Abstract
Pediatric cardiomyopathies are heterogeneous groups of serious disorders of the heart muscle and are responsible for significant morbidity and mortality among children who have the disease. While enormous improvements have been made in the treatment and survival of children with congenital heart disease, parallel strides have not been made in the outcomes for cardiomyopathies. Thus, ancillary therapies, such as nutrition and nutritional interventions, that may not cure but may potentially improve cardiac function and quality of life, are imperative to consider in children with all types of cardiomyopathy. Growth failure is one of the most significant clinical problems of children with cardiomyopathy with nearly one-third of children with this disorder manifesting some degree of growth failure during the course of their illness. Optimal intake of macronutrients can help improve cardiac function. In addition, several specific nutrients have been shown to correct myocardial abnormalities that often occur with cardiomyopathy and heart failure. In particular, antioxidants that can protect against free radical damage that often occurs in heart failure and nutrients that augment myocardial energy production are important therapies that have been explored more in adults with cardiomyopathy than in the pediatric population. Future research directions should pay particular attention to the effect of overall nutrition and specific nutritional therapies on clinical outcomes and quality of life in children with pediatric cardiomyopathy.
Introduction
Pediatric cardiomyopathies are heterogeneous groups of serious disorders of the heart muscle and are responsible for significant morbidity and mortality among children who have the disease. The incidence of pediatric cardiomyopathy is approximately between 1.13 and 1.24 cases per 100,000 children 18 years of age and younger, with the highest incidence among children less than one year of age [1-3]. The incidence tends to be higher among African American and Hispanic children in the US [2]. Despite its overall low incidence, cardiomyopathies result in some of the worst pediatric cardiology outcomes and are responsible for nearly one-half of all pediatric heart transplants [4]. Nearly one-third of all children diagnosed with pediatric cardiomyopathy prior to one year of age will die within one year of diagnosis [5], and 40% receive heart transplants within two years. Among those who live beyond the first year, the five-year survival is nearly 85% [6]. Despite these overall dismal statistics, the clinical course and outcomes of cardiomyopathy vary among patients, from complete recovery to death, even among those with similar functional types of cardiomyopathy.
The World Health Organization classifies cardiomyopathies into four distinct functional categories: 1) dilated cardiomyopathy, where the heart muscle fibers stretch, causing a chamber of the heart to enlarge, thus weakening the heart's ability to pump blood; 2) hypertrophic cardiomyopathy a functional type of cardiomyopathy occurs among older children and adults [7]. In hypertrophic cardiomyopathy, the growth or arrangement of muscle fibers is abnormal, leading to a thickening of the heart walls and reduction in size of the pumping chamber that may obstruct the blood flow; 3) restrictive cardiomyopathy, where the walls of the ventricles stiffen and lose their flexibility. When this occurs, the heart cannot fill adequately with blood and eventually the heart loses its ability to pump properly; and 4) arrythmogenic right ventricular cardiomyopathy. Arrythmogenic right ventricular cardiomyopathy is characterized by the replacement of myocytes in the right ventricle with fatty, fibrous tissue [8]. It has been found that mutations in genes that encode cell junction proteins can cause arrythmogenic right ventricular cardiomyopathy [9]. Distinctions between these cardiomyopathies are critical because differences in etiologies and outcomes vary by the functional type of cardiomyopathy. In general, the cause of PCM remains primarily unknown, yet genetic causes are likely to be a factor in most pediatric patients with recent studies demonstrating a large familial component [2,3]. Thus, as more becomes known of the causes and natural history of pediatric cardiomyopathy, there will be a greater ability to determine etiology-specific therapeutics that will positively impact outcomes.
While enormous improvements have been made in the treatment and survival of children with congenital heart disease, parallel strides have not been made in the outcomes for cardiomyopathies. Heart transplantation remains the standard of care for children with progressive disease. The percentage of children with cardiomyopathy who received a heart transplant has not declined over the past 10 years and cardiomyopathy remains the leading cause of transplantation for children over one year of age [10]. Nearly 40 percent of children who present with symptomatic cardiomyopathy receive a heart transplant or die [11,12]. Furthermore, the time to transplant or death for children with cardiomyopathy has not improved during the past 35 years, and the most economically advanced nations have no better outcomes than developing nations [10]. Cardiomyopathies have an associated cost of nearly $200 million/year in adults and children in the United States alone [13]. Improvements in technology and medicine have contributed to an improved survival for children having heart transplants, however, it has not resulted in either a normal life span, or quality of life. Recent medical research indicates that new treatments may soon be available. It has been suggested that advancements in stem cell research may be beneficial to children with cardiomyopathies [14]. Thus, ancillary therapies, such as nutrition and optimizing nutritional interventions, that may not cure but may potentially improve cardiac function and quality of life are imperative to consider in children with all types of cardiomyopathy.
Nutritional Status of Children with Cardiomyopathy
Growth problems are common in many pediatric illnesses [15-19]. Normal growth in children is considered an important clinical indicator of health. Chronic illness in children leads to an imbalance of energy where there is more energy expended secondary to the disease and less devoted to normal metabolic processes (i.e. – growth). Growth failure can be due to a variety of factors and it is often multifactorial. Increased energy expenditure secondary to chronic disease processes (hyperthyroidism, congestive heart failure, chronic infections, to name a few), gastrointestinal malabsorption, chronically low and suboptimal dietary intake, or psychosocial problems are the 4 most common and cited causes of poor growth in the child with chronic disease. The etiology of malnutrition in children with chronic illness is likely due to a combination of 2 or more of these factors.
Not only is growth an indicator of active and poorly controlled disease, but there is emerging evidence that progressive declines in nutritional status are linked closely and independently with deteriorating organ functions, morbidity and mortality in a variety of disorders [20,21]. The best example of this is the first discovery of pneumoncystis carinii pneumonia in otherwise healthy, but severely malnourished children in developing nations [22]. Furthermore, investigators have shown that improving nutritional status in malnourished and chronically ill children relate to improved survival and decreased utilization of health care resources through lower hospitalization rates [23]. In another study evaluating cardiac outcomes in chronically ill children [24], nutritional status was a strong and independent predictor of mortality and cardiac function. Thus, better nutritional status of the child (or adult) is related to the optimal functioning of the heart and other organ systems and eventual clinical outcomes [25, 26].
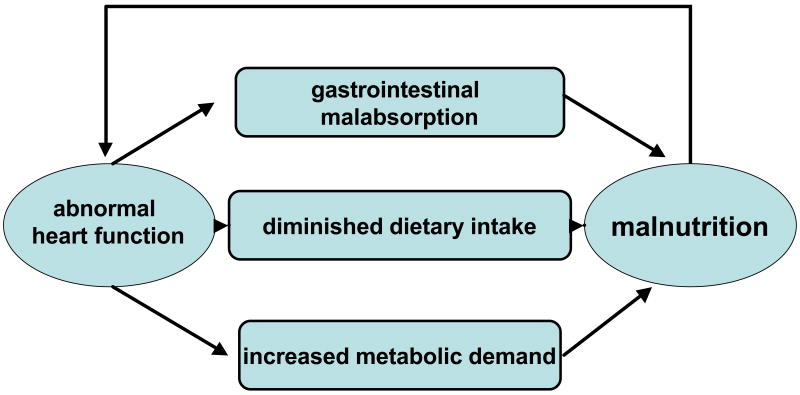
Growth failure is one of the most significant clinical problems of children with cardiomyopathy with nearly one-third of children with this disorder manifesting some degree of growth failure during the course of their illness. In a recent international study in Brazil [25], Azevedo determined that weight z-score was positively and independently correlated with survival in a chart review of 165 children with idiopathic dilated cardiomyopathy between 1979 and 2003. However, short of this restrospective chart review, there is a dearth of information on nutritional correlates to cardiac outcomes and function in children with cardiomyopathy. We have shown previously that in other models of chronic illness in children (human immunodeficiency virus infection), that cardiac muscle mass does not always waste in proportion to skeletal muscle [27]. This suggests that neurohormonal influences may affect cardiac status to a greater extent than skeletal muscle. The underlying cause of growth failure is usually due to persistent congestive heart failure as a result of an overall poor response to medical treatment. Significant cardiac dysfunction in these children can result in increased metabolic demands, decreased food intake and malabsorption of important nutrients. Growth failure or malnutrition in children can lead to problems in virtually every organ system, with many of the effects only partially reversible. Thus cardiomyopathy may lead to growth problems, but growth problems can lead to further complications that may directly or indirectly impact on heart function, leading to a vicious downward cycle (Figure 1). These clinical manifestations indicate that growth patterns may be an important predictor of the outcomes among CM patients or an important indicator of the severity of their cardiomyopathy. If this is so, then it becomes apparent that clinicians should be vigilant regarding treatment issues surrounding growth. The relationship between growth patterns and echocardiographic findings is unknown.
Figure 1. Vicious Downward Cycle of Nutrition in Pediatric Cardiomyopthy.
The Effect of Nutrients on Cardiac Endpoints in Heart Failure
Promotion of adequate nutrition begins with early detection of children at risk for malnutrition. Optimal nutrition is critical in providing children the means to recover from their illness and to withstand the detrimental metabolic effects of aggressive therapies. In theory, patients with heart failure who have either insufficient fat mass (BMI <10% for age and sex) or excess fat mass (BMI ≥ 85% for age and sex) should have poorer outcomes than patients with a BMI in the reference range (between 10% and 95%). Although excess body fat may increase the risk of developing heart failure, evidence suggests that it may be beneficial once heart failure develops. One mechanism may be that increased body fat provides a metabolic reserve that allows overweight and obese patients to tolerate the metabolic/catabolic stress associated with heart failure pathology for a longer time [28]. Another potential mechanism is related to the hypothesized differences in proinflammatory cytokine activity between underweight and overweight/obese individuals with heart failure [29]. In addition to releasing proinflammatory cytokines, adipose tissue is a source of anti-inflammatory cytokines. However, it is not known whether the potential positive effects of excess body fat in patients with heart failure vary depending on body fat distribution. Our current recommendations focus on decreasing body fat accumulation due to the known adverse effects and secondary diseases the may develop from obesity. However, more studies are needed in order to tease out the complex interaction between obesity and active heart failure with particular focus on the pediatric population that has been often difficult to study owing to limited numbers of children available to study.
