Abstract
Social recognition memory underlies many forms of rodent interaction and can be easily tested in the laboratory. Sex differences in aspects of this memory have been reported among young adults, and some studies indicate an age-related decline among male rats. In contrast, neither the impact of natural fluctuations in ovarian hormones nor the performance of aged female rats on social recognition memory has been previously evaluated. In experiments 1 and 2, the social recognition memory of young adult female Long-Evans rats (age 3-5 mos.) was compared during proestrus and estrus, and performance was found to be stable across estrous cycle phases. In experiment 3, the social recognition memory of young adults as compared to aged (16.5-19.5 mos.) rats was tested using the social discrimination procedure, following delays of 15, 45, 90 or 120 minutes. The estropausal status of aged female rats was tracked during the experiment but was not found to influence memory ability. Males of both ages investigated juveniles (both novel and familiar) more than did females, although despite this difference, both sexes demonstrated robust memory. Interestingly, only young adult females were capable of demonstrating memory following the longest delay. Collectively, our findings indicate that the pattern of age-related changes in social recognition memory is subtle and that aging does not greatly alter the behavioral sex differences observed among young adults.
Keywords: social discrimination, estrous cycle, estropause, sex differences
Introduction
Social recognition memory is the ability to recognize familiar conspecifics and is critical for many forms of social interaction including reproduction, establishment of dominance hierarchies, and pair bond formation in monogamous species. In rodents, social recognition is made on the basis of chemosensory cues present in the anogenital area, which collectively compose an “olfactory signature” [1]. The social recognition ability of rodents can be observed in the laboratory by measuring the reduction in the amount of time spent investigating the same individual across repeated exposures. Tested in this way, females demonstrate sustained memory over much longer delays compared to males, despite the fact that they engage in less initial investigatory behavior [2, 3].
Behavioral differences between male and female rodents in social recognition memory rely in part on sexually dimorphic neural pathways, including the extrahypothalamic systems of the neuropeptides arginine-vasopressin and oxytocin [4, 5]. For instance, the density of vasopressinergic projections from the bed nucleus of the stria terminalis (BNST) and the medial amygdala to the lateral septum is greater in males compared to females [6]. This sex difference can be abolished by either neonatal castration of males or by neonatal administration of testosterone to females, reflecting the organizational role of androgens in this pathway [7]. Testosterone can also exert an activational effect on this system, since the density vasopressin fibers from the BNST gradually decreases following castration of adult males, and can be restored by testosterone administration [8]. Male social recognition is highly dependent on vasopressin; memory for a juvenile is blocked following administration of a vasopressin receptor antagonist [9, 10], an effect which is not observed if the male has been castrated [11]. In contrast, females' ability to demonstrate social recognition memory is unaffected by blocking of vasopressin signaling [2].
Sex differences in performance on other tests of memory, such as object recognition and spatial working memory, have been consistently reported in young adult rats [12, 13]. Interestingly, the pattern of age-related decline on some of those tests is also sexually dimorphic [14-17]. Some studies [18-21], but not all [22], have found that social recognition memory declines with age in male rats. It is not known if a gender-specific age-related decline is evident in social recognition, as the social recognition memory ability of aged male and female rats has never been compared. In fact, to our knowledge, no previous study has examined aging of social recognition memory in females rodents.
The comparison of young and aged animals of both sexes within the same study is ideal; however, because replacement estrogen to ovariectomized female rats influences some aspects of social recognition memory [23] and because certain types of memory have been found to fluctuate across the rat estrous cycle [e.g., 24, 25], it was first necessary to determine whether social recognition memory ability fluctuates across the rat estrous cycle (experiments 1 and 2). During reproductive senescence, aging female rats undergo a loss of cyclicity but (in contrast to humans) continue to maintain constant, moderate levels of ovarian hormones [26, 27]. Around 12-14 months, females enter one of two “estropausal” states; typically, they alternate between them for some months before settling in one or the other and then (if they live long enough) become anestrus. We have shown that persistent diestrus (PD) females have significantly more plasma progesterone than persistent estrus females, whereas there is a trend for plasma estrogen levels to be higher among persistent estrus (PE) females [27]. The differences in hormonal profiles between these states merited their being tracked during experiment 3.
When testing the social recognition memory of aged rodents, the standard habituation-dishabituation procedure may not be appropriate. For instance, although aged male rats appeared to show intact memory, as indicated by a reduction in investigation of a familiar conspecific across repeated exposures (habituation), they actually failed to return to high levels of investigatory behavior when a second, novel conspecific was subsequently introduced (failure to dishabituate) [18]. This issue could potentially be avoided if aged animals were tested using the social discrimination procedure, in which the familiar and novel juveniles are presented simultaneously, rather than on separate trials. The social discrimination procedure has a number of advantages: the number of trials is cut in half, each trial contains its own internal control, and the sexual and aggressive behavior of adult males during testing is greatly reduced [28]. Therefore, experiment 3 was designed to determine whether the sex differences previously observed using the standard habituation procedure are present when using the social discrimination procedure, and if so whether these would be preserved during aging or if, as with other tests of memory, there are sex differences in the pattern of aging of social recognition memory.
Experiment 1: Social recognition memory of young adult females
Methods
Subjects
Subjects were young adult female Long-Evans rats (aged 3-5 months), descended from Simonsen Laboratory (Gilroy, CA) stock. Animals were single housed in standard clear Plexiglas laboratory cages, with food and water available ad libitum. The vivarium was maintained on a 12-hr light-dark cycle with the lights on at 0700. The estrous cycle phase of the subjects was monitored across the experiment via daily vaginal lavages (always in the afternoon). Beginning at weaning (24-25 days), animals were handled weekly until experimental training began, at which time females were lavaged daily.
Vaginal Lavages
Vaginal lavages were collected daily and viewed by low power light microscopy [27, 29]. All vaginal cell samples were obtained and classified by the same investigator. Diestrus smears consisted of primarily lymphocytes, in combination with a small number of nucleated epithelial cells and cornified epithelial cells; proestrus smears consisted of primarily nucleated epithelial cells; and estrus smears consisted of primarily cornified epithelial cells.
