Abstract
The MultiSite Gateway cloning system, based on site-specific recombination, enables the assembly of multiple DNA fragments in predefined order, orientation, and frame register. To streamline the construction of recombinant genes for functional analysis in plants, we have built a collection of 36 reference Gateway entry clones carrying promoters, terminators, and reporter genes, as well as elements of the LhG4/LhGR two-component system. This collection obeys simple engineering rules. The genetic elements (parts) are designed in a standard format. They are interchangeable, fully documented, and can be combined at will according to the desired output. We also took advantage of the MultiSite Gateway recombination sites to create vectors in which two or three genes can be cloned simultaneously in separate expression cassettes. To illustrate the flexibility of these core resources for the construction of a wide variety of plant transformation vectors, we generated various transgenes encoding fluorescent proteins and tested their activity in plant cells. The structure and sequence of all described plasmids are accessible online at http://www.psb.ugent.be/gateway/. All accessions can be requested via the same Web site.
The ability to assemble complex recombinant DNA molecules becomes increasingly important because biologists have begun to investigate systematically the functions of the many genetic elements identified in recently sequenced and annotated genomes (Brasch et al., 2004; Hilson, 2006). Several cloning systems are available for the transfer of segments between double-stranded DNA molecules that bypass the multistep protocols involving restriction enzyme and ligase reactions. These systems are based on recombinases that recognize specific DNA sequences long enough not to occur by chance but short enough not to interfere with the function of the cloned elements. They include the univector plasmid fusion (Liu et al., 1998), in-fusion cloning (Benoit et al., 2006), and the Gateway recombinational cloning system commercialized by Invitrogen and arguably the most popular nowadays. Gateway is based on the DNA sequences and enzymes that catalyze the insertion and excision of the λ phage DNA into and from the Escherichia coli chromosome (Hartley et al., 2000).
Binary T-DNA vectors used for Agrobacterium tumefaciens-mediated transformation of plant cells are large plasmids and are cumbersome to manipulate in classical restriction/ligation schemes. Therefore, several research teams rapidly developed Gateway versions of such vectors to streamline ectopic gene expression, gene silencing, and promoter studies in transgenic plants (Wesley et al., 2001; Karimi et al., 2002; Curtis and Grossniklaus, 2003). These original vector sets were later complemented with constructs that express protein fusions carrying fluorescent, purification, or epitope tags (for review, see Earley et al., 2006; Hilson, 2006; Karimi et al., 2007).
Most recently, the MultiSite Gateway technology has been developed for the simultaneous cloning of multiple DNA fragments in a versatile format (Cheo et al., 2004; Sasaki et al., 2004; Magnani et al., 2006), and plant binary T-DNA vectors have been devised for MultiSite Gateway protocols (Karimi et al., 2005). In this context, two, three, or more fragments, flanked by compatible attL and/or attR sites in so-called entry clones, can be recombined in predefined order and orientation into a destination vector in a single LR clonase reaction. The resulting plasmid is an expression clone suitable for functional assays and, in the present applications, for plant cell transformation.
Resources created for recombinational cloning should be designed with simple engineering rules in mind: Genetic elements (parts) should be constructed according to standard formats, easily combined according to the desired output, interchangeable, and fully documented. Taking advantage of the MultiSite Gateway system, we created entry clones, destination vectors, and expression clones to facilitate the assembly of plant genetic elements. We illustrate how these core resources can be used as flexible modules to create a wide variety of plant transformation vectors and we demonstrate the functionality of several MultiSite-derived transgenes in planta.
RESULTS AND DISCUSSION
Genetic Elements in Entry Clones
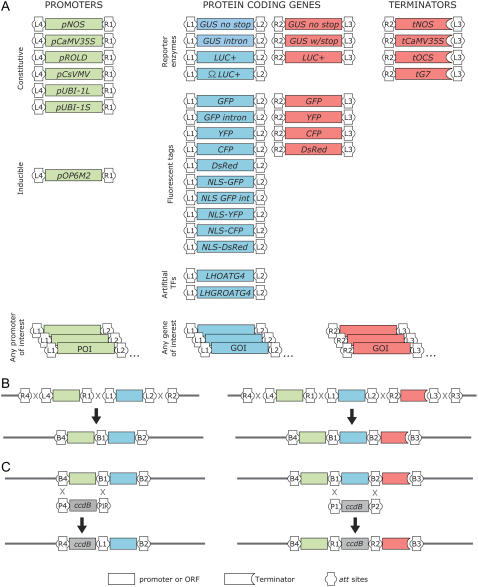
To streamline the construction of plant transgenes, we have built a collection of versatile entry clones containing commonly used plant genetic elements in a format compatible with two- or three-fragment MultiSite Gateway destination cassettes. In this framework, from 5′ to 3′ relative to transcription, the promoters or enhancer motifs are flanked by the attL4 and attR1 recombination sites, open reading frames (ORFs) by the attL1 and attL2 or attR2 and attL3 sites, and terminators by the attR2 and attL3 sites. These entry clones are recombined into plant binary T-DNA destination vectors carrying recipient ccdB cassettes flanked by the attR4-attR2 or attR4-attR3 sites (Karimi et al., 2005; Fig. 1A).
Figure 1.
Building blocks, DNA assembly, and replacement strategies for MultiSite Gateway cloning. A, Entry clones, available either as listed in Table I or potentially added to characterize sequences of interest. B, Assembly of expression clones by recombination of two (left) or three (right) entry clones with a destination vector in LR clonase reactions. C, Examples for the replacement of genetic elements by the counterselectable ccdB cassette within expression clones for the creation of modular destination vectors in reverse BP reactions. Box shape annotation is as indicated at the bottom of C. Green, blue, and red boxes mark sequences flanked by the att4-att1, att1-att2, and att2-att3 pairs, respectively. Gray boxes represent ccdB counterselectable cassettes. Gray lines indicate the backbone of binary T-DNA vectors.