Macronutrients
Children with cardiomyopathy, with varying degrees of congestive heart failure, need to receive adequate calories to compensate for their heart failure (that typically increases basal energy expenditure) as well as to provide additional calories for normal growth. As mentioned previously, children in heart failure often do not grow along expected standards for age and sex and poor growth may either contribute to poor cardiac function or be one result of it. Optimal caloric intake is usually estimated to be approximately 110 – 125% of the estimated energy requirement (EER) (Table) [30] for age and sex. However, greater or fewer calories may be required depending on the child's growth as a response to their intake; as normal nutrition is the balance between energy intake and energy utilization. Children who are in congestive heart failure often have increased metabolic rates due to the increased work of breathing, eating, or other routine activities of daily living. They may also malabsorb critical nutrients as a result of heart failure leading to gut edema. Furthermore, anorexia, due to a variety of reasons including a proinflammatory state (cachexia) or delayed gastric emptying secondary to increased edema may be a factor that contributes to suboptimal dietary intake. Physical activity level also has to be accounted for, with physical activity being indirectly related to the level of congestive heart failure [31]. However, physical inactivity may contribute the digression in heart function leading to worsening heart failure.
Table 1. Important Nutrients in Cardiomyopathy.
| Roles | Recommended daily intake | Dietary sources | |
|---|---|---|---|
| Calories | Provide energy for all metabolic processes and to support growth
Increased metabolic rate secondary to recurrent infections, increased muscle activity, and need for rapid growth. |
Healthy weight: calorie levels based on the Estimated Energy Requirements (EER) and activity levels from the Institute of Medicine Dietary Reference Intakes (DRI) Macronutrients Report, 2002.
Estimate catch-up growth needs in growth failure: by determining ideal body weight for height and using by indirect calorimetry or using calorie levels based on EER for that weight. Children in heart failure often require 10% to 50% more calories due to increased metabolic rates. |
For mild to moderate undernutrition, ad libitum oral feedings are appropriate, and caregivers should be advised to increase the caloric intake by increasing the caloric density of both liquids and solids.
Once a nutritional problem becomes chronic and the patient presents with severe growth failure (BMI<5% or weight/height<5%), or when the oral supplementation is no longer sufficient, an aggressive nutrition support plan including gastrostomy or intravenous alimentation needs to be advised |
| Protein (g/d) | Serves as the major structural component of all cells in the body, and functions as enzymes in membranes, as transport carriers, and some hormones. | Protein requirements are based on an increase in needs. RDA for protein may be increased by 50-100%
FTT: DRI protein for age × ideal weight for height (kg) / actual weight RDA/AI* Children 1-3 y: 13 4-8 y: 19 males 9-13 y: 34 > 14 y: 52 Females 9-13 y: 34 > 14 y: 46 |
From animal sources – “complete protein”: meat, poultry, fish, eggs, milk, cheese, and yogurt.
From plants: legumes, grains, nuts, seeds, and vegetables. |
| Carbohydrate | Source of calories to maintain body weight.
Primary energy source for the brain |
55 to 60% of the total calories
Children and adults 130 g/d Added sugars should comprise no more than 25% of total calories consumed. |
Starch and sugar are the major types of carbohydrates. Grains and vegetables (corn, pasta, potatoes, breads), are sources of starch. Natural sugars are found in fruits and juices. Sources of added sugars are soft drinks, candy, fruit, drinks, and desserts. |
| Fat (g/d) | Energy source and when found in foods, is a source of n-6 and n-3 polyunsaturated fatty acids. Its presence in the diet increases absorption of fat soluble vitamins and precursors such as vitamin A and pro-vitamin A carotenoids. |
AMDR
Children 1-3 y: 30-40 4-8 y: 25-35 Males and females: > 9 y: 25-35 |
Butter, margarine, vegetable oils, whole milk, visible fat on meat and poultry products, invisible fat in fish, shellfish, some plant products such as seeds and nuts, and bakery products. |
|
n-3 polyunsaturated fatty acids (linolenic acid)
(g/d) |
Possible favorable effect on left ventricular function | Children
1-3 y: 0.7 4-8 y: 0.9 males 9-13 y: 1.2 > 14 y: 1.6 Females 9-13 y: 1.0 > 14 y: 1.1 |
Oily fish such as sardines, mackerel, herring, trout, tuna, and salmon. Other sources include flaxseed, soy, canola oil |
|
n-6 polyunsaturated fatty acids (linoleic acid)
(g/d) |
Essential component of structural membrane lipids, involved with cell signaling, and precursor of eicosanoids. | Children
1-3 y: 7 4-8 y: 10 males 9-13y: 12 14-18 y: 17 Females 9-13 y: 10 14-18 y: 12 |
Nuts, seeds, and vegetable oils such as soybean, safflower, and corn oil. |
|
Fibers
(g/d) |
Reduces risk of coronary heart disease, assists in maintaining normal blood glucose levels. | Children
1-3 y: 19 4-8: 25 Males 9-13y: 31 14-18 y: 38 Females 9-18 y: 26 |
Soluble fibers: oatmeal, legumes, and some fruits and vegetables with pectin |
| Water (ml/d) | Essential for maintaining vascular volume. | Total fluid requirements:
1-10kg: 100ml/kg 10-20kg: 1000 ml + 50 ml/kg for each kg above 10 kg >20kg: 1500 ml + 20 ml/kg for each kg above 20 kg Water restriction may be recommended in advanced stages. |
All beverages, including water, as well as moisture in foods (high moisture foods include watermelon, meats, soups, etc.). |
| Vitamins and minerals | Antioxidants have an important role to play in protecting mitochondria and cells from reactive oxygen intermediates.
Deficiency in both macro and micronutrients may contribute to the wasting process once triggered. Patients are usually receiving loop diuretics which increase urinary excretion of micronutrients. |
Using multiple micronutrient supplementations has been shown to improve left ventricular ejection fraction and quality of life. | |
| B1 - Thiamine | Coenzyme in many physiologic functions including carbohydrate metabolism and maintenance of myelin necessary for proper nerve and muscle function. | Children
1-3 y: 0.4 4-8 y: 0.5 Males 9-13 y: 0.7 14-18 y: 1.0 Females 9-13 y: 0.7 14-18 y: 0.9 |
Fortified cereals, meat; meat and fish; dried beans, soy foods and peas; whole grains |
| B3 - Niacin | Functions in many biological redox reactions including intracellular respiration, fatty acid synthesis and glucose oxidation.
Niacin decreases blood levels of cholesterol and lipoprotein, which may reduce the risk of atherosclerosis. |
Children
1-3 y: 5 4-8 y: 6 Males 9-13 y: 9 > 14 y: 12 Females 9-13 y: 9 14-18 y: 11 |
Dairy products, meat, poultry, fish, fortified cereals, and peanuts. |
| B6 (mg/d) | Improvement of endothelial function by reducing homocysteine levels, which is associated with increased oxidative stress. | Children
1-3 y: 0.5 4-8 y: 0.6 Males 9-13 y: 1.0 > 14 y: 1.3 Females 9-13 y: 1.0 14-18 y: 1.8 |
Fortified cereals, beans, meat, poultry, fish, and some fruits and vegetables |
| B12 (μg/d) | Improvement of endothelial function by reducing homocysteine levels. | 1-3 y: 0.9
4-8 y: 1.2 9-13 y: 1.8 >14 y: 2.4 |
Fish, meat, poultry, eggs, milk, milk products, and fortified breakfast cereals |
| Folate (μg/d) | 1-3 y: 150
4-8 y: 200 9-13 y: 300 > 14 y: 400 |
Prepared breakfast cereals, beans, and fortified grains. | |
| Vitamin A (μg/d) | Important for normal vision, gene expression, reproduction, embryonic development, growth and immune function. | Children
1-3 y: 300 4-8 y: 400 Males 9-13 y: 600 >14 y: 900 Females 9-13 y: 600 >14 y: 700 Note: 1 RAE= 1 μg retinol, 12 μg β-carotene |
Many breakfast cereals, juices, dairy products, and other foods are fortified with vitamin A. Many fruits and vegetables, and some supplements, also contain beta-carotene and other vitamin A precursors, which the body can turn into vitamin A |
| Pro vitamin A carotenoids | Possible antioxidant activity. Associated with decreased risk of some cardiovascular events. | Not determinable due to lack of data of adverse effects. | Some fruits (papaya, peach, melon), some tubers (squash, yam, sweet potato), yellow/orange vegetables (carrots, peppers), green leafy vegetables. |
| Vitamin C (mg/d) | Important antioxidant and also helps maintain tissue levels of vitamins A and E, which also serve as antioxidants. | Children
1-3 y: 15 4-8 y: 25 Males 9-13 y: 45 14-18 y: 75 Females 9-13 y: 45 14-18 y: 65 |
Citrus fruits or juices, berries, green and red peppers, tomatoes, broccoli, and spinach. Many breakfast cereals are also fortified with vitamin C. |
| Vitamin E (mg/d) | Functions primarily as a chain-breaking antioxidant that prevents propagation of lipid peroxidation. | Children
1-3 y: 6 4-8 y: 7 Males 9-13 y: 11 14-18 y: 15 Females 9-13 y: 11 > 14 y: 15 |
Vegetable oils, nuts, green leafy vegetables, and fortified cereals |
| Vitamin D (μg/d) | Essential for calcium absorption from the intestine. | Children
1-8 y: 5 Males and females >9 y: 5 |
Fortified foods such as milk and breakfast cereals. |
| Calcium (mg/d) | In addition to bone metabolism, calcium plays a role in muscle contraction. | Children
1-3 y: 500 4-8 y: 800 Males and females 9-18 y: 1300 |
Dairy products are the mains source of calcium in the U.S. diet. Other sources include green vegetables, calcium-set tofu, some legumes, canned fish, seed, nuts, and certain fortified food products. |
| Zinc (mg/d) | Act as a component of antioxidant enzymes | Children
1-3 y: 3 4-8: 5 Males 9-13y: 8 > 14 y: 11 Females 9-13y: 8 14-18 y: 9 |
Oysters, red meat, poultry, beans, nuts, certain seafood, whole grains, fortified breakfast cereals, and dairy products |
| Copper | Component of enzymes in iron metabolism | Children
1-3 y: 34015 4-8 y: 440 Males and females 9-13 y: 700 14-18 y: 890 |
Organ meats, seafood, nuts, seeds, wheat bran cereals, whole grain products, cocoa products |
| Magnesium (mg/d) | Act as a component of antioxidant enzymes. May be involved in skeletal (and cardiac) | Children
1-3 y: 80 4-8: 130 Males 9-13y: 240 14-18 y: 410 Females 9-13y: 240 14-18 y: 360 |
Green leafy vegetables, some legumes (beans and peas), nuts and seeds, and whole, unrefined grains |
| Selenium | Antioxidant protection in concert with vitamin E | Children
1-3 y: 20 4-8: 30 Males and females 9-13y: 40 14-18 y: 55 |
cereals, meat, eggs, dairy products, human milk, and infant formula, which are good sources of highly available Se and are of low risk of providing excess amounts of Se. |
| Sodium (g/d) | Sodium restriction prevents exacerbations of heart failure and can reduce the dose of diuretic therapy | Children
1-3 y: 1.0 4-8: 1.2 Males 9-13y: 1.5 14-18 y: 1.5 Females 9-18y: 1.5 |
Processed foods to which sodium chloride (salt)/benzoate/phosphate have been added; salted meats, nuts, cold cuts; margarine; butter; salt added to foods in cooking or at the table. |
| Other nutrition supplements | |||
| Carnitine | Essential for the transport of long-chain fatty acids from cytoplasm into the sites of β-oxidation within the mitochondrial matrix | No DRI or RDA established.