Social Recognition Test
Adult female subjects were introduced to a juvenile (21-27 days) in their home cage and allowed to investigate it until either 5 minutes had elapsed or 30s had passed during which time the subject did not investigate the juvenile. After a 100 minute delay, the subject was presented with either the juvenile it had previously investigated (familiar juvenile) or a juvenile that it had never investigated (novel juvenile). Time spent investigating the juvenile was recorded for both the introduction and the recognition portions of the procedure. Investigation was considered to be any olfactory investigation of the juvenile by the adult subject, but consisted predominantly of olfactory investigation of the ano-genital area. In order to determine whether the sex of the juvenile influenced the adult females' behavior, 12 females were exposed to male juveniles, and a separate group of 7 females were exposed to female juveniles. In this repeated measures design, each female was tested during both proestrus and estrus, and the performance was compared within-subjects.
Statistical Analysis
Data obtained from subjects that were exposed to male juveniles and the data from subjects exposed to female juveniles were analyzed separately. A one-way repeated measures analysis of variance (ANOVA) was conducted (with estrous cycle phase run as the repeated measure) to examine the effect of estrous cycle phase on the amount of time spent investigating the male juvenile during the introduction. A 2 × 2 (cycle phase (proestrus vs. estrus) × trial type (familiar vs. novel juvenile)) repeated measures ANOVA was conducted on the amount of time spent investigating during the recognition phase of the procedure. p<.05 was considered significant.
Results
There was a trend towards females investigating the male juvenile less during proestrus than during estrus (F1,11=4.4, p<.06; proestrus: 22 +/-2.5 seconds, estrus: 33 +/-4.2 seconds). Although the juvenile males were not sexually mature, it was observed that the adult females in proestrus responded to them with both proceptive and receptive (lordosis) sexual behavior. Perhaps for this reason, adult females did not demonstrate recognition of juvenile males (F1,11=.67, p=.4). Neither was there an effect of cycle phase (F1,11=1.4, p=.3) or an interaction between trial type and cycle phase (F1,11=1.1, p=.3).
When adult females were exposed to juvenile females, they spent less time investigating on the introduction during proestrus than during estrus (F1,6=8.7, p<.03) (figure 1A). Adult females successfully demonstrated recognition memory for juvenile females; novel juveniles were investigated more than familiar (F1,6=15.9, p<.01) (figure 1B). Estrous cycle phase did not influence this memory, as there was no effect of cycle phase (F1,6=.05, p=.8) and no interaction between trial type and phase (F1,6=.005, p=.9).
Figure 1.
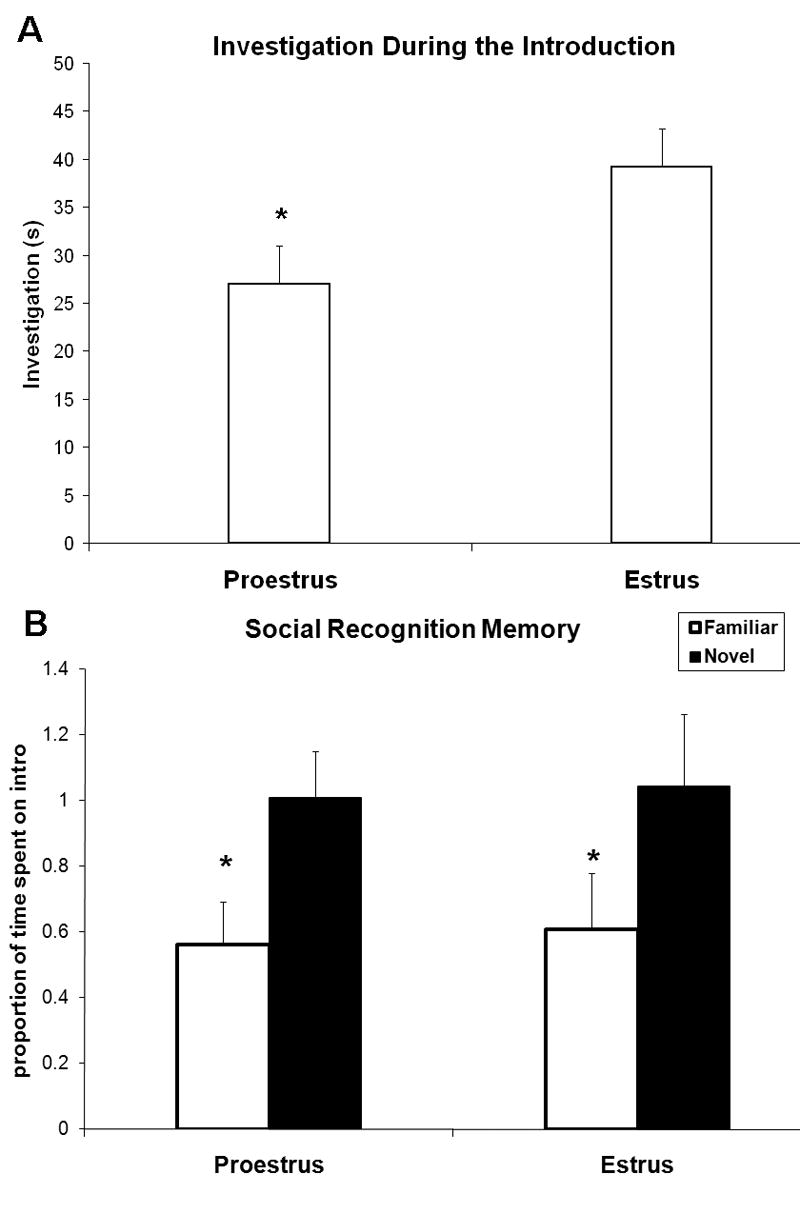
Experiment 1 (n=7). A Young adult females spent less time investigating when introduced to a juvenile female during proestrus compared to estrus (*p<.03). B. Young adult females (n=7) demonstrated equivalently robust social recognition memory of juvenile females during proestrus and estrus (*main effect of familiarity p<.01). Time spent investigating during the recognition portion is presented as a proportion of time spent investigating during the introduction.