By convention, the names of all entry clones are structured in the same way: the letters pEN are followed by the letter and number L4, L1, or R2, indicating which att site is placed at the 5′ end of the corresponding genetic element, the abbreviation of the element as listed in Table I, and the letter and number R1, L2, or L3, indicating which att site is placed at its 3′ end. For clarity, name parts are separated by hyphens.
Table I.
Genetic elements in entry clones
| Class | Element(s) | Abbreviationsb | Recipient pDONR | Entry Clone | att Sites |
|---|---|---|---|---|---|
| Promoters | pNOS | 1 | pDONR P4-P1R | pEN-L4-1-R1 | attL4-attR1 |
| pCaMV 35S | 2 | pDONR P4-P1R | pEN-L4-2-R1 | attL4-attR1 | |
| pROLD | 3 | pDONR P4-P1R | pEN-L4-3-R1 | attL4-attR1 | |
| pCsVMV | 4 | pDONR P4-P1R | pEN-L4-4-R1 | attL4-attR1 | |
| pUBI-1L, first exon and intron | UBIL | pDONR P4-P1R | pEN L4-UBIL-R1 | attL4-attR1 | |
| pUBI-1S | UBIS | pDONR P4-P1R | pEN-L4-UBIS-R1 | attL4-attR1 | |
| Reporter enzymes | GUS, no stop | S | pDONR 221 | pEN-L1-S-L2 | attL1-attL2 |
| GUS, intron | SI | pDONR 221 | pEN-L1-SI-L2 | attL1-attL2 | |
| GUS, no stop | S | pDONR P2R-P3 | pEN-R2-S-L3 | attR2-attL3 | |
| GUS, with stop | S* | pDONR P2R-P3 | pEN-R2-S*-L3 | attR2-attL3 | |
| LUC+ | L+ | pDONR 221 | pEN-L1-L+-L2 | attL1-attL2 | |
| Ω leader, LUC+ | OL+ | pDONR 221 | pEN-L1-OL+-L2 | attL1-attL2 | |
| LUC+ | L+ | pDONR P2R-P3 | pEN-R2-L+-L3 | attR2-attL3 | |
| Fluorescent tags | GFP | F | pDONR 221 | pEN-L1-F-L2 | attL1-attL2 |
| GFP, intron | FI | pDONR 221 | pEN-L1-FI-L2 | attL1-attL2 | |
| NLS, GFP, intron | NFI | pDONR 221 | pEN-L1-NFI-L2 | attL1-attL2 | |
| ER, GFP | ERF | pDONR P4-P3 | pEN-L4-ERF-L3 | attL4-attL3 | |
| YFP | Y | pDONR 221 | pEN-L1-Y-L2 | attL1-attL2 | |
| CFP | C | pDONR 221 | pEN-L1-C-L2 | attL1-attL2 | |
| DsRed | R | pDONR 221 | pEN-L1-R-L2 | attL1-attL2 | |
| NLS-GFP | NF | pDONR 221 | pEN-L1-NF-L2 | attL1-attL2 | |
| NLS-YFP | NY | pDONR 221 | pEN-L1-NY-L2 | attL1-attL2 | |
| NLS-CFP | NC | pDONR 221 | pEN-L1-NC-L2 | attL1-attL2 | |
| NLS-DsRed | NR | pDONR 221 | pEN-L1-NR-L2 | attL1-attL2 | |
| GFP | F | pDONR P2R-P3 | pEN-R2-F-L3 | attR2-attL3 | |
| YFP | Y | pDONR P2R-P3 | pEN-R2-Y-L3 | attR2-attL3 | |
| CFP | C | pDONR P2R-P3 | pEN-R2-C-L3 | attR2-attL3 | |
| DsRed | R | pDONR P2R-P3 | pEN-R2-R-L3 | attR2-attL3 | |
| Mini 35S, NLS, GFP, introna | M2NFI | pDONR 221 | pEN-L1-M2NFI-L2 | attL1-attL2 | |
| Terminators | tNOS | 6 | pDONR P2R-P3 | pEN-R2-6-L3 | attR2-attL3 |
| tCaMV 35S | 7 | pDONR P2R-P3 | pEN-R2-7-L3 | attR2-attL3 | |
| tOCS | 8 | pDONR P2R-P3 | pEN-R2-8-L3 | attR2-attL3 | |
| tG7 | 9 | pDONR P2R-P3 | pEN-R2-9-L3 | attR2-attL3 | |
| Two-component elements | LHOATG4 | – | pDONR 221 | pEN-L1-LHOATG4-L2 | attL1-attL2 |
| LHGROATG4 | – | pDONR 221 | pEN-L1-LHGROATG4-L2 | attL1-attL2 | |
| pOp6M2 | – | pDONR P4-P1R | pEN-L4-pOp6M2-R1 | attL4-attR1 |
This entry clone carries a hybrid sequence with a minimal CaMV 35S promoter upstream of an ORF coding for a NLS fused to GFP and interrupted by an intron.
Abbreviation used in plasmid names.
Promoters
Widely used promoters for ectopic expression in plant species were transferred into pDONR P4-P1R (Table I). The cauliflower mosaic virus (CaMV) 35S promoter with duplicated enhancer region that is highly active in most transgenic plant tissues (Benfey and Chua, 1990) was fused at its 3′ end with a portion of the tobacco mosaic virus Ω (O) leader sequence to promote translation of the downstream gene (Gallie, 2002; pEN-L4-2-R1). The NOPALINE SYNTHASE (NOS) promoter (pEN-L4-1-R1) is expressed in a wide range of plant cell types and is relatively weaker than the CaMV 35S promoter (Sanders et al., 1987). The ROLD promoter of Agrobacterium rhizogenes (pEN-L4-3-R1) is highly active in roots and calli of most dicotyledonous plant species (Leach and Aoyagi, 1991). The cassava vein mosaic virus (CsVMV) promoter (pEN-L4-4-R1) has been shown to direct highly constitutive gene expression in transgenic plants, similar to the CaMV 35S promoter (Verdaguer et al., 1998). The maize (Zea mays) ubiquitin promoter alone (pEN-L4-UBIS-R1) or including the first exon and the first intron of the ubiquitin gene (pEN-L4-UBIL-R1) are highly active in monocotyledonous plants (Christensen et al., 1992), and the sequence including the intron yields the highest level of gene expression in maize (Vain et al., 1996).
