Abstract
The purpose of this study was to evaluate the reliability of previously published findings on hand preferences in chimpanzees by evaluating hand use in a second colony of captive chimpanzees. We assessed hand preferences for a coordinated bimanual task in 116 chimpanzees (Pan troglodytes) at the University of Texas M. D. Anderson Cancer Center and compared them to previously published findings in captive chimpanzees at the Yerkes National Primate Research Center. The new sample showed significant population-level right handedness, which is consistent with previously published findings in the Yerkes chimpanzees. Combined data on the 2 chimpanzee colonies, revealed a significant effect of rearing history on hand preference, with wild-caught chimpanzees showing less right-handedness than captive-born mother-reared chimpanzees. We discuss the results in terms of the role of early environment on the development of laterality.
Keywords: laterality, chimpanzees, handedness, rearing effects, replication
The study of handedness in nonhuman primate has been a topic of renewed interest over the past 15 years. In contrast to historical views, several recent reviewers have argued that population-level handedness is evident in some species of nonhuman primates for specific tasks (Bradshaw and Rogers, 1993; Fagot and Vauclair, 1991; MacNeilage et al., 1987; Ward and Hopkins, 1993). Population-level handedness refers to the extent that a significant proportion of a sample population shows a specific directional hand preference for a task or set of tasks. The bulk of studies on handedness in nonhuman primates have focused on establishing whether a given species shows population-level handedness and, to a lesser extent, what effect certain postural or subject characteristics, e.g., sex or age, may have on the expression of hand use. One important factor in considering whether nonhuman primates exhibit population-level handedness is the extent to which effects are replicable across different laboratories and research settings, including comparisons of captive and wild subjects.
Of specific interest to our study is the extent to which population-level handedness might be present in a second sample of captive chimpanzees tested on a measure of hand use identical to one previously employed in another population of chimpanzees. With specific reference to chimpanzees, evidence of population-level handedness has largely come from the chimpanzees housed at the Yerkes National Primate Research Center (YERKES) (Hopkins and Pearson, 2000). Population-level right handedness occurs in the YERKES colony for behaviors such as throwing (Hopkins et al., 1993), bipedal reaching (Hopkins, 1993), bimanual feeding (Hopkins, 1994) and coordinated bimanual actions (Hopkins, 1995; Hopkins et al., 2001; Hopkins and Cantalupo, in press). Attempts to study similar kinds of motor behaviors in other chimpanzee samples have not always confirmed the findings in the YERKES colony, though the trends or patterns of results are comparable, particularly for adult ape subjects (Colell et al., 1995a, 1995b). In comparing findings between wild and captive populations, there is little consistency in results. In wild chimpanzees, there is little, if any, evidence of population-level handedness for tool-use (Boesch, 1991; McGrew and Marchant, 1991; Sugiyama et al., 1993) and spontaneous behaviors (Marchant and McGrew, 2001; McGrew and Marchant, 1996). The lack of consistent findings between captive and wild chimpanzees has led some to suggest that the results reported in the YERKES colony are due to the apes being reared in a right-handed human environment and this in someway has biased their hand use (McGrew and Marchant, 1997). In terms of the lack of consistent findings between captive settings, Palmer (2002) suggested that the reporting or recording of results in the YERKES colony might be biased due to inherent knowledge of the experimenters of these hypotheses. Palmer (2002) also suggested that the number of observations used to record hand use are not balanced and biases the effects toward population-level right-handedness in the YERKES chimpanzees.
In response to the criticism that the number of responses influences the representation of hand use, Hopkins and Cantalupo (in press) have shown that collecting the same number of responses for each subject had no effect on the expression of hand use for a coordinated bimanual use task in chimpanzees. Hopkins and Cantalupo (in press) have further argued that one important difference between the studies involving the YERKES chimpanzees in comparison to other studies in captive chimpanzees is sample size. Nearly all studies of the YERKES chimpanzees have had relatively large sample sizes (N >100) compared to other studies (N < 50). The larger sample sizes in the YERKES colony provide for more statistical power, which may account for the detection of significant population-level effects (Hopkins, 1999). Lastly, there is no evidence of differential hand use in the YERKES colony for any of the above stated measures when comparing apes reared by humans contrasted with those raised by chimpanzees. If raising the apes in a human environment were the potential explanation for the observed population-level right handedness, it would be predicted that apes reared by humans should be more right-handed than those raised by chimpanzees.
