Abstract
A universal vector (IL-60 and auxiliary constructs), expressing or silencing genes in every plant tested to date, is described. Plants that have been successfully manipulated by the IL-60 system include hard-to-manipulate species such as wheat (Triticum duram), pepper (Capsicum annuum), grapevine (Vitis vinifera), citrus, and olive (Olea europaea). Expression or silencing develops within a few days in tomato (Solanum lycopersicum), wheat, and most herbaceous plants and in up to 3 weeks in woody trees. Expression, as tested in tomato, is durable and persists throughout the life span of the plant. The vector is, in fact, a disarmed form of Tomato yellow leaf curl virus, which is applied as a double-stranded DNA and replicates as such. However, the disarmed virus does not support rolling-circle replication, and therefore viral progeny single-stranded DNA is not produced. IL-60 does not integrate into the plant's genome, and the construct, including the expressed gene, is not heritable. IL-60 is not transmitted by the Tomato yellow leaf curl virus's natural insect vector. In addition, artificial satellites were constructed that require a helper virus for replication, movement, and expression. With IL-60 as the disarmed helper “virus,” transactivation occurs, resulting in an inducible expressing/silencing system. The system's potential is demonstrated by IL-60-derived suppression of a viral-silencing suppressor of Grapevine virus A, resulting in Grapevine virus A-resistant/tolerant plants.
A common technology for the introduction and expression of foreign genes in plants is Agrobacterium-mediated transformation (Horsch et al., 1985). Another general approach for introducing and expressing foreign genes involves physical means, such as bombardment of DNA-coated inert beads into the plant (Klein et al., 1987; Sanford et al., 1987). Viral vectors have also been engineered for the delivery of genetic material and the expression of recombinant proteins in plants (Chapman et al., 1992; Scholthof et al., 1996; Shivprasad et al., 1999; Yusibov et al., 1999; Mallory et al., 2002; Pogue et al., 2002; Porta and Lomonossoff, 2002; Gleba et al., 2004; Istomina et al., 2004). With DNA plant viruses as vectors, the size of the insert is very limited, and when a viral gene is replaced by a foreign one to accommodate larger inserts, replication and movement are hampered and expression is restricted to the affected cells only (Shepherd, 1989; Ugaki et al., 1991; Stanley, 1993; Tamilselvi et al., 2004; Carrillo-Trip et al., 2006). In the case of RNA viruses, cloning of a foreign sequence downstream of a duplicated viral subgenomic promoter brings about expression of the foreign gene. In many cases, the foreign gene is, however, gradually deleted (Dawson et al., 1989). Viral-mediated delivery and expression of foreign genes are nontransgenic; therefore, they have been designated as “transient” (Scholthof et al., 1996; Marillonnet et al., 2005).
Tomato yellow leaf curl virus (TYLCV) is a monopartite geminivirus (Stanley, 1985), the genomic organization of which has been described previously (Argüello-Astorga et al., 1994; Hanley-Bowdoin et al., 2000). Briefly, TYLCV carries six overlapping open reading frames (ORFs) transcribed bi-directionally from an intergenic region (IR) serving as the viral origin of replication and as a bi-directional promoter. Two ORFs are expressed in the viral orientation (V1 and V2) and the other four in the complementary orientation (C1–C4). Each gene product appears to participate in more than one function. The viral single-stranded DNA (ssDNA) enters the plant and is converted to double-stranded DNA (dsDNA; replicative form) by the host machinery. The dsDNA replicates and expresses viral mRNAs and proteins. Viral genes are not involved in this stage (dsDNA to dsDNA) of replication, which depends solely on the host genes' activity. At the next stage of replication, the multitude of viral dsDNAs serve as templates for rolling-circle replication of progeny ssDNA. ORF C1 (also termed rep) is essential for the initiation of rolling-circle-type replication, bringing about the production of ssDNA from dsDNA templates. The progeny ssDNA molecules are then encapsidated, transported to other tissues, or transmitted to other plants. The viral ORFs V1, V2, C4, and probably C2 are implicated in symptom appearance and disease severity. In summary: five of the viral ORFs have no direct role in viral DNA replication or expression, whereas the sixth ORF (rep) is involved only in the rolling-circle phase (conversion of dsDNA to progeny ssDNA). Movement and pathogenicity, however, require the activity of viral gene products.
Three viral gene products are implicated in viral movement within the plant: the coat proteins (CPs) V1, V2, and C4 (Rojas et al., 2001). These three proteins control geminiviral DNA trafficking to the nucleus, out of the nucleus to the cell periphery, and through plasmodesmata to neighboring cells (Rothenstein et al., 2007). These proteins interact with each other, and different protein complexes are involved in the various stages of mobilization (Kumar et al., 2006). Host proteins have also been implicated in geminiviral trafficking in plants (Selth et al., 2004; Carvalho et al., 2006; Florentino et al., 2006).
Early on, conflicting reports were already being published with respect to the form of the geminiviral DNA that shuttles between the nucleus and the cytoplasm and across the plasma membrane, suggesting ssDNA (Pascal et al., 1994) or dsDNA (Noueiry et al., 1994) as the moving forms. However, more recently, dsDNA as well as ssDNA have been shown to interact with movement proteins, and dsDNA is suggested to be the predominant form in which the virus moves within the plant (Rojas et al., 1998; Hehnle et al., 2004).
Many (but not all) geminiviruses are considered to be phloem restricted, but this tropism is not strict. Closely related viruses may differ in their tissue tropism (Wege et al., 2001), and in other cases tropism changes with the developmental stage of the plant (Sudarshana et al., 1998). TYLCV is generally considered to be phloem restricted, but some reports have indicated spread to the mesophyll as well (Michelson et al., 1994). It has been conclusively shown in bipartite begomoviruses that the viral “common region” (the equivalent of the TYLCV-IR) and the viral BL1, BR1, and Ala-2 (the equivalent of TYLCV V1, V2, and C2, respectively) determine tropism (Morra and Petty, 2000). Tropism of Indian cassava mosaic virus was attributed to the AV2 (equivalent of TYLCV C2) gene product (Rothenstein et al., 2007).
TYLCV infection of tomato (Solanum lycopersicum) is harmful and causes serious agricultural/economic problems (Czosnek and Laterrot, 1997; Czosnek et al., 2001). TYLCV cannot be mechanically inoculated; it is transmitted by the whitefly Bemisia tabaci (Brown and Czosnek, 2002). The viral DNA of an Israeli isolate of TYLCV has been cloned and sequenced (Navot et al., 1991; GenBank accession no. X15656). This viral DNA can be delivered into plants by bombardment, albeit transiently and locally. Agroinoculation of geminiviral DNA as an entity that is longer than one genome in length causes systemic infection (Grimsley et al., 1987; Czosnek et al., 1993).
Geminiviral sequences have been found in the genomes of several host plants (Ashby et al., 1997). Recently, sequences downstream of the IR have been shown to be rescued from the genome, replicated as autonomous entities, and mobilized in plants following geminiviral infection (Morilla et al., 2006).
One plant reaction to viral infection is the stimulation of viral-specific RNA silencing triggered by the appearance of replicative forms of viral dsRNA (e.g. Baulcombe, 2005; Burgyan, 2006). Viruses counter-react by producing silencing suppressors (e.g. Voinnet et al., 1999; Qu and Morris, 2005; Wang and Metzlaff, 2005; Chellappan et al., 2005), and the equilibrium between silencing and suppression of silencing is a determinant of pathogenecity. Absence of a silencing suppressor has been shown to result in normal, healthy-looking plants despite infection (Fagoaga et al., 2006).
Grapevine virus A (GVA) is a vitivirus affecting grapevines and is transmissible to Nicotiana benthamiana. A GVA infectious clone has been produced, leading to a functional analysis of the viral genome (Galiakparov et al., 1999, 2003b). ORF 5 of GVA translates to a 10-kD nucleic-acid-binding protein (p10) that affects pathogenesis (Galiakparov et al., 2003a) and has been identified as the silencing suppressor of that virus (Zhou et al., 2006).
We engineered a symptomless deletion clone of TYLCV and, as discussed further on, chose to insert a plasmid within the rep gene. Long (at least 5 kb) inserts can thus be introduced into TYLCV-DNA. The virus-plasmid vector and the foreign gene inserted into it move systemically and are expressed in plants. The engineered construct is easily introduced into the plant and it replicates in both plant and Escherichia coli cells. A target gene could be silenced by expressing dsRNA. In addition, we engineered a series of artificial satellite DNAs activated to express or silence target genes following virus infection. By substituting the native virus with IL-60-BS, expression/silencing could be achieved without causing disease. We also induced viral resistance using a unique silencing approach.