Experiment 2: Social recognition memory of young adult females: introductory exposure held constant
The results of experiment 1 indicated that, although the degree of memory exhibited on proestrus was equivalent to that on estrus, proestrus females accomplished this after significantly less exposure to the juvenile during the introduction. One possible interpretation of this data is that social recognition memory is more efficient during proestrus compared to estrus. In the following experiment, therefore, we asked whether social recognition memory would be superior during proestrus compared to during estrus if the amount of investigation during the introduction was held constant.
Methods
Subjects
16 adult females were tested in this study. Because the results of experiment 1 showed that adult female rats do not show recognition of juvenile males in this paradigm, only juveniles females were used in this study. Housing, handling, and daily lavaging procedures were otherwise identical to experiment 1.
Social Recognition Test
Adult females were exposed to a juvenile female and were allowed 15 seconds of total investigation before being removed from the home test cage and placed in a separate cage with fresh bedding (to avoid exposure to the juvenile's scent during the delay period). It has been shown that such a cage change does not disturb the social memory of Long-Evans rats [30]. Total investigation included direct investigation of the juvenile (predominantly olfactory investigation of the ano-genital area) as well as investigation of juvenile-exposed bedding. Pilot trials indicated that, with this limited exposure on the introduction, adult females do not demonstrate memory for the familiar juvenile 100 minutes later (the delay period used in experiment 1). Therefore, recognition was tested 60 minutes following the introduction.
Statistical Analysis
A one-way repeated measures ANOVA was conducted (with estrous cycle phase run as the repeated measure) to examine the effect of estrous cycle phase on the amount of time spent investigating the juvenile during the introduction. A 2 × 2 repeated measures ANOVA (estrous phase (proestrus vs. estrus) × trial type (familiar vs. novel juvenile) was conducted on the amount of time spent investigating the juvenile during the recognition phase of the procedure. p<.05 was considered significant.
Results
Young adult females required equivalent amounts of time to achieve 15 seconds of investigation during the introduction whether in proestrus or estrus (figure 2A). Novel juveniles were investigated more than familiar (F1,15=42.8, p<.001), indicating significant social recognition memory (Figure 2B). However, there was no effect of cycle phase (F1,15=.12, p=.7) and no interaction of cycle phase and trial type (F1,15=.66, p=.4).
Figure 2.
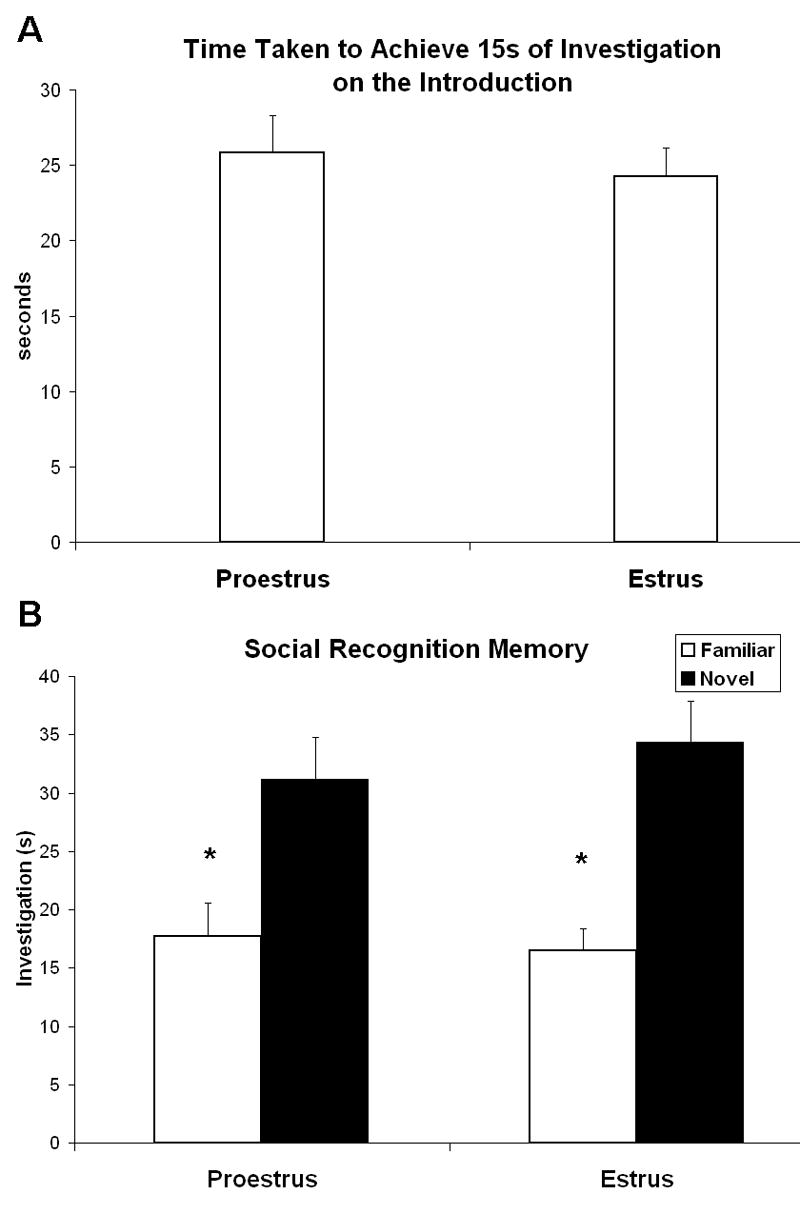
Experiment 2 (n=16). A. Estrous cycle phase did not influence the amount of time taken by young adult females to achieve 15 seconds of investigation when introduced to a juvenile female. B. When investigation of the juvenile during the introduction was held constant at 15 seconds, young adult females still demonstrated equivalently robust social recognition memory during proestrus and estrus (*main effect of familiarity p<.001).