The purpose of our study was to address empirically the question of handedness replication in a separate population of captive chimpanzees. Specifically, we sought to investigate the distribution of hand preference for a coordinated bimanual task in a comparable sample size of chimpanzees housed at the Department of Veterinary Sciences of The University of Texas M. D. Anderson Cancer Center in Bastrop, TX (BASTROP) for comparison with the findings reported in the YERKES colony. If hand preferences are consistent between sample populations then comparable results should be found in the BASTROP sample. Moreover, the effects of differential rearing on the expression of handedness should be comparable between chimpanzee samples.
METHOD
Subjects and Housing
The BASTROP subjects are 116 chimpanzees housed at the Department of Veterinary Sciences of The University of Texas M. D. Anderson Cancer Center The comprise 63 females and 53 males ranging from 6 to 40 years old (Mean = 21.07 yrs, s.d. = 10.35). The chimpanzees were housed in a variety of social and physical settings including pairs in indoor-outdoor runs; small, mixed-sex groups (n = 3 –7) in Primadomes; and, large, multimale–multifemale groups (n = 8 –16) in enriched, outdoor corrals. The corrals are 22 m in diameter and have grass as ground cover, climbing structures and other movable enrichment objects, and each one has an indoor area (Riddle et al., 1982). Primadomes are 10.7m in diameter and contain either grass, sand, or aspen chips as ground cover, and climbing structures and enrichment objects that are similar to those in the corrals. The indoor areas for Primadome-housed individuals are conventional indoor/outdoor runs totaling 2 × 4 × 6.1 × 2.4 m. Indoor/outdoor runs have concrete floors, raised resting boards, barred ceilings, some movable enrichment objects, and cinder-block walls.
Of the 63 female subjects, 25 were mother-reared, 11 were nursery-reared, 25 were wild-caught, and the rearing histories of the remaining 2 are unclear. Among the 53 male subjects, 27 were mother-reared, 8 were nursery-reared, 14 were wild caught, and 4 have unclear rearing histories. Mother-reared chimpanzees were reared by their biological mothers for >30 days. Nursery-reared subjects were brought to and raised in the nursery before they reached 31 days of age. Wild caught chimpanzees were captured in Africa. In order to simplify data analysis, chimpanzees with unknown rearing histories were grouped with the wild-caught apes since they were likely to be wild-caught, but we could not confirm their origin.
Procedure
We assessed hand preference via a task designed to elicit coordinated bimanual actions, referred to as the tube task. (Hopkins, 1995; Hopkins et al., 2001). Briefly, we smeared peanut butter on the inside edges of poly-vinyl-chloride (PVC) tubes ca. 25 cm long and 2.5 cm in diameter. The peanut butter was on both ends of the PVC pipe and was enough down the tube so that the chimpanzees could not lick the contents completely off, but instead had to use their fingers to extract it. We handed PVC tubes to the BASTROP subjects in their intact social groups in their home enclosures and used focal sampling to collect data from individuals. We recorded the hand (right or left) and finger (1–5) used to extract the peanut butter for each probe sequence—(finger in, finger out, finger licked)—by the experimenter. We collected data until the subjects either 1) dropped the tube, 2) stopped extracting peanut butter for 10 sec, or 3) returned the PVC tube to the experimenter. The 10-sec limit did not include instances in which the subjects were locomoting with the PVC pipe. We specifically applied this time limit when the chimpanzee had the tube in hand, was stationary, and was not attempting to feed, usually due to the absence of any remaining peanut butter.