Conditionally essential nutrients. |
Animal products like meat, fish, poultry, and milk. |
| Taurine | Nonessential amino acid that participates in controlling cellular calcium levels | Meat and fish | |
| Creatine phosphate | Primary high-energy phosphate reservoir of the heart and skeletal muscle. | Meat and fish | |
| Coenzyme Q10 | Critically necessary for oxidative energy production and cardiac function.
Role as a rate-limiting carrier for the flow of electrons through complexes I, II and III of the mitochondrial respiratory chain. |
Widespread throughout all food groups | |
BMR = Basal Metabolic Rate
EER = Estimated Energy Requirement
FTT = Failure to Thrive
DRI = Dietary Reference Intake.
AMDR = Acceptable Macronutrient Distribution Range – is the range of intake for a particular energy source that is associated with reduced risk of chronic disease while providing intakes of essential nutrients.
Food and Nutrition Board, Institute of Medicine, National Academies of Sciences. Retrieved June 20, 2007 from http://www.nap.edu
There is little information regarding the role of dietary macronutrients in the development or prevention of left ventricular hypertrophy or heart failure in children with cardiomyopathy. Dietary guidelines aimed at prevention of cardiovascular disease emphasize the importance of consuming a low-fat/high carbohydrate diet; however recent findings suggest that reducing fat intake and increasing carbohydrate consumption does not lower the risk of heart disease [32] in adults. Little information is available for children, yet it is becoming increasingly recognized that the root of adult cardiovascular disease may begin in childhood.
Studies of animal models have shown that a high-fat diet attenuated the hypertension-induced increase in left ventricular mass, cardiomyocyte hypertrophy, left ventricular chamber markers of cardiac dysfunction, and induction of molecular markers of cardiac hypertrophy and dysfunction [33-35]. However there are no data from humans to support extending this observation into clinical practice. The interactions among fat and carbohydrate intake, salt intake, hypertension, and cardiac size and function are complex and difficult to decipher in vivo. The reduced left ventricular hypertrophy with a high-fat/low-carbohydrate diet could be because of less insulin stimulation of cardiomyocyte growth. Dietary intake of carbohydrates, particularly sugars, determines the exposure of the heart to insulin and insulin-like growth factor. In addition, it is not clear if a low-sugar/high complex carbohydrate/low-fat diet could be just as effective at preventing left ventricular hypertrophy and contractile dysfunction in hypertension.
Nonpharmacological factors, often nutrition related, can influence the course of heart failure. There is general agreement that a diet high in sodium is potentially harmful in congestive heart failure, as it may cause fluid overload and potentially contribute to acute decompensation. Besides preventing exacerbations of heart failure, sodium reduction can reduce the dose of diuretic therapy. Water restriction may also be important, especially in advanced stages.
Micronutrients
Although provision of optimal calories and protein is important for growth and optimal cardiac function in children with cardiomyopathy, it is not always sufficient to optimize cardiac function. In adults with congestive heart failure, high protein feedings and a marked positive energy balance does not always correct the significant metabolic problems that occur in heart failure [36]. Thus, specific nutritional deficiencies specific for heart failure may play a role in optimizing or helping correct the failure. Children with cardiomyopathy and heart failure, similar to adults, may require greater than standard intakes of certain micronutrients in order to optimize the cardiac function [36,37]. Furthermore, serum levels of micronutrients may not necessarily reflect adequacy of these nutrients at the tissue level. The following discussion is of micronutrient, mineral and other nutritional deficiencies that are known to be problematic in patients with heart failure or may be therapeutic in improving cardiac function if given to patients as an ancillary intervention. These nutrients are either broadly categorized as antioxidants or nutrients known to affect myocardial energy production. However, several of these nutrients have more than one cellular role.
Antioxidants
Free radicals are products of oxygen metabolism and their rate of production is usually equal to their metabolism under normal circumstances. In certain clinical situations of stress, the production of these free radicals is greater than their normal clearance. At that point, the host's endogenous antioxidant system plays a major role to prevent or limit the deleterious effects of free radicals and in children with cardiomyopathy specifically, control further myocardial damage. Endogenous antioxidants include enzymatic antioxidants (e.g., zinc in superoxide dismutase or selenium in glutathione peroxidase), free radical scavengers (e.g., vitamins A, C or E) and metal chelators. Sources of antioxidants include the diet or through the use of specific nutritional supplements. Increased free radical formation and reduced antioxidant defenses [38,39] found in patients with heart failure can result from a combination of insufficient dietary intake and excessive utilization of specific antioxidants without adequate recycling or replacement. Recognizing and correcting multiple vitamin marginal deficiencies may be the key to the treatment of many heart failure patients. It has been recommended that individuals strive to achieve a higher intake of dietary antioxidants by increasing consumption of fruits, vegetables, and whole grains.
As a general rule, food and lifestyle factors that trigger the acute phase response should be avoided. This comprises, for example, excess of carbohydrates or saturated fat, alcohol, and smoking. Food that counteracts inflammatory processes can generally be recommended, for example fatty fish for its content of omega-3 fatty acids and possible favorable effect on left ventricular function. There are no clinical trials demonstrating the benefit of omega-3 fatty acid supplementations in patients with heart failure. However, the omega-3 fatty acids, eicosapentanoic acid and docosahexanoic acid, are essential nutrients. Thus, assuring adequate intake is necessary to meet nutritional requirements. The American Heart Association recommends 2 meals of fish, preferably fatty fish, per week and the use of vegetable oils high in α-linolenic acid such s canola, flaxseed, soybean, and walnut [40].
Injury from free radicals can contribute to coronary artery disease, myocardial infarction and cardiac dysfunction in some forms of cardiomyopathy in both humans and animals [41]. Free radicals can have both cytotoxic effects on the myocardium and also act as negative inotropes [42]. In models of congestive heart failure, antioxidants are elevated in cardiac hypertrophy and lower in cardiac failure [43,44]. For example, administration of vitamin E in one experimental model in Syrian Hamsters with end-stage cardiomyopathy showed optimization of alpha-tocopherol levels and improved glutathione peroxidase activity [45]. However, despite some experimental evidence, few studies have shown supplementation with antioxidants to have a significant impact on treatment of heart failure. The following section outlines experimental evidence that is available on the effects of specific antioxidants on cardiac conditions in patients with heart failure. Please note the dearth of information regarding their effects on children.
Vitamin A
Vitamin A can be found in 2 forms; preformed vitamin A (retinol) and carotenoids. Vitamin A is an antioxidant that can decrease oxidative stress. In experimental animal models, some have shown that vitamin A, taken as one of several antioxidants, prevents NF-kappaB activation, reduces mitochondrial cytochrome c release, decreases caspase activity, attenuates cardiomyocyte secretion of inflammatory cytokines, and improves myocardial contractile function [46]. In the neonatal rat heart, 9-cis-retinoic acid, stimulated transcription from the GLUT4 glucose transporter promoter (whose expression may be critical for the survival of cardiac myocytes in situations of stress) [47]. However, its role in the treatment of heart failure is unknown, and some clinical studies have found it to have no benefits in treating heart failure [48].
Vitamin E
Vitamin E is an antioxidant that can be detected in lower concentrations in patients with congestive heart failure [49]; however there is little evidence that shows the benefits in improving myocardial function with exogenous supplementation [50-54]. There have been few human trials, but one randomized, placebo controlled study in adults with congestive heart failure showed no effect on quality of life, norepinephrine levels and other neurotransmitters [55].
Taurine
Children with heart failure have increased levels of intracellular and mitochondrial calcium that can depress myocardial energy production and increase oxidative stress. Taurine, an amino acid, helps regulate calcium flux through the cells [56]. Taurine is the most abundant free amino acid in cardiac muscle cells. Taurine can be synthesized from methione and cysteine and is not essential. However, the activity of certain enzymes to systhesize taurine from these other amino acids is low in humans, thus the majority of taurine in the body is derived from foods including seafood and meat [57]. Proinflammatory cytokines can also decrease tissue taurine levels. With inflammatory conditions often accompanying heart failure, taurine supplementation may play a role in controlling oxidative stress as well of optimizing myocardial energy production [58]. For example, one study showed that heart tissue is more susceptible to adriamycin toxicity when taurine levels are low [59]. Studies regarding the benefit of taurine administration in various heart conditions have been promising [57,60,61]. However, taurine's potential beneficial effects in children have had limited attention.