RESULTS
A TYLCV-Based Vector and Its Derivatives Replicate and Spread in Plants without Causing Disease
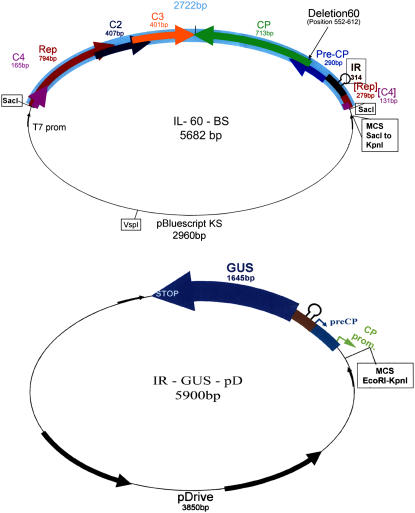
We produced a full-length clone of the aforementioned Israeli strain of TYLCV (GenBank accession no. X15656). A 1.8-genome-long construct, when agroinoculated into tomato plants, caused severe infection. The viral CP is not involved in geminivirus-DNA replication (Noris et al., 1998; Soto et al., 2005), and its role in viral movement requires sequences at the C terminus of the protein (Noris et al., 1998). However, plants inoculated with TYLCV carrying a point mutation in its CP ORF did not develop symptoms (Wartig et al., 1997). Therefore, in an attempt to obtain a symptomless clone, we chose to delete a stretch of 60 nucleotides (positions 552–612 of the aforementioned TYLCV-DNA), causing the removal of 20 amino acids (positions 27–46) near the N terminus of TYLCV-CP. The resultant clone (termed IL-60) replicated and spread in tomato plants following agroinoculation, but the plants remained symptomless for the duration of observation (until fruit set). As already mentioned, TYLCV can accommodate only short inserts: longer sequences can be inserted only at the expense of viral ones. A vector requires only dsDNA replication, and the rolling-circle phase is unnecessary. Therefore, we inserted a plasmid (pBluescript II KS+) within the rep gene (see “Materials and Methods”). The structure of the resultant virus-plasmid construct (termed IL-60-BS) is shown in Figure 1A. Plasmid insertion interrupted rep by placing a spacer between the second and third motifs required for the initiation of rolling-circle replication (see below). In addition, the insertion of the plasmid spacer brought about a frameshift of rep's motif III. Rolling-circle replication was thus abolished, as indicated by the absence of ssDNA in IL-60-BS-treated plants. IL-60-BS and all other constructs described below are shuttle vectors, propagated in plants as well as in E. coli cells.
Figure 1.
Diagrammatic illustration of IL-60-BS and constructs stimulated by it. Top, IL-60-BS. The arrows represent viral genes and their orientation. The thin line represents the inserted plasmid pBluescript II KS+. The stem-loop structure represents the IR. The location of the 60-bp deletion is marked. Bottom, An example of a construct that is stimulated by TYLCV infection or IL-60-BS administration. The thick rectangles represent TYLCV sequences. The thick arrow represents a reporter gene. The bold arrows represent the inserted plasmid pDrive. Thin arrows represent pT7 and SP6 promoters. [See online article for color version of this figure.]
The virus-plasmid vector IL-60-BS, as well as its derivatives carrying genes for GUS or GFP, were first introduced into tomato, a natural host of TYLCV. PCR analyses indicated the presence of vector in all tested leaves, as well as in flowers, fruits, and roots. As shown in Figure 2, the vector was found in parts remote from the point of injection (at least four leaves above it) as early as 3 d postinjection (p.i.) and persisted throughout the plant's life span (12 months p.i.).
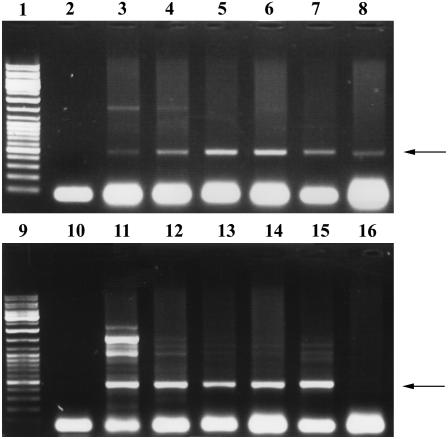
Figure 2.
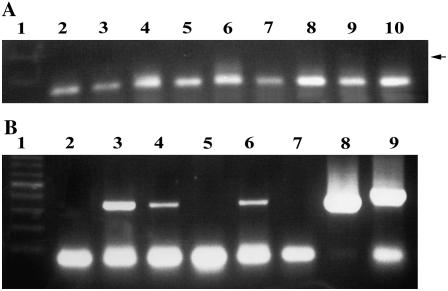
Time-course detection of the virus-plasmid vector. PCR was performed with primers 939/940 (Table I) delineating the virus-plasmid junction of IL-60-BS. Lanes 1 and 9, Size markers; lane 2, negative control (DNA was extracted from noninjected plants); lanes 10 and 16, negative controls (PCR was conducted without a template); lane 11, positive control with IL-60-BS as template. Tomato DNA was extracted from the same plant at the following times p.i.: 1 d (lane 3), 3 d (lane 4), 7 d (lane 5), 14 d (lane 6), 1 month (lane 7), 2 months (lane 8), 3 months (lane 12), 4 months (lane 13), 6 months (lane 14), and 12 months (lane 15). The arrows indicate the position of the expected 495-bp product.
The presence of vector sequences in remote tissues for long periods and their detection by Southern-blot analysis (Fig. 3) indicated its replication and spread in almost all of the injected plants (>90%). As shown below, the vector also replicated and spread in various other plants belonging to several different families, including monocots and woody trees.
Figure 3.
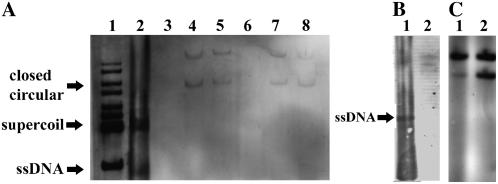
A, Southern-blot analysis of DNA extracted from TYLCV-infected tomato plants and from plants injected with IL-60-BS (2 months p.i.). The probe was a PCR product of a segment of the TYLCV-CP ORF. The DNA extracts in the various lanes were: size markers (lane 1), DNA extract (uncleaved) from TYLCV-infected tomato (lane 2), DNA extracts (uncleaved) from IL-60-BS-injected tomatoes (lanes 4 and 5), and DNA extracts (BglII-cleaved) from IL-60-BS-injected tomatoes (lanes 7 and 8). Lane 3 is empty and lane 6 is a negative control (uncleaved DNA from noninjected plant). The DNA forms of TYLCV (lane 2) are depicted on the left. Note that ssDNA does not appear in the IL-60 lanes. B, Southern-blot analysis as in A of DNA extracted from TYLCV-infected tomato. Lane 1, Untreated DNA; lane 2, S1-nuclease-treated DNA. C, Southern-blot analysis as in A. DNA was extracted from IL-60-BS-treated tomato. Lane 1, Untreated DNA; lane 2, DNA treated with S1 nuclease prior to electrophoresis.
IL-60 Does Not Integrate into the Plant Genome and Does Not Produce ssDNA
Total DNA was extracted from tomato leaves remote from the point of IL-60-BS injection and was subjected to Southern-blot analysis without cleaving or shearing. The membranes were reacted with a digoxigenin-labeled probe corresponding to part of the TYLCV-CP gene (Fig. 3). Two major bands were detected, as with TYLCV (presented as a positive control). In samples from leaves of vector-administered plants, the two corresponding bands were of a larger size than those of TYLCV alone, due to the presence of the plasmid. Southern analysis is far less sensitive than PCR, and, therefore, positive reactions in remote tissues after long periods cannot be attributed to residual, dilute samples of the DNA originally administered to the plant. The absence of a ssDNA band in the IL-60-BS lanes is indicative of the lack of ssDNA viral progeny, due to inactivation of rep and the consequent inability to initiate the rolling-circle phase.