We tested each BASTROP subject on ≥ 2 occasions following the general procedures described by Hopkins et al. 2001. Specifically, for one test, the subject was required to take the tube with its left hand. For the remaining test, the subject was required to take the tube with the right hand. The order of presentation of the tubes to either the left or right hand was randomized across subjects. If, at the end of the 2 test sessions, ≥30 responses were not performed by a subject, which occurred for 15 subjects, we performed 2 additional tests with the hand used to take the tube balanced across the two tests. Testing occurred in the outdoor portion of the runs, the Primadomes, and the corrals, and no individual was were separated for testing purposes. We conducted test sessions ca. twice per week to prevent habituation to the task. Most subjects attained the minimum criterion of 30 responses in the first 2 test sessions. We retested the 15 chimpanzees that required additional testing sessions to reach the minimum score criterion ≤3 mo of their initial sessions.
We recorded hand use while removing the peanut butter in 2 ways. First, we recorded bouts of right and left hand use. We separated bouts of hand use by any event that would result in a potential change in the use of one hand or the other. We separated bouts by either the chimpanzee’s movement to a different area to resume feeding or when the subject rotated the tube in order to access the peanut butter from the opposite end. We only recorded a bout change when the tube was physically rotated and not when a subject simply rotated its wrist in order to access the peanut butter in the tube. In addition to bouts, we also recorded the frequency of hand use each time they removed peanut butter from the tube. Each time the chimpanzee reached into the tube with a finger, extracted peanut butter and brought it to mouth, we recorded the hand used as left or right.
Data Analysis
We characterized hand preferences several different ways. First, we calculated a bout handedness index (BHI) for each of the 2 test sessions (BHI1, BHI2) as well as for the overall number of bouts (SUM-BHI) by subtracting the number of left bouts from the number of right bouts and dividing by the total number of bouts. Second, as with the bout data, we calculated a frequency handedness index (FHI) for each of the two test sessions (FHI1, FHI2) as well as for the overall frequency (SUM-FHI) via the same overall formula. Third, we calculated mean handedness indices (MEAN-FHI, MEAN-BHI) by averaging the FHI1, FHI2 and the BHI1, BHI2 scores, respectively, in order to rule out the possibility that different frequencies from one test session to the other did not skew individual handedness and one direction or another. Fourth, based on the total left and right hand frequencies, we used z-scores to evaluate whether the hand preferences of individual subjects deviated significantly from chance. This is the procedure most frequently used in nonhuman primate studies (Hopkins, 1999). We classified subjects with z-scores >1.96 or <–1.96 as right- and left-handed, respectively. We classified all other subjects as ambiguously-handed. For all analyses, α is p < .05. For all handedness indices, positive values reflect righthand biases and negative values reflect left hand biases. The absolute value of the handedness score reflects the magnitude of hand preference.
RESULTS
Reliability in Hand Use
We calculated handedness indices for the bouts and frequencies of hand use for the first and second test sessions as well as for the overall use in order to assess consistency in hand preference. For both bouts (r = .520, df = 111, p < .01) and frequencies (r = .533, df = 111, p < .01), the correlations are positive and significant, indicating that hand use for bouts and frequencies are consistent across test sessions in the BASTROP chimpanzees. Moreover, the handedness index for the overall bouts and frequencies are positive and significantly correlated (r = .925, df = 111, p < .01) indicating that the two different levels of analysis were measuring the same degree of hand use.
Population-Level Handedness
The mean handedness index scores and associated t-test values for the SUM-FHI, SUM-BHI, BHI1, BHI2, FHI1, and FHI2 are in Table I. We performed one-sample t-tests on each distribution of scores and population-level right handedness for all sets of scores. In terms of the z-scores based on the frequencies of left and right hand use, there are 31 left-handed, 33 ambiguously-handed and 52 right-handed chimpanzees, respectively in the BASTROP sample. This distribution differs significantly from chance as revealed by a chi-square goodness-of-fit test [X2(2) = 6.95, p < .03, N = 116]. Subsequent chi-square tests indicated that the number of right-handed subjects differs significantly from the number of left-handed [X2(1) = 5.31, p < .03, N = 85] and ambiguously-handed subjects [X 2(1) = 4.25, p < .03, N = 79]. Because the results are comparable across all measures (Table I), we used only the SUM-FHI scores in subsequent analyses.
Table I.
