Abstract
The motivation for radiation cross-linking of ultra-high molecular weight polyethylene (UHMWPE) is to increase its wear resistance to be used as bearing surfaces for total joint arthroplasty. However, radiation also leaves behind long-lived residual free radicals in this polymer, the reactions of which can detrimentally affect mechanical properties. In this review, we focus on the radiation cross-linking and oxidative stability of first and second generation highly cross-linked UHMWPEs developed in our laboratory.
Keywords: Radiation, cross-linking, antioxidant, joint arthroplasty, wear resistance
Introduction
The most accepted bearing couple in joint arthroplasty today is a cobalt/chromium/molybdenum alloy (CoCr) articulating against UHMWPE. This articulating couple is based on the original design of Dr. Charnley developed on the 1960's comprising a ‘hard’ metallic component articulating against a ‘soft’ polymeric component. Although total joint arthroplasty using this bearing couple has been one of the most successful operations of the last century, long term resorption of the bone around the implants (osteolysis) was observed, limiting the longevity of the arthroplasty. One major cause of this phenomenon is the immune reactions associated with the wear debris primarily generated from the UHMWPE component [1, 2].
UHMWPE has a composite structure with a highly ordered crystalline lamellae embedded in a randomly oriented amorphous matrix (Fig 1). Due to its high molecular weight, typically on the order of 2−6 million g/mol, it has high crystallinity (50−60%) and shape memory. Its high mechanical strength under load and its favorable fatigue resistance are attributed to its composite structure, where, in addition to the crystalline content and associated strength, the mobility of the chains in the amorphous phase provides ductility and plasticity to the polymer. The stresses to which the polymer is exposed are easily dissipated through plastic deformation. On the other hand, polyethylene wear is associated with increased plasticity. Increased plasticity allows the chains to be oriented in the direction of the applied stresses, and weakens the material in the transverse direction, leading to the breakup of particles, especially under the multi-directional motion of the joints [3].
Fig 1.
A transmission electron micrograph (a) and schematic (b) depicting the semi-crystalline morphology of UHMWPE.
Radiation cross-linking by high dose irradiation was proposed to increase the wear resistance of the polymer in general. Cross-linking of the polymer chains, which only occurs in the amorphous phase in polyethylene, introduces junctions between the polymer chains, reducing the mobility. Thus, it was hypothesized that wear would be reduced through a decrease in plasticity and thus orientation of the polymer.
Adhesive/abrasive wear properties of joint materials are typically tested in vitro using wear simulators. For the hip, the motion of the femoral neck on the acetabular surface in the joint is simulated on a bidirectional pin-on-disc machine (POD), where pins of UHMWPE are loaded on polished CoCr surfaces in a rectangular crossing pattern and the wear of the pins are determined gravimetrically at certain number of cycles [4, 5]. In a more clinically relevant test, joint simulators are used where the load and kinematics patterns are superimposed and actual implant devices are tested under these conditions [6-9]. Most of these tests are done in a synovial fluid analog (bovine serum) [10-12]. The results of these tests have been a good predictor of in vivo behavior. Clinically, wear is most often determined by calculating the penetration of the hard component in the soft polymeric surface from X-rays [13-15].
Early attempts were made at using gamma radiation to cross-link UHMWPE for bearing surfaces in total joint arthroplasty by using very high dose irradiation (1000 kGy; [16-19]). The wear rate of these surfaces was reduced compared to conventional UHMWPE (gamma sterilized in air). Radiation cross-linking in the presence of a sensitizing gas environment such as acetylene was also shown to increase the efficiency of cross-linking [20-22].
Alternative to ionizing radiation, cross-linking by silane chemistry was also proposed as a means to decrease the creep of the UHMWPE [23-25]. Typically, hip implants undergo a ‘bedding in’ period of one to two years, where the observed penetration of the femoral head into the acetabular socket is mainly due to the creep of the polymer. After this period, a steady wear rate is the major cause of further penetration. The average clinical penetration rates of 22-mm diameter acetabular cups made of silane cross-linked UHMWPE [26] articulating against ceramic femoral heads was 0.022 mm/year after the initial creep was complete. This value was significantly lower than the wear rate observed with conventional UHMWPE.