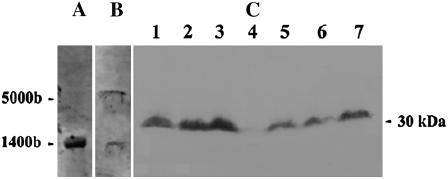
BglII does not cleave within IL-60-BS. If the vector had been integrated into the plant genome, then cleavage with BglII would have resulted in bands longer than those in the uncleaved samples. The bands obtained from cleaved and uncleaved samples were of the same size (Fig. 3), indicating that the vector had not been integrated into the plant genome. These bands were insensitive to digestion with S1-nuclease, confirming their dsDNA nature (Fig. 3C). Northern and western analyses indicated that viral genes are expressed in plants treated with IL-60-BS (Fig. 4). The transcription termination site of the RNA transcribed from ORFs V1 and V2 has not been determined. In fact, the transcription of long transcripts in both orientations has been implicated in the formation of the dsRNA required for gene silencing of geminiviruses (Bisaro, 2006). The approximately 1,440-bp-long transcript probably represents the native termination site at the juncture of the two opposing transcripts. However, in the case of Il-60-BS, a leaky terminator may lead to full-circle transcription, resulting in the appearance of an approximately 5,000-bp RNA.
Figure 4.
Expression of TYLCV genes in a plant treated with IL-60-BS. A, Northern-blot analysis of RNA extracted from TYLCV-infected tomato. B, Northern-blot analysis of RNA extracted from IL-60-BS-treated tomato. Membranes in A and B were reacted with a cloned segment of TYLCV-CP. RNA extracts from noninfected, nontreated plants did not react with the probe (not shown). C, Western-blot analysis. Lanes 2 and 3, Positive controls; proteins were extracted from TYLCV-infected tomatoes. Lane 4, Negative control. Proteins extracted from uninfected, untreated, tomato. Lanes 1 and 5 to 7, Protein was extracted from IL-60-BS-treated plants. The membrane was reacted with antibodies to TYLCV.
Expression of Foreign Genes Inserted in IL-60-BS
IL-60-BS-GUS and IL-60-BS-GFP (see “Materials and Methods”) were introduced into tomato plants. Replication of the constructs was monitored by PCR using primers of the reporter genes (167/408 for GUS and 345/895 for GFP; Table I). Positive reactions with DNA templates from leaves further up from the point of injection were observed as early as 3 d p.i. (an example is shown in Fig. 5A). GUS activity was detected by staining (Jefferson et al., 1987) and GFP by fluorescence (Blumenthal et al., 1999). TYLCV is a phloem-limited virus. Indeed, gene expression in tomato was initially observed in the plant's vascular system (Fig. 5). However, as described below, the vector gradually spread to mesophyll cells and eventually to the entire plant.
Table I.
Details of primers used in the various described PCR assays
| Primer Designation | Sequence 5′→3′ | Description | Use |
|---|---|---|---|
| 966 (reverse) | attgggctgtttccatagggc | Bases 928 to 908 of IL-60 | Distinguishing IL-60 from TYLCV |
| 977 (forward) | gaaggctgaacttcgacag | Bases 530 to 548 of IL-60 | |
| 939 (forward) | agagacaccgattcatttcaac | Bases 1 to 21 of IL-60-BS | Spanning the junction between Bluescript and IL-60 |
| 940 (reverse) | gcggataacaatttcacacag | Bases 826 to 845 of BS | |
| 167 (reverse) | cagcgtaagggtaatgcgag | Bases 2,468 to 2,449 of GenBank acc. M14641 | GUS-specific primers |
| 408 (forward) | gaacaacgaactgaactggcagac | Bases 1,867 to 1,890 of GenBank acc. M14641 | |
| 345 (reverse) | tgtgtggacaggtaatgg | Bases 694 to 669 of GenBank acc. U87974 | GFP-specific primers |
| 895 (forward) | ggccgaattcagtaaaggagaag | Bases 77 to 99 of GenBank acc. U87974 | |
| PDS (forward) | cagccgctttgatttctcc | Bases 934 to 953 of GenBank acc. M88683 | Preparation of tandem sense:antisense repeats of PDS |
| PDS (reverse) | cacaccttgctttctcatcc | Bases 1,133 to 1,114 of GenBank acc. M88683 | |
| 18S-rRNA (forward) | aggaattgacggaagggcac | Bases 1,142 to 1,446 of GenBank acc. AJ236016 | Load control for RT-PCR |
| 18S rRNA (reverse) | gtgcggcccagaacatctaag | Bases 1,466 to 1,446 of GenBank acc. AJ236016 |
Figure 5.
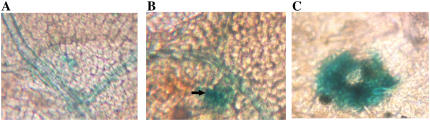
IL-60-BS-derived expression of a reporter gene in tomato plants. A, Expression of GUS in tomato 1 month p.i. B, Expression of GUS in tomato 2 months p.i. C, Enlargement of a section of B (shown by an arrow) exhibiting GUS spread outside of the vesicular system.
IL-60-BS-Derived Constructs Are Not Heritable and Are Not Transmitted by the Whitefly B. tabaci
Tomato plants carrying IL-60-BS-GUS and expressing GUS produced fruits. Seeds were collected from those fruits, and progeny (F1) plants were grown alongside their parents. We self-pollinated three GUS-expressing primary plants and checked 108 F1 progeny plants for the presence of GUS and of IL-60-BS by amplifying the junction between TYLCV and the plasmid (Table I). In addition, we checked 60 F2 progeny plants and 10 F3 plants. Whereas the parent plants reacted positively in all of the PCR tests and still carried and expressed GUS, only one F1 plant faintly expressed GUS. The F2 progeny plants of the GUS-expressing F1 plant did not express GUS. Overall, only one out of 178 progeny plants was found to carry IL-60-BS-GUS, and this was not carried on to further generations. An example of PCR analysis with primers for GUS is presented in Figure 6A. The weak GUS expression found in the single progeny plant (Fig. 6A, lane 2) may have been the result of “mechanical” contamination with the vector.
Figure 6.
A, An example of PCR analysis for GUS in progeny (F1) tomato plants. IL-60-GUS was introduced into the parent plant 12 months earlier. The parent plant still expressed GUS. Lane 1, Size markers; lanes 2 to 10, template DNA was extracted from various progeny plants of the GUS-expressing parent. The arrow marks the expected position of the PCR product. B, IL-60-BS is not transmitted by B. tabaci. PCR was conducted with primers 966/977 (Table I) distinguishing between IL-60-BS and TYLCV (the TYLCV band is 60 bp longer than the IL-60-BS band). Lane 1 exhibits size markers. In lanes 3, 4, and 6, template DNA was extracted from the IL-60-BS-carrying source plants on which the insects were fed. In lanes 2, 5, and 7, template DNA was extracted from the plants to which the insects were transferred. In lane 8, PCR was performed with cloned IL-60-BS as template (positive control). In lane 9, template DNA was extracted from a TYLCV-infected plant (positive control). All sections of the figures show results obtained from two levels of the very same gel; hence, the positive controls shown in lanes 8 and 9 of Figure 6B are also applicable to Figure 6A.
Transmission assays with B. tabaci were carried out as described in “Materials and Methods.” Briefly, the insects were fed on tomato plants carrying IL-60-BS and were later transferred to untreated plants. PCR assays were carried out with DNA extracted from the donor and recipient plants. Similar assays were carried out with TYLCV-infected plants (positive controls for the validity and efficiency of the assay). As shown in Figure 6B, the recipient plants did not carry IL-60-BS. As a general rule, 15 insects per plant are sufficient for TYLCV transmission. Suspecting that IL-60-BS may still be transmissible, albeit at a lower efficiency, in one experiment we colonized approximately 150 Bemisia individuals per plant and still did not find the construct in the recipient plants.
Transactivation of a Reporter Gene Placed Adjacent to the IR by a Disarmed Helper Virus and Partial Host Range of IL-60-BS
pIR-X are a series of constructs in which the IR of TYLCV is placed in the plasmid pDrive, enabling the insertion of foreign genes downstream of it (Fig. 1B; “Materials and Methods”). The satellites pIR-GUS and pIR-GFP did not replicate or express the reporter gene in protoplasts. Therefore, satellites of the structure pIR-X could not, by themselves, replicate and express in plants but could be propagated in E. coli. However, co-administration of IL-60-BS and pIR-GUS or pIR-GFP resulted in the replication of both constructs and strong GUS and GFP expression in plants (Figs. 7 and 8, respectively). As already mentioned, plants injected with only pIR-GUS did not express GUS. After 14 d, the plants were challenged with a wild-type TYLCV. Extensive GUS expression was noted within a few days, but the plants developed severe disease symptoms. Challenge inoculation with IL-60-BS, however, induced expression without causing any symptoms. As shown in Figure 7, expression of a gene placed under the control of IR becomes visible following challenge infection with a helper-disarmed virus. Because IR-derived expression requires only host factors, it is conceivable that the foreign gene is expressed by itself in the few cells it has entered and that the helper virus enables its movement throughout the plant, rendering its expression readily detectable.
