Abstract
Concern is mounting regarding the human health and environmental effects of bisphenol A (BPA), a high-production-volume chemical used in synthesis of plastics. We have reviewed the growing literature on effects of low doses of BPA, below 50 mg/kg/day, in laboratory exposures with mammalian model organisms. Many, but not all, effects of BPA are similar to effects seen in response to the model estrogens diethylstilbestrol and ethinylestradiol. For most effects, the potency of BPA is approximately 10 to 1,000-fold less than that of diethylstilbestrol or ethinylestradiol. Based on our review of the literature, a consensus was reached regarding our level of confidence that particular outcomes occur in response to low-dose BPA exposure. We are confident that adult exposure to BPA affects the male reproductive tract, and that long-lasting, organizational effects in response to developmental exposure to BPA occur in the brain, the male reproductive system, and metabolic processes. We consider it likely, but requiring further confirmation, that adult exposure to BPA affects the brain, the female reproductive system, and the immune system, and that developmental effects occur in the female reproductive system.
Keywords: Behavior, Neuroendocrine, Endocrine Disruptors, Immune System, Metabolism, Mouse, Rat, Reproduction
INTRODUCTION
Bisphenol A (BPA) is used as the monomer to manufacture polycarbonate plastic, the resin that lines most food and beverage cans, dental sealants, and as an additive in other plastics (Figure 1, Table 1) [1]. BPA is one of the highest volume chemicals produced worldwide; global BPA production capacity in 2003 was 2.2 million metric tons (over 6.4 billion pounds), with a 6-10% growth in demand expected per year [2]. Heat and either acidic or basic conditions accelerate hydrolysis of the ester bond linking BPA monomers, leading to release of BPA and the potential for human and environmental exposure. Studies conducted in Japan [3] and in the USA [4] have shown that BPA accounts for the majority of estrogenic activity that leaches from landfills into the surrounding ecosystem.
Figure 1.
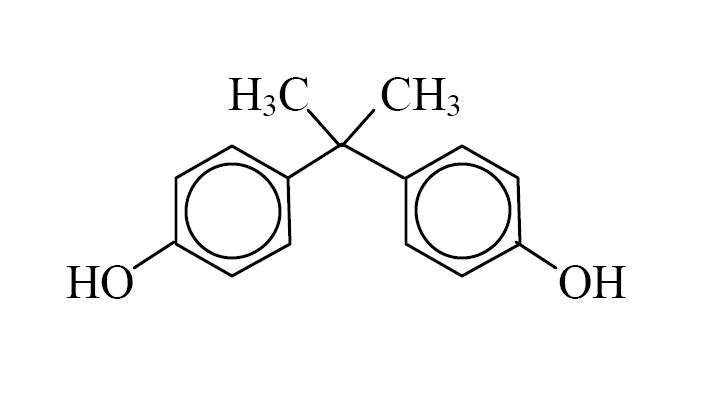
Chemical structure of bisphenol A (BPA).
Table 1.
| Molecular formula | (CH3)2C(C6H4OH)2 |
|---|---|
| Molecular weight | 228.29 |
| Water solubility | 120-300 mg/L |
| log kow | 3.40 |
| Melting point | 150-155 °C |
| Boiling point | 220 °C (4 mm Hg) |
| LD50*, rat, oral | 3,300-4,240 mg/kg [199] |
| LD50, mouse, oral | 2,500-5,200 mg/kg [1] |
| LD50, fish (Pimephales promelas), 96 hr | 4.6 mg/L [1] |
acute lethal dose, 50 % of population
BPA has been demonstrated in both in vivo and in vitro experiments to act as an endocrine disrupting chemical (EDC) (reviewed in [5, 6]). There is extensive evidence that BPA is an estrogen-mimicking chemical, although recent findings have revealed that BPA is a selective estrogen receptor modulator (SERM), since BPA and the potent endogenous estrogen 17β-estradiol (E2) do not always show identical effects, and in some studies BPA has been shown to antagonize the activity of E2 [7]. There is evidence that, similar to other estrogens, BPA can bind to androgen receptors and inhibit the action of androgen [8]. In addition, there is evidence for an anti-thyroid hormone effect of BPA [9]. However, effects of BPA mediated by binding to androgen and thyroid hormone receptors appear to require higher doses than those required to elicit estrogenic or antiestrogenic responses [7].