Thus, the hypothesis tested positive that cross-linking increased the wear resistance of UHMWPE and presented an improvement to address one major limitation of total joint arthroplasty.
First generation cross-linked UHMWPE
Most UHMWPE medical devices are terminally sterilized by gamma irradiation (25−40 kGy). Ionizing radiation induces radiolytic cleavage of the polymer chains throughout the polymer with the formation of C and H free radicals. Because of the high molecular weight of UHMWPE, recombination reactions along the backbone are favored limiting chain scission. The decay of the remaining free radicals is through recombination of the free radicals on different chains to form cross-links. However, some free radicals become trapped in the crystalline regions or in the folds of the crystalline lamellae for prolonged periods of time [27, 28]. Due to the fixed distance between the crystal chains and the immobility of the chains in the crystallites, these free radicals are thought to migrate along the chains to the crystalline/amorphous region and react with the diffused oxygen over time.
The primary alkyl and allyl free radicals on the polymer chains react with oxygen to form peroxy free radicals. These very reactive free radicals then stabilize themselves by abstracting hydrogens from nearby chains and becoming hydroperoxides. The decay of the hydroperoxides over time degrade the molecular weight. In addition, the abstraction of hydrogens creates a new primary alkyl free radical that feeds the oxidation reaction, leading to more severe degradation and more free radicals [29, 30]. This cascading oxidative reaction of the free radicals is the cause of oxidative embrittlement through the local re-crystallization of the newly formed degraded short chains (Fig 2). The molecular weight degradation accompanying embrittlement manifests itself in increased wear [31] and also fatigue-induced wear mechanisms such as delamination (Fig 3). Therefore, the elimination or deactivation of these free radicals becomes a crucial aspect in treating UHMWPE for total joint implants in addition to wear resistance.
Fig 2.

Increased crystallinity and embrittlement (as evidenced by the ‘white banding’ of an irradiated and oxidatively unstable UHMWPE surgically explanted component (adapted from [40]).
Fig 3.
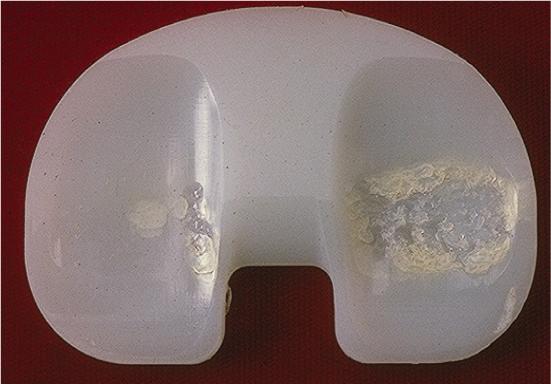
Delamination of a UHMWPE tibial component associated with oxidative embrittlement.
The amount of oxidative degradation is determined by calculating the amount of carbonyl-containing species in UHMWPE by spectroscopy. Also, an oxidation index can be calculated by normalizing the area under the curve of the carbonyl absorbance by a skeletal UHMWPE absorbance that does not change over time [32]. Similarly, the amount of hydroperoxides can be determined by staining with nitric oxide [33, 34] and this can be designated as ‘oxidative potential’, since the decay of hydroperoxides into carbonyl containing species eventually causes the oxidation index to increase. The oxidation resistance of UHMWPEs are assessed and compared in vitro by exposing them to oxygen at high temperature under accelerated conditions; for example at 70°C at 5 atm of pure oxygen for two weeks, and comparing their oxidation indices.