Figure 7.
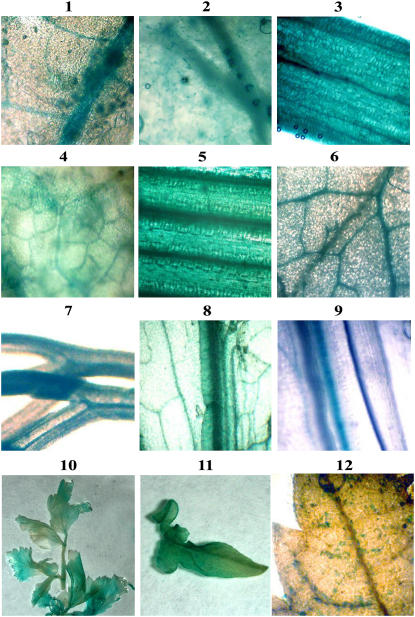
Examples of expression in plants following transactivation of pIR-GUS by IL-60-BS. Frames 1 to 9 show microscopic images of GUS-expressing plants. Expression is demonstrated in the following plants: tomato (frame 1), tobacco (frame 2), onion (frame 3), squash (frame 4), wheat (frame 5), cabbage (frame 6), dill (frame 7), parsley (frame 8), and lettuce (frame 9). GUS expression was corroborated by PCR (data not shown). Frames 10 and 11 show macroscopic images of GUS-expressing plants: a whole parsley plantlet (frame 10), a whole tomato leaf (frame 11), and a grapevine leaf (frame 12).
Figure 8.
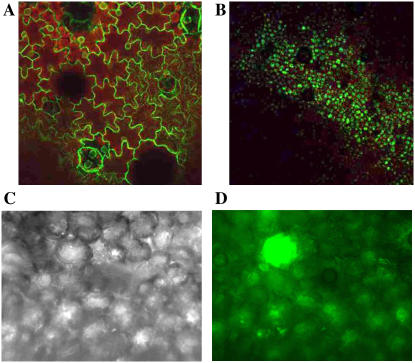
GFP expression in N. benthamiana following co-administration of IL-60-BS and pIR-GFP. A, Expression of GFP, driven by the 35S promoter, in transgenic tobacco. B, Expression of GFP from IL-60-BS + pIR-GFP 3 weeks p.i. (images A and B were photographed through a fluorescence binocular). C and D, pIR-GFP-driven fluorescence in N. benthamiana leaf tissue as seen in a dark-field inverted microscope. Image in frame D was programmed to show GFP fluorescence in green.
The expression and movement of a gene placed under the control of TYLCV-IR can thus be transactivated at a later time by challenge inoculation with a helper geminivirus. The disarmed IL-60-BS is able to transactivate in a harmless manner. In fact, these constructs are TYLCV satellites, as they depend on TYLCV or IL-60-BS as helper viruses for replication, movement, and expression.
The host range of TYLCV is quite broad, infecting plants belonging to several botanical families (Mansour and Al-Musa, 1992). IL-60-BS and pIR-GUS were co-administered to plants belonging to various botanical families [Solanaceae, Cucurbitaceae, Umbelliferae, Liliacae, Apiaceae, Gramineae (Poaceae), Rosaceae, Musaceae, Vitacaea, Rutaceae, and Cruciferae]: tomato, tobacco (Nicotiana tabacum), petunia (Petunia hybrida), cabbage (Brassica oleracea), Arabidopsis (Arabidopsis thaliana), lettuce (Lactuca sativa), summer squash (Cucurbita pepo), onion (Allium cepa), parsley (Petroselinum crispum), wheat (Triticum durum), pepper (Capsicum annuum), carrot (Daucus carota), banana (Musa acuminate), and dill (Antheum graveolens). The constructs were also introduced into the following woody plants: rose (Rosa hybrida), grapevine (Vitis vinifera), olive (Olea europaea), and orange (Citrus sinensis) seedlings. GUS expression was followed by PCR with GUS-amplifying primers (167/408; Table I) and by staining. GUS expression was observed in all of these plants (partial results are presented in Fig. 7). GUS was observed within 3 d in several plants (tomato, tobacco, and dill), while some other plants required longer periods (up to 3 weeks) before GUS expression was noted. The macroscopic pictures (Fig. 7, frames 10 and 11) indicate that GUS expression was widespread in all plant tissues. Every plant species that was thus treated expressed GUS and GFP (host range for GFP expression not shown). This host range exceeds that of native TYLCV and includes dicots and monocots and herbaceous and woody plants.
In many plants, expression of the reported gene was initially restricted to the vascular system; however, eventually the expressing construct moved to non-phloem tissues. In some plants (wheat, onion, dill, and others; Fig. 7, frames 3, 5, and 7), expression was already found outside the vascular system immediately following injection.
Comparative Quantification of the Level of Expression in IL-60-Derived Systems
The co-administration of IL-60-BS and pIR-GUS (or pIR-GFP) appeared, to the naked eye, to be a better expressing system than IL-60-BS-GUS (or IL-60-BS-GFP) alone. The levels of GUS and GFP expression, when co-administered with IL-60-BS, were tested quantitatively (see “Materials and Methods”) relative to their expression in transgenic plants driven by the strong plant promoter 35S (Fig. 8). In both cases, IL-60-BS-derived expression was comparable to that of 35S-derived expression. Expression levels of GUS in the different tested cases were approximately 0.25- to 2-fold those in the control transgenic plants. The actual rates for GUS, obtained by the 4-methylumbelliferyl glucuronide assay (fluorescence units per microgram protein per hour) were 3.7 for 35S-derived expression in tobacco, 2.9 for IL-60-BS-derived expression in N. benthamiana, 0.9 in petunia, and 6.6 in onion. The rates for GFP (fluorescence units per cell) were 1.67 for 35S-GFP and 1.81 for pIR-GFP co-injected with Il-60-BS (both in tobacco). While in transgenic plants, GFP expression was localized in the cytoplasm, expression in the IL-60-BS-driven system was localized in the vacuole. This level of expression is comparable to other viral vectors; the level of expression from the currently best-known plant virus vector is about 2-fold higher than that reported here (discussed further on).
Silencing of Endogenous Gene Expression by the Il-60-BS-Derived Systems
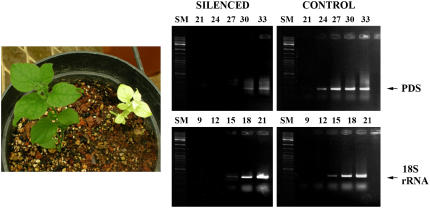
Two types of constructs were developed for silencing purposes. A construct of tandem sense:antisense repeats of a segment of the gene for phytoene desaturase (PDS) was inserted into pIR-GUS, replacing GUS with the PDS repeats (see “Materials and Methods”). The resultant construct (IR-PDS repeats) was induced to transcribe the PDS repeats by injecting it, along with IL-60-BS, into tomato, petunia, and N. benthamiana plants. The resultant RNA was expected to fold back on itself to make dsRNA and thus induce PDS silencing. Within 1 to 4 weeks (depending on the plant species), some leaves started to turn yellow (in tomato) or white (in N. benthamiana and petunia) due to chlorophyll bleaching resulting from PDS silencing. Quantitative reverse transcription (RT)-PCR indicated approximately 64-fold inhibition of expression (Fig. 9). Another silencing method consisted of inserting a target sequence between two opposing IR promoters (IR-X-RI), leading to the production of dsRNA upon transactivation with virus or IL-60-BS. PDS silencing was also achieved in this manner (data not shown).
Figure 9.
Left, PDS silencing in N. benthamiana following dual injection of pIR-PDS repeats along with IL-60-BS. The picture was taken 3 weeks p.i. Right, Semiquantitative RT-PCR demonstrating silencing. The PDS band appeared first in cycle 24 when RNA was extracted from the control plants and in cycle 30 with RNA from treated plants. Load control was performed with 18S-rRNA. SM, Size markers.
