal cell wall (Nawrath, 2006), could trigger this phenomenon. A more detailed comparison between the dso and cer5 mutant phenotypes might aid in identifying the factor promoting postgenital organ fusions because cer5 shows similar alterations in waxes compared to dso but does not exhibit fusion phenotypes.
In recent years, an increasing number of cuticle-related phenotypes and processes have been described (Nawrath, 2006). It is apparent that cuticle-associated proteins not only play a role in plant development, but they are also very active in the plant response to different stress conditions. Our study suggests that DSO plays a vital role in stress response programs mediated by the cuticle, including salt stress and wounding, as its expression was induced under these conditions. Supportive evidence for the importance of DSO function in response to stress, particularly salt stress, was provided by experiments showing that dso-1 seedlings are highly sensitive to salt treatment. Preliminary characterization of a gene trap line corresponding to DSO also showed that it is also induced by multiple stresses, including ABA, high salt, and Glc (Alvarado et al., 2004). Like DSO, the expression of CER6 encoding a VLCFA-condensing enzyme was enhanced by osmotic stress and the presence of ABA (Hooker et al., 2002).
The role of DSO in stress response could be explained by the need to alter surface structure upon water, salinity, and mechanical stress (Shepherd and Wynne Griffiths, 2006). Leaf transpiration has stomatal and cuticular components. Transpiration through the cuticle is largely determined by surface characteristics such as wax thickness and wax microstructure. Wax deposition that occurs rapidly within a few days is often a response to water stress, and stress-resistant plants often have thicker waxes compared to susceptible ones. Increased wax deposition upon exposure to salinity stress was reported for several plants, including salt-sensitive jojoba (Simmondsia chinensis), and seems to be primarily a response to water deficit (Mills et al., 2001). Moreover, in leaves of salt-sensitive jojoba, wax deposition is induced by exogenous ABA (Mills et al., 2001). Finally, mechanical stress such as wounding due to strong wind, rain drops, and leaf-to-leaf contact could also induce the formation of wax for reforming leaf structure (Shepherd and Wynne Griffiths, 2006). More in relation to ABA and stress response, dso-1 mutant plants displayed reduced root branching number. Lateral root formation is essential for the adaptation of plants to changing environmental challenges such as increased osmotic stress and salinity. Growing evidence suggests that ABA is involved in the regulation of root branching (De Smet et al., 2006). Interestingly, GUS expression driven by the DSO 5′ upstream region indicated DSO expression throughout lateral root development.
This study adds another piece to the puzzle of how plants assemble their outermost surface. Nevertheless, the mechanism of cuticle monomer transport from their site of synthesis to the membrane and further to the extracellular domain remains unclear.
MATERIALS AND METHODS
Plant Material and Growth Conditions
All plants, including the transgenic lines, were grown in the climate room at 20°C, 70% relative humidity, and a 16-h-light/8-h-dark cycle and were in the Arabidopsis (Arabidopsis thaliana) ecotype Columbia. Salk T-DNA insertion line SALK_072079 was obtained from the European Arabidopsis Stock Centre (Alonso et al., 2003). T-DNA insertion was identified in the fifth exon using oligonucleotides designed by the iSECT tool (Signal T-DNA Express Web site). DSO RNAi F1 plant seeds were stratified for 2 to 3 d at 4°C and subsequently sown on Murashige and Skoog plates supplemented with 50 μg/mL kanamycin and grown in a culture room under continuous light conditions at 20°C. Two-week-old seedlings were subsequently transferred to soil. For the root-branching experiment, 1-week-old transgenic plants displaying the typical fusion phenotypes were transferred to vertically placed Murashige and Skoog plates for additional 2 weeks growth. Only lateral roots branching out from the main root were counted. For the salt stress assay, 2-week-old seedlings were transferred to Murashige and Skoog plates supplemented with 200 mm NaCl for an additional 1 week growth. Plant survival was monitored during 1 week after application of the salt stress.