Co-enzyme Q10 (Ubiquinone)
Co-enzyme Q10 (ubiquinone) is a vitamin-like substance that is present in all human cells and responsible for energy production by facilitating the actions of the mitochondria. It is a rate-limiting carrier for the flow of electrons through complexes I, II and III of the mitochondrial respiratory chain and is also an endogenous lipophilic antioxidant. Those organs with the highest energy requirements, including the heart, have the highest Co-enzyme Q10 concentrations [62-64]. It is a powerful antioxidant and stabilizes membranes. Ubiquinone is present in varying amounts in all food groups, thus body stores may be partially supplied by diet. Oral absorption is slow but it is enhanced with lipids. There is a large hepatic first pass effect so that only 2-5% of an oral dose is taken up by the myocardium. Adults with congestive heart failure can have lower concentrations of Co-enzyme Q10 in their myocardium as determined by biopsy [65]. Low levels have also been associated with higher rates of mortality [66]. Studies of Co-enzyme Q10 supplementation have been contradictory with some showing improvement in functional status, clinical symptoms, and hospitalizations [67,68], while others showing no benefit [69-71. However, a meta-analysis of published reports [72] supported a hemodynamic benefit.
Vitamin C
Vitamin C is another powerful antioxidant that also has a role in vitamin E metabolism. Vitamin C levels are reduced in heart failure [73]. Vitamin C may have an important role in modifying apoptosis [74-77]. It also decreases TNF secretion, thereby improving inflammation [78]. Large doses have been shown to improve vasomotor function in patients with heart failure by possibly increasing nitric oxide production [78]. In NHANES I, subjects with a high dietary intake of antioxidants (including vitamin C) had a significantly lower all-cause mortality and in particular from coronary heart disease [79]. However, in subsequent prospective, randomized clinical trials in high-risk populations, vitamin C showed no benefit [80]. Acute vitamin C administration restored peripheral endothelial function in patients with coronary artery disease to normal values, but not in heart failure, especially in dilated cardiomyopathy. Thus, factors other than oxidative stress (eg, cytokines) can contribute to endothelial dysfunction in patients with heart failure [81]. Compiling the evidence, vitamin C may hold promise in altering peripheral endothelial function, however, few, if any studies have been performed in children with cardiomyopathy.
Nutrients that Affect Myocardial Energy Production
Thiamine (Vitamin B1)
Thiamine is a water-soluble vitamin and is synthesized by plants and other microorganisms, yet humans cannot synthesize it themselves. Thiamine is important for carbohydrate metabolism and a deficiency is found in up to 93% of patients with heart failure [82-87]. Loop diuretcs, among other factors, including malnutrition and poor overall nutritional status has been linked to thiamine deficiency [82-85]. Symptoms for congestive heart failure are common and can often be reversed with adequate supplementation [88].
L-Carnitine
L-Carnitine, an amino-acid derivative that helps the transport of long-chain fatty acids from the cytoplasm into the sites of [beta]-oxidation within the mitochondrial matrix. Furthermore, carnitine binds toxic acyl groups and releases free coenzyme A. Susequently, these acylcarnitines can diffuse freely out of the cell and be eliminated through the urine. Carnitine also indirectly activates pyruvate dehydrogenase, the rate-limiting enzyme for glucose oxidation [89, 90]; this, in turn, improves the coupling between glycolysis and glucose oxidation, thereby reducing the lactate and hydrogen burden on the myocyte. L-carnitine and its derivates play an important role in myocardial energy production. Carnitine stores can be replenished from endogenous synthesis from lysine and methionine, as well as from dietary intake. Patients with genetically determined deficiency develop both cardiac and skeletal dysfunction, which can be improved by carnitine administration [91]. Carnitine deficiency can also be an acquired state in individuals with established congestive heart failure, with levels reported to be depleted by as much as 50% [92]. Plasma levels may be as much as 3 – 5 times those of intracardiac levels, thus plasma levels are not a good measure of tissue concentrations. Patients in congestive heart failure generally exhibit a marked depletion (up to 50%) of both free and total carnitine [89. 92]. L-carnitine supplementation [89, 92, 93] can result in overall improvement in the cardiac status and quality of life of both animals and patients with myocardial dysfunction. A multicentered, randomized, placebo-controlled, double-blind clinical trial [94] showed a significant beneficial effect, including a reduction in adverse cardiac remodeling, when L-carnitine was taken for 12 months after myocardial infarction. Furthermore, improved 3 year survival was also found in patients given daily doses of L-carnitine [95]. Similar to other nutrients, it is clear that more studies will be needed to determine the potential benefits of this nutrient. Furthermore, there continues to be a dearth of studies in the pediatric population with heart failure.
Creatine
Creatine phosphate is the substrate for phosphate transfer to ADP to form ATP by the enzymatic activity of creatine kinase. Creatine is synthesized in the liver and spleen from arginine, glycine and methionine. The concentration of creatine in the myocardiocyte is determined by adrenergic drive [96], thus with heart failure, the concentration of creatine in the cell may be diminished [96, 97]. Creatine can improve calcium homeostasis [98] and survival of myocytes in culture.
Creatine supplements increases skeletal muscle creatine, and this may be most beneficial during short-term exercise to improve muscle strength, endurance and metabolism by reducing lactate [99-101]. Thus, creatine supplementation may not be as beneficial under normal situations. There have been few, if any studies on creatine's effects on heart failure, with notably none in children.
Other Nutrients
Vitamin D/Calcium
Adequate intake (Table) of calcium and vitamin D needs to be insured, as these nutrients are critical to optimize cardiac function. The childhood diet is typically deficient in both of these nutrients with intakes of only 50% of the DRI widely reported. In animal models, rats fed a vitamin D deficient diet developed poor cardiac function that was reversed with supplementation of vitamin D [102].
Folate/Vitamin B12
Folate is required to convert homocysteine to methione. Folate deficiency is frequently detected in patients with heart failure [103] and often this is coincident with low folate dietary intake [104]. Vascular endothelial function may be improved by folate [105]. Similarly, vitamin B12 deficiency is also linked to higher homocysteine levels. However, studies show that neither folate nor vitamin B12 improves intrinsic cardiac function, yet there may be greater effects on peripheral vasculature [106].
Magnesium
Magnesium deficiency can occur in up to 30% of patients with heart failure [107-109]. Medications, such as loop and thiazide diuretics contribute to urinary magnesium loss. Magnesium deficiency is associated with sodium retention and increased ventricular ectopy [110-112] with associated reduced cardiac contractility and increased peripheral vascular resistance [113, 114].
Zinc
Zinc is another antioxidant and its deficiency is related to apoptosis of the myocardiocyte [115]. Several medicines including angiotensin converting enzyme inhibitors, angiotensin II antagonists and thiazide diuretics can increase urinary zinc [116, 117]. However, it is unclear if zinc deficiency is related to or can improve heart failure.
Selenium
Selenium is a trace mineral that can be found in small amounts in the soil and food. Depending on the region of the world, foods grown in certain areas may have sufficient or poor concentrations of selenium owing to soil content. Meat and seafood have the greatest concentrations of selenium. Selenium deficiency has been associated with congestive cardiomyopathy (Keshan disease), skeletal myopathy, osteoarthropathy (Kashin–Beck disease), anemia, immune system alterations, increased risk of cancer, cardiovascular disease, hair and nail changes, infertility, and abnormalities in thyroid hormone metabolism in humans [118]. Selenium's greatest role is its action as a cofactor for the antioxidant enzyme, glutathione peroxidase which removes hydrogen peroxide and the deleterious lipid hydroperoxides generated by oxygen-derived species. Glutathione peroxidase deficiency contributes to endothelial dysfunction a major contributing factor in heart failure [119], in various conditions such as hyperhomocysteinemia [120]. This suggests that homocysteine may be involved in heart failure associated endothelial dysfunction through a peroxide-dependent oxidative mechanism. Selenium also plays a role in the control of thyroid hormone metabolism [121] by affecting synthesis and activity of de-iodinases, enzymes converting thyroxin into the biologically active triiodothyronine [122]. Thus, selenium (through its role in selenoenzymes, thyroid hormones, and interactions with homocysteine and endothelial function) appears to be a major mediator in several pathways potentially contributing to or possibly preventing heart failure.
The first case of endemic selenium deficiency was described in 1935 in Keshan County, in China. Clinical features were acute and/or chronic episodes of cardiogenic shock and/or congestive heart failure. Selenium supplementation may stop progression of the cardiac disease but is less successful at reversing the existing cardiac damage [123]. The daily recommended intake of selenium is 20 – 55 ug/day, depending on the age of the child. (30) (Table). Selenium deficiency in developed nations is more often seen in chronically ill, malnourished patients with malabsorption and in unsupplemented total parenteral nutrition (TPN)-dependent patients [124, 125]. Selenium deficiency also is encountered when nutrient-limited diets are used such as patients with phenylketonuria [126] and the ketogenic diet.
Assessment of selenium status is difficult because no optimal method is known. Dietary assessment is inaccurate, and selenium content depends on where the food was grown (soil content), which is usually unknown. Selenium can be measured in serum, plasma, whole blood, erythrocytes, urine, and hair. Serum and plasma concentrations correlate well with dietary intake and absorption and are a good indicator of short-term selenium status. Whole-blood and erythrocyte selenium levels reflect longer-term status. Activity of glutathione peroxidase is a well-accepted functional assay of selenium sufficiency.