Creating GVA Resistance/Tolerance in N. benthamiana
Posttranscriptional gene silencing has been reported to play a role in the plant's reaction to infection, and equilibrium between silencing and silencing suppression determines pathogenecity. We therefore attempted to produce nontransgenic resistance/tolerance by silencing the GVA-silencing suppressor p10, thereby arresting the virus's ability to exercise counter-silencing measures. The entire ORF 5 of GVA (coding for p10) was placed between two opposing IRs, as described in “Materials and Methods,” producing IR-p10-RI. This construct was injected into N. benthamiana plants along with IL-60-BS. Five days later, the plants were inoculated with GVA. Symptom appearance was monitored daily. While the control (noninjected) plants developed symptoms within a week, plants treated with IR-p10-RI + IL-60-BS were divided into two groups: asymptomatic plants and plants with very mild symptoms (Fig. 10). Semiquantitative RT-PCR indicated an approximately 60-fold reduction of p10 expression in symptomless treated plants, as compared to untreated plants (Fig. 10B), and an approximately 30-fold reduction in plants exhibiting mild symptoms (data not shown). Thus, suppression of the viral silencing suppressor gave rise to plants that are GVA resistant or tolerant.
Figure 10.
Silencing of GVA-p10. A, Phenotypic appearance of N. benthamiana plants. I, Noninfected, untreated plant. II, Plant treated with IR-p10-RI and inoculated with GVA 5 d post-treatment. III, Untreated plant inoculated with GVA. Pictures were taken 10 d after GVA inoculation. B, Results of quantitative RT-PCR analysis of the plants shown in I. Samples were withdrawn from PCR reactions at three-cycle intervals (cycles 18–30 for p10 and 12–24 for 18S-rRNA). Lanes 1 to 5, RNA was extracted from plant II depicted in frame A, pretreated with IR-p10-RI, and infected with GVA for 18 d; lanes 6 to 10, RNA was similarly extracted from plant III depicted in frame A, untreated, and GVA infected. Expression of 18S-rRNA, serving as internal control, indicated that the same amount of RNA, extracted from both plants, was amplified.
DISCUSSION
This article describes a platform for expression or silencing in plants that is fast responding and applicable to all plants tested to date. Thus, studies in hard-to-manipulate plants, such as wheat, pepper, and grapevine, can now be performed within days (2–3 weeks in woody plants). The IR-carrying segments of TYLCV fused to a foreign gene and introduced into plants (pIR-X) are stable, even though they do not replicate or spread in the plant. Their stability may be attributed to the inherent IR sequence. A helper geminivirus may be introduced at a later time, contributing transactivating factors and inducing the spread of, and expression from, the IR-carrying segment. In fact, pIR-X and IR-X-RI constructs are satellites, depending on a helper virus for replication, expression, and movement (see also Morilla et al., 2006). If the challenging entity is IL-60-BS, the outcome is harmless. This provides an inducible expression system. It also appears that double-injection of the aforementioned IR-carrying segment with IL-60-BS brings about stronger expression of the foreign gene, apparently due to its proximity to the IR. The level of expression from the strong plant promoter 35S ranges from 1% to 3% of the plant's soluble proteins (Liu et al., 1994; De Jaeger et al., 2002; Krishnan et al., 2002; Kang et al., 2006). We determined an up to 2-fold increase of expression in the IL-60-BS-derived system compared to that of 35S-derived expression, yielding roughly up to 6% of the soluble protein. In comparison, expression rate in plants from the best known viral (Tobacco mosaic virus) vector is approximately 10% of the soluble protein (Shivprasad et al., 1999). The IL-60-BS-driven system, therefore, expresses at about one-half the level of the Tobacco mosaic virus-derived vector.
By expressing constructs leading to the formation of dsRNA, the system can also induce silencing of a target gene. Insertion of short inverted repeats of PDS downstream of IR resulted in PDS silencing. GVA-resistant/tolerant plants were created by placing ORF 5 (p10) of GVA between two opposing IRs.
We modified the native TYLCV genome to achieve the desired functional alterations, i.e. abolishing symptoms but maintaining spreading capability. The CP of geminiviruses plays no role in viral DNA replication but is involved in viral movement, systemic spread, and symptom development in the plant (Liu et al., 1994; Wartig et al., 1997; Unseld et al., 2004). These characteristics have been mapped to the C-terminal part of the CP (Noris et al., 1998). Because one of the critical goals in constructing the vector was the retention of its spreading capacity, we altered the N-terminal part of the CP. We deleted 60 nucleotides of the TYLCV, causing the removal of 20 amino acids from the native viral CP. The resultant CP still carried a bipartite nuclear localization signal (amino acids 1–20), although a third part (KRR at positions 41–43) of what may have been a tripartite nuclear localization signal was removed. Deletion at the N terminus of CP also resulted in a deletion of 45 amino acids at the C terminus of the overlapping ORF V2 (“pre-coat”). Motif searches available at PROSITE (such as ELM and MotifScan) indicated that the deleted sequence includes a number of protein:protein recognition motifs, such as SH2, SH3, PDZ, and a motif recognized by SUMO (a ubiquitin-like protein) for modification. Apparently, TYLCV pre-coat functions within higher-order protein complexes, and the removal of these motifs interferes with the scaffolding of the aforementioned putative complexes. The rep gene product of geminiviruses is involved in rolling-circle replication (Saunders et al., 1991), i.e. the conversion of the gene-expressing dsDNA replicative form to ssDNA progeny. Recognition of, and binding to, the origin of replication, as well as initiation of rolling-circle replication by nicking at the origin, are all attributed to the N terminus of rep (Campos-Olivas et al., 2002). For use as a biotechnological tool, only the replication of and expression from dsDNA (which rely solely on host factors) are required, and the conversion of ssDNA to dsDNA as well as the synthesis of single-stranded progeny virus are immaterial. Therefore, we cloned the plasmid component of the vector within the N-terminal part of rep. IL-60-BS was constructed such that the plasmid, inserted at position 279 of TYLCV, interrupted the rep protein at position 93. The catalytic domain of rep is composed of three motifs. Motif III carries an α-helix (positions 99–106), including the catalytic Tyr (Y103; Y101 in the reported isolate of TYLCV), which is required for nicking (Campos-Olivas et al., 2002). The insertion of the plasmid at this position also interrupted ORF C4, which is involved in symptom expression (Rigden et al., 1994; Selth et al., 2004), thus contributing to the disarming of the virus. The aforementioned alterations are all consistent with a disarmed dsDNA construct that is capable of replicating (dsDNA to dsDNA) by attracting the host machinery to its origin of replication and retaining its mobility, but with no ability to produce progeny viral ssDNA. In fact, a plant episome was engineered that, along with the bacterial plasmid component, could shuttle between bacteria and plants. As already described, the current thinking is that the virus moves within the plant principally as dsDNA (Rojas et al., 1998; Hehnle et al., 2004), although the involvement of ssDNA has not been ruled out. The presently described system is devoid of ssDNA, thus supporting viral movement in a dsDNA form.
The previously suggested size limitation of inserts in geminivirus expression vectors (Stanley, 1993; Carrillo-Tripp et al., 2006) probably resulted from their reliance on ssDNA for movement, replication, and encapsidation. IL-60-derived structures are composed entirely of dsDNA forms and thus may have circumvented this limitation. Indeed, size limitations on the movement of a geminivirus-derived dsDNA have been reported to be between 2 and 9 kb (Rojas et al., 1998) and are compatible with the size range of insert acceptance by the IL-60-derived forms.
As already mentioned, the IR-carrying segment is stable, and the introduction of a helper geminivirus will allow its spread and expression, thereby providing an inducible expression system. By expressing constructs leading to the formation of dsRNA, the system can also silence a target gene.
The agricultural use of genomically modified plants is a matter of public debate, and in many countries it is prohibited by law or regulation. The main concerns voiced against the use of transgenic plants are the fear of inappropriate selection of the transgenic lineage (due to masked deleterious positional effects), possible cross-fertilization with weeds and other crops, further genome alterations due to recombination (especially when copies of endogenous genes are added), and possible transduction of the foreign sequences to plant and soil microorganisms. Introduction of antibiotic-resistant genes to food and the environment is also a major concern. The IL-60-derived systems remain active after removal of the bacterial origin of replication and the ampicillin-resistance gene (data not shown), these alterations making them potentially biosafe. Geminiviruses are not seed transmissible (Kashina et al., 2003). Analysis of the progeny of GUS-expressing plants indicated that the cloned trait is not inherited. The single occasion in which GUS expression was noted in a progeny plant was probably due to vector contamination of the seed cortex, as has been observed previously with several viruses (e.g. tomato mosaic virus; Hadas et al., 2004). However, even on this rare occasion, the vector was not inherited by further generations. The presently reported vector forms are not insect transmissible, even when the plants are colonized with a large number of insect vectors. Being noninheritable, fear of cross-fertilization is minimized. The IL-60-derived constructs do not integrate into the host genome, and thus the concern of deleterious positional effects is irrelevant. Recombination events take place at the meiotic stage of DNA replication (i.e. in the gametes), while the vector's replication occurs in somatic cells. In conclusion, we offer a new technology that might ease public and legislative environmental concerns. Secretion of the inserted gene product into the vacuole may be advantageous if, for example, the expression platform described herein were to be developed for the “biofarming” of large amounts of proteins or of proteins that are deleterious to the plant's well-being.