Mean handedness scores, standard errors, and t-test values
| Measure | Mean | SE | t- value | p |
|---|---|---|---|---|
| SUM-FHI | .129 | .052 | 2.44 | .016 |
| FHI1 | .125 | .063 | 1.98 | .050 |
| FHI2 | .145 | .061 | 2.39 | .018 |
| MEAN-FHI | .136 | .054 | 2.54 | .012 |
| SUM-BHI | .089 | .043 | 2.06 | .042 |
| BHI1 | .106 | .053 | 2.00 | .048 |
| BHI2 | .107 | .055 | 1.96 | .053 |
| MEAN-BHI | .107 | .045 | 2.28 | .024 |
Note. df = 115 for each analysis.
Because the number of observations contributing to each handedness index value varied across subjects, we ran a correlation between sample size and the SUM-FHI score. and found no significant association (r = −09, df = 114, n.s.). Figure 1 is a funnel plot scatter-plot depicting the association between sample size and hand use for the BASTROP sample. Palmer (2002) suggested that funnel-plots should be used to evaluate hand preference distributions in relation to sample size instead of correlation coefficients because they are more sensitive to irregularities in the data. In particular, funnel plots allow for evaluation of whether they conform to certain parameters of sampling bias in a distribution of scores. Palmer (2002) suggested that funnel plots be included in handedness studies, particularly in ones in which different numbers of observations are collected from subjects. In the funnel-graph, the distribution is relatively heterogeneous in terms of individual hand preference scores in relation to sample size (Fig. 1). Thus, the observed pattern of population-level right handedness in the BASTROP sample does not appear to be due to differing numbers of observations among individuals.
Fig. 1.
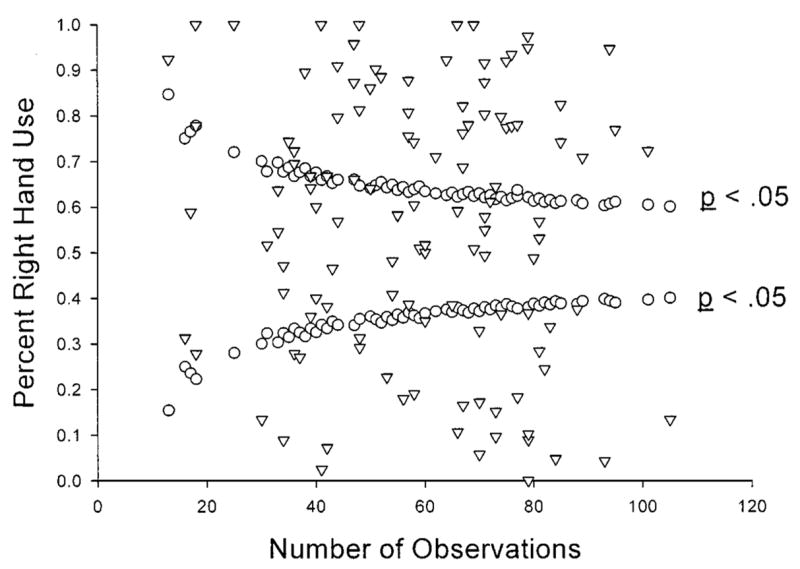
Funnel-graph of the percentage right hand use for all subjects plotted against the minimum number of responses needed to be classified as right- or left-handed based on z-scores. All data points falling within the funnel portion of the graph represent subjects classified as ambiguously-handed.
Sex and Rearing Effects
We assessed the influence of sex and rearing history on the overall handedness score via a factorial analysis of covariance with the SUM-FHI score serving as the dependent variable while sex and rearing history served as between group variables. Age was the covariate. There is no significant main effect or interaction. We performed the same analysis on the strength of hand preference. For this analysis, the absolute value of each SUM-FHI score served as the dependent measure. There is no significant main effect or interaction.
Comparison with Previous Findings in the YERKES Chimpanzees
To assess whether there are differences in the degree of handedness as a function of housing facility, we compared data from the BASTROP chimpanzees (n = 116) to findings on 110 YERKES chimpanzees on the same task via the identical procedure (Hopkins et al., 2001). We compared the samples on both direction and strength of hand preference. We performed 2 separate analyses of co-variance via the SUM-FHI and the absolute value of the SUM-FHI as the dependent measures for each analysis. Colony (BASTROP, YERKES), sex (male, female) and rearing history (mother-reared, human-reared, wild-caught) served as between group variables. Subject age served as a covariate. With respect to directional biases in hand use, there is no significant main effect or interaction involving the colony variable. However, there is a significant main effect for rearing history [F (2,213) = 4.16, p < .02]. Post hoc analysis using Tukey’s Honestly Significant Difference indicates that mother-reared chimpanzees have significantly higher SUM-FHI scores than wild-caught chimpanzees. (fig. 2). For strength of hand preference there is no significant main effect or interaction.
