Abstract
Background
Older people are prone to problems related to use of medicines. As they tend to use many different medicines, monitoring pharmacotherapy for older people in primary care is important.
Aim
To determine which procedure for treatment reviews (case conferences versus written feedback) results in more medication changes, measured at different moments in time. To determine the costs and savings related to such an intervention.
Design of study
Randomised, controlled trial, randomisation at the level of the community pharmacy.
Setting
Primary care; treatment reviews were performed by 28 pharmacists and 77 GPs concerning 738 older people (≥75 years) on polypharmacy (>five medicines).
Method
In one group, pharmacists and GPs performed case conferences on prescription-related problems; in the other group, pharmacists provided results of a treatment review to GPs as written feedback. Number of medication changes was counted following clinically-relevant recommendations. Costs and savings associated with the intervention at various times were calculated.
Results
In the case-conference group significantly more medication changes were initiated (42 versus 22, P = 0.02). This difference was also present 6 months after treatment reviews (36 versus 19, P = 0.02). Nine months after treatment reviews, the difference was no longer significant (33 versus 19, P = 0.07). Additional costs in the case-conference group seem to be covered by the slightly greater savings in this group.
Conclusion
Performing treatment reviews with case conferences leads to greater uptake of clinically-relevant recommendations. Extra costs seem to be covered by related savings. The effect of the intervention declines over time, so performing treatment reviews for older people should be integrated in the routine collaboration between GPs and pharmacists.
Keywords: geriatrics; pharmaceutical care; pharmacotherapy; therapeutics; prescriptions, drug
INTRODUCTION
Many older people suffer from chronic diseases for which medicines should be used. Older patients are more prone to problems related to their medicines because of the higher number they use, and because of a decline in cognitive and physical functioning. A previous study found that two-thirds of all older people have problems using their medicines correctly; and that these problems could lead to a deterioration in clinical condition for one of four older patients.1 Another study by the current authors found that there are prescription-related points of concern, possibly leading to a deterioration in clinical condition, in the pharmacotherapy of almost all older patients studied; for example, using diazepam, a benzodiazepine with a long half-life and hence unsuitable for use by older people. These problems were considered to be of direct clinical relevance in 30% of patients.2 The current intervention study focuses on prescribing medicines for older patients, rather than on user-related problems.
Monitoring pharmacotherapy for older people in primary care is important. One possible approach is the use of treatment reviews for individual patients by trained professionals (for example, GPs, clinical or community pharmacists, or two healthcare professionals of different professional backgrounds together). While earlier studies have shown that treatment reviews can be useful,3–6 supplementary studies are still needed to evaluate the comparative effectiveness of various models for treatment reviews.7
How this fits in
The literature supports the usefulness of treatment reviews for older people. Supplementary studies are needed to evaluate the comparative effectiveness of various models for mediation review. This study examined whether treatment reviews with case conferences lead to more medication changes than treatment reviews with written feedback. Furthermore, this study examined whether additional costs of a more time-consuming intervention could be covered by supplementary savings on medicine costs. Results indicate that performing treatment reviews with case conferences leads to greater uptake of clinically-relevant recommendations. Extra costs seem to be covered by related savings.
This study compared two procedures for treatment review by a team consisting of a community pharmacist and a GP. In one group (termed the case-conference group) the pharmacist and GP personally discussed problems, as identified in the pharmacotherapy of the patient through academic detailing or case conferences, and drew up a pharmaceutical care plan for each patient. In the other group (termed the written-feedback group) the pharmacist passes the results of a treatment review to the GP as written feedback. The former procedure may produce more and better results, but also could be more time consuming and costly, and require more organisational activity.
Effects and cost differences were determined at 6 and 9 months after the intervention. Furthermore, yearly savings in medicine costs for each year the medication change persisted were determined. The investigators were particularly interested in the medication changes made in response to clinically-relevant recommendations made by the pharmacist to the GP.
METHOD
Study design
The study was a clustered, randomised, controlled trial. Pharmacists in both intervention arms conducted treatment reviews. Written feedback only was given to the GPs in one group. In the other group, the pharmacist and the GP had personal contact in a case conference in which a pharmaceutical care plan was drawn up for each patient. Treatment reviews were performed between March and May 2004.
Randomisation across the intervention arms was at the level of the community pharmacy. Each pharmacy included GPs in only one of the intervention groups, so that contamination of the effect was prevented.
The unit of analysis was the GP (who prescribed the medicines that were evaluated in the treatment review). Patients were considered to be nested within general practices. On the basis of a power calculation using an intra-cluster correlation coefficient of 0.03 (deduced from the researchers' in-depth analysis of polypharmacy for older people2), and with the aim to detect a difference of 10% in medication changes following the recommendations between the groups,8 an estimated sample of at least 20 pharmacies, each associated with three GPs with 10 participating patients for each GP, was required.