Experiment 3: Aging of social recognition memory: influence of sex
Methods
Subjects
Subjects were 42 Long-Evans hooded rats, descended from Simonsen Laboratory (Gilroy, CA) stock, of the following sexes and ages: young adult (3-5 months) males (n=10) and females (n=10) and aged (16.5-19.5 months) males (n= 6) and females (n=16). Aged females included in the study were in one of three reproductively senescent states (determined by daily vaginal lavages, see below): persistent estrus (n= 7), persistent diestrus (n= 4), or irregular noncyclicity (n=5). Aged rats of both sexes had been used as breeders in the Department of Psychology's animal care facility (retired around 12 months of age).
Animals were housed in standard clear Plexiglas laboratory cages. All males were housed in the same room as the young females, and all aged females were housed in a separate, female-only room. Food and water were available ad libitum to all subjects, and both colony rooms were maintained on a 12-hr light-dark cycle with the lights on at 0700. All animals were handled on an average of once a week (beginning at the time of retirement from breeding for aged animals) until experimental training began, at which time females were lavaged daily and males were handled in a comparable fashion.
Vaginal Lavages
Vaginal lavages were collected daily (each afternoon) for all female subjects as in experiment 1. Among the aged females, those whose vaginal samples demonstrated a predominance of cornified cells (60% or more, as in young estrus) for a minimum of ten consecutive days were considered to be in a state of persistent estrus. Aged females whose vaginal samples demonstrated a predominance of lymphocytes mixed with a variety of other cell types (as in young diestrus), for a minimum of ten consecutive days were considered to be in a state of persistent diestrus. We have shown that females in persistent diestrus have significantly more plasma progesterone than those in persistent estrus, whereas there is a trend for plasma estrogen levels to be higher among persistent estrus females [27]. Aged females whose vaginal samples indicated an alteration between these two states were classified as irregularly noncyclic.
Social Discrimination Procedure
During the introduction, which took place in the animal's home cage, adult subjects were presented with a same-sex juvenile (21-27 days old) and were allowed to investigate until investigation had been engaged in for a total of 30s. The amount of time elapsed before reaching 30s of direct investigation during the introduction, which may indicate the subject's degree of interest in the juvenile, was recorded. At this time, the subject was removed from its cage and placed in a clean cage with fresh bedding, to ensure that access to the juvenile's scent was not possible during the delay period. After a delay (see below), the subject was simultaneously presented with the familiar juvenile (investigated during the introduction) or a novel juvenile (never before investigated). The amount of time spent investigating each juvenile was recorded for three minutes. Juveniles' coats had been colored (at least 20 minutes in advance of testing) with non-permanent, non-toxic markers, so that the experimenter could keep track of the familiar versus novel juvenile.
Animals were habituated to the social discrimination procedure on three consecutive days, with one trial (consisting of an introduction, delay (30 minutes), and discrimination) occurring each afternoon between 1-4 p.m. Testing followed a break of two days during which the animals were left undisturbed. Testing consisted of four consecutive days of training, with one trial per day (between 1-4 p.m.). However, the length of the delay was either 15, 45, 90, or 120 minutes. Each subject was tested with each of the delay intervals once, in a randomized order. Occasionally, an animal would fail to reach 30s of investigation time after three minutes had elapsed on the introduction. When this occurred, the delay and discrimination occurred as usual, but the data was excluded from the analysis and a make-up trial with the appropriate delay interval between introduction and discrimination was conducted at the end of testing. All behavioral procedures were videotaped.
Statistical Analysis
A 2 × 2 (age × sex) ANOVA was conducted on the average amount of time elapsed before reaching 30s of investigation during the introduction on the 4 test days. A 2 × 4 × 2 × 2 mixed measures ANOVA (discrimination (familiar vs. novel juvenile) × delay (15, 45, 90, 120) × age (young adult vs. aged) × sex (male vs. female) was conducted on the amount of time spent investigating during the three minutes of discrimination. In this analysis, discrimination and delay were run as within-subjects (repeated) factors and sex and age as between-subjects factors. (Aged females in persistent estrus, persistent diestrus, and those irregularly cycling were combined into a single group after determining that estropausal state did not influence any dependent variable, nor did it interact with any other independent variable). Post hoc analyses including planned comparisons were conducted using the least significant difference (LSD) method. All analyses were conducted using SPSS statistical software, and p<.05 was considered significant.
Results
Introduction
Both sex and age influenced the amount of time taken by the subjects to obtain 30s of direct investigation during the introduction (figure 3A). Females took longer than males (F1,164=29.1, p<.001), reflecting more and/or longer pauses between bouts of investigation than males. This sex difference was present among both young adults (young females > young males, p<.001) and aged rats (aged males > aged females, p<.01). There was a trend for young adult animals to obtain 30s of investigation faster than aged animals (F1,164=3.6, p<.06). This was primarily due to the significantly less amount of time required by young males when compared to aged males (p<.04), as there was no difference on this measure between young females and aged females (p=.73).
Figure 3.