Conclusions
Little is understood regarding the role of growth and nutrition in pediatric cardiomyopathy as a predictor of its outcomes. Understanding the link between nutrition and outcomes in pediatric cardiomyopthy would be useful in classifying patients into appropriate prognostic categories that will aid in the identification of patients who would benefit most from transplant or other types of medical treatment. Furthermore, understanding the role of growth and nutrition in predicting outcomes in pediatric cardiomyopathy may also focus attention on early and aggressive nutritional interventions for these children that may ultimately prevent or delay progressive decline in heart function or eventual heart transplantation. There is evidence that nutrition can also be used as specific therapy toward optimizing cardiac function by decreasing the effects of free radicals or augmenting myocardial energy production. However, many scientific studies are contradictory in adults with heart failure and there is a disappointingly few studies among children with cardiomyopathy. Future efforts should focus on collaborative descriptive and interventional studies that further define the role of nutrition and nutritional interventions on cardiac-specific endpoints as well as quality of life in children with cardiomyopathy.
Acknowledgments
Supported by NIH NHLBI grant RO1 HL53392 and the Children's Cardiomyopathy Foundation, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nugent AW, Daubeney PE, Chondros P, et al. The epidemiology of childhood cardiomyopathy in Australia. N Engl J Med. 2003 Apr 24;348(17):1639–1646. doi: 10.1056/NEJMoa021737. [DOI] [PubMed] [Google Scholar]
- 2.Lipshultz SE, Sleeper LA, Towbin JA, et al. The incidence of pediatric cardiomyopathy in two regions of the United States. N Engl J Med. 2003 Apr 24;348(17):1647–1655. doi: 10.1056/NEJMoa021715. [DOI] [PubMed] [Google Scholar]
- 3.Grenier MA, Osganian SK, Cox GF, et al. Design and implementation of the North American Pediatric Cardiomyopathy Registry. Am Heart J. 2000;139(2 Pt 3):S86–95. doi: 10.1067/mhj.2000.103933. [DOI] [PubMed] [Google Scholar]
- 4.Boucek MM, Faro A, Novick RJ, et al. The Registry of the Internation Society of Heart and Lung Transplantation: Fourth Official Pediatric Report-2000. J Heart Lung Transplant. 2001;20:39–52. doi: 10.1016/s1053-2498(00)00243-6. [DOI] [PubMed] [Google Scholar]
- 5.Colan SD, Spevak PJ, Parness IA, et al. Cardiomyopathies. In: Fyler DC, editor. Nadas' Pediatric Cardiology. New York: Balfus and Hanley; 1992. [Google Scholar]
- 6.Burch M, Siddiqi SA, Celermajer DD, et al. Dilated cardiomyopathy in children: determinants of outcome. British Heart Journal. 1994;72:246–250. doi: 10.1136/hrt.72.3.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maron BJ, Tajik AJ, Ruttenberg HD, et al. Hypertrophic cardiomyopathy in infants: Clinical features and natural history. Circulation. 1982;65(1):7–17. doi: 10.1161/01.cir.65.1.7. [DOI] [PubMed] [Google Scholar]
- 8.Towbin JA, Bowles NE. The failing heart. Nature. 2002 Jan 10;415(6868):227–33. doi: 10.1038/415227a. [DOI] [PubMed] [Google Scholar]
- 9.Thiene G, Becker AE, Buja LM, Fallon JT, McManus BM, Schoen FJ, Winters GL. Toward a cardiovascular pathology training report on the forum held in Vancouver, March 6, 2004, Society for Cardiovascular Pathology. Cardiovasc Pathol. 2005 Nov Dec;14(6):312–9. doi: 10.1016/j.carpath.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Boucek MM, Edwards LB, Keck BM, et al. The Registry of the International Society for Heart and lung Transplantation: sixth official pediatric report-2003. J Heart Lung Transplant. 2003;22:636–652. doi: 10.1016/s1053-2498(03)00184-0. [DOI] [PubMed] [Google Scholar]
- 11.Bilgic A, Ozbarlas N, Ozkutlu S, et al. Cardiomyopathies in children: clinical, epidemiological and prognostic evaluation. Japanese Heart Journal. 1990;31:789–797. doi: 10.1536/ihj.31.789. [DOI] [PubMed] [Google Scholar]
- 12.Lipshultz SE. Ventricular dysfunction clinical research in infants, children and adolescents. Progr Pediatric Cardiol. 2000 Nov 4;12(1):1–28. doi: 10.1016/s1058-9813(00)00076-x. [DOI] [PubMed] [Google Scholar]
- 13.Evans RW. Economic and social costs of heart transplantation. Heart Transplant. 1982;1:243–251. [PubMed] [Google Scholar]
- 14.Strauss A, Lock JE. Pediatric cardiomyopathy--a long way to go. N Engl J Med. 2003 Apr 24;348(17):1703–1705. doi: 10.1056/NEJMe030027. [DOI] [PubMed] [Google Scholar]
- 15.Blecker U, Mehta DI, Davis R, et al. Nutritional problems in patients who have chronic disease. Pediatric Reviews. 2000;21(1):29–32. doi: 10.1542/pir.21-1-29. [DOI] [PubMed] [Google Scholar]
- 16.Karp RJ, Bachrach SJ, Moskowitz S. Malnutrition in chronic illness of childhood with special reference to pulmonary disease. Clin Chest Medicine. 1980;1(3):375–383. [PubMed] [Google Scholar]
- 17.Kelly DA. Nutrition and growth in patients with chronic liver disease. Indian Journal of Pediatrics. 1995;62(5):533–544. doi: 10.1007/BF02761872. [DOI] [PubMed] [Google Scholar]
- 18.Kohaut EC. Chronic renal disease and growth in childhood. Current opinions in Pediatrics. 1995;7(2):171–175. doi: 10.1097/00008480-199504000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Yip R, Scanlon K. The burden of Malnutrition: a population perspective. Journal of Nutrition. 1994 10:2043s–2046s. doi: 10.1093/jn/124.suppl_10.2043S. [DOI] [PubMed] [Google Scholar]
- 20.Franssen FM, Wouters EF, Schols AM. The contribution of starvation deconditioning and ageing to the observed alterations in peripheral skeletal muscle in chronic organ diseases. Clinical Nutrition. 2002;21(1):1–14. doi: 10.1054/clnu.2001.0485. [DOI] [PubMed] [Google Scholar]
- 21.Keusch GT. The history of nutrition: malnutrition, infection, and immunity. Journal of Nutrition. 2003;133(1):336s–340s. doi: 10.1093/jn/133.1.336S. [DOI] [PubMed] [Google Scholar]
- 22.Hughes WT, Price RA, Sisko F, et al. Protein-calorie malnutrition. A host determinant for Pneumocystis carnii. American Journal of Dis Child. 1974;128(1):44–52. doi: 10.1001/archpedi.1974.02110260046008. [DOI] [PubMed] [Google Scholar]
- 23.Miller TL, Awnetwant EL, Evans S, et al. Gastrostomy tube supplementation fo HIV-infected children. Pediatrics. 1995;96:696–702. [PubMed] [Google Scholar]
- 24.Al-Attar I, Orav EJ, Exil V, et al. Pedictors of cardiac morbidity and related mortality in children with acquired immunodeficiency syndrome. Journal of the American College of Cardiology. 2003;41(9):1598–1605. doi: 10.1016/s0735-1097(03)00256-0. [DOI] [PubMed] [Google Scholar]
- 25.Azevedo VM, Albanesi-Filho FM, Santos MA, et al. The impact of malnutrition on idiopathic dilated cardiomyopathy in children. J Pediatrics (Rio J) 2004;80(3):211–216. Portuguese. [PubMed] [Google Scholar]
- 26.Leitch CA. Growth, nutrition and energy expenditure in pediatric heart failure. Prog Pediatr Cardiol. 2000;11(3):195–202. doi: 10.1016/s1058-9813(00)00050-3. [DOI] [PubMed] [Google Scholar]
- 27.Miller TL, Orav EJ, Colan SD, Lipshultz SE. Nutritional status and cardiac mass and function in children infected with the human immunodeficiency virus. Am J Clin Nutr. 1997 Sep;66(3):660–4. doi: 10.1093/ajcn/66.3.660. [DOI] [PubMed] [Google Scholar]
- 28.Davos CH, Doehner W, Rauchhaus M, Cicoira M, Francis DP, Coats AJ, Clark AL, Anker SD. Body mass and survival in patients with chronic heart failure without cachexia: the importance of obesity. J Card Fail. 2003 Feb;9(1):29–35. doi: 10.1054/jcaf.2003.4. [DOI] [PubMed] [Google Scholar]
- 29.Horwich TB, Fonarow GC, Hamilton MA, MacLellan WR, Woo MA, Tillisch JH. The relationship between obesity and mortality in patients with heart failure. J Am Coll Cardiol. 2001 Sep;38(3):789–95. doi: 10.1016/s0735-1097(01)01448-6. [DOI] [PubMed] [Google Scholar]
- 30.Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, Fluoride (1997). Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12 (1998). Dietary Reference Intakes for vitamin C, Vitamin E, Selenium, and Carotenoids (2000). Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc (2001). Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (2002). Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate (2004). Food and Nutrition Board, Institute of Medicine, National Academies of Sciences. Retrieved June 20, 2007 from http://www.nap.edu