Study population: participating healthcare professionals and their patients
In this intervention study, Service Apotheek Nederland, a franchise organisation supporting independent community pharmacies in their professional activities, supported this research with their organisational skills. All pharmacies registered with Service Apotheek Nederland (n = 120) were invited to participate in the study. Participating pharmacies each contacted three GPs (convenience sample). Ten home-dwelling older people (aged ≥75 years), registered by one GP, who were using at least five prescription medicines continuously at the start of the study were selected at random from a large database in which community pharmacy dispensing data are collected.
Pharmacy codes for these patients were presented on a secure web page that was only accessible to the participating pharmacist. The pharmacist had to exclude older patients who were terminally ill, deceased, lived in a home for older people, younger than 75 years, or used fewer than five medicines continuously; this could be done online. If patients were excluded, new patients were presented on the web page as long as these patients met inclusion criteria.
Computerised screening tool for detecting suboptimal prescribing for older people
The Foundation for Pharmaceutical Statistics (SFK) collects pharmacy dispensing data from 90% of the community pharmacies in the Netherlands. The SFK gathers these data in an anonymous format: only patient codes are recorded to safeguard the privacy of patients. This allows the SFK to reconstruct utilisation patterns of individual patients without any danger of exposing patients' identities.9
A computerised screening tool was designed to search the SFK records for suboptimal prescribing for older people. This computerised screening tool was an aid for participating pharmacists: they could obtain the results of these searches (as performed in January 2004) on a secured website. Because the pharmacists have to send dispensing files from the pharmacy system to the SFK database at the end of a month, the SFK always has a delay in the database. The searches were performed in the database including the data of January 2004. Searches were not updated during the study period. Pharmacists received a graphical representation of the pharmacy record of each participant and a list of potential problems identified by the computerised screening tool. This information lists the Anatomical Therapeutic Chemical code10 of the medicine(s) causing the problem, a description of the problem, and some directions for potential improvement.
Intervention
All participating pharmacists were invited to attend a training session dealing with problems related to medication use in older people and treatment reviews. After this training session, pharmacists were randomised and the pharmacists in both intervention groups performed treatment reviews with the support of the computerised screening tool. They had to decide which of the recommendations highlighted by the computerised screening tool should be given to the GP, and whether additional recommendations concerning the pharmacotherapy of these patients should be highlighted.
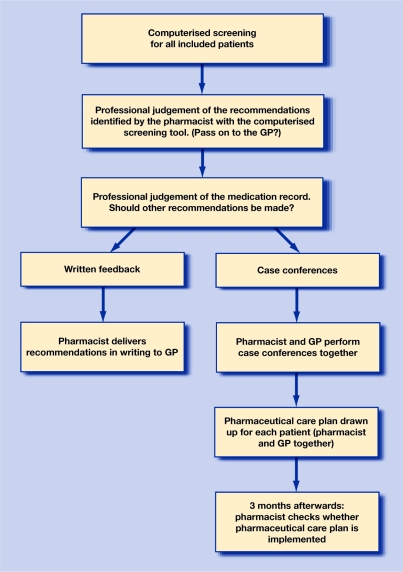
The two intervention groups differed in their organisational models (Figure 1):
Written-feedback group: pharmacists listed all recommendations per patient and delivered them to the GP's office. The pharmacist did not follow up cases.
Case-conference group: the pharmacist and GP discussed all recommendations with each other, including other concerns about the patients (if any). The pharmacist and the GP together filled in a standardised pharmaceutical care plan, in which they addressed who was responsible for the activities in this plan. Three months later the pharmacist checked whether these activities had been carried out.
Figure 1.
Flow diagram of the intervention study comparing two procedures for medication reviews of older people on polypharmacy in primary care.
Variables and instruments
Medication changes following clinically-relevant recommendations
Researchers determined how many clinically-relevant recommendations were made by the pharmacist; and determined the number of medication changes consistent with these recommendations that could be detected in the medication records. To verify that differences in the number of medication changes were not caused by variation in the number of recommendations made, a secondary outcome measure was used that relates the number of recommendations followed to the number of recommendations made: the percentage of clinically-relevant recommendations leading to medication changes.
A copy of the list of recommendations that the pharmacist made for each patient was sent to the research team. Six and 9 months after treatment reviews, pharmacy records and drug-dispensing profiles (graphical representations of the pharmacy records) were received by the research team from the pharmacies. Two experienced pharmacy assistants determined whether the recommendations had led to medication changes, whether these medication changes had been maintained, and whether results of the recommendations as proposed could be identified in drug-use profiles and pharmacy records (for example, is the drug used for the right indication? Is blood pressure checked regularly?). If the researchers could not determine whether action had been taken in response to a recommendation, the particular recommendation was considered to have not been acted on.
After 9 months, researchers recorded whether medication changes that were present at 6 months had been maintained. It could not be assumed that medication changes initiated more than 6 months after the treatment reviews were caused by the intervention, so these medication changes were not included.