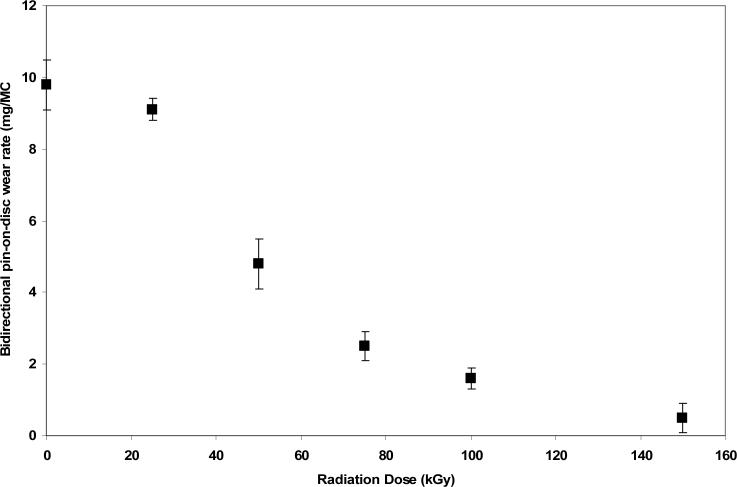
First generation cross-linked UHMWPEs were developed to provide an alternative to gamma sterilized UHMWPE, which had high wear and also showed high oxidation in vivo. Wear resistance was shown to be directly related to increased crosslink density [4, 5]; the cross-linking was saturated and the wear resistance reached a steady value at about 100 kGy of ionizing radiation (Fig 4).
Fig 4.
POD wear rates of e-beam cross-linked and subsequently melted UHMWPE as a function of radiation dose (adapted from [4]).
The mechanism of reduction of wear through cross-linking is related to a reduction in plastic deformation as mentioned above. This reduction in plastic deformation also reduces the ability of the polymer to dissipate stresses from locations where stress is concentrated and cracks may be formed and propagated. In fact, the mechanical strength, elongation-at-break, impact strength and fatigue crack propagation resistance of UHMWPE are all decreased as a function of increasing absorbed radiation dose [35, 36]. For this reason, current clinically available highly cross-linked UHMWPEs are cross-linked at doses ranging from 50 to 100 kGy to optimize both wear resistance and fatigue strength [37].
The residual free radicals are addressed by post-irradiation thermal treatment in first generation highly cross-linked UHMWPE. One method uses heating above the melting point of the cross-linked polymer subsequent to irradiation. This melts the crystalline regions and allows the recombination of the trapped free radicals in these regions. Once, the polymer is re-crystallized, the residual free radicals have been eliminated and this wear resistant UHMWPE is also oxidatively stable [38]. One downside of post-irradiation melting is that it further decreases the fatigue strength of UHMWPE, already reduced by radiation cross-linking [36]. This is due to a decrease in crystallinity accompanying the melting step; cross-linked UHMWPE is not able to crystallize to the same levels as uncross-linked UHMWPE.
Another method of thermal treatment is annealing below the melting point after radiation cross-linking. Although the residual free radical concentration is reduced in this manner, they are not completely eliminated as with melting and this cross-linked polymer is still susceptible to oxidation. In fact, high levels of oxidation have been observed with irradiated and annealed UHMWPE both in vitro accelerated aging studies [39] and in surgically explanted components [40, 41].
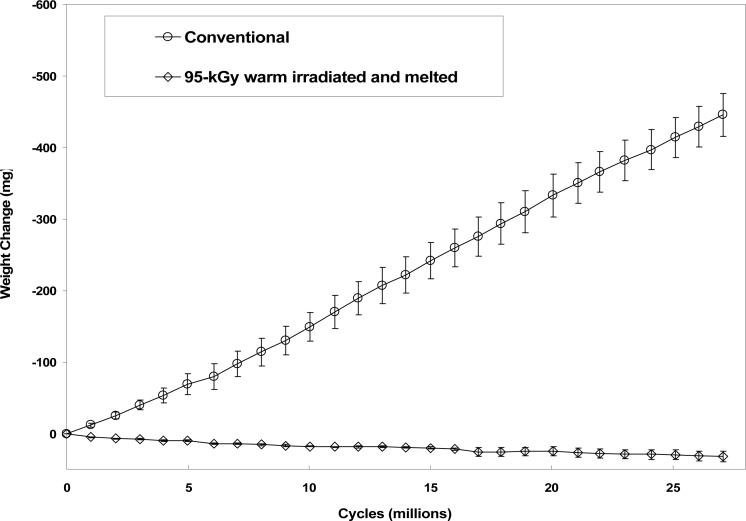
Radiation cross-linked and subsequently melted UHMWPEs have been in clinical use since 1998 and showed marked decrease in wear compared to conventional, gamma sterilized UHMWPE (Fig 6; [42, 43]). These highly cross-linked UHMWPEs show great promise in minimizing the occurrence of osteolysis in the longer term.