Promoter entry clones formatted in such attL4-promoter-attR1 cassettes can be recombined with any sequence available in another entry clone carrying an attL1 site at its 5′ end with regard to transcription as described (Hope et al., 2004; http://www.psb.ugent.be/SAP/; Fig. 1B). This panel of promoters enables strong ectopic expression of multiple genes in a wide variety of plant species. Because their sequences are different, they can be used to transcribe multiple transgenes in the same plant, decreasing the risk of silencing.
Reporters
Classical plant reporter genes were cloned into pDONR 221 and pDONR P2R-P3 for a wide range of applications, including plant promoter analysis and protein fusion localization (Table I). In all cases, the reporter ORF was positioned in the Gateway reading frame A according to the manufacturer's guidelines. The gene coding for the E. coli GUS enzyme (Jefferson et al., 1987) was captured in the entry clones pEN-L1-S-L2, pEN-L1-SI-L2, and pEN-R2-S*-L3. In pEN-L1-S-L2, the original stop codon was removed from the GUS ORF region to enable translation of GUS as an N-terminal tag added to another protein. In pEN-L1-SI-L2, the GUS ORF contains the PIV2 intron that originates from the potato (Solanum tuberosum) st-ls1 gene and that has been shown to be correctly spliced in Arabidopsis (Arabidopsis thaliana), tobacco (Nicotiana tabacum), poplar (Populus sp.), common bean (Phaseolus vulgaris), and tepary bean (Phaseolus acutifolius; Vancanneyt et al., 1990; Kapila et al., 1997). Intron-containing reporter genes are required when transformed plant cells must be marked unambiguously and distinguished from bacteria, such as Agrobacterium strains, cocultivated with target plant tissues, and in which reporter background expression may be observed (Vancanneyt et al., 1990; Lewin et al., 1998). In pEN-R2-S*-L3, the GUS ORF includes the stop codon and can be cloned in frame with any other attL1-ORF-attL2 for the expression of a GUS C-terminal tag.
The firefly LUCIFERASE (LUC) gene is extensively used as a reporter for in vivo transcriptional regulation studies (Millar et al., 1992). The LUC ORF (luciferase+ or L+, a luciferase with adapted codon usage for enhanced expression; http://www.promega.com/) was captured in the entry clones pEN-L1-L+-L2, pEN-L1-OL+-L2, and pEN-R2-L+-L3 (Table I). In pEN-L1-OL+-L2, the Ω leader was included upstream of the L+ ORF.
Fluorescent proteins (FPs) are molecular reporters whose presence and subcellular localization can be monitored dynamically in living cells. They can be expressed alone or as tags fused to other proteins (Hanson and Köhler, 2001). Several FP-coding genes were captured as entry clones in diverse formats suitable for various applications: enhanced GFP (F), enhanced CYAN FP (CFP; C), enhanced YELLOW FP (YFP; Y), and RED FP (DsRed; R; CLONTECH; http://www.clontech.com/). These genes were cloned into pDONR 221 as simple ORFs (pEN-L1-FP-L2 series) or preceded in frame by the nuclear localization signal (NLS) sequence from a putative tobacco transcription factor (Grebenok et al., 1997; pEN-L1-NFP-L2 series) and into pDONR P2R-P3 as simple ORFs (pEN-R2-FP-L3 series; see Table I for a complete list of FP entry clones). In addition, the PIV2 intron was introduced into the GFP ORF to generate pEN-L1-FI-L2 (see above).
Because the original stop codon was removed from all FP and NLS-FP ORFs, any pEN-L1-FP-L2/pEN-L1-NFP-L2 or pEN-R2-FP-L3 clone can be recombined in a MultiSite LR clonase reaction with any other ORF for the expression of proteins carrying an N- or C-terminal FP tag, respectively. Alternative stop codons in the three frames are located downstream of the Gateway cassette in all the binary T-DNA destination vectors constructed for MultiSite cloning (Karimi et al., 2005). For example, the NLS-FP ORF from a pEN-L1-NFP-L2 entry clone can be fused in frame with the FP ORF from a pEN-R2-FP-L3 for the expression of a double FP molecule (Fig. 2A). Such large double fluorescent tags are shuttled and retained into the nucleus and yield a higher signal than single tags when transcribed under the control of specific promoters. In the same framework, an NLS-FP can be expressed as a fusion with other reporters such as GUS (Quaedvlieg et al., 1998; Fig. 2A).
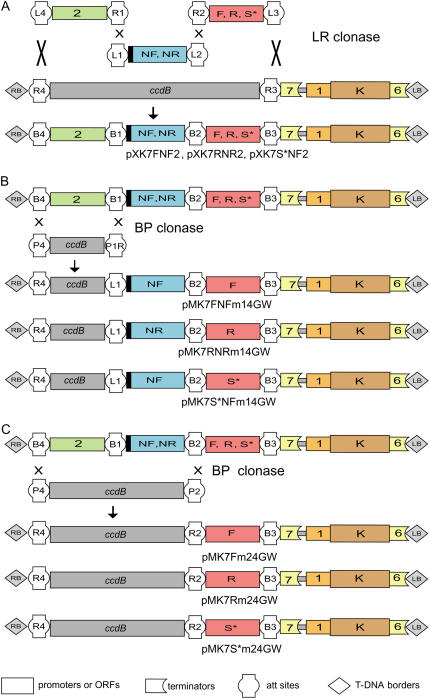
Figure 2.