Clinical relevance of recommendations by pharmacists
Clinical relevance was assessed for all recommendations (as they were identified by the computerised screening tool or by pharmacists) that were communicated to GPs. Recommendations were classified as:
Clinically relevant: recommendation will lead to improvement in the general health of the patient.
Potentially relevant: for example, relevance depending on the medical condition of the individual patient.
Clinically irrelevant: recommendation will not lead to improvement in the general health of the patient.
For most of the recommendations identified by the computerised screening tool, clinical relevance was based on an earlier in-depth analysis of pharmacotherapy,2 while for some of the remaining problems it was based on the literature.11,12 For the recommendations that pharmacists identified themselves, clinical relevance was based on the previous in-depth analysis2 or was determined by an expert panel during the present study.
The expert panel consisted of a GP, a community pharmacist, a geriatrician, and a clinical pharmacist who were chosen because of their experience in geriatric pharmacology. The expert panel did not include individual members of the participating pharmacies or general practices. Clinical relevance was determined using a consensus method. Panel members had to fill in their individual opinions regarding the clinical relevance of each recommendation. Panel members received overviews in which their own scores were compared with those of the other panel members. During a consensus meeting on the telephone, panel members discussed all differences until they reached consensus about the clinical relevance of each particular recommendation.
Changes in costs of medicines used
For each medication change, the difference in medication costs at 6 and 9 months after the treatment review was determined. Researchers determined the differences in medication costs if the medication changes, persisting until 9 months after the intervention, would persist for another 12 months. Differences in medicine costs were determined by adding the costs of the medicines and the dispensing fees for the pharmacy following legal regulations (15-day prescriptions for all new medicines, followed by 3-month prescriptions for all medicines, except hypnotics for which the legal maximum prescription period is 30 days). Supplementary costs caused by wastage were not included in the calculations.
Time consumed by the intervention
Participating healthcare professionals were asked to keep a separate time log of all intervention activities for each patient. This time log was used to calculate the costs of the treatment reviews (at an assumed rate of €50 per hour for each healthcare professional, analogous to another study in the Netherlands13).
No reimbursement was given for participation in the study. Participating pharmacists were invited to a training session in which medication-related problems and the process of treatment reviews concerning older people were dealt with. Pharmacists were offered free use of the computerised screening tool.
Data analysis
Baseline characteristics of the participating healthcare professionals and participants in both intervention groups were compared using χ2 tests for dichotomous values and Student's t-tests for differences in means of numerous values.
The type of problem, directions for improvement, origin (computerised screening tool or pharmacist), and clinical relevance for each recommendation passed on to the GP were entered into a Microsoft® Access database. Whether recommendations were acted on, partially acted on, or not acted on at all at the various times of measurement, was also recorded in this database. Actual changes in costs associated with the interventions were also recorded in this database. The database was analysed using SPSS (version 12.0).
Because patients in this study were nested within general practices, researchers analysed the number of medication changes following clinically-relevant recommendations at the level of GP. For each GP the total number of recommendations acted on was determined. Because the number of recommendations followed by the different GPs was not normally distributed, differences between groups were determined using χ2 statistics at this level. Before χ2 statistics could be performed, the number of recommendations followed was categorised (zero, one, or more than one recommendation followed). Multilevel (mixed-model) analysis was performed to determine whether differences in costs and in percentages of recommendations followed were present. A model with a random intercept and all other variables fixed was used. Multilevel analyses were performed using SAS (version 8.2).14
RESULTS
Study population
Of the 120 pharmacies invited, 29 (with 84 accompanying GPs; three pharmacies could only find two GPs to take part in the study) were included in the randomisation. Four GPs were subsequently excluded for different reasons. During the intervention period, one pharmacy in the written-feedback group dropped out (along with three accompanying GPs) because they could not find the time to deliver pharmacy dispensing data of patients to the research team.
Data for 28 pharmacies were gathered and added to the database (13 written feedback and 15 case conferences) accompanied by data for 77 GPs. A total of 738 patients were included (351 in the written-feedback group and 387 in the case-conference group).
Before the 6-month assessments, but after the treatment review, 37 patients were excluded for various reasons (Supplementary Figure 1). In the period between 6 and 9 months, 16 participants were excluded.
Table 1 shows characteristics of the participating pharmacists, GPs, and their patients. The patient and pharmacy characteristics for the written-feedback group and the case-conference group were comparable. Of the GP characteristics, only the number of single-handed practices was unevenly distributed among intervention groups.
Table 1.
Characteristics of participating pharmacies, GPs, and patients.
| Characteristics | Written feedback | Case conferences |
|---|---|---|
| Pharmacies, n | 13 | 15 |
| Pharmacists employed in the pharmacy, n | 2.0 | 1.9 |
| Older patients (≥75 years) at the pharmacy, % | 8.9 | 13.4 |
| Pharmacies with 350 or more prescriptions a day, %a | 58.3 | 71.4 |
| GPs, n | 37b | 40c |
| Single-handed practices, % | 59.3 | 32.4e |
| Patients in practice, nd | 3168 | 3279 |
| Patients aged ≥75 years in general practice, % | 10.6 | 13.5 |
| Patients, n | 351 | 387 |
| Age of patients, years | 81 | 81 |
| Males, % | 34.9 | 40.6 |
| Mean number of medicines used by participants (as measured by computerised screening tool), n | 7.3 | 7.1 |
Student's t-test for differences in continuous values and χ2 statistics for binominal values.