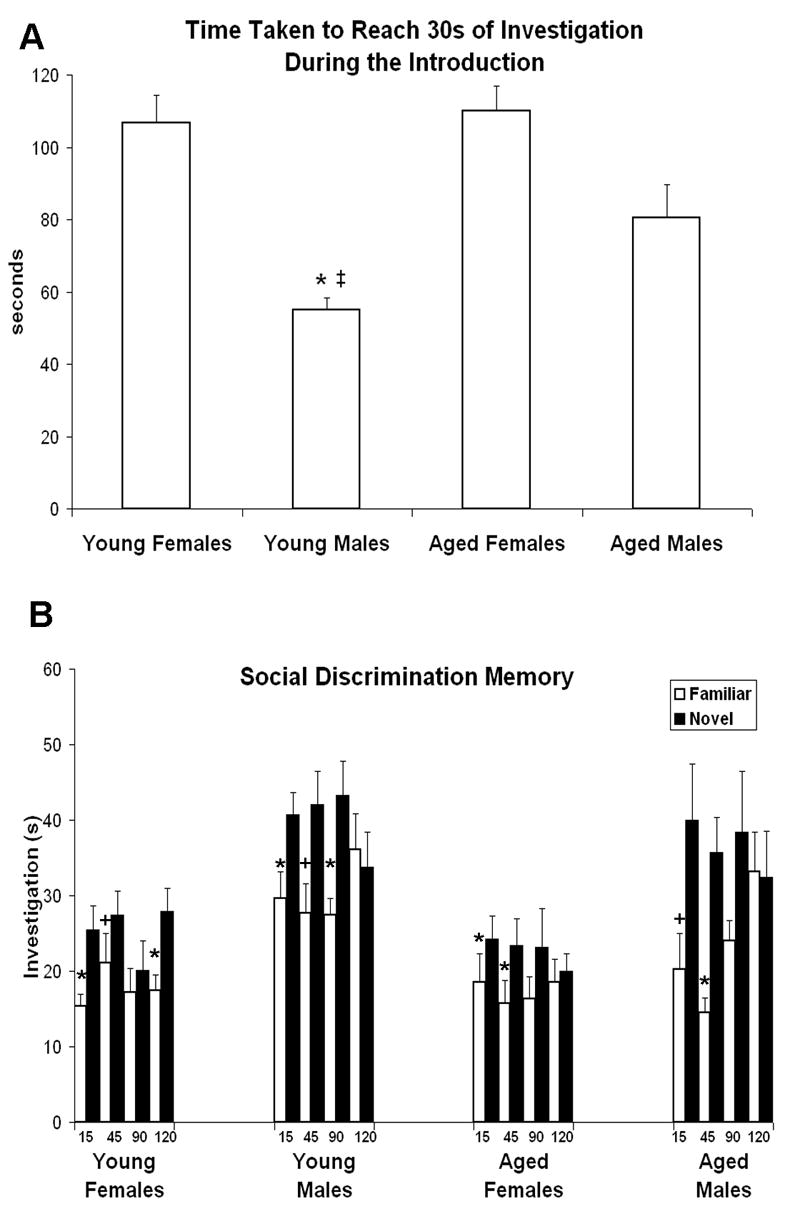
Experiment 3 (n=42; young adult males (n=10) and females (n=10), aged males (n=6) and females n=12). A. Both sex and age influenced the amount of time taken by animals to obtain 30 seconds of investigation when introduced to a juvenile of the same sex: females took longer than males (p<.001) and aged animals took longer than young adults (p<.06). * males<females of the same age (p<.01 for both). ‡ young males < aged males (p<.04). B. Social recognition memory of young adult and aged animals of both sexes, as tested by the social discrimination procedure (main effect of familiarity p<.001). Males spent more time investigating juveniles (both novel and familiar) than did females, regardless of age (main effect of sex p<.001). Discrimination between novel and familiar juveniles worsened with longer delays (main effect of delay p<.01). The ability to discriminate interacted with both delay length and sex (p<.02), with all groups capable of discriminating at the shortest delay but only young adult females able to discriminate at the longest delay (see text for details). *over white bars indicates significant novel>familiar comparison following the indicated delay. +over white bars indicates a statistical trend towards novel>familiar comparison following the indicated delay.
Discrimination
Overall, subjects discriminated between the novel and familiar juveniles, as indicated by a greater amount of time spent investigating the novel juvenile (F1,114=40.9, p<.001) (figure 3B). As expected, discrimination ability and delay length interacted, with discrimination worsening with longer delays (F3,114=4.3, p<.01). There was a main effect of sex, with males spending more time investigating juveniles (both novel and familiar) than females (F1,38=24.8, p<.001), whereas age did not significantly impact the amount of time spent investigating juveniles (F1,38=2.1, p=.16). There was a trend towards an interaction between discrimination ability and sex (F1,38=3.5, p<.07), and a significant three-way interaction between discrimination ability, length of delay, and sex (F3,114=3.6, p<.02).
In order to further explore the data and these interactions, a separate 2 × 4 ANOVA (discrimination (familiar vs. novel juvenile) × delay (15, 45, 90, 120)) was conducted for each of the four groups. All groups demonstrated the ability to discriminate between novel and familiar juveniles (young adult females, F1,9=8.6, p<.02; young adult males F1,9=8.9, p<.02; aged females F1,15=8.7, p<.01; aged males F1,5=11.3, p<.02). Planned comparisons revealed that after a 15 minute delay, all groups spent more time investigating the novel compared to the familiar juvenile (young females p<.021, young males p<.035, aged females p<.01, aged males p<.07). After a 45 minute delay, there was a trend towards significant discrimination for young females and males (p<.09 for both), an effect which reached significance for both aged females (p<.02) and aged males (p<.01). The direction of the means was the same for all four groups after 90 minutes, although the effect only reached significance for young adult males (p<.02). After 120 minutes, only young females discriminated (p<.001), whereas the other three groups spent an equal amount of time investigating the novel and the familiar juvenile.
Discussion
Experiments 1 and 2
The results of experiments 1 and 2 indicate that social recognition memory remains stable across the female rat estrous cycle. In experiment 1, the degree of memory exhibited was similar on proestrus and estrus, although the amount of introductory investigation of juveniles was less on proestrus. This raised the possibility that social recognition memory is more efficient during proestrus as compared to estrus and that, if the amount of introductory investigation was held constant across all trials, the subsequent memory ability of females might be shown to be superior on proestrus relative to estrus. This hypothesis was not supported by the results of experiment 2, in which introductory investigation time was limited to 15s for each trial but there was again no difference in memory as a result of cycle phase.
A separate point concerning the relevance of the estrous cycle to performance on this task is that when testing adult female rats using a social recognition memory paradigm, female juveniles must be used as stimulus conspecifics. In experiment 1, exposure to sexually immature, weanling age juvenile males (age 21-27 days) induced proestrus females to engage in sexual behaviors, including in some instances lordosis. During the recognition portion of the trial, adult females continued to investigate both novel and familiar male juveniles at the level of introductory investigation. As a result, the ability of this test to detect memory for the male juvenile was compromised. Therefore, in experiments 2 and 3, adults were always presented with same-sex juveniles.
Experiment 3
Our findings show that male rats investigate juveniles (both novel and familiar) more than females when tested using the social discrimination procedure, confirming a sex difference in either interest or motivational factors that has been reported for the standard test of social recognition memory [2, 3]. This sex difference is known to rely on circulating testosterone—males castrated as adults behave similarly to intact females in this regard, and testosterone administration to either castrated males or adult females results in levels of investigation seen in intact males [3, 31]. Additionally, we have established that young adult females maintain the ability to discriminate between novel and familiar juveniles for longer intervals than do young adult males, just as they do in the standard social recognition memory paradigm [2].
