Abstract
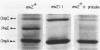
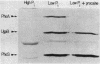
The envZ11 missense mutation in the regulatory gene envZ pleiotropically repressed synthesis of OmpF, alkaline phosphatase, and several proteins of the maltose regulon. Procaine treatment of wild-type cells resulted in the same phenotype through an envZ+-mediated mechanism. Here we show that envZ11-procaine act differently on the mal and pho regulons. In the mal system, the expression of the positive regulator gene malT, measured as beta-galactosidase activity of a malT-lac+ operon fusion, was drastically reduced by procaine treatment or by the envZ11 mutation. In contrast, expression of the positive regulator of the pho regulon phoB was not reduced by procaine treatment. The products of the regulatory genes phoM, phoR, and phoU were also not required for procaine action. Procaine and envZ11 inhibited expression of only two products of the pho regulon, alkaline phosphatase and the PhoE porin. The conclusion that envZ11-procaine act differently on the mal and the pho regulons is supported by our ability to isolate second-site mutations with a Mal+ PhoA- phenotype in an envZ11 strain.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alphen W. V., Lugtenberg B. Influence of osmolarity of the growth medium on the outer membrane protein pattern of Escherichia coli. J Bacteriol. 1977 Aug;131(2):623–630. doi: 10.1128/jb.131.2.623-630.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benner D., Müller N., Boos W. Temperature-sensitive catabolite activator protein in Escherichia coli BUG6. J Bacteriol. 1985 Jan;161(1):347–352. doi: 10.1128/jb.161.1.347-352.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman E., Beckwith J. Analysis of the regulation of Escherichia coli alkaline phosphatase synthesis using deletions and phi80 transducing phages. J Mol Biol. 1975 Aug 5;96(2):307–316. doi: 10.1016/0022-2836(75)90350-2. [DOI] [PubMed] [Google Scholar]
- Chapon C. Role of the catabolite activator protein in the maltose regulon of Escherichia coli. J Bacteriol. 1982 May;150(2):722–729. doi: 10.1128/jb.150.2.722-729.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossart P., Gicquel-Sanzey B. Regulation of expression of the crp gene of Escherichia coli K-12: in vivo study. J Bacteriol. 1985 Jan;161(1):454–457. doi: 10.1128/jb.161.1.454-457.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freundlieb S., Boos W. Alpha-amylase of Escherichia coli, mapping and cloning of the structural gene, malS, and identification of its product as a periplasmic protein. J Biol Chem. 1986 Feb 25;261(6):2946–2953. [PubMed] [Google Scholar]
- GAREN A., ECHOLS H. Properties of two regulating genes for alkaline phosphatase. J Bacteriol. 1962 Feb;83:297–300. doi: 10.1128/jb.83.2.297-300.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett S., Taylor R. K., Silhavy T. J., Berman M. L. Isolation and characterization of delta ompB strains of Escherichia coli by a general method based on gene fusions. J Bacteriol. 1985 May;162(2):840–844. doi: 10.1128/jb.162.2.840-844.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett S., Taylor R. K., Silhavy T. J. Isolation and characterization of chain-terminating nonsense mutations in a porin regulator gene, envZ. J Bacteriol. 1983 Oct;156(1):62–69. doi: 10.1128/jb.156.1.62-69.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granett S., Villarejo M. Regulation of gene expression in Escherichia coli by the local anesthetic procaine. J Mol Biol. 1982 Sep 15;160(2):363–367. doi: 10.1016/0022-2836(82)90181-4. [DOI] [PubMed] [Google Scholar]
- Granett S., Villarejo M. Selective inhibition of carbohydrate transport by the local anesthetic procaine in Escherichia coli. J Bacteriol. 1981 Aug;147(2):289–296. doi: 10.1128/jb.147.2.289-296.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan C. D., Wanner B., Inouye H. Analysis of regulation of phoB expression using a phoB-cat fusion. J Bacteriol. 1983 Nov;156(2):710–717. doi: 10.1128/jb.156.2.710-717.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M. N., Silhavy T. J. Genetic analysis of the ompB locus in Escherichia coli K-12. J Mol Biol. 1981 Sep 5;151(1):1–15. doi: 10.1016/0022-2836(81)90218-7. [DOI] [PubMed] [Google Scholar]
- Hall M. N., Silhavy T. J. The ompB locus and the regulation of the major outer membrane porin proteins of Escherichia coli K12. J Mol Biol. 1981 Feb 15;146(1):23–43. doi: 10.1016/0022-2836(81)90364-8. [DOI] [PubMed] [Google Scholar]
- Hengge R., Boos W. Maltose and lactose transport in Escherichia coli. Examples of two different types of concentrative transport systems. Biochim Biophys Acta. 1983 Aug 11;737(3-4):443–478. doi: 10.1016/0304-4157(83)90009-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazdunski C., Baty D., Pagès J. M. Procaine, a local anesthetic interacting with the cell membrane, inhibits the processing of precursor forms of periplasmic proteins in Escherichia coli. Eur J Biochem. 1979 May 2;96(1):49–57. doi: 10.1111/j.1432-1033.1979.tb13012.x. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Van Alphen L. Molecular architecture and functioning of the outer membrane of Escherichia coli and other gram-negative bacteria. Biochim Biophys Acta. 1983 Mar 21;737(1):51–115. doi: 10.1016/0304-4157(83)90014-x. [DOI] [PubMed] [Google Scholar]