Two pharmacists did not answer the question about daily number of prescriptions, evenly divided over both intervention groups.
Twenty-seven questionnaires from GPs in the written-feedback group were received (73% response).
Thirty-seven questionnaires from GPs in the case-conference group were received (93% response).
Seven GPs did not answer the question about number of patients in practice: three in the feedback in writing group, four in the group with case conferences.
P<0.05.
Number and clinical relevance of recommendations as presented to GPs
Participating pharmacists made a total of 1569 recommendations regarding the pharmacotherapy of 624 participating patients; no recommendation was given for 114 (15.4%) patients. The computerised screening tool identified 62.0% of the recommendations that were passed on to the GPs; the pharmacists themselves identified 38.0%. Pharmacists in the case-conference group identified significantly more recommendations themselves than the pharmacists in the written-feedback group (41.7% versus 34.2%, P = 0.003).
Table 2 shows the number and types of recommendations presented to GPs. The recommendations were categorised by clinical relevance. Some recommendations with limited clinical relevance were made (3.4%); for example, prolonged use of vitamin preparations without an indication. Many recommendations with potential clinical relevance were made (77.3%); for example, performance of regular checks. Most clinically-relevant recommendations were about prescribing the correct geriatric dosage (104 recommendations), followed by prescribing medicines considered unsuitable for use by older people (99 recommendations), and prescribing omissions (34 recommendations).
Table 2.
Numbers, types, and clinical relevance of medication recommendations from pharmacists to GPs.
| Feedback in writing (% within category of clinical relevance) | Case conferences (% within category of clinical relevance) | ||||||
|---|---|---|---|---|---|---|---|
| Type of recommendation | Not clinically relevant | Potentially clinically relevant | Clinically relevant | Not clinically relevant | Potentially clinically relevant | Clinically relevant | Example of clinically relevant recommendation |
| Dose unsuitable for patients | 1 (4.8) | 51 (9.5) | 62 (42.2) | 0 | 73 (10.8) | 42 (26.9) | Use of more than 20 mg of temazepam daily |
| Medicines unsuitable for patients aged ≥75 years | 2 (9.5) | 18 (3.3) | 35 (23.8) | 0 | 14 (2.1) | 64 (41.0) | Use of glibenclamide, which can cause prolonged hypoglycaemia |
| Medicine not useful at al | 7 (33.3) | 27 (5.0) | 17 (11.6) | 4 (12.5) | 32 (4.7) | 15 (9.6) | Use of meprobamate, for which safer alternatives are available |
| Prescribing omissions | 4 (19.0) | 29 (5.4) | 16 (10.9) | 5 (15.6) | 43 (6.4) | 18 (11.5) | Omitting to prescribe osteoporosis prophylaxis for a patient using high doses of corticosteroids for a long period |
| Incorrect duration of therapy | 2 (9.5) | 10 (1.9) | 7 (4.8) | 0 | 6 (0.9) | 3 (1.9) | Prolonged use of hypnotics |
| Unnecessary therapeutic duplication | 0 | 39 (7.3) | 3 (2.0) | 1 (3.1) | 40 (5.9) | 5 (3.2) | Simultaneous use of different benzodiazepines |
| Form of medication unsuitable for older people | 0 | 12 (2.2) | 3 (2.0) | 2 (6.3) | 16 (2.4) | 0 | Use of immediate release nifedipine capsules |
| Suitable for the indication? | 0 | 53 (9.9) | 2 (1.4) | 0 | 111 (16.4) | 3 (1.9) | Patient uses an oestrogen preparation regularly used for complaints caused by menopause |
| Adherence (too much or too little use) | 1 (4.8) | 57 (10.6) | 1 (0.7) | 1 (3.1) | 74 (10.9) | 2 (1.3) | Patient uses more oxazepam than prescribed |
| Contraindication known | 2 (9.5) | 9 (1.7) | 1 (0.7) | 6 (18.8) | 12 (1.8) | 3 (1.9) | Use of amiodarone by patient suffering from hypothyreosis |
| Drug-drug interaction known | 1 (4.8) | 60 (11.2) | 0 | 0 | 60 (8.9) | 0 | – |
| Treatment of side effect of other medicine | 0 | 84 (15.6) | 0 | 2 (6.3) | 77 (11.4) | 0 | – |
| Others | 1 (4.8) | 88 (16.4) | 0 | 11 (34.4) | 118 (17.5) | 1 (0.6) | Use of a combination of amlodipine and losartan for a patient with oedema. Stop amlodipine and increase losartan dosage |
| Total | 21 (100) | 537 (100) | 147 (100) | 32 (100) | 676 (100) | 156 (100) | – |
| Number of records per patient | 0.06 | 1.53 | 0.42 | 0.08 | 1.75 | 0.40 | – |
| Total number | 705 | 864 | – | ||||
| Number of records per patient | 2.01 | 2.23 | P = 0.059, difference = 0.229, 95% CI = −0.467 to −0.009 | ||||
The mean number of recommendations per patient made by the pharmacists in the case-conference group seemed to be higher than that in the group with written feedback; however, this difference was not statistically significant (P = 0.059).