Aged subjects of both sexes were competent at discriminating between novel and familiar juveniles following the shorter delays. One obvious difference between the present study and earlier ones, some of which have reported age-related impairments in this type of memory for males, is the use of the social discrimination procedure. Using the standard testing procedure, others have found that aged males successfully habituated to a familiar stimulus animal but then subsequently failed to dishabituate to a novel one [18]. By presenting both stimulus animals simultaneously, it may be that the subject's preference for the novel over the familiar animal is more easily demonstrated. Alternatively, other methodological differences between studies, such as the strain and age of the subjects, could also be contributing to discrepancies in findings. One group that used Wistar rats found that social recognition memory was impaired beginning at age twelve months [19, 20]. Puzzlingly, another study found that habituation to a familiar juvenile is greater in seven and eleven month old male Wistar rats compared to three month old adults [22]. The only study apart from the present to use Long-Evans hooded rats found, as we have, that social memory was largely preserved during aging in male rats of this strain (although it was more susceptible to the disruptive effects of interference) [32].
Olfactory deficits are among the earliest symptoms of age-related neurodegenerative disorders, including Alzheimer's disease, Parkinson's disease, and Huntington's chorea; in fact, olfactory dysfunction often appears in preclinical individuals and is therefore being advanced as a predictive measure for neurodegenerative diseases [33, 34]. Age-related changes in rat olfaction have not been well studied and the few published studies have used only male subjects. Some have found that non-social odor discrimination is impaired in aged Wistar rats, raising the possibility that observed age-related social recognition deficits may be more sensory than mnemonic in nature [19, 20]. Even within a strain results can differ, however, as another group compared olfactory sensitivity and non-social odor discrimination ability at various time points across the Wistar rat lifespan and found that, although olfactory sensitivity was reduced with age, odor discrimination learning remained intact [35]. Such findings illustrate that, at least under some circumstances, olfactory sensitivity and olfactory learning can follow distinct age-related patterns of change.
In general, the performance of aged animals in the present study did not seem to be profoundly impacted by the aging process. It is tempting to speculate that the neural substrates for social recognition are relatively more resistant to changes associated with the aging process than those that underlie performance on tasks that require spatial navigation (which does show marked age-related declines [36, 37]). Some studies may support this interpretation [35, 36], but continued work in this area is necessary.
This is the first time that the performance of aged male rats on the social discrimination procedure has been tested, and the social recognition memory of aged female rats has never before been evaluated using any procedure. Aged male rats continued to spend more time overall investigating juveniles (both novel and familiar) than did aged female rats. However, the superior memory ability of females was not preserved during aging—unlike young adult females, aged females were not able to discriminate between novel and familiar juveniles after 120 minutes. Similarly, in a study which tested the spatial working memory ability of aged rats, aged females were more profoundly impaired than aged males [14]. In contrast, when spatial reference memory is tested using the Morris water maze, males have consistently showed greater age-related impairments than females [15-17]. Collectively these findings indicate that sex differences in the pattern of age-related cognitive decline are dependent on the type of memory being tested, possibly because different mnemonic abilities rely on different brain regions which could reflect the aging process in a sex-specific manner.
The neural basis of sex differences in the pattern of age-related cognitive decline has not been well explored because, until very recently, studies have examined only aged males. Our previous work has consistently demonstrated sex-specific neuroanatomical changes with age, and while the pattern of changes is region-specific, overall it appears that the male rat brain is more affected by the aging process than is the female's. In area CA1 of hippocampus, a neural region known to play a role in memory, young adult males have larger pyramidal neurons than do young adult females; however, this sex difference disappears during aging as males show an age-related loss of dendritic branches but females do not [37]. In the primary motor cortex, no sex difference exists among young adults, but males lose dendritic spines from pyramidal neurons with age whereas females do not, resulting in a sex difference favoring females during aging [38]. Age-related reductions in both dendritic branching and dendritic spine density on pyramidal neurons in the medial prefrontal cortex were found for both sexes, but with greater losses for males [27]. The prefrontal cortex is a region that is crucially involved in maintaining memory across a delay [e.g., 39, 40]. Although at this point speculative, it is possible that altered prefrontal and/or hippocampal neuronal architecture contributes to the reduced ability of aged animals to demonstrate social recognition memory following a longer delay, as observed in the present study. Interestingly, in none of these studies have we found a neuroanatomical difference between aged females of different estropausal states, consistent with the lack of behavioral differences between these groups found in the present study.
The relative competence of aged males on this task and the fact they continued to investigate juveniles more than aged females was initially somewhat curious, given the established dependence of these performance variables on circulating testosterone in males [3, 31], and that testosterone levels are reduced in aged male rats [41]. However, among young males the effect of castration on social recognition memory is temporary—7 days following surgery, males were impaired, but 14 days postoperative they were not [11]. In the same study, a vasopressin antagonist was no longer able to impact social recognition memory castrated males, even after their ability to perform on the task returned. The authors argue that, following castration, males undergo a gradual shift from relying on a vasopressin-dependent neural circuit (whose function relies on testosterone) to a vasopressin-independent neural circuit (such as that employed by females [2]). Thus it remains possible that when aging male rats first begin to experience a decline in testosterone, they also experience a concomitant decline in social recognition memory, but that they subsequently gradually regain competency on the task. Alternatively, the age-related decline in testosterone (about 25% in Long-Evans rats [41]), may not be sufficient to impact social recognition memory.