Fig 6.
Hip simulator wear rates of 95-kGy warm irradiated and melted UHMWPE and conventional UHMWPE acetabular liners with 28 mm inner diameter (adapted from [38]).
Second generation cross-linked UHMWPE
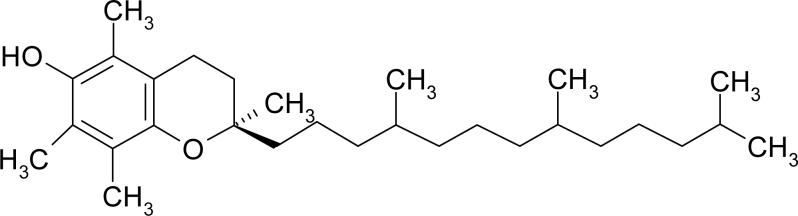
We developed a second generation highly cross-linked UHMWPE by stabilizing the residual free radicals by vitamin E (Fig 5), a well-known chain breaking antioxidant. Vitamin E hinders the peroxidation of lipids in the body [44, 45], which is a similar desired function in irradiated UHMWPE. It acts by donating a hydrogen to the alkyl/allyl and peroxy free radicals and prevents cascading oxidation reactions [46].
Fig 5.
Chemical structure of α-tocopherol (Vitamin E).
There are two methods that can be used to incorporate vitamin E in UHMWPE; the first is by blending vitamin E, a viscous liquid with UHMWPE resin powder and consolidating them together before molding. The second is to diffuse vitamin E in molded and radiation cross-linked UHMWPE. The advantage of blending is the ease of obtaining a uniform concentration of vitamin E. The disadvantage is that the cross-linking efficiency of UHMWPE is reduced in the presence of vitamin E with increasing vitamin E concentration [47]. This is presumably due to vitamin E donating a proton to and deactivating the primary free radicals intended for crosslinking the polymer chains. Also, since vitamin E is present during consolidation and subsequently during irradiation, some vitamin E activity is lost. Processing in this manner would require a careful optimization of low vitamin E concentration and high radiation dose required to obtain both high cross-link density and enough vitamin E activity to prevent oxidation in UHMWPE in the long-term. While diffusion of vitamin E in cross-linked UHMWPE avoids these aspects, it requires additional time to incorporate vitamin E throughout thicker components due to the time and temperature dependence of diffusion [48]. Vitamin E diffusion throughout UHMWPE of desired thickness is achieved by doping at high temperature in pure vitamin E followed by homogenization at high temperature in inert gas [48, 49].
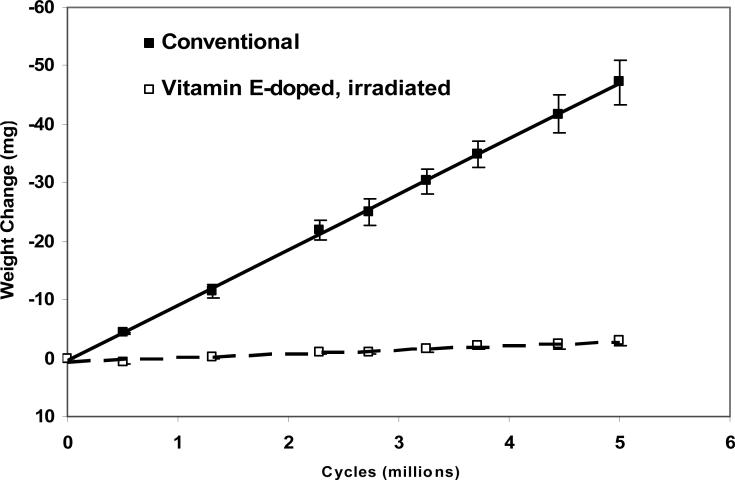
The advantage of this UHMWPE over conventional, gamma sterilized UHMWPE is both its wear and oxidation resistance. The high cross-link density imparted by high dose irradiation improves the wear resistance substantially (Fig 7; [49]) and due to the oxidative stability imparted by vitamin E, oxidative aggravation of wear is not expected. Secondly, by using vitamin E stabilization, the crystallinity of the radiation cross-linked UHMWPE is retained and the fatigue properties of this UHMWPE are improved over irradiated and melted UHMWPE [31, 49].