In addition to the use of the IL-60 system for expression, it can be employed for silencing. We demonstrated a case of inducible silencing of an endogenous gene (PDS). We proceeded by building a system in which virus infection stimulates the silencing of one of its own genes, bringing about resistance/tolerance. The treated plants become resistant/tolerant within a few days of injection, as compared to conventional breeding which, after many years of development, has been only partially successful.
MATERIALS AND METHODS
Clones and Constructs
For reasons discussed earlier, we deleted 60 bp (positions 552–612) of a full-length clone of the Israeli strain of TYLCV (Navot et al., 1991), producing IL-60. Deletion was carried out by inverse PCR using primers directed outward from the ends of the deleted segment. In contrast to the native TYLCV, agroinoculation of tomato (Lycopersicon esculentum) plants with a multimeric form of IL-60 resulted in systemic, but symptomless, infection.
The plasmid Bluescript II KS+ was SacI-linearized and ligated to the SacI site of IL-60. The resultant 5,682-bp-long virus-plasmid construct was termed IL-60-BS. This construct replicates in Escherichia coli cells, and milligram quantities of it can be obtained by a simple midi-prep procedure for plasmid isolation. The plasmid was introduced into position 2,443 of IL-60, thus interrupting the latter's rep gene by placing a 2,960-bp insert near its 5′ end. The inserted spacer also caused a frameshift, so that the two parts of rep were in different reading frames. Surprisingly, a construct bearing a single length of viral genome can thus be administered directly into plants without Agrobacterium mediation, and the construct replicates and spreads in the affected plants.
Reporter genes were introduced into IL-60-BS as follows. IL-60-BS was cleaved with EcoRV (which cleaves solely within the multiple cloning site of BS). The coding region of the reporter gene GUS (bases 1,466–3,274; GenBank accession no. M14641) was PCR-amplified adding a SacI restriction site to one end and a SalI site to the other. All of the restriction sites were blunt-ended with T4-DNA polymerase, and the GUS was inserted into the EcoRV site of the vector. A construct containing the coding sequence of the GFP (bases 1–797; GenBank accession no. U87974) was inserted into IL-60-BS in exactly the same way. The resultant constructs were termed IL-60-BS-GUS and IL-60-BS-GFP.
In addition, constructs carrying the plasmid pDrive instead of Bluescript were prepared and behaved similarly to the aforementioned constructs.
The IR serves as the origin of replication as well as a strong bi-directional promoter for the expression of all viral genes. A satellite construct consisting of the IR, pre-coat ORF, and a short part of the 5′ untranslated region of CP was fused to the aforementioned coding sequence of GUS. This construct was termed IR-GUS and was force-cloned into pDRIVE with SacI and SalI. The resultant construct was termed pIR-GUS. Various other sequences were also inserted by replacing GUS to produce pIR-X. These constructs cannot replicate in plants, but can be induced to replicate, move, and be expressed by TYLCV infection or IL-60-BS injection.
Two sets of constructs were used for silencing. For PDS silencing, the GUS part of IR-GUS was replaced by an inverted-repeat segment of part of the tomato gene for PDS, prepared by amplifying tomato DNA with PDS primers (Table I). The resultant 199-bp product was TA cloned into the plasmid pDrive. The plasmid was then cleaved with BamHI and XbaI, and the resultant fragments were self-ligated, resulting in tandem inverted repeats of various lengths (multiplications of 199 bp). Following electrophoresis, a fragment of approximately 400 bp was extracted from the gel. This fragment is a tandem repeat of the PCR product, one repeat in a sense orientation and the other in an antisense orientation. The PDS fragment was inserted into the IR-carrying pDRIVE (see preparation of IR-GUS) that had been digested with BamHI and XbaI.
Another construct for silencing consisted of two IR segments of TYLCV placed in opposite orientations at both ends of the multiple cloning site of the plasmid pDrive, and the construct was termed IR-X-RI. To silence GVA-p10, the viral ORF 5 (bases 7,011–7,287; GenBank accession no. AF007415) was PCR-amplified and TA cloned into pDrive. IRs were then inserted at both ends of p10 (between the KpnI and PstI sites upstream of p10 and between the HindIII and SalI sites downstream of it).
Propagation of the Virus-Plasmid Vectors and Their Administration to Plants
E. coli cells were transformed with the pertinent virus-plasmid construct, propagated under ampicillin selection, and the construct was extracted by standard procedures (Sambrook and Russel, 2001). A stem, or leaf petiole, of a recipient plant was punctured with a hypodermic needle. A capillary tube was inserted into the resultant hole, and approximately 200 ng of DNA (in 100 μL) was pipetted into the tube until fully soaked up by the plant. For large-scale application, samples were delivered into plants by the aforementioned BIM-LAB instrument.
Molecular Analyses
Southern, northern, western, PCR, and quantitative RT-PCR analyses were carried out according to standard procedures (Sambrook and Russel, 2001). GUS and GFP activities were detected according to published procedures (Jefferson et al., 1987; Blumenthal et al., 1999). Because tomato plants carry geminivirus sequences in their genome (Ashby et al., 1997), PCR with virus primers may not reflect the presence of vector sequences. It was also necessary to ascertain that PCR products had been obtained from vector templates and not from a possible virus infection. To avoid false detection due to plant viral sequences or natural virus infection, every PCR test was carried out with primers amplifying IL-60 segments and, in addition, with at least two sets of primers: (1) primers distinguishing IL-60 from TYLCV; and (2) primers amplifying the reporter gene inserted into IL-60-BS or pIR-GUS, or primers flanking the plasmid-virus junction of IL-60. Primer details are presented in Table I.
Probes for Southern and northern analyses were labeled by the PCR-digoxigenin procedure (Roche Molecular Biochemicals). Chemiluminescent probes for western blots were prepared with the SuperSignal West Pico kit (Pierce). All probes were prepared according to the respective manufacturer's protocol.
Quantitative PCR was carried out by removing aliquots from an ongoing PCR of a target gene (or cDNA) at different cycles and determining the threshold of band appearance. A similar assay, with the same templates, was carried out with primers for a constitutive gene, and the threshold of its band appearance was determined. Each treatment threshold was given an arbitrary quantitative value according to the formula Δct = 2−(ct target gene − ct constitutive gene), Ct being the cycle threshold. The relative quantitative increase/decrease of templates between control and treated plants was estimated from the ratio of their respective Δcts.
GUS staining and GFP fluorescence were monitored as described earlier. GFP images were photographed without a filter to detect any native fluorescence derived from leaf damage and then with a filter (Leica MZ FL III, GFP2). Levels of GFP expression in various treated plants were compared by measuring GFP fluorescence intensity per cell. These determinations were calculated from the microscopic images by the Image Pro 3 program of Media Cybernetics. Levels of GUS expression were determined by 4-methylumbelliferyl glucuronide assay (Jefferson et al., 1987) and expressed as fluorescence intensity per microgram protein per hour.
Insect Transmission Assay
Whiteflies (Bemisia tabaci; 15–100 per plant) were fed on tomatoes carrying IL-60-BS for 48 h. The insects were then placed in one chamber of a dual-chamber container (the two chambers were separated by stretched Parafilm, the other chamber containing 12.5% Suc). Following 48 h feeding on Suc (through the membrane), the insects were transferred to nontreated plants. Similarly, as a positive control, insects were fed on TYLCV-infected plants, then on Suc and then on the recipient plant. The presence of TYLCV or IL-60-BS was followed by PCR.
Acknowledgments
The study was carried out in the framework of Minerva's Otto Warburg Center for Plant Biotechnology. We thank the Wolfson Foundation for the use of facilities contributed to the Plant Science Institute.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Ilan Sela (sela@agri.huji.ac.il).
Some figures in this article are displayed in color online but in black and white in the print edition.
Open Access articles can be viewed online without a subscription.