Generation of Plant Transformation Constructs and Transgenic Arabidopsis
For generating the DSO RNAi construct, a 298-bp genomic fragment was amplified with sense (5′-AAAAAGCAGGCTCATATGTGACCCAAGACGATAAC-3′) and antisense (5′-AGAAAGCTGGGTGCAGAAGCACTATCAAGACCAC-3′) oligonucleotides, and integrated into pDONR201 using the Gateway cloning system (Invitrogen). The LR Clonase (Invitrogen) was then used to recombine this fragment into pK7GWIWG2(I) binary vector (Karimi et al., 2002). For overexpression, the full-length DSO cDNA was amplified and inserted into BJ36 (Moore et al., 1998), under control of the 35S CaMV promoter, and subsequently cloned into the pMLBART binary vector. For plant transformation, inflorescences were dipped into Agrobacterium tumefaciens strain GV3101 carrying the transgene construct as described (Clough and Bent, 1998).
Toluidine Blue and Nile Red Staining and Visualization of the Leaf Vasculature
The method for examination of cuticular integrity was performed as described (Tanaka et al., 2004). For the leaf vasculature visualization, leaves of 4-week-old plants were bleached for 24 h in ethanol:acetic acid (6:1), immersed in 70% ethanol for 1 h, mounted in chloralhydrate mixture (chloralhydrate:glycerol:water, 8:1:2), and observed using standard light microscopy. Nile Red staining was performed as described previously (Pighin et al., 2004).
Gene Expression Analyses Using Semiquantitative RT-PCR
Two to four rosette leaves of 3- to 4-week-old plants were used for total RNA isolation. Total RNA was isolated using the RNAeasy Plant Mini kit (QIAGEN) according to the manufacturer's protocol. Isolated total RNA was treated by DNAse according to the manufacturer's protocol (Promega). Total RNA (500 ng/1 μg) was transcribed to cDNA using oligo(dT)15 and AMV reverse transcriptase (Chimerix). For the PCR reaction, 1 to 5 μL of was used as a template for a 20-μL PCR reaction with the following primers: DSO sense (5′-ATGTTACTCCTTGGGTCAGAG-3′), antisense (5′-ATTTCGGCACAATGCAAAC-3′) with expected band size of 399 bp; CER5 sense (5′-TGGGATGGAAGTGAGAAAGG-3′), antisense (5′-GAGCCAAGATCGATGTGTAG-3′) with expected band size 193 bp; and WBC13 sense (5′-GGGGATTGTCACAGAAAGGA-3′), antisense (5′-TGACCCGACACAAATGGATA-3′) with expected band size 156 bp. PCR was started with a 94°C denaturation step for 2 min, followed by 45 s at 94°C, 45 s at 58°C, 1 min at 72°C, and 5 min of final elongation. The following amounts of amplification cycles were used: 27 for DSO, 33 for WBC13, and 36 for CER5.
DSO Promoter Analysis Using GUS or GFP as Reporter Gene
The DSO 5′ upstream region (2,394 bp, termed pDSO) was amplified using antisense (5′-NcoI-AAACCATGGCTCTTAAACCAAAACAGAGGATT-3′) and sense (5′-BamHI-TTTAAGAATTAATTGTCTAAATAAC-3′) oligonucleotides, excised with appropriate restriction enzymes, and subcloned into the pMAX vector containing the GUS coding sequence and the NOS terminator. The pDSO fragment together with the GUS gene was excised with PacI and AscI and cloned into the binary vector pBIN+ (van Engelen et al., 1995). GUS staining and embedding was performed according to Pekker et al. (2005). For the promoter-directed GFP expression experiment, a two-component LHG4-10OP transactivation system was used as described (Moore et al., 1998). The DSO 5′ upstream region (4,417 bp) was amplified using sense (5′-BamHI-AGGATCCCTCTTAAACCAAAACAGAGG-′3) and antisense (5′-PstI-CTGCAGGGTAAGTAATTTAGCAATTG-′3) oligonucleotides, and placed 5′ to the LHG4 gene in BJ36. The BJ36 was cut with NotI and subcloned into pART27. To transactivate the GFP, this construct was transformed into a 10OP:GFP line. Seeds of the F2 GFP-positive plants were dissected for observation of embryos, and GFP signal was observed either using standard fluorescent microscopy or confocal microscopy (excitation at 488 nm, emission was at 500–530 nm for GFP and 620–750 nm for chlorophyll).