Fig 7.
Hip simulator wear rates of 85-kGy irradiated, vitamin E-doped UHMWPE and conventional UHMWPE acetabular liners with 28 mm inner diameter (adapted from [49]).
We used real-time aging on the shelf, and in water to determine the free radical decay and oxidation in radiation cross-linked, vitamin E-diffused and terminally gamma sterilized UHMWPE [50]. The alkyl and allyl free radicals induced by irradiation in vitamin E-stabilized, irradiated UHMWPE decayed over time, especially upon exposure to oxygen. They evolved into oxygen-centered free radicals that are exhibited in a sharp singlet peak in electron spin resonance, similar to irradiated but unstabilized UHMWPE. However, despite this transition being accompanied by increasing levels of oxidation in unstabilized UHMWPE, vitamin E-stabilized, irradiated UHMWPE did not show any appreciable oxidation after 7 months.
Conclusions
Radiation cross-linking of UHMWPE has been a great advance in total joint arthroplasty bearing surfaces and shows promise in decreasing the occurrence of osteolysis related to the wear of UHMWPE. We have reviewed briefly how cross-linking together with the stabilization of residual free radicals and the optimization of the crystalline morphology of UHMWPE can be modified to obtain a wear, oxidation and fatigue resistant bearing surface.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
PACS Category 60: Condensed Matter: Structural, mechanical and thermal properties
References
- 1.Cooper R, McAllister C, Borden L, Bauer T. J. Arthroplasty. 1992;7:285. doi: 10.1016/0883-5403(92)90050-z. [DOI] [PubMed] [Google Scholar]
- 2.Kovacik MW, Gradisar IA, Jr., Haprian JJ, Alexander TS. Clin. Orthop. Relat. R. 2000;379:186. doi: 10.1097/00003086-200010000-00022. [DOI] [PubMed] [Google Scholar]
- 3.Edidin AA, Pruitt L, Jewett CW, Crane DJ, Roberts D, Kurtz SM. J.Arthroplasty. 1999;14:616. doi: 10.1016/s0883-5403(99)90086-4. [DOI] [PubMed] [Google Scholar]
- 4.Muratoglu OK, Bragdon CR, O'Connor DO, Jasty M, Harris WH, Gul R, McGarry F. Biomaterials. 1999;20:1463. doi: 10.1016/s0142-9612(99)00039-3. [DOI] [PubMed] [Google Scholar]
- 5.Muratoglu OK, Bragdon CR, O'Connor DO, Jasty M, Harris WH, Gul R, McGarry F. Biomaterials. 2003;24:1527. doi: 10.1016/s0142-9612(99)00039-3. [DOI] [PubMed] [Google Scholar]
- 6.Scales J, Kelly P, Goddard D. Friction torque studies of total joint replacements. The use of a simulator. Ann. Rheum. Dis. 1969;28(Suppl):30. [PMC free article] [PubMed] [Google Scholar]
- 7.Dumbleton J, Miller D, Miller E. Wear. 1972;20:165. [Google Scholar]
- 8.Bragdon C, O'Connor D, Lowenstein J, Jasty M, Syniuta W. Proc. Inst. Mech. Eng. [H] 1996;210:157. doi: 10.1243/PIME_PROC_1996_210_408_02. [DOI] [PubMed] [Google Scholar]
- 9.O'Connor DO, Bragdon CR, Lowenstein JD, Ramamurti B, Burke DW, Harris WH. World Tribology Congress. Mechanical Engineering Publications Ltd.; London: 1997. A 12 station, upright hip simulator wear testing machine employing oscillating motion replicating human gait cycle. [Google Scholar]
- 10.Ahlroos T, Saikko V. Wear. 1997;211:113. [Google Scholar]