Chemicals classified as endocrine disruptors include not only hormone-mimics or antagonists that act via binding to receptors, but also chemicals that can interfere with hormone synthesis and clearance, as well as other aspects of tissue metabolism. Experiments have shown that BPA influences enzyme activity and thus metabolism in various tissues. Another mechanism of endocrine disruption is the alteration of hormone receptor expression, and experiments described below have shown that BPA alters hormone receptor numbers and hormone receptor gene activity in target tissues.
In this review, we summarize the recent literature on low-dose effects of BPA in laboratory animals. The majority of the studies used rats and mice; only a few used other mammalian species. We conclude with a series of statements expressing our level of confidence concerning various effects of BPA in laboratory animals at low doses.
Rationale for experimental design, analysis and reporting criteria
Endocrinology experiments with laboratory animals are particularly vulnerable to confounding effects. Among other difficulties, treatment effects can be masked by hormonally active components of feed, water, or caging; and species and strains differ greatly in their sensitivity to different hormonally active compounds. Therefore, proper reporting of experimental design is critical to evaluation of studies in the literature. Below, we discuss several of the most important aspects of experimental design, which we considered when evaluating the studies discussed in this review.
Animal model
Published results must identify precisely the animal model and supplier being used. For example, Sprague-Dawley rats from different commercial breeders cannot be assumed to be the same, since it is an outbred stock. In particular, the outbred Sprague-Dawley CD rat from Charles River Laboratories [Crl:CD(SD)] has very low sensitivity to exogenous estrogens, and after more than 50 years of selective breeding by Charles River for large body size and litter size, it would be inappropriate to identify these rats as just Sprague-Dawley (Table 2). In contrast to the Crl:CD(SD) rat, male and female CD-1 (ICR) mice are highly sensitive to exposure to low doses of BPA during development as revealed by over 20 published studies (reviewed below) reporting significant effects of low doses of BPA in this outbred stock. This high sensitivity of the CD-1 mouse to BPA is predicted by the high responsiveness to positive control estrogens: E2, ethinylestradiol and diethylstilbestrol (DES), as revealed by both the in vivo studies discussed here and other studies of CD-1 cells and organs in primary culture [10-12].
Table 2.
Lack of effects reported in low-dose in vivo bisphenol A (BPA) research using the Charles River Laboratories Sprague-Dawley (Crl:CD(SD)) rat strain.
| Crl:CD(SD) RAT
STUDIES: | |
|---|---|
| Effect | No Effect |
| 0 (0%) | 13 (100%) |
The marked difference in sensitivity of different animal models used in toxicological, pharmacological and endocrinological research is just one of many reasons why it is essential that experiments include appropriate positive controls, which is discussed in more detail in following sections. With regard to examination of the in vivo estrogenic activity of BPA, which is the subject of this review, the sensitivity of the endpoint of interest in the chosen animal model should be characterized with a positive control such as E2 (appropriate if administration is by injection or subcutaneous capsule, due to very limited oral absorption of E2) or either DES or ethinylestradiol (appropriate if chemicals are administered orally, since they are orally active at very low doses). For example, in the CD-1 mouse, which is the animal model used by the U.S. National Toxicology Program, an appropriate positive control dose of ethinylestradiol or DES to detect a response to BPA within the low-dose range (below 50 mg/kg/day) would be an oral dose not greater than 5 μg/kg/day. This suggestion is based on numerous reports that for responses mediated by nuclear estrogen receptors, estimates of BPA potency in CD-1 mice range between 10 – 1,000-fold less than either ethinylestradiol or DES, depending on the specific response being measured [10, 12-16]. Also, doses of DES above 5 μg/kg/day can result in opposite effects relative to lower doses; this has been shown following developmental exposure for the prostate [16, 17] and uterus [18]. High doses of these positive control estrogenic chemicals are thus not appropriate as a positive control for low-dose effects of BPA or other estrogenic endocrine disrupting chemicals.