References
- Argüello-Astorga GR, Guevara-Gonzalez RG, Herrera-Estrella LR, Rivera-Bustamante RF (1994) Geminivirus replication origins have a group-specific organization of iterative elements: a model for replication. Virology 203 90–100 [DOI] [PubMed] [Google Scholar]
- Ashby MK, Warry A, Bejarano ER, Khshoggi A, Burrell M, Lichtenstein CP (1997) Analysis of multiple copies of geminiviral DNA in the genome of four closely related Nicotiana species suggest a unique integration event. Plant Mol Biol 35 313–321 [DOI] [PubMed] [Google Scholar]
- Baulcombe D (2005) RNA silencing. Trends Biochem Sci 30 290–293 [DOI] [PubMed] [Google Scholar]
- Bisaro DM (2006) Silencing suppression by geminivirus proteins. Virology 344 158–168 [DOI] [PubMed] [Google Scholar]
- Blumenthal A, Kuznetzova L, Edelbaum O, Raskin V, Levy M, Sela I (1999) Measurements of GFP fluorescence in plants: quantification, correlation to expression, rapid screening and differential gene expression. Plant Sci 142 93–99 [Google Scholar]
- Brown JK, Czosnek H (2002) Whitefly transmission of plant viruses. In RT Plumb, ed, Advances in Botanical Research, Vol 36. Academic Press, New York, pp 65–100
- Burgyan J (2006) Induced RNA silencing and suppression: defence and counter-defence. J Plant Pathol 88 233–244 [Google Scholar]
- Campos-Olivas R, Louis JM, Clèrot D, Gronenborn B, Gronenborn AM (2002) The structure of a replication initiator unites diverse aspects of nucleic acid metabolism. Proc Natl Acad Sci USA 99 10310–10315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo-Tripp J, Shimada-Beltran H, Riviera-Bustamante R (2006) Use of geminiviral vectors for functional genomics. Curr Opin Plant Biol 9 209–215 [DOI] [PubMed] [Google Scholar]
- Carvalho MF, Turgeon R, Lazarowitz SG (2006) The geminivirus nuclear shuttle protein NSP inhibits the activity of AtNSI, a vascular-expressed Arabidopsis acetyltransferase regulated with sink-to-source transition. Plant Physiol 140 1317–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman S, Kavanagh T, Baulcomb D (1992) Potato virus X as a vector for gene expression in plants. Plant J 2 549–557 [DOI] [PubMed] [Google Scholar]
- Chellappan P, Vanitharani R, Fauquet CM (2005) MicroRNA-binding viral protein interferes with Arabidopsis development. Proc Natl Acad Sci USA 102 10381–10386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czosnek H, Ghanim M, Morin S, Rubinstein G, Fridman V, Zeidan M (2001) Whiteflies: vectors, and victims (?), of geminiviruses. Adv Virus Res 57 291–322 [DOI] [PubMed] [Google Scholar]
- Czosnek H, Kheyrpour A, Gronnenborn B, Remetz E, Zeidan M, Altman A, Rabinowitch HD, Vidavsky S, Kedar N, Gafni Y, et al (1993) Replication of tomato yellow leaf curl virus (TYLCV) DNA in agroinoculated leaf-disks from selected tomato genotypes. Plant Mol Biol 22 995–1005 [DOI] [PubMed] [Google Scholar]
- Czosnek H, Laterrot H (1997) A worldwide survey of tomato yellow leaf curl viruses. Arch Virol 142 1391–1406 [DOI] [PubMed] [Google Scholar]
- Dawson WO, Lewandowski DJ, Hilf ME, Bubrick P, Raffo AJ, Shaw JJ, Grantham GL, Desjardins PR (1989) A tobacco mosaic virus-hybrid expresses and loses an added gene. Virology 172 285–292 [DOI] [PubMed] [Google Scholar]
- De Jaeger G, Scheffer S, Jacobs A, Zanbre M, Zobell O, Goossens A, Depicker A, Angenon G (2002) Boosting heterologous protein production in transgenic dicotyledonous seeds using Phaseolus vulagaris regulator sequences. Nat Biotechnol 20 1265–1268 [DOI] [PubMed] [Google Scholar]
- Fagoaga C, Lopez C, de Mendoza AH, Moreno P, Navarro L, Flores R, Pena L (2006) Posttranscriptional gene silencing of p23 silencing suppressor of citrus tristeza virus confers resistance to the virus in transgenic Mexican lime. Plant Mol Biol 60 153–165 [DOI] [PubMed] [Google Scholar]
- Florentino LH, Santos AA, Fontenelle MR, Pinheiro GL, Zerbini FM, Baracat-Pereira MC, Fontes EPB (2006) A PERK-like receptor kinase interacts with the geminivirus nuclear shuttle protein and potentiates viral infection. J Virol 80 6648–6656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galiakparov N, Tanne E, Sela I, Gafny R (1999) Infectious RNA transcripts from grapevine virus A clone. Virus Genes 19 235–242 [DOI] [PubMed] [Google Scholar]
- Galiakparov N, Tanne E, Mawassi M, Gafny R, Sela I (2003. a) ORF 5 of grapevine virus A encodes a nucleic acid-binding protein and affects pathogenesis. Virus Genes 27 257–262 [DOI] [PubMed] [Google Scholar]
- Galiakparov N, Tanne E, Sela I, Gafny R (2003. b) Functional analysis of the grapevine virus A genome. Virology 306 42–50 [DOI] [PubMed] [Google Scholar]
- Gleba Y, Marillonnet S, Klimyuk V (2004) Engineering viral expression vectors for plants: the ‘full virus’ and the ‘deconstructed virus’ strategies. Curr Opin Plant Biol 7 182–188 [DOI] [PubMed] [Google Scholar]
- Grimsley N, Hohn T, Davies JW, Hohn B (1987) Agrobacterium-mediated delivery of infectious maize streak virus into maize plants. Nature 325 177–179 [Google Scholar]
- Hadas R, Pearlsman M, Gefen T, Lachman O, Hadar E, Sharabany G, Antignus Y (2004) Indexing system for tomato mosaic virus (ToMV) in commercial tomato seed lots. Phytoparasitica 32 421–424 [Google Scholar]
- Hanley-Bowdoin L, Settlage SB, Orozco BM, Nager S, Robertson D (2000) Geminiviruses: models for plant DNA replication, transcription, and cell cycle regulation. Crit Rev Biochem Mol Biol 35 105–140 [PubMed] [Google Scholar]
- Hehnle S, Wege C, Jeske H (2004) Interaction of DNA with movement proteins of geminiviruses revisited. J Virol 78 7698–7706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsch RB, Fry JE, Hoffmann NL, Eichholtz D, Rogers SG, Fraley RT (1985) A simple and general method for transferring genes into plants. Science 227 1229–1231 [DOI] [PubMed] [Google Scholar]
- Istomina EA, Snegireva PB, Shiyan AN (2004) Construction of a full-length cDNA of tobacco mosaic virus strain V-69 genome. Russ J Genet 40 1356–1363 [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MV (1987) GUS fusion: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6 3901–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang TJ, Kim BG, Yang JY, Yang MS (2006) Expression of synthetic cholera toxin B subunit in tobacco using ubitiquin promoter and bar gene as a selectable marker. Mol Biotechnol 32 203–210 [DOI] [PubMed] [Google Scholar]
- Kashina BD, Mabagala RB, Mpunami AA (2003) Biomolecular relationships among isolates of tomato yellow leaf curl Tanzania virus. Phytoparasitica 31 188–199 [Google Scholar]
- Klein TM, Fromm M, Weissinger A, Thomas D, Schaaf S, Sletten M, Sanford JC (1987) High velocity microprojectiles for delivering nucleic acids into living cells. Nature 327 70–73 [Google Scholar]
- Krishnan R, McDonald KA, Dandekar AM, Jackman AP, Falk B (2002) Expression of recombinant trichosanthin, a ribosome-inactivating protein, in transgenic tobacco. J Biotechnol 97 69–88 [DOI] [PubMed] [Google Scholar]
- Kumar PP, Usha R, Zrachya A, Levy Y, Spanov H, Gafni Y (2006) Protein-protein interactions and nuclear trafficking of coat protein and beta C1 protein associated with Bhendi yellow vein mosaic disease. Virus Res 122 127–136 [DOI] [PubMed] [Google Scholar]
- Liu D, Raghothama KG, Hasegawa PM, Bressan RA (1994) Osmotin overexpression in potato delays development of disease symptoms. Proc Natl Acad Sci USA 91 1888–1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallory AC, Parks G, Endres MW, Baulcombe D, Bowman LH, Pruss GJ, Vance VB (2002) The amplicon-plus system for high-level expression of transgenes in plants. Nat Biotechnol 20 622–625 [DOI] [PubMed] [Google Scholar]