- 11.Walker PS, Blunn GW, Perry JP, Bell CJ, Sathasivam S, Andriacchi TP, Paul JP, Haider H, Campbell PA. Clin. Orthop. Relat. R. 2000;372:290. doi: 10.1097/00003086-200003000-00032. [DOI] [PubMed] [Google Scholar]
- 12.Bell J, Tipper JL, Ingham E, Stone MH, Fisher J. Proc. Inst. Mech. Eng. [H] 2001;215:259. doi: 10.1243/0954411011533661. [DOI] [PubMed] [Google Scholar]
- 13.Martell JM, Berdia S. J. Bone Joint Surg. Am. 1997;79:1635. doi: 10.2106/00004623-199711000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Bragdon CR, Malchau H, Yuan X, Perinchief R, Karrholm J, Borlin N, Estok DM, Harris WH. J. Orthop. Res. 2002;20:688. doi: 10.1016/S0736-0266(01)00171-1. [DOI] [PubMed] [Google Scholar]
- 15.McCalden RW, Naudie DD, Yuan X, Bourne RB. J. Bone Joint Surg. Am. 2005;87:2323. doi: 10.2106/JBJS.E.00223. [DOI] [PubMed] [Google Scholar]
- 16.Oonishi H, Takayama Y, Tsuji E. Radiat. Phys. Chem. 1992;39:495. [Google Scholar]
- 17.Oonishi H, Takayama Y, Tsuji E. The low wear of cross-linked polyethylene socket in total hip prostheses. In: Wise DL, et al., editors. Encyclopedic handbook of biomaterials and bioengineering. Part A: Materials. Marcel Dekker; New York: 1995. p. 1853. [Google Scholar]
- 18.Oonishi H, Kadoya Y, M S. J. Biomed. Mater. Res. B. 2001;58:167. doi: 10.1002/1097-4636(2001)58:2<167::aid-jbm1003>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 19.Oonishi H, Clarke I, Masuda S, Amino H. J. Arthroplasty. 2001;16:129. doi: 10.1054/arth.2001.28371. [DOI] [PubMed] [Google Scholar]
- 20.Grobbelaar CJ, DuPlessis TA, Marais F. J Bone Joint Surg BR. 1978;60:370. doi: 10.1302/0301-620X.60B3.681412. [DOI] [PubMed] [Google Scholar]
- 21.Grobbelaar CJ, Weber FA, Spirakis A, DuPlessis TA, Cappaert G, Cakic JN. S. Afr. J. Surg. 1999;11:140. [Google Scholar]
- 22.Klein PG, Gonzalez-Orozco JA, Ward IM. Polymer. 1991;32:1732. [Google Scholar]
- 23.Atkinson JR, Dowling JM, Cicek RZ. Biomaterials. 1980;1:89. doi: 10.1016/0142-9612(80)90005-8. [DOI] [PubMed] [Google Scholar]
- 24.Atkinson JR, Cicek RZ. Biomaterials. 1983;4:267. doi: 10.1016/0142-9612(83)90026-1. [DOI] [PubMed] [Google Scholar]
- 25.Atkinson JR, Cicek RZ. Biomaterials. 1984;5:326. doi: 10.1016/0142-9612(84)90030-9. [DOI] [PubMed] [Google Scholar]
- 26.Wroblewski BM, Siney PD, Dowson D, Collins SN. J. Bone Joint Surg. BR. 1996;78:280. [PubMed] [Google Scholar]
- 27.Jahan MS, Thomas DE, Trieu HH, Haggard WO, Conta RL, Parr JE. Investigation of free radicals in shelf-aged polyethylene tibial components. Wright Medical. 1996 [Google Scholar]
- 28.Jahan MS, King MC, Haggard WO, Sevo KL, Parr JE. Radiat. Phys. Chem. 2001;62:141. [Google Scholar]
- 29.Rabek J, Ranby B. Photochemical oxidation reactions of synthetic polymers. In: Kinell P, Ranby B, editors. ESR Applications to Polymer Research. Almqvist-Wiksell Forlag AB; Stockholm: 1973. [Google Scholar]
- 30.Al-Malaika S, Scott G. Atmospheric Oxidation and Antioxidants. Elsevier Science Publishers B.V.; Amsterdam: 1993. Autoxidation; p. 45. [Google Scholar]
- 31.Oral E, Wannomae KK, Hawkins NE, Harris WH, Muratoglu OK. Biomaterials. 2004;25:5515. doi: 10.1016/j.biomaterials.2003.12.048. [DOI] [PubMed] [Google Scholar]