The pharmacy assistants could not determine from the pharmacy records whether 883 of the recommendations presented in Table 2 (56.3% of all recommendations) had been acted on or not. Most problems concerned doubts about the correctness of the indication and doubts whether routine checks were performed.
In the comparisons that followed, only the 303 recommendations with direct clinical relevance were included. For 18 recommendations problems were solved or the particular medicine had been discontinued before the intervention started; these recommendations were excluded. Another 16 recommendations were excluded from the 6-month measurement because the particular patients were excluded from the study. Thus, 269 recommendations were included in the analysis of the 6-months measurement. A further five recommendations were similarly excluded for the 9-months measurement, leaving 264 recommendations.
Medication changes following clinically-relevant recommendations made by pharmacists
Table 3 presents the number of medication changes following clinically-relevant recommendations. Significantly more medication changes were initiated in the case-conference group than in the written-feedback group (42 versus 22, P = 0.02). This difference is also present in the maintained medication changes at 6 months after the treatment reviews (36 versus 19; P = 0.02). For medication changes maintained until 9 months after the treatment review, the difference between the groups was no longer significant (33 versus 19, P = 0.07).
Table 3.
Number of medication changes following recommendations with clinical relevance.
| Medication changes | |||
|---|---|---|---|
| Time | Written feedback, n | Case conferences, n | χ2P-value |
| Measurement at 0–6 months and 6 months | 128 | 141 | – |
| 0–6 months after medication reviews (initiated medication changes, all attempts, whether or not sustained) | 22 | 42 | 0.016 |
| 6 months after medication review (sustained changes) | 19 | 36 | 0.022 |
| Measurement at 9 months | 126 | 138 | – |
| Changes sustained until 9 months after medication review | 19 | 33 | 0.070 |
Percentage of clinically-relevant recommendations followed was examined as a secondary outcome measure. Significantly higher percentages of these recommendations led to initiated medication changes in the case-conference group than in the written-feedback group (29.8% versus 17.2%, P = 0.02). This was also seen for the percentage of maintained medication changes 6 months after the treatment reviews (25.5% versus 14.8%, P = 0.03). At the 9-month measurement there was no statistically significant difference (23.9% versus 15.1%, P = 0.08).
Cost evaluation
Pharmacists in the case-conference group spent significantly more time on this intervention (with accompanying increase in costs) than pharmacists in the written-feedback group. This was also true for GPs who kept time logs. Unfortunately, only 37.0% of all GPs kept time logs: 42.5% in the case-conference group and 30.5% in the written-feedback group.
Changes in patient pharmacy records that were attributed to the treatment review led to a modest decrease in medicine costs. Although these benefits were slightly greater in the case-conference group, this difference did not reach statistical significance (Table 4).
Table 4.
Costs and savings caused by the intervention (multilevel statistics); all recommendations are included.
| Cost changes per patient, €a | ||||
|---|---|---|---|---|
| Written feedback (n) | Case conferences (n) | P-value | Difference (95% CI) | |
| Costs caused by time expenses on the total process of medication review | ||||
| GPb | 6.22 (97) | 8.68 (163) | – | – |
| Pharmacist | 9.69 (350)c | 15.03 (387) | 0.001 | 5.27 (2.21 to 8.34) |
| Savings due to medication changes at 9 months after intervention | −4.33 (320) | −7.78 (365) | 0.357 | 3.44 (−3.89 to 10.77) |
| Yearly savings in medicine costs for each year that medication change persists | −7.79 (320) | −12.24 (365) | 0.443 | 4.44 (−6.90 to 12.81) |
| Net expenses at 9 months after medication review (including pharmacy costs and savings on medicines) | 5.52 (319) | 7.23 (365) | 0.655 | 1.72 (−5.80 to 9.23) |
| Net expenses at 9 months after medication review (including pharmacy and GP costs and savings on medicines) | 14.22 (89) | 13.71 (153) | – | – |
Positive number = expenses, negative number = savings.
From GPs, time logs for only 37% of all participants were received.
For one patient the time log from the pharmacy got lost, so this patient was excluded from this analysis.