Creation of modular destination vectors via reverse BP clonase reaction. A, Combined genetic elements p35S (pEN-L4-2-R1), NLS-GFP (pEN-L1-NF-L2), and GFP (pEN-R2-F-L3), or p35S (pEN-L4-2-R1), NLS-DsRed (pEN-L1-NR-L2), and DsRed (pEN-R2-R-L3), or p35S (pEN-L4-2-R1), NLS-GFP (pEN-L1-NF-L2), and GUS (pEN-R2-S*-L3) were merged and introduced via a MultiSite LR clonase reaction into a destination vector (pK7m34GW) carrying the attR4-ccdB-attR3 Gateway cassette, yielding the expression clones pK7FNF2, pK7RNR2, and pK7S*NF2, respectively. B, Recombination of these clones with pDONR P4-P1R in a BP recombination reaction to restore the attR4-ccdB-attL1 Gateway cassette and to create modular destination vector series for promoter analysis. C, Recombination of the same expression clones with pDONR P4-P2 to restore the attR4-ccdB-attR2 Gateway cassette and to create another series of modular destination vectors for translational fusion with a genomic fragment including promoter and coding sequence. Box shape annotation is as indicated at the bottom of C. Abbreviations specify the following sequences: 1, pNOS; 2, pCaMV 35S; 6, tNOS; 7, tCaMV 35S; F, GFP; NF, NLS-GFP; NR, NLS-DsRed; R, DsRed; S*, GUS with stop codon; K, NPTII kanamycin resistance selectable marker.
Any simple or fused ORF carrying an attL1 at its 5′ end may be recombined in the same reaction with any promoter of choice available as an attL4-promoter-attR1 entry clone, such as those described above. Also, any reporter in an attR2-reporter-attL3 format can be recombined with a promoter or ORF built as an attL1-promoter-attL2 entry clone.
Lastly, we have included in the reporter series an attL1-attL2 cassette containing, from 5′ to 3′, a minimal 35S promoter (46 bp; Benfey et al., 1990) followed by the ORF coding for an intron-containing NLS-GFP intron (pEN-L1-M2NFI-L2). This reporter cassette enables the direct addition of enhancer elements (captured in an attL4-attR1 entry clone; as singleton, multimers, or embedded in a larger promoter region) to a transcriptional reporter.
Terminators
To avoid combining identical sequences in different transgenes carried in the same plant, several 3′ polyadenylation sequences were cloned in pDONR P2R-P3 (Table I), originating either from the NOS gene (pEN-R2-6-L3), the CaMV 35S (pEN-R2-7-L3), the OCTOPINE SYNTHASE gene (OCS; pEN-R2-8-L3), or the GENE7 (G7; pEN-R2-9-L3; Velten and Schell, 1985).
Two-Component System for Transcriptional Regulation
Gene activation systems may be encoded in two distinct components: (1) a transcription factor whose regulated expression determines the activity of (2) a cognate promoter, itself controlling the transcription of a gene of interest. Such configurations are particularly appealing to rapidly assign a well-characterized transcription pattern (corresponding to the expression domain of the transcription factor) to a variety of loci, either by transformation or by crossing a driver line with recipient lines.
Components formatted for MultiSite cloning include two artificial transcription factors: LhG4 (or LHOATG4) and LhGR2 (or LHGROATG4; Table I). Both are optimized for expression in plant tissues and contain the yeast (Saccharomyces cerevisiae) GAL4 activation domain fused to the bacterial LacI repressor DNA-binding domain (Moore et al., 1998). LhG4 is constitutively active (Rutherford et al., 2005); LhGR2 contains in addition the mammalian glucocorticoid receptor domain (Craft et al., 2005) enabling its induction by a glucocorticoid (usually dexamethasone) supplied in watering solution, in culture medium, or by organ painting. In the presence of dexamethasone that associates with the glucocorticoid receptor domain, the chimeric LhGR factor is targeted to the nucleus where it becomes active. The LhG4 and LhGR2 ORFs are available as entry clones (pEN-L1-LHOATG4-L2 and pEN-L1-LHGROATG4-L2) for construction of an activator locus. The artificial inducible promoter including six operator sites (pOp6) and a minimal CaMV 35S promoter (M2) is available as a separate entry clone (pEN-L4-pOp6M2-R1) for the construction of a responder locus.
Expression Clones
The sequence-validated DNA segments captured as entry clones constitute a flexible source of reference elements that can be assembled at will in expression clones. An expression clone is produced in an LR clonase reaction that resolves all compatible attL and attR sites within one or several entry clones and a destination vector into the corresponding attB sites. Together with several recipient binary T-DNA destination vectors carrying one of three plant selectable markers (Karimi et al., 2005), the series of promoter, reporter, and terminator entry clones described above can be combined with one's favorite sequences for the rapid construction of plant transgenes (Fig. 1B).
By convention, the name of such expression clones created in a MultiSite LR clonase reaction starts with the letters pX followed by the letter K, H, or B, indicating whether the plant selectable marker in the binary vector T-DNA codes for kanamycin, hygromycin, or Basta resistance, respectively, then by a succession of letters and numbers describing the newly assembled expression unit according to the abbreviations of the elements listed in Table I and ordered relative to the T-DNA left border.
To illustrate the flexibility of this cloning strategy, we have built various FP fusion expression clones (Fig. 2A) based on the pK7m34GW destination vector, including pXK7FNF2 (pEN-L4-2-R1 × pEN-L1-NF-L2 × pEN-R2-F-L3), pXK7RNR2 (pEN-L4-2-R1 × pEN-L1-NR-L2 × pEN-R2-R-L3), and pXK7S*NF2 (pEN-L4-2-R1 × pEN-L1-NF-L2 × pEN-R2-S*-L3).
Typically, we validate the structure of new expression clones in either of two ways: (1) the overall plasmid structure can be verified by analyzing the DNA size pattern resulting from digestion with two different restriction enzymes (or restriction enzyme combinations); or (2) each attB junction (between every element originating from the entry clones and the recipient backbone destination vector) can be amplified by PCR and size verified with PCR primers located within the cloned elements or the vector backbone and directed toward the att sequences. Such primers can be used recurrently for validating any combination involving the elements they derive from.