SEM
Stems (second internodes from the bottom) were collected from wild-type and dso-1, dso-2, or dso-3 plants after 5 to 6 weeks of growth. Leaves were collected and fixated with glutaraldehyde using standard protocols and dried using critical point drying. Samples were mounted on aluminum stubs and sputter-coated with gold. SEM was performed using an XL30 ESEM FEG microscope (FEI) at 5 to 10 kV.
TEM
Leaves and stems from 50-d-old plants were collected and processed using a standard protocol (Weigel and Glazebrook, 2002). The spurr resin-embedded samples were sectioned (70 nm) using an ultramicrotome (Leica) and observed with a Tecnai T12 TEM (FEI).
Subcellular Localization of DSO and Confocal Microscopy
For examination of the DSO protein subcellular localization, the fragment containing the DSO 5′ upstream region (4,417 bp) and the DSO cDNA were translationally fused to GFP at N′ termini. The DSO cDNA was amplified using sense (5′-BamHI-TTTGGATCCCTACCATCTGCGAGCTCCATC-′3) and antisense (5′-XhoI-AAACTCGAGAATGGAGATAGAAGCAAGCAG-′3) oligonucleotides, excised with BamHI and XhoI, and cloned into the 10OP∷N′-GFP in the BJ36 vector (Moore et al., 1998). The DSO 5′ upstream region was amplified using sense (5′-KpnI-AAAGGTACCTAAGAATTAATTGTCTAAATAAC-′3) and antisense (5′-XhoI-TTTCTCGAGCTCTTAAACCAAAACAGAGGATT-′3) oligonucleotides, and cut with KpnI and XhoI. The 10OP∷N′-GFP:DSO in BJ36 was cut with SalI, NotI, and a modified pBlueScript SK+ vector (Stratagene Cloning Systems) cut with NotI and KpnI. After a triple ligation, the obtained vector was cut with PacI and AscI, and cloned into the pBIN+ binary vector (van Engelen et al., 1995). Protoplasts from epidermis-enriched stem segments were prepared as described elsewhere (Sheen, 2001). Fluorescence was observed by an Olympus CLSM500 microscope with an argon laser at 488 nm for excitation, and images for GFP and chlorophyll signals were collected through 505 to 525 nm for GFP and 620 to 750 nm for chlorophyll and FM4-64. FM4-64 plasma membrane staining was performed as described elsewhere (Zheng et al., 2005). When the stem cross sections were analyzed using confocal microscopy, the presence of the GFP signal was verified using the laser photobleach approach. Time-lapse images were taken before the bleach and immediately after photobleaching, to assess the recovery of the GFP signal (Supplemental Movie S1).
Wax and Cutin Analysis
For wax analysis, the second internodes of 7-week-old plants (n = 5) or leaves of 4- to 5-week-old plants were cut and immersed twice in 5 mL of hexane for 30 s at room temperature. The obtained solution containing the cuticular waxes was spiked with 2 μg of tetracosane (Fluka) as an internal standard and analyzed as described (Kurdyukov et al., 2006a). The extracted stem area was calculated based on the measurements of stem length and upper and lower diameter. Leaf area was assessed using the NIH ImageJ software. For cutin analysis, 4-week-old mature leaves (n = 3–4 for the wild type and 25 for dso-2 or dso-3) were photographed and their areas measured using the NIH ImageJ software. Soluble lipids were extracted from samples by dipping in 10 mL of methanol:chloroform (1:1, v/v) mixture for 14 d (solvent was changed daily). Leaf material was dried, weighed (about 25–30 mg), and used for analysis as described (Kurdyukov et al., 2006a).
Generation of cer5-1/dso-2 Double Mutants
Plants exhibiting a mild dso-2 phenotype were crossed with cer5-1 plants. Seeds from F2 plants with a dso-2 phenotype were selected, and the double mutants were identified in the F3 generation based on the additive phenotype that segregated in close to a 3:16 ratio and PCR to verify the presence of the 35S CaMV promoter fragment and the DSO cDNA using the following oligonucleotides: sense, 35S-out-CAATCCCACTATCCTTCG, and antisense, DSO TGTCTGCTTGCTTCTATCTC (expected band size 361 bp).