Feed, water, and housing
The exact feed used must be identified. Ideally, the estrogenicity of the feed should be characterized, since estrogenic components have been demonstrated to occur in both soy-based and non-soy-based animal feeds. There is also the possibility of variation in estrogenic activity between different lots of feed commonly used in toxicological research [19]. If possible, the same lot of feed (same mill date) should be used throughout an experiment.
The type of caging should be carefully selected to avoid estrogenic contamination of experimental animals. In particular, polycarbonate cages and water bottles should not be used, since they will leach uncontrolled concentrations of BPA to the experimental animals [20]. Polypropylene cages have been used successfully in studies of estrogens in mice, and polysulfone cages are available to replace polycarbonate; polysulfone is a co-polymer containing BPA and sulfone, but it is reported to be more resistant to degradation at high temperatures relative to polycarbonate [20]. Similarly, the source of drinking water must be free of BPA and other estrogens; reverse osmosis and carbon filtration is often necessary to achieve this requirement. In addition, if BPA is delivered in drinking water, the water must be free of chlorine or other reactive ions. Adding BPA to chlorinated water results in formation of tetrachlorobisphenol A.
Method of dosing
In all cases the precise method of dosing the animals and the time of dosing should be identified. Methods of administration of BPA include: 1. Oral (p.o.): by gavage, by adding BPA to feed or drinking water, or by feeding the chemical in oil; 2. Injection: subcutaneous (s.c.), intraperitoneal (i.p.), intracisternal, or intramuscular (i.m.) routes; and 3. Implantation of Silastic® capsules or Alzet® minipumps that lead to steady-state exposures. The rationale for the dosing method must also be stated. For example, minipump implants model continuous exposure and avoid the first-pass metabolism of BPA in the liver that results from oral exposure.
The positive control chosen must be compatible with the selected route of exposure. For example, E2 has very low activity when administered orally. For studies in which BPA is administered orally, DES or ethinylestradiol are appropriate positive controls. When BPA is administered in constant release capsules, E2 would be an appropriate positive control, while an estrogen agonist with a long half-life would be inappropriate. Route of administration influences the rate of metabolism of BPA, at least in adults [21], and there is some evidence for a higher contribution from ingestion of BPA in humans relative to inhalation or absorption through the skin [22]. However, the report that BPA levels in plasma collected from pregnant women in Germany show a range encompassing two orders of magnitude, between 0.1 and 10 parts per billion [23], suggests the likely possibility of variable exposure to multiple sources of BPA. Thus, although oral delivery appears to be most relevant for extrapolation to humans, all delivery methods may reveal effects of BPA.
Design and Analysis Issues
When animals are assigned to groups, the litter must be taken into account. It is well established that for both outbred stocks and inbred strains, the litter is a significant source of variation and needs to be accounted for in assigning animals to groups. Ideally, one animal per litter should be used. In cases when this is not possible, there are statistical methods (such as including litter as a main effect variable and dividing the F value for treatment effects by the F value for litter effects) that can be used when more than one animal per litter is used, or litter can be used as a covariate in ANCOVA. The method used to avoid confounding litter effects must be reported.
For all experiments, the positive and negative controls must be clearly identified. Experiments using a replicate block design should include a positive and a negative control in each block, if possible. Experiments showing no effect and lacking positive control data cannot be interpreted.
When taking measurements from adult animals, care must be taken to normalize the reproductive state of the experimental animals. Males should be singly housed for 2-4 weeks before collection, in order to avoid physiological differences arising from differences in dominance status of the males. Normalization of adult female reproductive status can be achieved by assaying their estrous cycles and collecting females at the same stage, or by using ovariectomized females with or without hormone replacement [24].