Modular Destination Vectors Created via Reverse BP Clonase Reaction
An expression clone itself can serve as a template to create novel modular destination vectors by recombining one of its DNA segments flanked by attB sites with a cassette delimited by the matching attP sites (Fig. 1C). Such cassettes are carried by the usual pDONR vectors and contain the selectable CHLORAMPHENICOL RESISTANCE gene and the counterselectable ccdB gene. The fragment replacement is catalyzed in a normal BP clonase reaction and introduces back attL and/or attR sites within the selected plasmid (Cheo et al., 2004).
Modular destination vectors generated by reverse BP cloning are particularly advantageous when series of constructs need to be created in which only one of the elements varies. Once the variable segment is replaced by a ccdB cassette flanked by attL and/or attR sites, the resulting destination vector can be used to generate additional expression clones, bypassing the need to perform MultiSite Gateway LR clonase reactions that involve three or more plasmids and require complex assembly validation. Of course, similar Gateway destination vectors can be built via classical restriction/ligation strategies, but such cloning may be cumbersome to perform with large plasmids and they do not allow the downstream modular manipulation of elements in the resulting expression clones by alternating reverse BP and LR reactions.
By convention, the name of modular destination vectors created via reverse BP clonase reaction from an expression clone starts with the letters pM followed by the letter K, H, or B, then the letters and numbers describing the expression unit (see above). The newly inserted ccdB cassette that replaced one or more of the elements in the original expression clone is specified by the letter m (for MultiSite), two numbers (1 and 2, or 3 and 4) indicating its flanking att sites, followed by the letters GW (short for Gateway) listed according to their order relative to the T-DNA left border.
To illustrate reverse BP cloning, we created two series of modular destination vectors. The CaMV 35S promoter alone or together with the first adjacent NFP ORF was replaced in pK7FNF2, pK7RNR2, and pK7S*NF2 by the compatible Gateway ccdB cassette from pDONR P4-P1R or pDONR P4-P2, respectively (Fig. 2, B and C). These plasmids can be used for promoter analysis (NLS-GFP-GFP, NLS-RED-RED, and NLS-GFP-GUS) or translational fusion of a genomic region containing a promoter and a downstream coding sequence, with three different reporter genes.
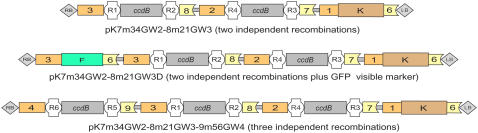
Destination Vectors with Two or Three Independent Gateway Expression Cassettes
Genetic analyses or biotechnological applications often require the ectopic expression of two or more transgenes (Halpin et al., 2001). To facilitate such manipulations, we have designed destination vectors carrying independent Gateway cassettes for the transcription of two or three sequences under the control of different strong plant promoters. In pK7m34GW2-8m21GW3, the T-DNA contains an attR1-ccdB-attR2 cassette between the ROLD promoter and OCS terminator and an attR4-ccdB-attR3 cassette between the CaMV 35S promoter and terminator (Fig. 3). DNA sequences available as attL1-gene1-attL2 and attL4-gene2-attL3 entry clones can be transferred simultaneously in this double vector (Tubb et al., 2005). Furthermore, in pK7m34GW2-8m21GW3D, a fluorescent reporter was introduced close to the right border of the T-DNA (Fig. 3). This marker enables visual screens for transformed GFP-expressing cells especially useful for studies in chimeric tissues, such as hairy roots or in vitro cultured calli. In pK7m34GW2-8m21GW3-9m56GW4, a third attR6-ccdB-attR5 cassette was added in the T-DNA between the CsVMV promoter and G7 terminator (Fig. 3). DNA sequences available as attL1-gene1-attL2, attL4-gene2-attL3, and attL6-gene3-attL5 entry clones can be transferred simultaneously in this triple vector. In our hands, LR clonase reactions recombining distinct entry clone sequences into these double or triple destination vectors were as successful as the contiguous assembly of two or three fragments in MultiSite LR reactions (Fig. 1B).
Figure 3.
Schematic representation of the T-DNA in Gateway binary destination vectors for the simultaneous ectopic expression of two or three genes. Box shape annotation is as indicated at the bottom of Figure 2C. Abbreviations specify the following sequences: 1, pNOS; 2, pCaMV 35S; 3, pROLD; 4, pCsVMV; 6, tNOS; 7, tCaMV 35S; 8, tOCS; 9, tG7; F, GFP; K, NPTII kanamycin resistance selectable marker.
Expression of Reporter and Fusion Proteins in Planta
mRNAs transcribed from MultiSite expression cassettes carry one or more attB sites in their 5′ untranslated region, coding sequence, or 3′ untranslated region. To demonstrate that such configurations do not impede protein synthesis in plant tissues, we tested the production of several FP fusions in stable Arabidopsis transformants and in transfected tobacco Bright Yellow-2 (BY-2) protoplasts.
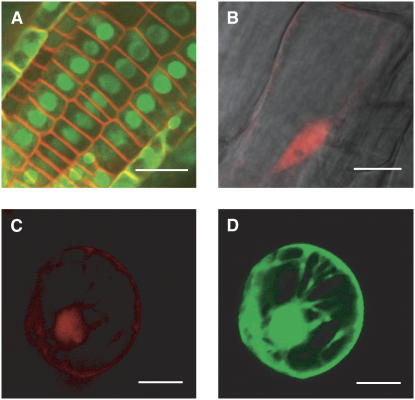
First, we created the pK7FNFI2 expression clone (p35S:NLS-GFPintron-GFP:t35S) in a MultiSite LR clonase reaction combining the elements in the entry clones pEN-L4-2-R1, pEN-L1-NFI-L2, and pEN-R2-F-L3 into the pK7m34GW destination vector. The pK7FNFI2 binary expression plasmid was introduced into A. tumefaciens (LBA4404) and transformed into Arabidopsis plants. Microscopic analysis of a kanamycin-resistant T1 seedling revealed a strong nuclear GFP signal in cells across the plant, also demonstrating the efficient intron splicing from the GFP-coding sequence (Fig. 4A). Similarly, T1 Arabidopsis plants transformed with the pK7RNR2 expression clone (p35S:NLS-DsRed-DsRed:t35S) displayed a clear red fluorescent signal strictly restricted to the nucleus (Fig. 4B).