Fig. 2.
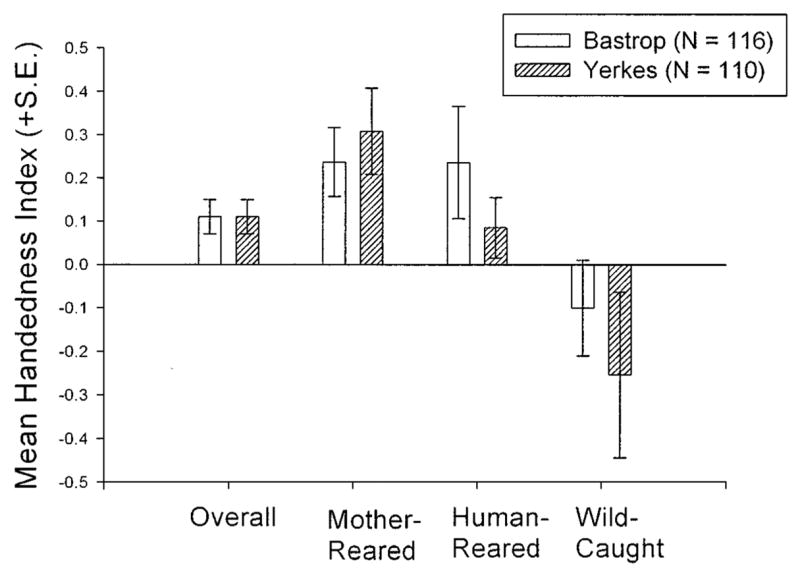
Mean SUM-FHI Scores (+s.e.) for the BASTROP and YERKES chimpanzee colonies.
DISCUSSION
Three primary findings emerged in our study. First, there is population-level right handedness in a second colony of captive chimpanzees for a coordinated bimanual action task. Second, hand use is consistent between tests, despite experimental efforts to bias hand use toward a bimodal distribution of hand use. Third, when the data were combined from the BASTROP and YERKES chimpanzees, we found significant rearing differences in hand preference, with mother-reared chimpanzees displaying significantly more right-handedness than that of wild-caught chimpanzees.
There is considerable consistency in the findings across the YERKES and BASTROP chimpanzee samples. The overall handedness scores are comparable and the general pattern of results is the same when subjects are compared by rearing condition in each colony. Our results indicate that population-level handedness in chimpanzees is not restricted to the YERKES colony, a finding that contradicts recent criticisms by Palmer (2002) and McGrew and Marchant (1997). One of the major strengths of our study derives from the fact that the same measure was employed in the YERKES and BASTROP colonies. In contrast, many other attempts to compare findings in different samples of chimpanzees have relied on different measures/procedures (Hopkins and Pearson, 2000; McGrew and Marchant, 1997). Often, comparisons among chimpanzee samples, as well as among different species, are difficult because of the use of various measures, procedures or statistical analyses (Hopkins, 1999). Our findings clearly reinforce the notion that for truly comparative investigations of handedness, comparable, if not identical, measures of hand use are needed in order to make meaningful inferences from the results.