- Mansour A, Al-Musa A (1992) Tomato yellow leaf curl virus: host range and virus-vector relationships. Plant Pathol 41 122–125 [Google Scholar]
- Marillonnet S, Thoeringer C, Kandiza R, Klimyuk V, Gleba Y (2005) Systemic Agrobacterium tumefaciens-mediated transfection of viral replicons for efficient transient expression in plants. Nat Biotechnol 23 718–723 [DOI] [PubMed] [Google Scholar]
- Michelson I, Zamir D, Czosnek H (1994) Accumulation and translocation of tomato yellow leaf curl virus (TYLCV) in a lycopersicon-esculentum breeding line containing the l-chilense TYLCV tolerance gene ty-1. Phytopathology 84 928–933 [Google Scholar]
- Morilla G, Castillo AG, Preiss W, Jeske H, Bejarano ER (2006) A versatile transcription-based system to identify cellular proteins involved in gemeinivirus replication. J Virol 80 3624–3633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morra MR, Petty ITD (2000) Tissue specificity of geminivirus infection is genetically determined. Plant Cell 12 2259–2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navot N, Pichersky E, Zeidan M, Zamir D, Czosnek H (1991) Tomato yellow leaf curl virus: a whitefly-transmitted geminivirus with a single genomic component. Virology 185 151–161 [DOI] [PubMed] [Google Scholar]
- Noris E, Vaira AM, Caciagli P, Masenga V, Gronenbom B, Accotto GP (1998) Amino acids in the capsid protein of tomato yellow leaf curl virus that are crucial for systemic infection, particle formation and insect transmission. J Virol 72 10050–10057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noueiry AO, Lucas WJ, Gilbertson RL (1994) Two proteins of a plant DNA virus coordinate nuclear and plasmodesmal transport. Cell 76 925–932 [DOI] [PubMed] [Google Scholar]
- Pascal E, Sanderfoot AA, Ward BM, Medville R, Turgeon R, Lazarowitz SG (1994) The geminivirus BR1 movement protein binds single-stranded DNA and localizes to the cell nucleus. Plant Cell 6 995–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogue GP, Lindbo JA, Garger SJ, Fitzmaurice WP (2002) Making an ally from an enemy: plant virology and the new agriculture. Annu Rev Phytopathol 40 45–74 [DOI] [PubMed] [Google Scholar]
- Porta C, Lomonossoff GP (2002) Viruses as vectors for the expression of foreign sequences in plants. Biotechnol Genet Eng Rev 19 245–291 [DOI] [PubMed] [Google Scholar]
- Qu F, Morris TJ (2005) Suppressors of RNA silencing encoded by plant viruses and their role in viral infection. FEBS Lett 579 5958–5964 [DOI] [PubMed] [Google Scholar]
- Rigden J, Krake L, Rezaian M, Dry I (1994) ORF C4 of tomato leaf curl geminivirus is a determinant of symptom severity. Virology 204 847–850 [DOI] [PubMed] [Google Scholar]
- Rojas MR, Jiang H, Salati R, Xoconostie-Cazares B, Sudarshana MR, Lucas WJ, Gilbertson RL (2001) Functional analysis of proteins involved in movement of the monopartite begomovirus tomato yellow leaf curl virus. Virology 291 110–125 [DOI] [PubMed] [Google Scholar]
- Rojas MR, Noueiry AO, Lucas WJ, Gilbertson RL (1998) Bean dwarf mosaic geminivirus movement proteins recognize DNA in a form- and size-specific manner. Cell 95 105–113 [DOI] [PubMed] [Google Scholar]
- Rothenstein D, Krenz B, Selchow O, Jeske H (2007) Tissue and cell tropism of Indian cassava mosaic virus (ICMV) and its AV2 (precoat) gene product. Virology 359 137–145 [DOI] [PubMed] [Google Scholar]
- Sambrook J, Russel DW (2001) Molecular Cloning, Ed 3. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- Sanford JC, Klein TM, Wolf ED, Allen N (1987) Delivery of substances into cells and tissues using a particle bombardment process. J Part Sci Technol 5 27–37 [Google Scholar]
- Saunders K, Lucy A, Stanley J (1991) DNA forms of the geminivirus African cassava mosaic virus consistent with a rolling circle mechanism of replication. Nucleic Acids Res 19 2325–2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholthof HB, Scholthof K-BG, Jackson AO (1996) Plant virus gene vectors for transient expression of foreign proteins in plants. Annu Rev Phytopathol 34 299–323 [DOI] [PubMed] [Google Scholar]
- Selth LA, Randles JW, Rezaian MA (2004) Host responses to transient expression of individual genes encoded by Tomato leaf curl virus. Mol Plant Microbe Interact 17 27–33 [DOI] [PubMed] [Google Scholar]
- Shepherd RJ (1989) Biochemistry of DNA plant viruses. In A Marcus, ed, The Biochemistry of Plants, Vol 15. Academic Press, New York, pp 536–616
- Shivprasad S, Pogue GP, Lewandowski DJ, Hidalgo L, Donson J, Grill LK, Dawson WO (1999) Heterologous sequences greatly affect foreign gene expression in tobacco mosaic virus-based vectors. Virology 255 312–323 [DOI] [PubMed] [Google Scholar]
- Soto MJ, Chen LF, Seo YS, Gilbertson RL (2005) Identification of regions of the beet mild curly top virus (family Geminiviridae) capsid protein involved in systemic infection, virion formation and leafhopper transmission. Virology 341 257–270 [DOI] [PubMed] [Google Scholar]
- Stanley J (1985) The molecular biology of geminiviruses. Adv Virus Res 30 139–177 [DOI] [PubMed] [Google Scholar]
- Stanley J (1993) Geminiviruses: plant-viral vectors. Curr Opin Genet Dev 3 91–96 [DOI] [PubMed] [Google Scholar]
- Sudarshana MR, Wang HL, Lucas WJ, Gilberton RL (1998) Dynamics of bean dwarf mosaic geminivirus cell-to-cell and long-distance movement in Phaseolus vulgaris revealed, using the green fluorescence protein. Mol Plant Microbe Interact 11 277–291 [Google Scholar]
- Tamilselvi D, Anad G, Swarup S (2004) A geminivirus AYVV-derived shuttle vector for tobacco BY2 cells. Plant Cell Rep 23 81–90 [DOI] [PubMed] [Google Scholar]
- Ugaki M, Ueda T, Timmermans MCP, Vieira J, Elliston KO, Messing J (1991) Replication of a geminivirus derived shuttle vector in maize endosperm cells. Nucleic Acids Res 19 371–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unseld S, Frischmuth T, Jeske H (2004) Short deletions in nuclear targeting sequences of African cassava mosaic virus coat protein prevents geminivirus twinned particle formation. Virology 318 90–101 [DOI] [PubMed] [Google Scholar]
- Voinnet O, Pinto YM, Baulcombe DC (1999) Suppression of gene silencing: a general strategy used by diverse DNA and RNA viruses of plants. Proc Natl Acad Sci USA 96 14147–14152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MB, Metzlaff M (2005) RNA silencing and antiviral defense in plants. Curr Opin Plant Biol 8 216–222 [DOI] [PubMed] [Google Scholar]
- Wartig L, Kheyr-Pour A, Noris E, De Kouchkovsky F, Jouanneau F, Gronenborn B, Jupin I (1997) Genetic analysis of the monopartite tomato yellow leaf curl geminivirus: roles of V1, V2, and C2 ORFs in viral pathogenesis. Virology 228 132–140 [DOI] [PubMed] [Google Scholar]
- Wege C, Saunders K, Stanley J, Jeske H (2001) Comparative analysis of tissue tropism of bipartite geminiviruses. J Phytopath-Phytopathologische Zeitscrift 149 359–368 [Google Scholar]
- Yusibov V, Shivprasad S, Turpen TH, Dawson W, Koprowski H (1999) Plant viral vectors based on tobamoviruses. Curr Top Microbiol Immunol 240 81–94 [DOI] [PubMed] [Google Scholar]
- Zhou Z, Dell'Orco M, Saldarelli P, Minafra A, Martelli GP (2006) Identification of an RNA-silencing suppressor in the genome of grapevine virus A. J Gen Virol 87 2387–2395 [DOI] [PubMed] [Google Scholar]