Figure 4.
Production of FPs in plant cells transformed with MultiSite Gateway expression units. A, Nuclear localization of GFP in Arabidopsis root epidermal cells transformed with pKFNF2 (p35S:NLS-GFPintron-GFP:t35S). Cells were stained with a membrane-selective FM dye (in red). Size bar = 20 μm. B, Nuclear localization of DsRed in an Arabidopsis root epidermal cell transformed with pK7RNR2 (p35S:NLS-RED-RED:t35S). Size bar = 10 μm. C and D, Simultaneous transient expression of nuclear-targeted DsRed (C) and ER-targeted GFP (D) in the same BY-2 protoplast transfected with pK7ERF2-8NR3. Bar = 50 μm.
Lastly, we verified that both Gateway expression cassettes were functional in the double vector pK7m34GW2-8m21GW3. For this purpose, we placed the nuclear DsRed (pEN-L1-NR-L2) and endoplasmic reticulum (ER) GFP (pEN-L4-ERF-L3) ORFs under the control of the ROLD and 35S promoters, respectively. The resulting expression clone, pK7ERF2-8NR3, was introduced into BY-2 protoplasts by polyethylene glycol/Ca2+ transfection. As expected, both the nuclear DsRed and ER GFP signals were detected 48 h after transfection (Fig. 4, C and D).
Perspectives
Since the advent of reliable cloning strategies that enable the exchange of oriented DNA fragments between plasmids and regardless of their sequence, clones carrying reference genetic elements have become highly valuable tools for the assembly of composite transgenes. This report describes the addition of 51 accessions (36 entry, nine destination, and six expression plasmids) to the Gateway collection created by the Department of Plant Systems Biology (Ghent, Belgium) for plant gene function analysis. On their own, these numbers demonstrate the breadth of this resource. But, the examples provided here mainly serve to illustrate how basic versatile genetic elements can be mixed and matched in the MultiSite Gateway framework. We encourage researchers to combine existing core Gateway vectors with their own sequences of interest, in other words, their own entry clones. In that regard, it is pertinent to note that publicly available genome-scale Arabidopsis sequence repertoires have been or are being built as Gateway entry clones, including ORFs, promoters, gene-specific sequence tags, and artificial microRNAs for gene knockdown (for review, see Hilson, 2006). Lastly, the expression unit of any plant vector can be transformed into a MultiSite Gateway destination cassette in which the building blocks (entry clone sequences) presented here can be inserted.
All listed vectors can be queried online at http://www.psb.ugent.be/gateway/ for structural descriptions, vector maps, and sequences. They can also be ordered via the same Web pages. This collection will continue to grow as sequences are added to the plant molecular biology toolbox. Novel accessions may include promoters defining interesting transcription domains or efficiently induced by specific chemicals, reporters with interesting properties, such as bright fluorescent moieties active at particular excitation or emission wavelengths, and enzymes coding for additional antibiotic resistance or suited for improved colorimetric assays. We welcome suggestions to further develop Gateway clone resources for the benefit of the plant research community as a whole.
MATERIALS AND METHODS
Bacterial Strains
The Escherichia coli host strains were either DH5α or DB3.1 (Invitrogen) grown at 37°C in Luria-Bertani broth medium (Gibco BRL) with appropriate antibiotics. Agrobacterium tumefaciens C58C1 (pMP90) was cultured in yeast (Saccharomyces cerevisiae) extract broth medium with 50 mg L−1 rifampicin and 20 mg L−1 gentamycin. Agrobacterium strains were transformed by electroporation (Mattanovich et al., 1989).
Plant Materials and Transformation
Arabidopsis (Arabidopsis thaliana) ecotype Columbia plants were transformed via A. tumefaciens floral dip (Clough and Bent, 1998). T1 seedlings were grown in vitro on kanamycin-selective medium (0.5× Murashige and Skoog microelements and macroelements; Duchefa Biochemie), 1% (w/v) Suc, pH 5.8, with 0.8% (w/v) agar at 21°C and under 16-h-light/8-h-dark photoperiods. For staining, resistant T1 seedlings were treated for 5 min in 50 μm membrane-selective FM4-64 dye (Invitrogen). Imaged seedlings were mounted between slide and cover glass and analyzed with a 100-m confocal microscope equipped with the software package LSM510 version 3.2 (Zeiss).
BY-2 tobacco (Nicotiana tabacum) cell suspensions were grown in BY-2 medium (Nagata et al., 1992) in the dark at 25°C on an orbital shaker (150 rpm). A 5-mL aliquot of saturated culture was used weekly to incubate fresh medium (100 mL in 500-mL flasks). BY-2 protoplasts were prepared from 100 mL of 3-d-old BY-2 cell suspension cultures, and polyethylene glycol/Ca2+ transfection was performed with 105 cells and 100 μg DNA/mL as described (De Sutter et al., 2005). Cells were analyzed 48 h after transfection with the confocal microscope.
PCR Amplification
The attB sequences were included in the oligonucleotides carrying the 5′ and 3′ gene-specific sequences designed for PCR amplification of the corresponding fragments. Oligonucleotide sequences are provided in Supplemental Table S1 for all genetic elements captured in entry clones and can be used as examples for the generation of additional clones. PCR reaction mix contained 0.5 μm of each primer, 25 ng template DNA, 10 mm each dNTP, 2.5 units of Platinum Pfx DNA polymerase (Invitrogen), 1× PCR buffer, and 1 mm MgSO4. The PCR profile included an initial step at 94°C for 5 min, 30 cycles at 94°C for 20 s, 55°C for 30 s, and 68°C from 1 to 5 min depending on the amplified DNA fragment size (2 min/kb), and a final extension at 68°C for 10 min.