The expenses and cost benefits of the intervention partially cancelled each other out. The extra savings in the case-conference group offset a part of the extra costs due to extra time needed by the pharmacists and GPs in this group. There was no significant difference in remaining net costs including pharmacy time expenses and savings on medicine costs between the two intervention groups. For the remaining net costs, including pharmacist time expenses, GP time expenses, and savings on medicine costs, no statistical testing was performed because of the small number of participants. The results shown in Table 4 do not seem to indicate differences in remaining costs including pharmacy-time, GP-time, and savings on medicine costs.
DISCUSSION
Summary of main findings
In this study, community pharmacists performed treatment reviews for 738 patients and feedback was given to GPs. Feedback in personal contact between the pharmacist and the GP (case conferences) led to significantly more medication changes following recommendations of clinical relevance.
Medicine costs were also influenced by the interventions. Both types of intervention showed modest savings regarding medicine costs. The slightly greater savings seem to cover extra costs caused by pharmacist and GP time expenses in the case-conference group.
When the effect of the intervention was examined over time, differences between the intervention groups were shown to decrease gradually.
Strengths and limitations of the study
The process of medication reviews was studied in a large sample of pharmacists and GPs by means of a cluster controlled trial, and a cost-evaluation was included. Despite these strengths, this study was not without limitations. Only medication changes were taken into account; therefore, a considerable number of recommendations with solutions other than medication changes were not examined. For example, the effect of some drug–drug interactions can be monitored by checking blood pressure or other parameters, but it was not checked whether such actions were taken. This loss of data undoubtedly reduced the ability to detect differences, and made this analysis fairly conservative.
Time logs were received from only 37% of participating GPs, and as the calculations that include GP time expenses are based on a small number of patients, they have to be interpreted with some reservations. Statistical tests were not performed on these figures.
GPs were chosen by the participating pharmacists (convenience sample). It is possible that only GPs with whom the pharmacists had the best professional relationships were included, which could have led to an over-rating of the effect of these interventions.
Comparison with existing literature
This study indicates that treatment reviews involving personal contact (case conferences) lead to more medication changes than an intervention including only written feedback. Studies considering improvement of prescribing practice report similar findings.15–18 As the extra costs of this approach seem to be covered by the extra savings on medicine costs, the case-conference approach is recommended in practice.
The persistence of medication changes over time was also investigated. Eighty-six per cent of the medication changes were maintained for at least 9 months after the treatment review in the written-feedback group, and 79% in the case-conference group. Other studies have shown that 84%19 and 64%20 of the interventions were maintained until 6 months after the intervention. Another study has shown that 90% of recommendations made by a clinical pharmacist remained implemented up to 1 year after the patient interview.21
Savings on medicine costs were also studied. Although older patients included in this study were using at least five prescription medicines, prescribing omissions were identified. Studies concerning the quality of pharmaceutical care have also observed omissions.2,22–26 In a number of cases, these prescribing omissions were for newer, more expensive medicines (for example, bisphosphonates and proton-inhibitors); therefore, it is not surprising that the medicine costs did not decrease much. Some studies have found differences in costs after medications reviews,13,19,27–30 while others have not.4,31–33
Implications for future research
At the time the study was planned, a number of trials were performed considering the effectiveness of treatment reviews. In the trials published at the time, no statistical difference in health status or patient satisfaction was found.3–6 As a result of the information from the literature and because the current study examined the differences between two procedures for treatment reviews (case conferences versus written feedback), it was decided not to make the study more complicated by measuring health status at patient level. However, positive trends in clinical outcomes were found in a later study by Sorensen et al.34
In a systematic review some evidence was found that pharmacist-led interventions incorporating a medication review are effective in reducing hospital admissions,35 and in a further study concerning older people living in care homes a reduction in the number of falls was found when performing clinical medication review.30
Further research is needed to examine the clinical consequences of treatment reviews and medication reviews. To measure the clinical consequences, health status, health-related patient satisfaction, numbers of falls, hospital admissions, and mortality rates could be used. These studies should include large populations of older people because medication is just one of the parameters that influences these clinical outcome measures. These kinds of studies have already been carried out for home-based interventions after hospital admissions to prevent re-admissions,36,37 showing variable and unexpected results.
Supplementary Material
Acknowledgments
The authors thank Service Apotheek Beheer BV, all participating pharmacists and GPs for their contribution to data collection. The authors thank A de Bruyn and H Leenders for their assistance during the data collection and data processing and R Akkermans for the performance of multilevel statistics. The authors thank YA Hekster, PharmD, PhD; PAF Jansen, MD, PhD; JR van der Laan, MD; and EC Weening PharmD for their contributions to the expert panel.