- Lundrigan M. D., Earhart C. F. Gene envY of Escherichia coli K-12 affects thermoregulation of major porin expression. J Bacteriol. 1984 Jan;157(1):262–268. doi: 10.1128/jb.157.1.262-268.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundrigan M., Earhart C. F. Reduction in three iron-regulated outer membrane proteins and protein a by the Escherichia coli K-12 perA mutation. J Bacteriol. 1981 May;146(2):804–807. doi: 10.1128/jb.146.2.804-807.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossal N. G., Heppel L. A. The release of enzymes by osmotic shock from Escherichia coli in exponential phase. J Biol Chem. 1966 Jul 10;241(13):3055–3062. [PubMed] [Google Scholar]
- Pugsley A. P., Schnaitman C. A. Identification of three genes controlling production of new outer membrane pore proteins in Escherichia coli K-12. J Bacteriol. 1978 Sep;135(3):1118–1129. doi: 10.1128/jb.135.3.1118-1129.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabourin D., Beckwith J. Deletion of the Escherichia coli crp gene. J Bacteriol. 1975 Apr;122(1):338–340. doi: 10.1128/jb.122.1.338-340.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma V., Reeves P. Genetic locus (ompB) affecting a major outer-membrane protein in Escherichia coli K-12. J Bacteriol. 1977 Oct;132(1):23–27. doi: 10.1128/jb.132.1.23-27.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw W. V. Chloramphenicol acetyltransferase from chloramphenicol-resistant bacteria. Methods Enzymol. 1975;43:737–755. doi: 10.1016/0076-6879(75)43141-x. [DOI] [PubMed] [Google Scholar]
- Silhavy T. J., Brickman E., Bassford P. J., Jr, Casadaban M. J., Shuman H. A., Schwartz V., Guarente L., Schwartz M., Beckwith J. R. Structure of the malB region in Escherichia coli K12. II. Genetic map of the malE,F,G operon. Mol Gen Genet. 1979 Jul 24;174(3):249–259. doi: 10.1007/BF00267797. [DOI] [PubMed] [Google Scholar]
- TORRIANI A., ROTHMAN F. Mutants of Escherichia coli constitutive for alkaline phosphatase. J Bacteriol. 1961 May;81:835–836. doi: 10.1128/jb.81.5.835-836.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. K., Hall M. N., Silhavy T. J. Isolation and characterization of mutations altering expression of the major outer membrane porin proteins using the local anaesthetic procaine. J Mol Biol. 1983 May 25;166(3):273–282. doi: 10.1016/s0022-2836(83)80085-0. [DOI] [PubMed] [Google Scholar]
- Tommassen J., Leunissen J., van Damme-Jongsten M., Overduin P. Failure of E. coli K-12 to transport PhoE-LacZ hybrid proteins out of the cytoplasm. EMBO J. 1985 Apr;4(4):1041–1047. doi: 10.1002/j.1460-2075.1985.tb03736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommassen J., Lugtenberg B. PHO-regulon of Escherichia coli K12: a minireview. Ann Microbiol (Paris) 1982 Mar-Apr;133(2):243–249. [PubMed] [Google Scholar]
- Villarejo M., Case C. C. envZ mediates transcriptional control by local anesthetics but is not required for osmoregulation in Escherichia coli. J Bacteriol. 1984 Sep;159(3):883–887. doi: 10.1128/jb.159.3.883-887.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandersman C., Moreno F., Schwartz M. Pleiotropic mutations rendering Escherichia coli K-12 resistant to bacteriophage TP1. J Bacteriol. 1980 Sep;143(3):1374–1383. doi: 10.1128/jb.143.3.1374-1383.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner B. L., Latterell P. Mutants affected in alkaline phosphatase, expression: evidence for multiple positive regulators of the phosphate regulon in Escherichia coli. Genetics. 1980 Oct;96(2):353–366. doi: 10.1093/genetics/96.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner B. L. Overlapping and separate controls on the phosphate regulon in Escherichia coli K12. J Mol Biol. 1983 May 25;166(3):283–308. doi: 10.1016/s0022-2836(83)80086-2. [DOI] [PubMed] [Google Scholar]
- Wanner B. L., Sarthy A., Beckwith J. Escherichia coli pleiotropic mutant that reduces amounts of several periplasmic and outer membrane proteins. J Bacteriol. 1979 Oct;140(1):229–239. doi: 10.1128/jb.140.1.229-239.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willsky G. R., Malamy M. H. Control of the synthesis of alkaline phosphatase and the phosphate-binding protein in Escherichia coli. J Bacteriol. 1976 Jul;127(1):595–609. doi: 10.1128/jb.127.1.595-609.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagil E., Bracha M., Lifshitz Y. The regulatory nature of the phoB gene for alkaline phosphatase synthesis in Escherichia coli. Mol Gen Genet. 1975;137(1):11–16. doi: 10.1007/BF00332537. [DOI] [PubMed] [Google Scholar]
- Zuckier G., Torriani A. Genetic and physiological tests of three phosphate-specific transport mutants of Escherichia coli. J Bacteriol. 1981 Mar;145(3):1249–1256. doi: 10.1128/jb.145.3.1249-1256.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]