- 31.Linde LM. Psychiatric aspects of congenital heart disease. Psychiatr Clin North Am. 1982;5:399–406. [PubMed] [Google Scholar]
- 32.Howard BV, Van Horn L, Hsia J, Manson JE, Stefanick ML, Wassertheil-Smoller S, Kuller LH, LaCroix AZ, Langer RD, Lasser NL, Lewis CE, Limacher MC, Margolis KL, Mysiw WJ, Ockene JK, Parker LM, Perri MG, Phillips L, Prentice RL, Robbins J, Rossouw JE, Sarto GE, Schatz IJ, Snetselaar LG, Stevens VJ, Tinker LF, Trevisan M, Vitolins MZ, Anderson GL, Assaf AR, Bassford T, Beresford SA, Black HR, Brunner L, Brzyski RG, Caan B, Chlebowski RT, Gass M, Granek I, Greenland P, Hays J, Heber D, Heiss G, Hendrix SL, Hubbell FA, Johnson KC, Kotchen JM. Low-fat dietary pattern and risk of cardiovascular disease: the Women's Health Initiative Randomized Controlled dietary Moification Trial. JAMA. 2006 Feb 8;295(6):655–66. doi: 10.1001/jama.295.6.655. [DOI] [PubMed] [Google Scholar]
- 33.Sparagna GC, Hickson-Bick DL, Buja LM, McMillin JB. A metabolic role for mitochondria in palmitate-induced cardiac myocyte apoptosis. Am J Physiol Heart Circ Physiol. 2000 Nov;279(5):H2124–32. doi: 10.1152/ajpheart.2000.279.5.H2124. [DOI] [PubMed] [Google Scholar]
- 34.Gudz TI, Tserng KY, Hoppel CL. Direct inhibition of mitochondrial respiratory chain complex III by cell-permeable ceramide. J Biol Chem. 1997 Sep 26;272(39):24154–8. doi: 10.1074/jbc.272.39.24154. [DOI] [PubMed] [Google Scholar]
- 35.Okere IC, Young ME, McElfresh TA, Chess DJ, Sharov VG, Sabbah HN, Hoit BD, Ernsberger P, Chandler MP, Stanley WC. Low carbohydrate/high-fat diet attenuates cardiac hypertrophy, remodeling, and altered gene expression in hypertension. Hypertension. 2006 Dec;48(6):1116–23. doi: 10.1161/01.HYP.0000248430.26229.0f. Epub 2006 Oct 23. [DOI] [PubMed] [Google Scholar]
- 36.Broqvist M, Arnqvist H, Dahlstrom U, Larsson J, Nylander E, Permert J. Nutritional assessment and muscle energy metabolism in severe chronic congestive heart failure: effects of long-term dietary supplementation. Eur Heart J. 1994;15:1641–50. doi: 10.1093/oxfordjournals.eurheartj.a060447. [DOI] [PubMed] [Google Scholar]
- 37.Sole MJ, Jeejeebhoy KN. Conditioned nutritional requirements: Therapeutic relevance to heart failure. Herz. 2002;27:174–9. doi: 10.1007/s00059-002-2360-0. PubMed SpringerLink. [DOI] [PubMed] [Google Scholar]
- 38.de Lorgeril M, Salen P. Diet as preventive medicine in cardiology. Curr Opin Cardiol. 2000 Sep;15(5):364–70. doi: 10.1097/00001573-200009000-00009. [DOI] [PubMed] [Google Scholar]
- 39.Keith M, Geranmayegan A, Sole MJ, Kurian R, Robinson A, Omran AS, Jeejeebhoy KN. Increased oxidative stress in patients with congestive heart failure. J Am Coll Cardiol. 1998 May;31(6):1352–6. doi: 10.1016/s0735-1097(98)00101-6. [DOI] [PubMed] [Google Scholar]
- 40.Kris-Etherton PM, Harris WS, Appel LJ. American Heart Association Nutrition Committee Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002 Nov 19;106(21):2747–57. doi: 10.1161/01.cir.0000038493.65177.94. No abstract available. Erratum in: Circulation. 2003 Jan 28;107(3):512. [DOI] [PubMed] [Google Scholar]
- 41.Kaul N, Siveski-Iliskovic N, Hill M, Slezak J, Singal PK. Free radicals and the heart. J Pharmacol Toxicol Methods. 1993 Oct;30(2):55–67. doi: 10.1016/1056-8719(93)90008-3. [DOI] [PubMed] [Google Scholar]
- 42.Prasad K, Kalra J, Bharadwaj L. Cardiac depressant effects of oxygen free radicals. Angiology. 1993 Apr;44(4):257–70. doi: 10.1177/000331979304400401. [DOI] [PubMed] [Google Scholar]
- 43.Dhalla AK, Singal PK. Antioxidant changes in hypertrophied and failing guinea pig hearts. Am J Physiol. 1994 Apr;266(4 Pt 2):H1280–5. doi: 10.1152/ajpheart.1994.266.4.H1280. [DOI] [PubMed] [Google Scholar]
- 44.Gupta M, Singal PK. Higher antioxidative capacity during a chronic stable heart hypertrophy. Circ Res. 1989 Feb;64(2):398–406. doi: 10.1161/01.res.64.2.398. [DOI] [PubMed] [Google Scholar]
- 45.Li RK, Sole MJ, Mickle DA, Schimmer J, Goldstein D. Vitamin E and oxidative stress in the heart of the cardiomyopathic Syrian hamster. Free Rad Biol Med. 1997;24:252–8. doi: 10.1016/s0891-5849(97)00224-4. [DOI] [PubMed] [Google Scholar]
- 46.Carlson D, Maass DL, White DJ, Tan J, Horton JW. Antioxidant vitamin therapy alters sepsis-related apoptotic myocardial activity and inflammatory responses. Am J Physiol Heart Circ Physiol. 2006 Dec;291(6):H2779–89. doi: 10.1152/ajpheart.01258.2005. [DOI] [PubMed] [Google Scholar]
- 47.Montessuit C, Papageorgiou I, Campos L, Lerch R. Retinoic acids increase expression of GLUT4 in dedifferentiated and hypertrophied cardiac myocytes. Basic Res Cardiol. 2006 Jan;101(1):27–35. doi: 10.1007/s00395-005-0567-y. [DOI] [PubMed] [Google Scholar]
- 48.Palace VP, Khaper N, Qin Q, Singal PK. Antioxidant potentials of vitamin A and carotenoids and their relevance to heart disease. Free Radic Biol Med. 1999;26:746–61. doi: 10.1016/s0891-5849(98)00266-4. [DOI] [PubMed] [Google Scholar]
- 49.Miwa K, Kishimoto C, Nakamura H, Makita T, Ishii K, Okuda N, Yodoi J, Sasayama S. Serum thioredoxin and alpha-tocopherol concentrations in patients with major risk factors. Circ J. 2005;69:291–4. doi: 10.1253/circj.69.291. [DOI] [PubMed] [Google Scholar]
- 50.The Heart Outcomes Prevention Evaluation Study Investigators. Vitamin E supplementation and cardiovascular events in high-risk patients. N Engl J Med. 2000;342:154–60. doi: 10.1056/NEJM200001203420302. [DOI] [PubMed] [Google Scholar]
- 51.Lonn E, Yusuf S, Hoogwerf B, Pogue J, Yi Q, Zinman B, Bosch J, Dagenais G, Mann JF, Gerstein HC. HOPE Study; MICRO-HOPE Study. Effects of vitamin E on cardiovascular and microvascular outcomes in high-risk patients with diabetes: results of the HOPE study and MICRO-HOPE substudy. Diabetes Care. 2002;25:1919–27. doi: 10.2337/diacare.25.11.1919. [DOI] [PubMed] [Google Scholar]
- 52.Lonn E, Bosch J, Yusuf S, Sheridan P, Pogue J, Arnold JM, Ross C, Arnold A, Sleight P, Probstfield J, Dagenais GR. HOPE and HOPE-TOO Trial Investigators. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA. 2005;293:1338–47. doi: 10.1001/jama.293.11.1338. [DOI] [PubMed] [Google Scholar]
- 53.Mann JF, Lonn EM, Yi Q, Gerstein HC, Hoogwerf BJ, Pogue J, Bosch J, Dagenais GR, Yusuf S. HOPE Investigators. Effects of vitamin E on cardiovascular outcomes in people with mild-to-moderate renal insufficiency: results of the HOPE study. Kidney Int. 2004;65:1375–80. doi: 10.1111/j.1523-1755.2004.00513.x. [DOI] [PubMed] [Google Scholar]
- 54.Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet. 1999;354:447–55. [PubMed] [Google Scholar]
- 55.Keith ME, Jeejeebhoy KN, Langer A, Kurian R, Barr A, O'Kelly B, Sole MJ. A controlled clinical trial of vitamin E supplementation in patients with congestive heart failure. Am J Clin Nutr. 2001;73:219–24. doi: 10.1093/ajcn/73.2.219. [DOI] [PubMed] [Google Scholar]
- 56.Azuma J, Sawamura A, Awata N. Usefulness of taurine in chronic congestive heart failure and its prospective application. Jpn Circ. 1992;56:95–9. doi: 10.1253/jcj.56.95. [DOI] [PubMed] [Google Scholar]
- 57.Schuller-Levis GB, Park E. Taurine: new implications for an old amino acid. FEMS Microbiol Lett. 2003;226:195–202. doi: 10.1016/S0378-1097(03)00611-6. [DOI] [PubMed] [Google Scholar]
- 58.Grimble RF, Jackson AA, Persaud C, Wride MJ, Delers F, Engler R. Cysteine and glycine supplementation modulate the metabolic response to tumor necrosis factor alpha in rats fed a low protein diet. J Nutrition. 1992;122:2066–73. doi: 10.1093/jn/122.11.2066. [DOI] [PubMed] [Google Scholar]
- 59.Schaffer SW, Allo S, Harada H, Mozaffari M. Potentation of myocardial ischemic injury by drug-induced taurine depletion. In: Huxtable RJ, Franconi F, Giotti A, editors. The biology of taurine. New York: Plenum Press; 1987. pp. 151–8. [DOI] [PubMed] [Google Scholar]
- 60.Kramer JH, Chovan JP, Schaffer SW. The effect of taurine on calcium paradox and ischemic heart failure. Am J Physiol. 1981;240:H238–46. doi: 10.1152/ajpheart.1981.240.2.H238. [DOI] [PubMed] [Google Scholar]
- 61.Oudit GY, Trivieri MG, Khaper N, Hussain T, Wilson GJ, Liu P, Sole MJ, Backx PH. Taurine supplementation reduces oxidative stress and improves cardiovascular function in an iron overload murine model. Circulation. 2004;109:1877–85. doi: 10.1161/01.CIR.0000124229.40424.80. [DOI] [PubMed] [Google Scholar]