Statistical Analysis
Data are presented as means ± sd. Statistical significance was determined by a Student's t test. P values <0.05 were considered to be statistically significant.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession number 30685555.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Gene expression analysis of DSO and related sequences in dso lines.
Supplemental Figure S2. Phylogenetic tree of the Arabidopsis WBC subfamily.
Supplemental Figure S3. Root-branching phenotype of dso plants.
Supplemental Figure S4. Cuticular lipids analysis of dso-2 and wild-type plants.
Supplemental Table S1. GC-MS analysis of cutin monomers of dso-3 and wild-type plants.
Supplemental Table S2. Phenotype penetration in the DSO loss-of-function lines.
Supplemental Table S3. Homology between the DSO/CER5 subclade of WBC-type ABC transporters.
Supplemental Movie S1. GFP signal recovery in pDSO∷GFP-DSO transgenic plants.
Supplementary Material
Acknowledgments
We thank Dr. Eyal Shimoni and Hanna Levanony for assistance with TEM; Dr. Eugenia Klein for help with SEM; Alexander Goldschmidt for the 10OP:GFP line and kind help with fluorescence microscopy; Max Itkin for the pMAX construct; Vladimir Kiss for assistance with confocal microscopy; and Guy Gafni for technical assistance.
This work was supported by the Adolfo and Evelyn Blum Career Development Chair (to A.A.), by the Israeli Ministry of Science (Eshkol fellowship for postdocs to D.P.), by the William Z. and Eda Bess Novick Young Scientist Fund (grant to A.A.), by the Y. Leon Benoziyo Institute for Molecular Medicine (to A.A.), by the United States-Israel Binational Agricultural Research and Development Fund (fellowship to S.S.-G.), by the Salk Institute (fellowship to S.S.-G.), by the National Research Initiative of the U.S. Department of Agriculture Cooperative State Research, Education, and Extension Service (grant to J.C.), and by the German Research Society (grant no. SCHR506/7–1 to L.S. and R.F.).
The author responsible for the distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Asaph Aharoni (asaph.aharoni@weizmann.ac.il).
Some figures in this article are displayed in color online but in black and white in the print edition.
The online version of this article contains Web-only data.
References
- Aharoni A, Dixit S, Jetter R, Thoenes E, van Arkel G, Pereira A (2004) The SHINE clade of AP2 domain transcription factors activates wax biosynthesis, alters cuticle properties, and confers drought tolerance when overexpressed in Arabidopsis. Plant Cell 16 2463–2480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301 653–657 [DOI] [PubMed] [Google Scholar]
- Alvarado MC, Zsigmond LM, Kovacs I, Cseplo A, Koncz C, Szabados LM (2004) Gene trapping with firefly luciferase in Arabidopsis. Tagging of stress-responsive genes. Plant Physiol 134 18–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becraft PW, Kang SH, Suh SG (2001) The maize CRINKLY4 kinase controls a cell autonomous differentiation response. Plant Physiol 127 486–496 [PMC free article] [PubMed] [Google Scholar]
- Berge KE, Tian H, Graf GA, Yu L, Grishin NV, Schultz J, Kwiterovich P, Shan B, Barnes R, Hobbs HH (2000) Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science 290 1771–1775 [DOI] [PubMed] [Google Scholar]
- Bonaventure G, Beisson F, Ohlrogge J, Pollard M (2004) Analysis of the aliphatic monomer composition of polyesters associated with Arabidopsis epidermis: occurrence of octadeca-cis-6, cis-9-diene-1,18-dioate as the major component. Plant J 40 920–930 [DOI] [PubMed] [Google Scholar]
- Chang G, Roth CB (2001) Structure of MsbA from E. coli: a homolog of the multidrug resistance ATP binding cassette (ABC) transporters. Science 293 1793–1800 [DOI] [PubMed] [Google Scholar]