Entry Clones
PCR products were purified with the High Pure PCR purification kit (Roche). Five microliters BP clonase reactions, including 30 ng of purified PCR product, 50 ng of donor vector, and 1 μL of enzyme, were incubated at 25°C overnight. After proteinase treatment, a fraction of the reaction was transformed into E. coli DH5α. For each construct, a few colonies were picked and the insert was sequenced. Only entry clones carrying a sequence of interest identical to the PCR template were selected for subsequent LR reactions. Information about the Gateway donor vectors (pDONR 221, pDONR P2R-P3, pDONR P4-P1R, pDONR P4-P3, and pDONR P5-P6) are available from the manufacturer (http://www.invitrogen.com/).
Expression Clones
Expression clones were created via MultiSite LR clonase reactions recombining inserts from entry clones within and with the recipient destination vector (Karimi et al., 2005). Recombination reactions were done in 10 μL total volume containing 2 μL LR II clonase plus (Invitrogen), 30 ng of each entry clone, and 70 ng of destination vector. The incubation and subsequent treatments were the same as those for BP reactions. The E. coli DH5α was transformed with 3 μL of the LR reaction using the heat shock transformation method.
Modular Destination Vectors
Modular destination vectors were created via reverse BP clonase reactions combining an expression clone and pDONR P4-P1R or pDONR P4-P2 to restore an attR4-ccdB-attL1 or attR4-ccdB-attR2 Gateway cassette, respectively. These reactions were catalyzed by BP clonase II and conditions were the same as described for the production of entry clones. To increase the efficiency of reverse BP recombination, the pDONR vectors were first linearized with the EcoRV restriction enzyme that cuts open the plasmids outside of the Gateway cassette. Transformed DB3.1 cells were selected on chloramphenicol plates. To test recombination with modular destination vectors, LR clonase reactions were done with pEN-L4-2-R1, pEN-L4-1-R1, and pEN-L4-3-R1 entry clones containing the CaMV 35S, NOS, and ROLD promoters, respectively.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Table S1. Primer pairs for PCR amplification and cloning of the genetic elements.
Supplementary Material
Acknowledgments
We thank Daniel Van Damme and David Talengera for kindly providing the pOp6 and maize ubiquitin promoter entry clones, respectively, Ian Moore for sharing elements of the LhG4/LhGR two-component system, Björn De Meyer for support with the plant Gateway Web pages and online database, Rebecca De Clercq for help in cloning, Wilson Ardiles for sequencing, Valya Vassileva and Ryan Whitford for guidance throughout confocal microscope analysis, and Martine De Cock and Ana I. Fernandez for help in preparing the manuscript.
This work was supported by the 6th European Integrated Projects AGRON-OMICS (grant no. LSHG–CT–2006–037704).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Pierre Hilson (pierre.hilson@psb.ugent.be).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Benfey PN, Chua NH (1990) The cauliflower mosaic virus 35S promoter: combinatorial regulation of transcription in plants. Science 250 959–966 [DOI] [PubMed] [Google Scholar]
- Benfey PN, Ren L, Chua N-H (1990) Tissue-specific expression from CaMV 35S enhancer subdomains in early stages of plant development. EMBO J 9 1677–1684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit RM, Wilhelm RN, Scherer-Becker D, Ostermeier C (2006) An improved method for fast, robust, and seamless integration of DNA fragments into multiple plasmids. Protein Expr Purif 45 66–71 [DOI] [PubMed] [Google Scholar]
- Brasch MA, Hartley JL, Vidal M (2004) ORFeome cloning and systems biology: standardized mass production of the parts from the parts-list. Genome Res 14 2001–2009 [DOI] [PubMed] [Google Scholar]
- Cheo DL, Titus SA, Byrd DRN, Hartley JL, Temple GF, Brasch MA (2004) Concerted assembly and cloning of multiple DNA segments using in vitro site-specific recombination: functional analysis of multi-segment expression clones. Genome Res 14 2111–2120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen AH, Sharrock RA, Quail PH (1992) Maize polyubiquitin genes: structure, thermal perturbation of expression and transcript splicing, and promoter activity following transfer to protoplasts by electroporation. Plant Mol Biol 18 675–689 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16 735–743 [DOI] [PubMed] [Google Scholar]
- Craft J, Samalova M, Baroux C, Townley H, Martinez A, Jepson I, Tsiantis M, Moore I (2005) New pOp/LhG4 vectors for stringent glucocorticoid-dependent transgene expression in Arabidopsis. Plant J 41 899–918 [DOI] [PubMed] [Google Scholar]
- Curtis MD, Grossniklaus U (2003) A Gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol 133 462–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Sutter V, Vanderhaeghen R, Tilleman S, Lammertyn F, Vanhoutte I, Karimi M, Inzé D, Goossens A, Hilson P (2005) Exploration of jasmonate signalling via automated and standardized transient expression assays in tobacco cells. Plant J 44 1065–1076 [DOI] [PubMed] [Google Scholar]
- Earley KW, Haag JR, Pontes O, Opper K, Juehne T, Song K, Pikaard CS (2006) Gateway-compatible vectors for plant functional genomics and proteomics. Plant J 45 616–629 [DOI] [PubMed] [Google Scholar]
- Gallie DR (2002) The 5′-leader of tobacco mosaic virus promotes translation through enhanced recruitment of eIF4F. Nucleic Acids Res 30 3401–3411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebenok RJ, Pierson E, Lambert GM, Gong F-C, Afonso CL, Haldeman-Cahill R, Carrington JC, Galbraith DW (1997) Green-fluorescent protein fusions for efficient characterization of nuclear targeting. Plant J 11 573–586 [DOI] [PubMed] [Google Scholar]