- 62.Kishi T, Okamoto T, Takahashi T, Goshima K, Yamagami T. Cardiostimulatory action of coenzyme Q homologues on cultured myocardial cells and their biochemical mechanisms. Clin Investig. 1993;71 8:S71–5. doi: 10.1007/BF00226844. [DOI] [PubMed] [Google Scholar]
- 63.Bentinger M, Dallner G, Chojnacki T, Swiezewska E. Distribution and breakdown of labeled coenzyme Q10 in rat. Free Radic Biol Med. 2003 Mar 1;34(5):563–75. doi: 10.1016/s0891-5849(02)01357-6. [DOI] [PubMed] [Google Scholar]
- 64.Shindo Y, Witt E, Han D, Epstein W, Packer L. Enzymic and non-enzymic antioxidants in epidermis and dermis of human skin, Invest. Dermatol. 1994;102:122–124. doi: 10.1111/1523-1747.ep12371744. [DOI] [PubMed] [Google Scholar]
- 65.Kitamura N, Yamaguchi A, Otaki M. Myocardial tissue level of co-enzyme Q10 in patients with cardiac failure. In: Folkers K, Yamamura Y, editors. Biomedical and Physical Aspects of Coenzyme Q. Vol. 4. Amsterdam: Elsevier; 1984. pp. 243–57. [Google Scholar]
- 66.Jameson S. Statistical data support prediction of death within 6 months on low levels of coenzyme Q10 and other entities. Clin Invest. 1993;71:S137–9. doi: 10.1007/BF00226855. [DOI] [PubMed] [Google Scholar]
- 67.Hofman-Bang C, Rehnqvist N, Swedberg K, et al. Coenzyme Q10 as an adjunctive treatment of chronic congestive heart failure. The Q10 study group. J Card Fail. 1995;2:101–7. doi: 10.1016/1071-9164(95)90011-x. [DOI] [PubMed] [Google Scholar]
- 68.Morisco C, Trimarco B, Condorelli M. Effect of coenzyme Q10 therapy in patients with congestive heart failure: a long-term multicentre randomised study. Clin Invest. 1993;71:S134–6. doi: 10.1007/BF00226854. [DOI] [PubMed] [Google Scholar]
- 69.Watson PS, Scalia GM, Galbraith A, et al. Lack of effect of coenzyme Q10 on left ventricular function in patients with congestive cardiac failure. J Am Coll Cardiol. 1999;33:1549–52. doi: 10.1016/s0735-1097(99)00064-9. [DOI] [PubMed] [Google Scholar]
- 70.Khatta M, Alexander BS, Krichten CM, et al. The effect of coenzyme Q10 in patients with congestive heart failure. Ann Intern Med. 2000;132:636–40. doi: 10.7326/0003-4819-132-8-200004180-00006. [DOI] [PubMed] [Google Scholar]
- 71.Permanetter B, Rossy W, Klein G, et al. Ubiquinone (coenzyme Q10) in the long-term treatment of idiopathic dilated cardiomyopathy. Eur Heart J. 1992;13:1528–33. doi: 10.1093/oxfordjournals.eurheartj.a060096. [DOI] [PubMed] [Google Scholar]
- 72.Soja AM, Mortenson SA. Treatment of congestive heart failure with Co-enzyme Q10 illuminated by meta-analysis of clinical trials. Mol Aspects Med. 1997;18(Suppl):S159–S168. doi: 10.1016/s0098-2997(97)00042-3. [DOI] [PubMed] [Google Scholar]
- 73.de Lorgeril M, Salen P, Accominotti M, Cadau M, Steghens JP, Boucher F, de Leiris J. Dietary and blood antioxidants in patients with chronic heart failure. Insights into the potential importance of selenium in heart failure. Eur J Heart Fail. 2001;3:661–9. doi: 10.1016/s1388-9842(01)00179-9. [DOI] [PubMed] [Google Scholar]
- 74.Guaiquil VH, Golde DW, Beckles DL, Mascareno EJ, Siddiqui MA. Vitamin C inhibits hypoxia-induced damage and apoptotic signaling pathways in cardiomyocytes and ischemic hearts. Free Radic Biol Med. 2004;37:1419–29. doi: 10.1016/j.freeradbiomed.2004.06.041. [DOI] [PubMed] [Google Scholar]
- 75.Fu YC, Chi CS, Yin SC, Hwang B, Chiu YT, Hsu SL. Norepinephrine induces apoptosis in neonatal rat cardiomyocytes through a reactive oxygen species-TNF alpha-caspase signaling pathway. Cardiovasc Res. 2004;62:558–6. doi: 10.1016/j.cardiores.2004.01.039. [DOI] [PubMed] [Google Scholar]
- 76.Rossig L, Hoffmann J, Hugel B, Mallat Z, Haase A, Freyssinet JM, Tedgui A, Aicher A, Zeiher AM, Dimmeler S. Vitamin C inhibits endothelial cell apoptosis in congestive heart failure. Circulation. 2001;104:2182–7. doi: 10.1161/hc4301.098284. [DOI] [PubMed] [Google Scholar]
- 77.Qin F, Yan C, Patel R, Liu W, Dong E. Vitamins C and E attenuate apoptosis, beta-adrenergic receptor desensitization, and sarcoplasmic reticular Ca2+ ATPase downregulation after myocardial infarction. Free Radic Biol Med. 2006 May 15;40(10):1827–42. doi: 10.1016/j.freeradbiomed.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 78.Shi W, Meininger CJ, Haynes TE, Hatakeyama K, Wu G. Regulation of tetrahydrobiopterin synthesis and bioavailability in endothelial cells. Cell Biochem Biophys. 2004;41:415–34. doi: 10.1385/CBB:41:3:415. [DOI] [PubMed] [Google Scholar]
- 79.Enstrom JE, Kanim LE, Klein MA. Vitamin C intake and mortality among a sample of the United States population. Epidemiology. 1992;3:194–202. doi: 10.1097/00001648-199205000-00003. [DOI] [PubMed] [Google Scholar]
- 80.MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:23–33. doi: 10.1016/S0140-6736(02)09328-5. [DOI] [PubMed] [Google Scholar]
- 81.Erbs S, Gielen S, Linke A, Mobius-Winkler S, Adams V, Baither Y, Schuler G, Hambrecht R. Improvement of peripheral endothelial dysfunction by acute vitamin C application: different effects in patients with coronary artery disease, ischemic, and dilated cardiomyopathy. Am Heart J. 2003 Aug;146(2):280–5. doi: 10.1016/S0002-8703(03)00184-4. [DOI] [PubMed] [Google Scholar]
- 82.Seligmann H, Halkin H, Rauchfleisch S, Kaufmann N, Motro M, Vered Z, Ezra D. Thiamine deficiency in patients with congestive heart failure receiving long-term furosemide therapy: a pilot study. Am J Med. 1991;91:151–5. doi: 10.1016/0002-9343(91)90007-k. [DOI] [PubMed] [Google Scholar]
- 83.Zenuk C, Healey J, Donnelly J, Vaillancourt R, Almalki Y, Smith S. Thiamine deficiency in congestive heart failure patients receiving long-term furosemide therapy. Can J Clin Pharmacol. 2003;10:184–8. [PubMed] [Google Scholar]
- 84.Brady JA, Rock CL, Horneffer MR. Thiamin status, diuretic medications, and the management of congestive heart failure. J Am Diet Assoc. 1995;95:541–4. doi: 10.1016/S0002-8223(95)00148-4. [DOI] [PubMed] [Google Scholar]
- 85.Kwok T, Falconer-Smith JF, Potter JF, Ives DR. Thiamine status of elderly patients with cardiac failure. Age Ageing. 1992;21:67–71. doi: 10.1093/ageing/21.1.67. [DOI] [PubMed] [Google Scholar]
- 86.Pfitzenmeyer P, Guilland JC, d'Athis P, Petit-Marneier C, Gaudet M. Thiamine status of elderly patients with cardiac failure including the effects of supplements. Int J Vitam Nutr Res. 1994;64:113–8. [PubMed] [Google Scholar]
- 87.Yue QY, Beerman B, Lindstrom B, Nyquist O. No difference in blood thiamine diphosphate levels between Swedish Caucasians patients with congestive heart failure treated with furosemide and patients without heart failure. J Intern Med. 1997;242:491–5. doi: 10.1111/j.1365-2796.1997.tb00022.x. [DOI] [PubMed] [Google Scholar]
- 88.Mendoza CE, Rodriguez F, Rosenberg DG. Reversal of refractory congestive heart failure after thiamine supplementation: a case report and review of the literature. J Cardiovasc Pharmacol Ther. 2003;8:313–6. doi: 10.1177/107424840300800409. [DOI] [PubMed] [Google Scholar]
- 89.Arsenian MA. Carnitine and its derivatives in cardiovascular disease. Prog Cardiovasc Dis. 1997;40:265–286. doi: 10.1016/s0033-0620(97)80037-0. [DOI] [PubMed] [Google Scholar]
- 90.Schonekess BO, Allard MF, Lopaschuk GD. Propionyl L-carnitine improvement of hypertrophied heart function is accompanied by an increase in carbohydrate oxidation. Circ Res. 1995;77:726–734. doi: 10.1161/01.res.77.4.726. [DOI] [PubMed] [Google Scholar]
- 91.Engel AG, Rebouche CJ. Carnitine metabolism and inborn errors. J Inherit Metab Dis. 1984;7 1:38–43. doi: 10.1007/BF03047372. [DOI] [PubMed] [Google Scholar]
- 92.Pepine CJ. The therapeutic potential of carnitine in cardiovascular disorders. Clin Ther. 1991;13:2–18. [PubMed] [Google Scholar]
- 93.Whitmer JT. L-carnitine treatment improves cardiac performance and restores high-phosphate pools in cardiomyopathic Syrian hamster. Circ Res. 1987;61:396–408. doi: 10.1161/01.res.61.3.396. [DOI] [PubMed] [Google Scholar]
- 94.Iliceto S, Scrutinio D, Bruzzi P, et al. Effects of L-carnitine administration on left ventricular remodeling after acute anterior myocardial infarction: the L-Carnitine Ecocardiografia Digitalizzata Infarto Miocardico (CEDIM) Trial. Am Coll Cardiol. 1995;26:380–387. doi: 10.1016/0735-1097(95)80010-e. [DOI] [PubMed] [Google Scholar]