When data from the two chimpanzee samples are combined there is a significant main effect for rearing condition, with captive-born, mother-reared chimpanzees showing significantly higher handedness scores than those of wild-caught subjects. Prima facie, our results support the general conclusion forwarded by McGrew and Marchant (1997) that population-level handedness is an artifact of chimpanzees being raised in a right-handed human environment. However, there are limitations to this interpretation that should be considered when attempting to explain the observed patterns of results. First, rearing status is, to some extent, confounded with subject age. In other words, there are no wild-caught younger subjects; all wild-caught subjects are relatively old (>23 years of age in the 2 samples). The mean age for wild-caught, captive-born mother-reared and captive-born human-reared chimpanzees is 32, 14, and 13 years, a difference that is significant F (2,224) = 185.14, p < .001. Thus, some of the effect may simply be due to age rather than rearing per se. Second, most, if not all, of the wild-caught chimpanzees were captured at a very young age (<2 yr) and therefore have been in captivity for the majority of their lives. Unless the argument can be made that chimpanzee hand preferences are fully developed by 2 years of age (Hopkins, 1994, 1995), then all of the wild-caught chimpanzees had ample opportunity to develop a right-side bias as a result of exposure to a human environment. In fact, the argument could be made that they should be more right-handed than the remaining chimpanzees because they have been exposed to human rearing and an artifactual environment for a substantially longer period of time (≥18 yrs, on average). Third, any explanation that rests on the assumption that human-rearing biases captive chimpanzees toward right-handedness must address why the mother-reared chimpanzees are more right-handed than the human-reared subjects. Common sense would suggest that the opposite pattern should emerge, since human-reared subjects had significantly higher levels of contact with human beings.
One possible explanation for the observed rearing effects is the potential relationship between perinatal stress and the development of hemispheric specialization, as manifest in behavioral and brain asymmetries. In rats, pre-and postnatal stress affects the development of behavioral and brain asymmetries (Denenberg, 1988; Tang and Verstynen, 2002). Westergaard and colleagues (2000, 2001) have reported some interesting findings concerning measures of early stress and hand preferences in monkeys. Specifically, measures of cortisol at 6 mo of age correlate with handedness at 6 and 12 mo of age in both capuchins (Westergaard et al., 2000) and rhesus monkeys (Westergaard et al., 2001). In both species, higher cortisol levels, which indicate greater stress, are associated with right hand use in reaching. It is important to emphasize that overall, the capuchins and rhesus monkeys showed population-level left handedness (Westergaard et al., 2000; 2001). Thus, higher cortisol levels were associated with nondominant, or atypical, handedness in them. From the standpoint of our results, the wild-caught subjects were all captured early in life, no doubt an extremely stressful experience for the subjects. The early stress associated with capture and subsequent maternal separation may have significantly altered the development of lateralization in them versus subjects born and raised in captivity. As has been argued elsewhere, differences between handedness findings in captive and wild populations are complex and are affected by multiple important factors. In order to make definitive comparisons between settings (Hopkins, 1999), studies that make use of procedures that bridge the methodological gap between wild and captive apes must be conducted.
In conclusion, the handedness findings in the BASTROP chimpanzee colony are consistent with those previously reported in the YERKES chimpanzees for a task measuring coordinated bimanual actions. Attempts to replicate handedness findings for the TUBE task between different captive populations of capuchins (Spinnozi et al., 1998; Westergard et al., 1996) and rhesus monkeys (Westergaard et al., 1996; Westergaard et al., 1998) has not yielded consistent results, which points to the importance of replication in handedness studies. The attempts to replicate the TUBE results in monkeys have not necessarily used the exact same procedures or subjects with comparable rearing histories, which similarly points to the importance of controlling for methodological and subject factors when comparing findings among settings. That the BASTROP and YERKES chimpanzees were tested via the same procedure, and had similar demographics in terms of subject variables, may have contributed to our positive results.
Finally, in addition to the important dimension of replication, testing additional chimpanzee colonies will collectively increase the sample sizes to levels that would allow for complex statistical analyses of various genetic and nongenetic models of handedness. Hopkins, Dahl and Pilcher (2001) conducted preliminary and largely descriptive behavioral-genetic analyses of handedness in the YERKES chimpanzees, but with increased samples sizes from other chimpanzee colonies, the potential use of more precise quantitative analyses that would provide estimates of the contributions of genetic and nongenetic factors to the expression of handedness in chimpanzees will be feasible. This would be invaluable data to test evolutionary theories that proclaim the genetic basis of handedness to be a uniquely human adaptation (Annett, 1985; Corballis, 1999; Warren, 1980).