Finally, the performance of aged female rats did not significantly vary according to estropausal status. On a test of spatial reference memory, the performance of females in persistent estrus is superior to that of females in persistent diestrus [17]. It is not possible to conclude, however, that the social recognition memory of aged female rats is unaffected by ovarian hormones. Female rats do not experience a loss of gonadal hormones during reproductive senescence; although cyclicity is halted, female rats continue to secrete moderate, constant levels of both estrogen and progesterone [27]. It is possible that, without the continued presence of ovarian hormones, female rats would be unable to successfully discriminate following even the shorter delays. In young adult females, social recognition memory ability is negatively affected by ovariectomy, and is restored by replacement estrogen [23]. Perhaps a threshold level of estrogen is required to maintain social recognition memory, and that relatively small differences in ovarian hormone levels are not sufficient to impact performance. The poor social recognition memory of young ovariectomized females but the equivalently robust memory of females in proestrus and estrus reported here are findings that collectively argue in favor of this hypothesis. Now that the baseline performance of intact aged females has been established, it will be possible to make meaningful comparisons in future studies between these animals and those that are ovariectomized and allowed to go through the aging process without ovarian hormones.
In summary, our findings demonstrate that male rats investigate juveniles (both novel and familiar) more than females when tested using the social discrimination procedure. Despite this, young adult females maintain the ability to discriminate between novel and familiar juveniles for longer than young adult males. These results confirm those obtained using more traditional tests of social recognition memory. The present study is the first to test the social recognition memory ability of aged females using any procedure, and to compare their memory to that of aged males. The sex difference in absolute amount of investigation still holds for aged animals, however, the superior memory of females following longer delays is not preserved during aging. Apart from this finding, social recognition memory ability does not seem to be greatly impacted by aging in either sex.
Acknowledgments
We thank Jessica Loweth, Beth Whitman, Joanna O'Neil and Reshma Mohiuddin for their assistance with collection of behavioral data. This work was supported by NIH AG18046, AG022499, and NSF IBN0136468. J.M. supported by NIMHD 07333.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Popik P, van Ree JM. Neurohypophyseal peptides and social recognition in rats. Prog Brain Res. 1998;119:415–36. doi: 10.1016/s0079-6123(08)61585-x. [DOI] [PubMed] [Google Scholar]
- 2.Bluthe RM, Dantzer R. Social recognition does not involve vasopressinergic neurotransmission in female rats. Brain Res. 1990;535(2):301–4. doi: 10.1016/0006-8993(90)91613-l. [DOI] [PubMed] [Google Scholar]
- 3.Thor DH. Testosterone and persistance of social investigation in laboratory rats. J Comp Physiol Psychol. 1980;94(5):970–6. doi: 10.1037/h0077831. [DOI] [PubMed] [Google Scholar]
- 4.de Vries GJ, Miller MA. Anatomy and function of extrahypothalamic vasopressin systems in the brain. Prog Brain Res. 1998;119:3–20. doi: 10.1016/s0079-6123(08)61558-7. [DOI] [PubMed] [Google Scholar]
- 5.Ferguson JN, Young LJ, Insel TR. The neuroendocrine basis of social recognition. Front Neuroendocrinol. 2002;23(2):200–24. doi: 10.1006/frne.2002.0229. [DOI] [PubMed] [Google Scholar]
- 6.de Vries GJ, Buijs RM, Swaab DF. Ontogeny of the vasopressinergic neurons of the suprachiasmatic nucleus and their extrahypothalamic projections in the rat brain--presence of a sex difference in the lateral septum. Brain Res. 1981;218(12):67–78. doi: 10.1016/0006-8993(81)90989-6. [DOI] [PubMed] [Google Scholar]
- 7.De Vries GJ, Best W, Sluiter AA. The influence of androgens on the development of a sex difference in the vasopressinergic innervation of the rat lateral septum. Brain Res. 1983;284(23):377–80. doi: 10.1016/0165-3806(83)90019-6. [DOI] [PubMed] [Google Scholar]
- 8.de Vries GJ, Buijs RM, Sluiter AA. Gonadal hormone actions on the morphology of the vasopressinergic innervation of the adult rat brain. Brain Res. 1984;298(1):141–5. doi: 10.1016/0006-8993(84)91157-0. [DOI] [PubMed] [Google Scholar]
- 9.Dantzer R, et al. Septal vasopressin modulates social memory in male rats. Brain Res. 1988;457(1):143–7. doi: 10.1016/0006-8993(88)90066-2. [DOI] [PubMed] [Google Scholar]
- 10.Dantzer R, et al. Modulation of social memory in male rats by neurohypophyseal peptides. Psychopharmacology (Berl) 1987;91(3):363–8. doi: 10.1007/BF00518192. [DOI] [PubMed] [Google Scholar]
- 11.Bluthe RM, Schoenen J, Dantzer R. Androgen-dependent vasopressinergic neurons are involved in social recognition in rats. Brain Res. 1990;519(12):150–7. doi: 10.1016/0006-8993(90)90073-k. [DOI] [PubMed] [Google Scholar]
- 12.Jonasson Z. Meta-analysis of sex differences in rodent models of learning and memory: a review of behavioral and biological data. Neurosci Biobehav Rev. 2005;28(8):811–25. doi: 10.1016/j.neubiorev.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Sutcliffe JS, Marshall KM, Neill JC. Influence of gender on working and spatial memory in the novel object recognition task in the rat. Behav Brain Res. 2007;177(1):117–25. doi: 10.1016/j.bbr.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 14.Markowska AL. Sex dimorphisms in the rate of age-related decline in spatial memory: relevance to alterations in the estrous cycle. J Neurosci. 1999;19(18):8122–33. doi: 10.1523/JNEUROSCI.19-18-08122.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lukoyanov NV, et al. Effects of age and sex on the water maze performance and hippocampal cholinergic fibers in rats. Neurosci Lett. 1999;269(3):141–4. doi: 10.1016/s0304-3940(99)00442-5. [DOI] [PubMed] [Google Scholar]