- 95.Risos I. Three-year survival of patients with heart failure caused by dilated cardiomyopathy and L-carnitine administration. Am Heart J. 2000;139:S120–3. doi: 10.1067/mhj.2000.103917. [DOI] [PubMed] [Google Scholar]
- 96.Nascimben L, Ingwall JS, Pauletto P, Friedrich J, Gwathmey JK, Saks V, Pessina AC, Allen PD. Creatine kinase system in failing and nonfailing human myocardium. Circulation. 1996;94:1894–901. doi: 10.1161/01.cir.94.8.1894. [DOI] [PubMed] [Google Scholar]
- 97.Neubauer S, Horn M, Cramer M, Harre K, Newell JB, Peters W, Pabst T, Ertle G, Hahn D, Ingwall JS, Kochsiek K. Myocardial phosphocreatine-to-ATP ratio is a predictor of mortality in patients with dilated cardiomyopathy. Circulation. 1997;96:2190–6. doi: 10.1161/01.cir.96.7.2190. [DOI] [PubMed] [Google Scholar]
- 98.Pulido SM, Passaquin AC, Leijendekker WJ, et al. Creatine supplementation improves intracellular Ca2+ handling and survival in mdx skeletal muscle cells. FEBS Lett. 1998;439:357–362. doi: 10.1016/s0014-5793(98)01399-4. [DOI] [PubMed] [Google Scholar]
- 99.Balsom PD, Soderlund K, Ekblom B. Creatine in humans with special reference to creatine supplementation. Sports Med. 1994;18:268–280. doi: 10.2165/00007256-199418040-00005. [DOI] [PubMed] [Google Scholar]
- 100.Casey A, Constantin-Teodosiu D, Howell S, et al. Creatine ingestion favorably affects performance and muscle metabolism during maximal exercise in humans. Am J Physiol. 1996;271:E31–E37. doi: 10.1152/ajpendo.1996.271.1.E31. [DOI] [PubMed] [Google Scholar]
- 101.Andrews R, Grenhaff P, Curtis S, Perry A, Cowley AJ. The effect of dietary creatine supplementation on skeletal muscle metabolism in congestive heart failure. Eur Heart J. 1998;19:617–622. doi: 10.1053/euhj.1997.0767. [DOI] [PubMed] [Google Scholar]
- 102.Weisshaar RE, Simpson RU. Involvement of vitamin D3 with cardiovascular function. Direct and indirect effects. Am J Physiol. 1987;253:E675–83. doi: 10.1152/ajpendo.1987.253.6.E675. [DOI] [PubMed] [Google Scholar]
- 103.Witte KK, Desilva R, Chattopadhyay S, Ghosh J, Cleland JG, Clark AL. Are hematinic deficiencies the cause of anemia in chronic heart failure? Am Heart J. 2004 May;147:924–30. doi: 10.1016/j.ahj.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 104.Gorelik O, Almoznino-Sarafian D, Feder I, Wachsman O, Alon I, Litvinjuk V, Roshovsky M, Modai D, Cohen N. Dietary intake of various nutrients in older patients with congestive heart failure. Cardiology. 2003;99:177–81. doi: 10.1159/000071246. [DOI] [PubMed] [Google Scholar]
- 105.Andersson SE, Edvinsson ML, Edvinsson L. Reduction of homocysteine in elderly with heart failure improved vascular function and blood pressure control but did not affect inflammatory activity. Basic & Clinical Pharmacology & Toxicology. 2005;97:306–10. doi: 10.1111/j.1742-7843.2005.pto_146.x. [DOI] [PubMed] [Google Scholar]
- 106.Andersson SE, Edvinsson ML, Edvinsson L. Reduction of homocysteine in elderly with heart failure improved vascular function and blood pressure control but did not affect inflammatory activity. Basic Clin Pharmacol Toxicol. 2005;97:306–10. doi: 10.1111/j.1742-7843.2005.pto_146.x. [DOI] [PubMed] [Google Scholar]
- 107.Wester PO, Dyckner T. Intracellular electrolytes in cardiac failure. Acta Med Scand Suppl. 1986;707:33–6. doi: 10.1111/j.0954-6820.1986.tb18112.x. [DOI] [PubMed] [Google Scholar]
- 108.Milionis HJ, Alexandrides GE, Liberopoulos EN, Bairaktari ET, Goudevenos J, Elisaf MS. Hypomagnesemia and concurrent acid-base and electrolyte abnormalities in patients with congestive heart failure. Eur J Heart Fail. 2002;4:167–73. doi: 10.1016/s1388-9842(01)00234-3. [DOI] [PubMed] [Google Scholar]
- 109.Cohen N, Almoznino-Sarafian D, Zaidenstein R, Alon I, Gorelik O, Shteinshnaider M, Chachashvily S, Averbukh Z, Golik A, Chen-Levy Z, Modai D. Serum magnesium aberrations in furosemide (frusemide) treated patients with congestive heart failure: pathophysiological correlates and prognostic evaluation. Heart. 2003;89:411–16. doi: 10.1136/heart.89.4.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Haigney MC, Berger R, Schulman S, Gerstenblith G, Tunin C, Silver B, Silverman HS, Tomaselli G, Calkins H. Tissue magnesium levels and the arrhythmic substrate in humans. J Cardiovasc Electrophysiol. 1997;8:980–6. doi: 10.1111/j.1540-8167.1997.tb00620.x. [DOI] [PubMed] [Google Scholar]
- 111.Gottlieb SS, Baruch L, Kukin ML, et al. Prognostic importance of the serum magnesium concentration in patients with congestive heart failure. J Am Coll Cardiol. 1990;16:827–31. doi: 10.1016/s0735-1097(10)80329-8. [DOI] [PubMed] [Google Scholar]
- 112.Eichorn EJ, Tandon PK, Di Bianco R. Clinical and prognostic significance of serum magnesium concentration in patients with severe CCF: the PROMISE study. J Am Coll Cardiol. 1993;21:634–40. doi: 10.1016/0735-1097(93)90095-i. [DOI] [PubMed] [Google Scholar]
- 113.Wester PO. Electrolyte balance in heart failure and the role for magnesium ions. Am J Cardiol. 1992;70:44–9C. doi: 10.1016/0002-9149(92)91357-a. [DOI] [PubMed] [Google Scholar]
- 114.Gottlieb SS. Importance of magnesium in congestive heart failure. Am J Cardiol. 1989;63:39–42G. doi: 10.1016/0002-9149(89)90218-x. [DOI] [PubMed] [Google Scholar]
- 115.Strassburger M, Bloch W, Sulyok S, Schuller J, Keist AF, Schmidt A, Wenk J, Peters T, Wlaschek M, Krieg T, Hafner M, Kumin A, Werner S, Muller W, Scharffetter-Kochanek K. Heterozygous deficiency of manganese superoxide dismutase results in severe lipid peroxidation and spontaneous apoptosis in murine myocardium in vivo. Free Radic Biol Med. 2005;38:1458–70. doi: 10.1016/j.freeradbiomed.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 116.Golik A, Zaidenstein R, Dishi V, Blatt A, Cohen N, Cotter G, Berman S, Weissgarten J. Effects of captopril and enalapril on zinc metabolism in hypertensive patients. J Am Coll Nutr. 1998;17:75–8. doi: 10.1080/07315724.1998.10720459. [DOI] [PubMed] [Google Scholar]
- 117.Koren-Michowitz M, Dishy V, Zaidenstein R, Yona O, Berman S, Weissgarten J, Golik A. The effect of losartan and losartan/hydrochlorothiazide fixed-combination on magnesium, zinc, and nitric oxide metabolism in hypertensive patients: a prospective open-label study. Am J Hypertens. 2005;18:358–63. doi: 10.1016/j.amjhyper.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 118.Arthur JR, Nicol F, Mitchell JH, et al. Selenium and iodine deficiencies and selenoprotein function. Biomed Environ Sci. 1997;10:129–35. [PubMed] [Google Scholar]
- 119.Levander OA. A global view of human selenium nutrition. Annu Rev Nutr. 1987;7:227–50. doi: 10.1146/annurev.nu.07.070187.001303. [DOI] [PubMed] [Google Scholar]
- 120.Schnabel R, lackner KJ, Rupprecht HJ, et al. Glutathione peroxidase 1 activity and homocysteine for cardiovascular risk prediction. Results from the Athero Gene Study. J Am Coll Cardiol. 2005;45:1631–7. doi: 10.1016/j.jacc.2005.02.053. [DOI] [PubMed] [Google Scholar]
- 121.Wu HY, Xia YM, Ha PC, Chen XS. Changes in myocardial thyroid hormone metabolism and alpha-glycerophosphate activity in rats deficient in iodine and selenium. Br J Nutr. 1997;78:671–6. doi: 10.1079/bjn19970182. [DOI] [PubMed] [Google Scholar]
- 122.Blankenberg S, Rupprecht HJ, Bickel C, et al. Glutathione peroxidase 1 activity and cardiovascular events in patients with coronary artery disease. N Engl J Med. 2003;349:1605–13. doi: 10.1056/NEJMoa030535. [DOI] [PubMed] [Google Scholar]
- 123.Reeves WC, Marchuard SP, Willis SE, et al. Reversible cardiomyopathy due to selenium deficiency. J Parent Enter Nutr. 1989;13:663–5. doi: 10.1177/0148607189013006663. [DOI] [PubMed] [Google Scholar]
- 124.Kien CL, Ganther HE. Manifestations of chronic selenium deficiency in a child receiving total parenteral nutrition. Am J Clin Nutr. 1983;37:319–21. doi: 10.1093/ajcn/37.2.319. [DOI] [PubMed] [Google Scholar]
- 125.Gramm HJ, Kopf A, Brätter P. The necessity of selenium substitution in total parenteral nutrition and artificial alimentation. J Trace Elem Med Biol. 1995;9:1–12. doi: 10.1016/S0946-672X(11)80002-7. [DOI] [PubMed] [Google Scholar]
- 126.Darling G, Mathias P, Oregan M, et al. Serum selenium levels in individuals on PKU diets. J Inherit Metab Dis. 1991;87:339. doi: 10.1007/BF01800019. [DOI] [PubMed] [Google Scholar]