- Chen X, Goodwin SM, Boroff VL, Liu X, Jenks MA (2003) Cloning and characterization of the WAX2 gene of Arabidopsis involved in cuticle membrane and wax production. Plant Cell 15 1170–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16 735–743 [DOI] [PubMed] [Google Scholar]
- De Smet I, Zhang H, Inzé D, Beeckman T (2006) A novel role for abscisic acid emerges from underground. Trends Plant Sci 11 434–439 [DOI] [PubMed] [Google Scholar]
- Faure JD, Vittorioso P, Santoni V, Fraisier V, Prinsen E, Barlier I, Van Onckelen H, Caboche M, Bellini C (1998) The PASTICCINO genes of Arabidopsis thaliana are involved in the control of cell division and differentiation. Development 125 909–918 [DOI] [PubMed] [Google Scholar]
- Franke R, Briesen I, Wojciechowski T, Faust A, Yephremov A, Nawrath C, Schreiber L (2005) Apoplastic polyesters in Arabidopsis surface tissues: a typical suberin and a particular cutin. Phytochemistry 66 2643–2658 [DOI] [PubMed] [Google Scholar]
- Hock T, Cottrill T, Keegan J, Garza D (2000) The E23 early gene of Drosophila encodes an ecdysone-inducible ATP-binding cassette transporter capable of repressing ecdysone-mediated gene activation. Proc Natl Acad Sci USA 97 9519–9524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker TS, Millar TA, Kunst L (2002) Significance of the expression of the CER6 condensing enzyme for cuticular wax production in Arabidopsis. Plant Physiol 129 1568–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenks MA, Tuttle HA, Eigenbrode SD, Feldmann KA (1995) Leaf epicuticular waxes of the Eceriferum mutants in Arabidopsis. Plant Physiol 108 369–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jetter R, Schäffer S, Riederer M (2000) Leaf cuticular waxes are arranged in chemically and mechanically distinct layers: evidence from Prunus laurocerasus L. Plant Cell Environ 23 619–628 [Google Scholar]
- Jin P, Guo T, Becraft PW (2000) The maize CR4 receptor-like kinase mediates a growth factor-like differentiation response. Genesis 27 104–116 [DOI] [PubMed] [Google Scholar]
- Karimi M, Inzé D, Depicker E (2002) GATEWAY vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci 7 193–195 [DOI] [PubMed] [Google Scholar]
- Kolattukudy P (2001) Polyesters in higher plants. Adv Biochem Eng Biotechnol 71 1–49 [DOI] [PubMed] [Google Scholar]
- Krolikowski KA, Victor JL, Wagler TN, Lolle SJ, Pruitt RE (2003) Isolation and characterization of the Arabidopsis organ fusion gene HOTHEAD. Plant J 35 501–511 [DOI] [PubMed] [Google Scholar]
- Kunst L, Samuels AL (2003) Biosynthesis and secretion of plant cuticular wax. Prog Lipid Res 42 51–80 [DOI] [PubMed] [Google Scholar]
- Kurata T, Kawabata-Awai C, Sakuradani E, Shimizu S, Okada K, Wada T (2003) The YORE-YORE gene regulates multiple aspects of epidermal cell differentiation in Arabidopsis. Plant J 36 55–66 [DOI] [PubMed] [Google Scholar]
- Kurdyukov S, Faust A, Nawrath C, Bar S, Voisin D, Efremova N, Franke R, Schreiber L, Saedler H, Metraux JP, et al (2006. a) The epidermis-specific extracellular BODYGUARD controls cuticle development and morphogenesis in Arabidopsis. Plant Cell 18 321–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurdyukov S, Faust A, Trenkamp S, Bar S, Franke R, Efremova N, Tietjen K, Schreiber L, Saedler H, Yephremov A (2006. b) Genetic and biochemical evidence for involvement of HOTHEAD in the biosynthesis of long-chain alpha-,omega-dicarboxylic fatty acids and formation of extracellular matrix. Planta 224 315–329 [DOI] [PubMed] [Google Scholar]
- Lolle SJ, Cheung AY (1993) Promiscuous germination and growth of wild-type pollen from Arabidopsis and related species on the shoot of the Arabidopsis mutant, fiddlehead. Dev Biol 155 250–258 [DOI] [PubMed] [Google Scholar]