- 16.Veng LM, Granholm AC, Rose GM. Age-related sex differences in spatial learning and basal forebrain cholinergic neurons in F344 rats. Physiol Behav. 2003;80(1):27–36. doi: 10.1016/s0031-9384(03)00219-1. [DOI] [PubMed] [Google Scholar]
- 17.Warren SG, Juraska JM. Sex differences and estropausal phase effects on water maze performance in aged rats. Neurobiol Learn Mem. 2000;74(3):229–40. doi: 10.1006/nlme.1999.3948. [DOI] [PubMed] [Google Scholar]
- 18.Guan X, Dluzen DE. Age related changes of social memory/recognition in male Fischer 344 rats. Behav Brain Res. 1994;61(1):87–90. doi: 10.1016/0166-4328(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 19.Prediger RD, Batista LC, Takahashi RN. Caffeine reverses age-related deficits in olfactory discrimination and social recognition memory in rats Involvement of adenosine A1 and A2A receptors. Neurobiol Aging. 2005;26(6):957–64. doi: 10.1016/j.neurobiolaging.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 20.Prediger RD, De-Mello N, Takahashi RN. Pilocarpine improves olfactory discrimination and social recognition memory deficits in 24 month-old rats. Eur J Pharmacol. 2006;531(13):176–82. doi: 10.1016/j.ejphar.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 21.Terranova JP, et al. Social olfactory recognition in rodents: deterioration with age, cerebral ischaemia and septal lesion. Behav Pharmacol. 1994;5(1):90–98. [PubMed] [Google Scholar]
- 22.Hlinak Z, Krejci I. Social recognition in male rats: age differences and modulation by MIF-I and Alaptide. Physiol Res. 1991;40(1):59–67. [PubMed] [Google Scholar]
- 23.Hlinak Z. Social recognition in ovariectomized and estradiol-treated female rats. Horm Behav. 1993;27(2):159–66. doi: 10.1006/hbeh.1993.1012. [DOI] [PubMed] [Google Scholar]
- 24.Warren SG, Juraska JM. Spatial and nonspatial learning across the rat estrous cycle. Behav Neurosci. 1997;111(2):259–66. doi: 10.1037//0735-7044.111.2.259. [DOI] [PubMed] [Google Scholar]
- 25.Markus EJ, Zecevic M. Sex differences and estrous cycle changes in hippocampus-dependent fear conditioning. Psychobiology. 1997;25:246–252. [Google Scholar]
- 26.Huang HH, et al. Patterns of sex steroid and gonadotropin secretion in aging female rats. Endocrinology. 1978;103(5):1855–9. doi: 10.1210/endo-103-5-1855. [DOI] [PubMed] [Google Scholar]
- 27.Markham JA, Juraska JM. Aging and sex influence the anatomy of the rat anterior cingulate cortex. Neurobiol Aging. 2002;23(4):579–88. doi: 10.1016/s0197-4580(02)00004-0. [DOI] [PubMed] [Google Scholar]
- 28.Engelmann M, Wotjak CT, Landgraf R. Social discrimination procedure: an alternative method to investigate juvenile recognition abilities in rats. Physiol Behav. 1995;58(2):315–21. doi: 10.1016/0031-9384(95)00053-l. [DOI] [PubMed] [Google Scholar]
- 29.Feder HH. Estrous cyclicity in mammals. In: Adler NT, editor. Neuroendocrinology of Reproduction. Plenum Press; New York: 1981. pp. 279–329. [Google Scholar]
- 30.Burman OH, Mendl M. Short-term social memory in the laboratory rat: its susceptibility to disturbance. Appl Anim Behav Sci. 2000;67(3):241–254. doi: 10.1016/s0168-1591(99)00120-3. [DOI] [PubMed] [Google Scholar]
- 31.Thor DH, Wainwright KL, Holloway WR. Persistence of attention to a novel conspecific: some developmental variables in laboratory rats. Dev Psychobiol. 1982;15(1):1–8. doi: 10.1002/dev.420150102. [DOI] [PubMed] [Google Scholar]
- 32.Taylor G, et al. Adult ontogeny of rat working memory of social interactions. J Gerontol A Biol Sci Med Sci. 1999;54(3):M145–51. doi: 10.1093/gerona/54.3.m145. [DOI] [PubMed] [Google Scholar]
- 33.Albers MW, Tabert MH, Devanand DP. Olfactory dysfunction as a predictor of neurodegenerative disease. Curr Neurol Neurosci Rep. 2006;6(5):379–86. doi: 10.1007/s11910-996-0018-7. [DOI] [PubMed] [Google Scholar]
- 34.Moberg PJ, et al. Olfactory recognition: differential impairments in early and late Huntington's and Alzheimer's diseases. J Clin Exp Neuropsychol. 1987;9(6):650–64. doi: 10.1080/01688638708405208. [DOI] [PubMed] [Google Scholar]
- 35.Kraemer S, Apfelbach R. Olfactory sensitivity, learning and cognition in young adult and aged male Wistar rats. Physiol Behav. 2004;81(3):435–42. doi: 10.1016/j.physbeh.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 36.Gallagher M, et al. Effects of aging on the hippocampal formation in a naturally occurring animal model of mild cognitive impairment. Exp Gerontol. 2003;38(12):71–7. doi: 10.1016/s0531-5565(02)00159-6. [DOI] [PubMed] [Google Scholar]
- 37.Markham JA, et al. Sexually dimorphic aging of dendritic morphology in CA1 of hippocampus. Hippocampus. 2005;15(1):97–103. doi: 10.1002/hipo.20034. [DOI] [PubMed] [Google Scholar]
- 38.Markham JA, Ward JE, Juraska JM. Abstract Viewer/Itenerary Planner. Washington, DC: Society for Neuroscience; 2001. Sex differences in the pattern of aging: spine density in the rat primary motor cortex. Online. Program 101.8. [Google Scholar]
- 39.Phillips AG, Ahn S, Floresco SB. Magnitude of dopamine release in medial prefrontal cortex predicts accuracy of memory on a delayed response task. J Neurosci. 2004;24(2):547–53. doi: 10.1523/JNEUROSCI.4653-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sloan HL, Good M, Dunnett SB. Double dissociation between hippocampal and prefrontal lesions on an operant delayed matching task and a water maze reference memory task. Behav Brain Res. 2006;171(1):116–26. doi: 10.1016/j.bbr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 41.Fentie IH, et al. Age-related decreases in gonadal hormones in Long-Evans rats: relationship to rise in arterial pressure. Endocrine. 2004;25(1):15–22. doi: 10.1385/ENDO:25:1:15. [DOI] [PubMed] [Google Scholar]