- 32.Kurtz SM, Muratoglu OK, Buchanan FJ, Currier B, Gsell R, Shen FW, Yau SS. Biomaterials. 2001;22:2875. doi: 10.1016/s0142-9612(01)00033-3. [DOI] [PubMed] [Google Scholar]
- 33.Carlsson DJ, Brousseau R, Zhang C, Wiles DM. Polym. Degrad. Stabil. 1987;17:303. [Google Scholar]
- 34.Greenbaum E, Muratoglu O, Harris W. Effect of radiation and thermal history on hydroperoxide content in UHMWPE.. Transactions of the 48th Annual Meeting of the Orthopaedic Research Society.; Dallas, TX. 2002. [Google Scholar]
- 35.Ries M, Pruitt L. Clin. Orthop. Relat. R. 2005;440:149. doi: 10.1097/01.blo.0000185310.59202.e5. [DOI] [PubMed] [Google Scholar]
- 36.Oral E, Malhi A, Muratoglu O. Biomaterials. 2006;27:917. doi: 10.1016/j.biomaterials.2005.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muratoglu OK, Vittetoe D, Rubash H. Damage of Implant Surfaces in Total Knee Arthroplasty. In: Callaghan J, et al., editors. The Adult Knee. Lippincott Williams and Wilkins; Philadelphia: 2002. [Google Scholar]
- 38.Muratoglu OK, Bragdon CR, O'Connor DO, Jasty M, Harris WH. 1999 HAP Paul Award. J Arthroplasty. 2001;16:149. doi: 10.1054/arth.2001.20540. [DOI] [PubMed] [Google Scholar]
- 39.Wannomae KK, Christensen SD, Freiberg AA, Bhattacharyya S, Harris WH, Muratoglu OK. Biomaterials. 2006;27:1980. doi: 10.1016/j.biomaterials.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 40.Wannomae K, Bhattacharyya S, Freiberg A, Estok D, Harris W, Muratoglu O. J Arthroplasty. 2006;21:1005. doi: 10.1016/j.arth.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 41.Currier B, Mayor M, Currier J, Lyford K, Collier J. Crossfire™ Retrievals-What can we learn?; Transactions of the 51st Annual Meeting of the Orthopaedic Research Society.; Washington, D.C.. 2005. [Google Scholar]
- 42.Digas G, Karrholm J, Thanner J, Malchau H, Herberts P. Clin Orthop Relat. R. 2004;429:16. [PubMed] [Google Scholar]
- 43.Martell JM, Verner JJ, Incavo SJ. J. Arthroplasty. 2003;18:9. doi: 10.1016/s0883-5403(03)00341-3. [DOI] [PubMed] [Google Scholar]
- 44.Packer L. Protective role of vitamin E in biological systems. Am J Clin Nutr. 1991;53:1050S. doi: 10.1093/ajcn/53.4.1050S. [DOI] [PubMed] [Google Scholar]
- 45.Packer L, Kagan VE. Vitamin E: the antioxidant harvesting center of membranes and lipoproteins. In: Packer L, Fuchs J, editors. Vitamin E in Health and Disease. Marcel Dekker; New York: 1993. p. 179. [Google Scholar]
- 46.Scott G. Antioxidants: Chain breaking mechanisms. In: Scott G, editor. Atmospheric Oxidation and Antioxidants. Elsevier Science Publishers B.V.; Amsterdam: 1993. p. 121. [Google Scholar]
- 47.Oral E, Greenbaum E, Malhi A, Muratoglu O. Biomaterials. 2005;26:6657. doi: 10.1016/j.biomaterials.2005.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oral E, Wannomae K, Muratoglu O. 51st Annual Meeting of the Orthopaedic Research Society.; Washington, D.C.. 2005. [Google Scholar]
- 49.Oral E, Christensen S, Malhi A, Wannomae K, Muratoglu O. J. Arthroplasty. 2006;21:580. doi: 10.1016/j.arth.2005.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oral E, Rowell S, Muratoglu O. Biomaterials. 2006;27:5580. doi: 10.1016/j.biomaterials.2006.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]