Acknowledgments
This work was supported in part by NIH grants RR-00165, U42-RR-15090, NS-42867, NS-36605 and HD-38051. Correspondence and reprint requests should be addressed to Dr. William Hopkins, Division of Psychobiology, Yerkes National Primate Research Center, 954 Gatewood Road, Atlanta, Georgia 30322. E-mail: Lrcbh@rmy.emory.edu or whopkins@berry.edu.
References
- Annett M. Left, Right, Hand and Brain: The Right-Shift Theory. Erlbaum; London: 1985. [Google Scholar]
- Bard KA. Responsive Care: Behavioral Intervention for Nursery-Reared Chimpanzees. 1996. Available from the Jane Goodall Institute, Ridgefield, CT, 06877. [Google Scholar]
- Boesch C. Handedness in wild chimpanzees. Int J Primatol. 1991;6:541–558. [Google Scholar]
- Bradshaw J, Rogers LJ. The Evolution of Lateral Asymmetries, Language, Tool Use and Intellect. Academic Press; San Diego: 1993. [Google Scholar]
- Colell M, Segarra MD, Sabater Pi J. Manual laterality in chimpanzees (Pan troglodytes) in complex tasks. J Comp Psychol. 1995a;109:298–307. doi: 10.1037/0735-7036.109.3.298. [DOI] [PubMed] [Google Scholar]
- Colell M, Segarra MD, Sabater Pi J. Hand preferences in chimpanzees (Pan troglodytes), bonobos (Pan paniscus) and orangutans (Pongo pygmaeus) in food-reaching and other daily activities. Int J Primatol. 1995b;16:413–434. [Google Scholar]
- Corballis MC. The genetics and evolution of handedness. Psychol Rev. 1997;104:714–727. doi: 10.1037/0033-295x.104.4.714. [DOI] [PubMed] [Google Scholar]
- Denenberg VH. Laterality in animals. Brain and behavioral asymmetries and the role of early experience. In: Molfese DL, Segalowitz SJ, editors. Brain Lateralization in Children: Developmental Implications. Guilford Press; New York: 1988. pp. 59–72. [Google Scholar]
- Fagot J, Vauclair J. Manual laterality in nonhuman primates: A distinction between handedness and manual specialization. Psychol Bull. 1991;109:76–89. doi: 10.1037/0033-2909.109.1.76. [DOI] [PubMed] [Google Scholar]
- Hook-Costigan MA, Rogers LJ. Hand preferences in New World primates. Int J Comp Psychol. 1997;9:173–207. [Google Scholar]
- Hopkins WD. Posture and reaching in chimpanzees (Pan) and orangutans (Pongo) J Comp Psychol. 1993;17:162–168. doi: 10.1037/0735-7036.107.2.162. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. Hand preferences for bimanual feeding in 140 captive chimpanzees (Pan troglodytes): Rearing and ontogenetic factors. Dev Psychobiol. 1994;27:395–407. doi: 10.1002/dev.420270607. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. Hand preferences for a coordinated bimanual task in 110 chimpanzees (Pan troglodytes): Cross-sectional analysis. J Comp Psychol. 1995;105:178–190. doi: 10.1037/0735-7036.109.3.291. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. On the other hand: Statistical issues in the assessment and interpretation of hand preference data in nonhuman primates. Int J Primatol. 1999;20:851–866. [Google Scholar]
- Hopkins WD, Pearson K. Chimpanzee (Pan troglodytes) handedness: Variability across multiple measures of hand use. J Comp Psychol. 2000;114:126–135. doi: 10.1037/0735-7036.114.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Cantalupo C. Does variation in sample size explain individual differences in hand preferences of chimpanzees (Pan troglodytes)? An empirical study and reply to Palmer (2002) Am J Phys Anthropol. doi: 10.1002/ajpa.10170. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Dahl JF, Pilcher D. Genetic influence on the expression of hand preferences in chimpanzees (Pan troglodytes): Evidence in support of the right shift theory and developmental instability. Psychol Sci. 2001;12:299–303. doi: 10.1111/1467-9280.00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Bard KA, Jones A, Bales S. Chimpanzee hand preference for throwing and infant cradling: Implications for the origin of human handedness. Curr Anthropol. 1993;34:786–790. [Google Scholar]