Supplementary information
Additional information accompanies this paper at: http://www.rcgp.org.uk/bjgp-suppinfo
Funding body
This study was funded by a research grant from the Royal Association for the Advancement of Pharmacy (KNMP)
Ethics committee
Before the start of this study the study protocol was applied at the Commissie Mensgebonden Onderzoek Regio Arnhem-Nijmegen (CMO-nr: 2003/242) for ethical approval. This board decided that based on the study protocol and the WMO regulations (regulations for research including human) no ethical approval was needed
Competing interests
Service Apotheek Nederland supported this study with their organisational skills
REFERENCES
- 1.Denneboom W, Dautzenberg MG, Grol R, Smet PA. User-related pharmaceutical care problems and factors affecting them: the importance of clinical relevance. J Clin Pharm Ther. 2005;30(3):215–223. doi: 10.1111/j.1365-2710.2005.00636.x. [DOI] [PubMed] [Google Scholar]
- 2.Denneboom W, Dautzenberg MG, Grol R, De Smet PA. Analysis of polypharmacy in older patients in primary care using a multidisciplinary expert panel. Br J Gen Pract. 2006;56(528):504–510. [PMC free article] [PubMed] [Google Scholar]
- 3.Hanlon JT, Weinberger M, Samsa GP, et al. A randomized, controlled trial of a clinical pharmacist intervention to improve inappropriate prescribing in elderly outpatients with polypharmacy. Am J Med. 1996;100(4):428–437. doi: 10.1016/S0002-9343(97)89519-8. [DOI] [PubMed] [Google Scholar]
- 4.Krska J, Cromarty JA, Arris F, et al. Pharmacist-led medication review in patients over 65: a randomized, controlled trial in primary care. Age Ageing. 2001;30(3):205–211. doi: 10.1093/ageing/30.3.205. [DOI] [PubMed] [Google Scholar]
- 5.Zermansky AG, Petty DR, Raynor DK, et al. Clinical medication review by a pharmacist of patients on repeat prescriptions in general practice: a randomised controlled trial. Health Technol Assess. 2002;6(20):1–86. doi: 10.3310/hta6200. [DOI] [PubMed] [Google Scholar]
- 6.Zermansky AG, Petty DR, Raynor DK, et al. Randomised controlled trial of clinical medication review by a pharmacist of elderly patients receiving repeat prescriptions in general practice. BMJ. 2001;323(7325):1340–1343. doi: 10.1136/bmj.323.7325.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Smet PA, Dautzenberg M. Repeat prescribing: scale, problems and quality management in ambulatory care patients. Drugs. 2004;64(16):1779–1800. doi: 10.2165/00003495-200464160-00005. [DOI] [PubMed] [Google Scholar]
- 8.Grimshaw JM, Thomas RE, MacLennan G, et al. Effectiveness and efficiency of guideline dissemination and implementation strategies. Health Technol Assess. 2004;8(6):iii–iv. 1–72. doi: 10.3310/hta8060. [DOI] [PubMed] [Google Scholar]
- 9.Teichert M, Baart F, De Smet PA, Peter AGM. Decline in prolonged hormone replacement therapy in women aged 45 years or more, and impact of a centralised database tool. Int J Pharm Pract. 2006;14(1):31–35. [Google Scholar]
- 10.World Health Organization. WHO Collaborating Centre for Drug Statistic Methodology. Geneva: World Health Organization; 2005. ATC Index with DDDs 2005. [Google Scholar]
- 11.Fick DM, Cooper JW, Wade WE, et al. Updating the Beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts. Arch Intern Med. 2003;163(22):2716–2724. doi: 10.1001/archinte.163.22.2716. [DOI] [PubMed] [Google Scholar]
- 12.van der Kuy A. Medisch Farmaceutische Voorlichting/Uitgave van de Commissie Farmaceutische Hulp van het College van zorgverzekeraars. The Dutch Health Insurance Board; 2004. Farmacotherapeutisch kompas. [Drug compendium] [Google Scholar]
- 13.Bos E. Polyfarmacieproject bespaart kosten [Polypharmacy project causes savings] Pharm Weekbl. 2005;140(34):1052–1053. [Google Scholar]
- 14.Litell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS system for mixed models. Cary, NC: SAS Institute Inc; 1999. [Google Scholar]
- 15.Pippalla RS, Riley DA, Chinburapa V. Influencing the prescribing behaviour of physicians: a metaevaluation. J Clin Pharm Ther. 1995;20(4):189–198. doi: 10.1111/j.1365-2710.1995.tb00648.x. [DOI] [PubMed] [Google Scholar]
- 16.Grimshaw JM, Shirran L, Thomas R, et al. Changing provider behavior: an overview of systematic reviews of interventions. Med Care. 2001;39(8 Suppl 2):II2–45. [PubMed] [Google Scholar]
- 17.Anderson GM, Lexchin J. Strategies for improving prescribing practice. CMAJ. 1996;154(7):1013–1017. [PMC free article] [PubMed] [Google Scholar]
- 18.Soumerai SB, McLaughlin TJ, Avorn J. Improving drug prescribing in primary care: a critical analysis of the experimental literature. Milbank Q. 1989;67(2):268–317. [PubMed] [Google Scholar]