- Lolle SJ, Hsu W, Pruitt RE (1998) Genetic analysis of organ fusion in Arabidopsis thaliana. Genetics 149 607–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie SM, Howells AJ, Cox GB, Ewart GD (2000) Sub-cellular localization of the white/scarlet ABC transporter to pigment granule membranes within the compound eye of Drosophila melanogaster. Genetica 108 239–252 [DOI] [PubMed] [Google Scholar]
- Mills D, Zhabg G, Benzioni A (2001) Effect of different salts and ABA on growth and mineral uptake in jojoba shoots grown in vitro. J Plant Physiol 158 1031–1039 [Google Scholar]
- Moore I, Galweiler L, Grosskopf D, Schell J, Palme K (1998) A transcription activation system for regulated gene expression in transgenic plants. Proc Natl Acad Sci USA 95 376–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrath C (2006) Unraveling the complex network of cuticular structure and function. Curr Opin Plant Biol 9 281–287 [DOI] [PubMed] [Google Scholar]
- Pekker I, Alvarez JP, Eshed Y (2005) Auxin response factors mediate Arabidopsis organ asymmetry via modulation of KANADI activity. Plant Cell 17 2899–2910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pighin JA, Zheng H, Balakshin LJ, Goodman IP, Western TL, Jetter R, Kunst L, Samuels AL (2004) Plant cuticular lipid export requires an ABC transporter. Science 306 702–704 [DOI] [PubMed] [Google Scholar]
- Pohl A, Devaux PF, Herrmann A (2005) Function of prokaryotic and eukaryotic ABC proteins in lipid transport. Biochim Biophys Acta 1733 29–52 [DOI] [PubMed] [Google Scholar]
- Pruitt RE, Vielle-Calzada JP, Ploense SE, Grossniklaus U, Lolle SJ (2000) FIDDLEHEAD, a gene required to suppress epidermal cell interactions in Arabidopsis, encodes a putative lipid biosynthetic enzyme. Proc Natl Acad Sci USA 97 1311–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryser U (1992) Ultrastructure of the epidermis of developing cotton (Gossypium) seeds: suberin, pits, plasmodesmata, and their implication for assimilate transport into cotton fibers. Am J Bot 79 14–22 [Google Scholar]
- Sanchez-Fernandez R, Davies TG, Coleman JO, Rea PA (2001) The Arabidopsis thaliana ABC protein superfamily, a complete inventory. J Biol Chem 276 30231–30244 [DOI] [PubMed] [Google Scholar]
- Schmutz A, Buchala AJ, Ryser U (1996) Changing the dimensions of suberin lamella of green cotton fibers with a specific inhibitor of the endoplasmic reticulum-associated fatty acid elongases. Plant Physiol 110 403–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnurr J, Shockey J, Browse J (2004) The acyl-CoA synthetase encoded by LACS2 is essential for normal cuticle development in Arabidopsis. Plant Cell 16 629–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonherr J (2006) Characterization of aqueous pores in plant cuticles and permeation of ionic solutes. J Exp Bot 57 2471–2491 [DOI] [PubMed] [Google Scholar]
- Schulz B, Frommer W (2004) Plant biology. A plant ABC transporter takes the lotus seat. Science 306 622–625 [DOI] [PubMed] [Google Scholar]
- Schulz B, Kolukisaoglu HU (2006) Genomics of plant ABC transporters: the alphabet of photosynthetic life forms or just holes in membranes? FEBS Lett 580 1010–1016 [DOI] [PubMed] [Google Scholar]
- Sheen J (2001) Signal transduction in maize and Arabidopsis mesophyll protoplasts. Plant Physiol 127 1466–1475 [PMC free article] [PubMed] [Google Scholar]
- Shepherd T, Wynne Griffiths D (2006) The effects of stress on plant cuticular waxes. New Phytol 171 469–499 [DOI] [PubMed] [Google Scholar]