- Hopkins WD, Fernandez-Carriba S, Wesley MJ, Hostetter A, Pilcher D, Poss S. The use of bouts and frequencies in the evaluation of hand preferences for a coordinated bimanual task in chimpanzees (Pan troglodytes): An empirical study comparing two different indices of laterality. J Comp Psychol. 2001;115:294–299. doi: 10.1037//0735-7036.115.3.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant LF, McGrew WC. Laterality of function in apes: A meta-analysis of methods. J Hum Evol. 1991;21:425–438. [Google Scholar]
- Marchant LF, McGrew WC. Laterality of limb function in wild chimpanzees of Gombe National Park: Comprehensive study of spontaneous activities. J Hum Evol. 1996;30:427–443. [Google Scholar]
- McGrew WC, Marchant LF. Chimpanzees, tools, and termites: Hand preference or handedness? Curr Anthropol. 1992;33:114–119. [Google Scholar]
- McGrew WC, Marchant LF. On the other hand: Current issues in and meta-analysis of the behavioral laterality of hand function in nonhuman primates. Yearbook Phys Anthropol. 1997;40:201–232. [Google Scholar]
- McGrew WC, Marchant LF. Ethological study of manual laterality in the chimpanzees of the Mahale mountains, Tanzania. Behaviour. 2001;138:329–358. [Google Scholar]
- MacNeilage PF, Studdert-Kennedy MG, Lindblom B. Primate handedness reconsidered. Behav Brain Sci. 1987;10:247–303. [Google Scholar]
- Palmer AR. Chimpanzee right-handedness reconsidered: Evaluating the evidence using funnel graphs. Am J Phys Anthropol. 2002;118:191–199. doi: 10.1002/ajpa.10063. [DOI] [PubMed] [Google Scholar]
- Rogers LJ, Andrews RJ. Comparative Vertebrate Lateralization. Oxford University Press; Oxford: 2002. [Google Scholar]
- Spinozzi G, Castornina MG, Truppa V. Hand preferences in unimanual and coordinated-bimanual tasks by tufted capuchin monkeys (Cebus apella) J Comp Psychol. 1998;112:183–191. [Google Scholar]
- Sugiyama Y, Fushimi T, Sakura O, Matsuzawa T. Hand preference and tool-use in wild chimpanzees. Primates. 1993;34:151–159. [Google Scholar]
- Tang AC, Verstynen T. Early life environment modulates ‘handedness’ in rats. Behav Brain Res. 2002;131:1–7. doi: 10.1016/s0166-4328(01)00330-8. [DOI] [PubMed] [Google Scholar]
- Ward JP, Hopkins WD. Primate laterality: Current behavioral evidence of primate asymmetries. Springer; New York: 1993. [Google Scholar]
- Warren JM. Handedness and laterality in humans and other animals. Physiol Psychol. 1980;8:351–359. [Google Scholar]
- Westergaard GC, Suomi SJ. Hand preference for a bimanual task in tufted capuchins (Cebus apella) and rhesus macaques (Macaca mulatta) J Comp Psychol. 1996;110:406–411. doi: 10.1037/0735-7036.110.4.406. [DOI] [PubMed] [Google Scholar]
- Westergaard GC, Champoux M, Suomi SJ. Hand preference in infant rhesus macaques (Macaca mulatta) Child Dev. 1997;68:387–393. doi: 10.1111/j.1467-8624.1997.tb01946.x. [DOI] [PubMed] [Google Scholar]
- Westergaard GC, Byrne G, Suomi SJ. Handedness and cortisol in tufted capuchin monkeys. Dev Psychobiol. 2000;36:213–217. doi: 10.1002/(sici)1098-2302(200004)36:3<213::aid-dev4>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Westergaard GC, Lussier ID, Suomi SJ. Stress correlates of hand preference in rhesus monkeys. Dev Psychobiol. 2001;38:116–122. doi: 10.1002/1098-2302(200103)38:2<110::aid-dev1003>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Westergaard GC, Champoux M, Suomi SJ. Plasma cortisol is associated with hand preference in rhesus monkeys. Dev Psychobiol. 2001;38:110–115. doi: 10.1002/1098-2302(200103)38:2<116::aid-dev1004>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]