Definition of “Low Dose”
Low-dose effects of environmental endocrine disrupting chemicals generally refer to effects being reported at doses lower than those used in traditional toxicological studies for risk assessment purposes. “Low dose” is also commonly used to refer to environmentally relevant doses, i.e., doses resulting in serum levels close to those observed in human serum. For BPA, prior to 1997, the lowest dose studied for risk assessment purposes was 50 mg/kg/day, which in the USA remains the currently accepted lowest observed adverse effect level (LOAEL) that was used to calculate the current EPA reference dose (and FDA acceptable daily intake or ADI dose) of 50 μg/kg/day; this presumed “safe” dose is estimated by dividing the LOAEL by three 10-fold safety factors (i.e. by 1000) [25]. Thus, we included in our analysis studies dosing with less than 50 mg/kg/day BPA (Tables 3 and 4).
Table 3.
Published studies since 1997 (reviewed in detail in Table 4) that reported low-dose effects of bisphenol A (BPA) in mice and rats resulting from exposure during development or during adulthood.
| Number of Studies (Total number of studies, including citations in other sections) | Observed adverse effects |
|---|---|
| 51 (51) | Altered brain physiology, structure, and behavior |
| 22 (27) | Altered male reproductive organs, including lower sperm production |
| 17 (19) | Altered female reproductive organs, including accelerated puberty |
| 8 (22) | Altered metabolism |
| 7 (7) | Altered immune system |
Table 4.
Published papers reporting biological effects in animal studies (excluding aquatic animals) for bisphenol A (BPA) in the low-dose exposure range (<50,000 μg/kg BW/day), sorted by tissue or endpoint examined. The information indicated in the column headings provides information in published studies. If information is not reported, this is indicated by “NR”. Day plug observed = GD 0. Mice GD 19 = PND 1. Rats GD 22 = PND1. Female = “F”; Male = “M”; DES = diethylstilbestrol.
| Reference | In Other Sections |
Animal | Sex | Exposure, Vehicle |
Time of Exposure |
Doses Tested, μg/kg BW/day (*P<0.05) |
Endpoints | Age at Collection |
Other Chemicals Tested |
|---|---|---|---|---|---|---|---|---|---|
| Effects on Brain Physiology | |||||||||
| Akingbemi, 2004 [41] |
Sex Diff., Testost., Metab. |
Long Evans Rat, CRL | M | Oral, Oil Oral Gavage |
PND 21-PND 35 GD 12-PND 21 |
2.4*, 10(*),100,000, 200,000
2.4*, 10*, 100,000*, 200,000 2.4* |
Serum LH, Testosterone, suppression Serum Estradiol Suppression Pituitary LHβ and ERα mRNA Expression; Body Weight; Seminal Vesicle Weight (see also In Vitro) | PND 35 PND 90 |
|
| Aloisi, 2001 [120] | Rat | F | Oral, Oil | adult | 40,000* | ERα | adult | ||
| Ceccarelli, 2007 [32] | Sprague-Dawley Rat Harlan Italy | F, M | Oral | PND 23 - 30 | 40* | Hypothalamic ERα | PND 27 PND 90 | ethinylestradiol | |
| Evans, 2004 [39] | Sheep, Poll Dorset | F | Injection, Oil | 4 weeks (prepuberty) - 11 weeks | 3,500* | Tonic LH Secretion; LH Pulse Frequency & Amplitude | 11 weeks | DES, Octylphenol | |