- Sieber P, Schorderet M, Ryser U, Buchala A, Kolattukudy P, Métraux JP, Nawrath C (2000) Transgenic Arabidopsis plants expressing a fungal cutinase show alterations in the structure and properties of the cuticle and postgenital organ fusions. Plant Cell 12 721–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H, Machida Y (2006) The cuticle and cellular interactions. In M Riederer, C Muller, eds, Biology of the Plant Cuticle. Blackwell Publishing, Oxford, pp 312–333
- Tanaka H, Onouchi H, Kondo M, Hara-Nishimura I, Nishimura M, Machida C, Machida Y (2001) A subtilisin-like serine protease is required for epidermal surface formation in Arabidopsis embryos and juvenile plants. Development 128 4681–4689 [DOI] [PubMed] [Google Scholar]
- Tanaka H, Watanabe M, Watanabe D, Tanaka T, Machida C, Machida Y (2002) ACR4, a putative receptor kinase gene of Arabidopsis thaliana, that is expressed in the outer cell layers of embryos and plants, is involved in proper embryogenesis. Plant Cell Physiol 43 419–428 [DOI] [PubMed] [Google Scholar]
- Tanaka T, Tanaka H, Machida C, Watanabe M, Machida Y (2004) A new method for rapid visualization of defects in leaf cuticle reveals five intrinsic patterns of surface defects in Arabidopsis. Plant J 37 139–146 [DOI] [PubMed] [Google Scholar]
- Todd J, Post-Beittenmiller D, Jaworski JG (1999) KCS1 encodes a fatty acid elongase 3-ketoacyl-CoA synthase affecting wax biosynthesis in Arabidopsis thaliana. Plant J 17 119–130 [DOI] [PubMed] [Google Scholar]
- van Engelen FA, Molthoff JW, Conner AJ, Nap JP, Pereira A, Stiekema WJ (1995) pBINPLUS: an improved plant transformation vector based on pBIN19. Transgenic Res 4 288–290 [DOI] [PubMed] [Google Scholar]
- Verbeke JA (1992) Fusion events during floral morphogenesis. Annu Rev Plant Physiol Plant Mol Biol 43 583–598 [Google Scholar]
- Vogg G, Fischer S, Leide J (2004) Tomato fruit cuticular waxes and their effects on transpiration barrier properties: functional characterization of a mutant deficient in a very-long-chain fatty acid beta-ketoacyl-CoA synthase. J Exp Bot 55 1401–1410 [DOI] [PubMed] [Google Scholar]
- Watanabe M, Tanaka H, Watanabe D, Machida C, Machida Y (2004) The ACR4 receptor-like kinase is required for surface formation of epidermis-related tissues in Arabidopsis thaliana. Plant J 39 298–308 [DOI] [PubMed] [Google Scholar]
- Weigel D, Glazebrook J, editors (2002) Arabidopsis: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- Wellesen K, Durst F, Pinot F, Benveniste I, Nettesheim K, Wisman E, Steiner-Lange S, Saedler H, Yephremov A (2001) Functional analysis of the LACERATA gene of Arabidopsis provides evidence for different roles of fatty acid omega-hydroxylation in development. Proc Natl Acad Sci USA 98 9694–9699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F, Goodwin SM, Xiao Y, Sun Z, Baker D, Tang X, Jenks MA, Zhou JM (2004) Arabidopsis CYP86A2 represses Pseudomonas syringae type III genes and is required for cuticle development. EMBO J 23 2903–2913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazaki K (2006) ABC transporters involved in the transport of plant secondary metabolites. FEBS Lett 580 1183–1191 [DOI] [PubMed] [Google Scholar]
- Yephremov A, Wisman E, Huijser P, Huijser C, Wellesen K, Saedler H (1999) Characterization of the FIDDLEHEAD gene of Arabidopsis reveals a link between adhesion response and cell differentiation in the epidermis. Plant Cell 11 2187–2201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Rowland O, Kunst L (2005) Disruptions of the Arabidopsis enoyl-CoA reductase gene reveal an essential role for very-long-chain fatty acid synthesis in cell expansion during plant morphogenesis. Plant Cell 17 1467–1481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu YQ, Xu KX, Luo B, Wang JW, Chen XY (2003) An ATP-binding cassette transporter GhWBC1 from elongating cotton fibers. Plant Physiol 133 580–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.