- Halpin C, Barakate A, Askari BM, Abbott JC, Ryan MD (2001) Enabling technologies for manipulating multiple genes on complex pathways. Plant Mol Biol 47 295–310 [PubMed] [Google Scholar]
- Hanson MR, Köhler RH (2001) GFP imaging: methodology and application to investigate cellular compartmentation in plants. J Exp Bot 52 529–539 [PubMed] [Google Scholar]
- Hartley JL, Temple GF, Brasch MA (2000) DNA cloning using in vitro site-specific recombination. Genome Res 10 1788–1795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilson P (2006) Cloned sequence repertoires for small- and large-scale biology. Trends Plant Sci 11 133–141 [DOI] [PubMed] [Google Scholar]
- Hope IA, Stevens J, Garner A, Hayes J, Cheo DL, Brasch MA, Vidal M (2004) Feasibility of genome-scale construction of promoter∷reporter gene fusions for expression in Caenorhabditis elegans using a multisite gateway recombination system. Genome Res 14 2070–2075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6 3901–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapila J, De Rycke R, Van Montagu M, Angenon G (1997) An Agrobacterium-mediated transient gene expression system for intact leaves. Plant Sci 122 101–108 [Google Scholar]
- Karimi M, De Meyer B, Hilson P (2005) Modular cloning in plant cells. Trends Plant Sci 10 103–105 [DOI] [PubMed] [Google Scholar]
- Karimi M, Depicker A, Hilson P (2007) Recombinational cloning with plant Gateway vectors. Plant Physiol 145 1144–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi M, Inzé D, Depicker A (2002) GATEWAY vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci 7 193–195 [DOI] [PubMed] [Google Scholar]
- Leach F, Aoyagi K (1991) Promoter analysis of the highly expressed rolC and rolD root-inducing genes of Agrobacterium rhizogenes: enhancer and tissue-specific DNA determinants are dissociated. Plant Sci 79 69–76 [Google Scholar]
- Lewin A, Jacob D, Freytag B, Appel B (1998) Gene expression in bacteria directed by plant-specific regulatory sequences. Transgenic Res 7 403–411 [Google Scholar]
- Liu Q, Li MZ, Leibham D, Cortez D, Elledge SJ (1998) The univector plasmid-fusion system, a method for rapid construction of recombinant DNA without restriction enzymes. Curr Biol 8 1300–1309 [DOI] [PubMed] [Google Scholar]
- Magnani E, Bartling L, Hake S (2006) From Gateway to MultiSite Gateway in one recombination event. BMC Mol Biol 7 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattanovich D, Rüker F, da Câmara Machado A, Laimer M, Regner F, Steinkellner H, Himmler G, Katinger H (1989) Efficient transformation of Agrobacterium spp. by electroporation. Nucleic Acids Res 17 6747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar AJ, Short SR, Hiratsuka K, Chua N-H, Kay SA (1992) Firefly luciferase as a reporter of regulated gene expression in higher plants. Plant Mol Biol Rep 10 324–337 [Google Scholar]
- Moore I, Gälweiler L, Grosskopf D, Schell J, Palme K (1998) A transcription activation system for regulated gene expression in transgenic plants. Proc Natl Acad Sci USA 95 376–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata T, Nemoto Y, Hasezawa S (1992) Tobacco BY-2 cell line as the “HeLa” cell in the cell biology of higher plants. Int Rev Cytol 132 1–30 [Google Scholar]
- Quaedvlieg NEM, Schlaman HRM, Admiraal PC, Wijting SE, Stougaard J, Spaink HP (1998) Fusions between green fluorescent protein and β-glucuronidase as sensitive and vital bifunctional reporters in plants. Plant Mol Biol 37 715–727 [DOI] [PubMed] [Google Scholar]
- Rutherford S, Brandizzi F, Townley H, Craft J, Wang Y, Jepson I, Martinez A, Moore I (2005) Improved transcriptional activators and their use in mis-expression traps in Arabidopsis. Plant J 43 769–788 [DOI] [PubMed] [Google Scholar]
- Sanders PR, Winter JA, Barnason AR, Rogers SG, Fraley RT (1987) Comparison of cauliflower mosaic virus 35S and nopaline synthase promoters in transgenic plants. Nucleic Acids Res 15 1543–1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Sone T, Yoshida S, Yahata K, Hotta J, Chesnut JD, Honda T, Imamoto F (2004) Evidence for high specificity and efficiency of multiple recombination signals in mixed DNA cloning by the Multisite Gateway system. J Biotechnol 107 233–243 [DOI] [PubMed] [Google Scholar]
- Tubb J, Groth AC, Leong L, Emery DW (2005) Simultaneous sequence transfer into two independent locations of a reporter vector using MultiSite Gateway Technology. Biotechniques 39 553–557 [DOI] [PubMed] [Google Scholar]
- Vain P, Finer KR, Engler DE, Pratt RC, Finer JJ (1996) Intron-mediated enhancement of gene expression in maize (Zea mays L.) and bluegrass (Poa pratensis L.). Plant Cell Rep 15 489–494 [DOI] [PubMed] [Google Scholar]
- Vancanneyt G, Schmidt R, O'Connor-Sanchez A, Willmitzer L, Rocha-Sosa M (1990) Construction of an intron-containing marker gene: splicing of the intron in transgenic plants and its use in monitoring early events in Agrobacterium-mediated plant transformation. Mol Gen Genet 220 245–250 [DOI] [PubMed] [Google Scholar]
- Velten J, Schell J (1985) Selection-expression plasmid vectors for use in genetic transformation of higher plants. Nucleic Acids Res 13 6981–6998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdaguer B, de Kochko A, Fux CI, Beachy RN, Fauquet C (1998) Functional organization of the cassava vein mosaic virus (CsVMV) promoter. Plant Mol Biol 37 1055–1067 [DOI] [PubMed] [Google Scholar]
- Wesley SV, Helliwell CA, Smith NA, Wang M, Rouse DT, Liu Q, Gooding PS, Singh SP, Abbott D, Stoutjesdijk PA, et al (2001) Construct design for efficient, effective and high-throughput gene silencing in plants. Plant J 27 581–590 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.