- 19.Britton ML, Lurvey PL. Impact of medication profile review on prescribing in a general medicine clinic. Am J Hosp Pharm. 1991;48(2):265–270. [PubMed] [Google Scholar]
- 20.Grymonpre RE, Williamson DA, Montgomery PR. Impact of a pharmaceutical care model for non-institutionalised elderly: results of a randomised, controlled trial. Int J Pharm Pract. 2001;9:235–241. [Google Scholar]
- 21.Petty DR, Zermansky AG, Raynor DK, et al. Clinical medication review by a pharmacist of elderly patients on repeat medications in general practice: pharmacist interventions and review outcomes. Health Technol Assess. 2002;6(20):39–46. doi: 10.3310/hta6200. [DOI] [PubMed] [Google Scholar]
- 22.Higashi T, Shekelle PG, Solomon DH, et al. The quality of pharmacologic care for vulnerable older patients. Ann Intern Med. 2004;140(9):714–720. doi: 10.7326/0003-4819-140-9-200405040-00011. [DOI] [PubMed] [Google Scholar]
- 23.Barakat K, Wilkinson P, Deaner A, et al. How should age affect management of acute myocardial infarction? A prospective cohort study. Lancet. 1999;353(9157):955–959. doi: 10.1016/S0140-6736(98)07114-1. [DOI] [PubMed] [Google Scholar]
- 24.Rochon PA, Gurwitz JH. Prescribing for seniors: neither too much nor too little. JAMA. 1999;282(2):113–115. doi: 10.1001/jama.282.2.113. [DOI] [PubMed] [Google Scholar]
- 25.Lipton HL, Bero LA, Bird JA, McPhee SJ. Under medication among geriatric outpatients: results of a randomized controlled trial. Annu Rev Gerontol Geriatr. 1992;12:95–108. [Google Scholar]
- 26.Gollub SB. Is intensive drug therapy appropriate for older patients? Lancet. 1999;353:940–941. doi: 10.1016/S0140-6736(99)90024-7. [DOI] [PubMed] [Google Scholar]
- 27.Boer WO, Mill van JWF, Mulder-Wildemors LMG, Tromp TFJ. De toegevoegde waarde van samenwerking huisarts-apotheker [The added value of cooperation between GPs and pharmacists] Pharmaceutisch Weekblad. 2002;137(44):1565–1569. [Google Scholar]
- 28.Mason JD, Colley CA. Effectiveness of an ambulatory care clinical pharmacist: a controlled trial. Ann Pharmacother. 1993;27(5):555–559. doi: 10.1177/106002809302700503. [DOI] [PubMed] [Google Scholar]
- 29.Rodgers S, Avery AJ, Meechan D, et al. Controlled trial of pharmacist intervention in general practice: the effect on prescribing costs. Br J Gen Pract. 1999;49(446):717–720. [PMC free article] [PubMed] [Google Scholar]
- 30.Zermansky AG, Alldred DP, Petty DR, et al. Clinical medication review by a pharmacist of elderly people living in care homes — randomised controlled trial. Age Ageing. 2006;35(6):586–591. doi: 10.1093/ageing/afl075. [DOI] [PubMed] [Google Scholar]
- 31.Sellors J, Kaczorowski J, Sellors C, et al. A randomized controlled trial of a pharmacist consultation program for family physicians and their elderly patients. CMAJ. 2003;169(1):17–22. [PMC free article] [PubMed] [Google Scholar]
- 32.Malone DC, Carter BL, Billups SJ, et al. An economic analysis of a randomized, controlled, multicenter study of clinical pharmacist interventions for high-risk veterans: the IMPROVE study. Impact of Managed Pharmaceutical Care Resource Utilization and Outcomes in Veterans Affairs Medical Centers. Pharmacotherapy. 2000;20(10):1149–1158. doi: 10.1592/phco.20.15.1149.34590. [DOI] [PubMed] [Google Scholar]
- 33.Furniss L, Burns A, Craig SK, et al. Effects of a pharmacist's medication review in nursing homes. Randomised controlled trial. Br J Psychiatry. 2000;176:563–567. doi: 10.1192/bjp.176.6.563. [DOI] [PubMed] [Google Scholar]
- 34.Sorensen L, Stokes JA, Purdie DM, et al. Medication reviews in the community: results of a randomised, controlled effectiveness trial. Br J Clin Pharmacol. 2005;58(6):649–664. doi: 10.1111/j.1365-2125.2004.02220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Royal S, Smeaton L, Avery AJ, et al. Interventions in primary care to reduce medication related adverse events and hospital admissions: systematic review and meta-analysis. Qual Saf Health Care. 2006;15(1):23–31. doi: 10.1136/qshc.2004.012153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holland R, Lenaghan E, Harvey I, et al. Does home based medication review keep older people out of hospital? The HOMER randomised controlled trial. BMJ. 2005;330(7486):293. doi: 10.1136/bmj.38338.674583.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stewart S, Pearson S, Luke CG, Horowitz JD. Effects of home-based intervention on unplanned readmissions and out-of-hospital deaths. J Am Geriatr Soc. 1998;46(2):174–180. doi: 10.1111/j.1532-5415.1998.tb02535.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.