| Facciolo, 2002 [34] | Sprague-Dawley Rat (CRL Italy) | F | Oral, Oil | GD 0 – PND 23 | 40, 400* | GABAA Receptors | PND 10, PND 23 | ||
| Funabashi, 2001 [117] | Rat | F | Injection, s.c. | adult | 50,000* | Hypothalamic Preoptic Area Progesterone Receptors | Estradiol, Butyl benzyl phthalate | ||
| Funabashi, 2004 [119] | Rat | F | Inection s.c. | adult | 40,000* | Frontal and Temporal Cortex change in Progesterone Receptor mRNA | adult | Octylphenol Nonylphenol | |
| Funabashi, 2003 [118] | Rat | F | Oral, Oil | adult | 4, 40, 400*, 4,000* | Hypothalamic Progesterone Receptors, POA and VMH | adult | Estradiol | |
| Kawai, 2007 [33] | ICR mice | M | Oral | GD 11-17 | 2* | Change in ERα And ERβ immnoactivity in dorsal raphe nucleus | PND 35 PND 63 PND 91 | ||
| Kawato, 2004 [42] | Wistar Rat | M | Oral, Water | GD 11-PND 21 | 0.1*, 1, 10, 50 mg/L in drinking water | Brain Steroidogenesis | 4 weeks | ||
| Khurana, 2000 [31] | Fischer 344 Rat | F, M | Injection, Oil | PND 1 - 5 | 15,000*, 75,000*
15,000* |
Hyperprolactinemia Pituitary ERα, ERβ Expression | PND 10-PND 30 | DES, Octylphenol | |
| MacLusky, 2005 [44] | Rat | F | Injection, Oil | adult | 40*, 120*, 400*
45* |
Hippocampal Pyramidal Neuron Synaptogenesis | adult | 17β-Estradiol, 17α-Estradiol | |
| Nikaido, 2004 [15] | Puberty, Metab. |
Crj:CD-1 (ICR) Mouse | F | Injection, DMSO | GD15-GD18 | 500*, 10,000*
500*, 10,000* 500*, 10,000* 500, 10,000* |
Cycle Length, Diestrus Reduced Corpora Lutea Mammary Gland Dev. Age at Vaginal Opening | 4,8,12,16 weeks and weeks 9-11 examined for estrous cycles, | DES, Genistein, Zearalenone, Resveratrol |
| Ramos, 2003 [30] | Prostate | Wistar-derived Rat | M | Osmotic Minipump | GD 7 – 22 | 25*, 250*
25*, 250* |
Ventral Prostate; HPG Axis ERβ | PND 15, 30, 120 | |
| Rubin, 2001 [37] | Metab. | Sprague Dawley Rat, Taconic | F | Oral, Water | GD6 – PND 21 | 100*, 1,200* | Body Wt., Cyclicity, LH | BW from PND2-110 Cycles 4 and 6 months | |
| Savabieasfahani, 2006 [40] | Suffolk Ewes | F | Sc injection in cottonseed oil into pregnant dams | GD30-GD90 | 5000* | Low birth weight Increased LH levels during 1st two months of postnatal life Extended breeding season Dampened magnitude of LH surge | 6weeks-40+ weeks of age | Methoxychlor | |
| Steinmetz, 1997 [121] | Rat | F | Implant | adult | 300* | Serum Prolactin; PRF Activity (see also In Vitro) | adult | Estradiol | |
| Talsness, 2000 [38] | Prostate, Metab. |
Sprague Dawley Rat, Harlan-Winkelmann | F, M | Oral, Oil | GD 6- GD 21 | 100*, 50,000*
100*, 50,000 100*, 50,000 |
Reproductive System Effects Body Weight, Estrus Cycle; AGD in Males | adult 3 months PND 3, 15, 21 | Ethinylestradiol |
| Zoeller, 2005 [9] | Sprague Dawley Rat, Zivic Miller | F, M | Oral, Feed Via treated wafer | GD 6-duration not defined | 1,000*, 10,000*, 50,000* | Serum Thyroxin; Dentate Gyrus RC3/Neurogranin Expression | PND 4, 8, 15, and 35 | ||
| Zsarnovszky, 2005 [43] | Sprague Dawley Rats | F, M | Injections into cerebellum | PND4- PND19 and adults 250-300g | Concentrations of 10-12- 10−6 M BPA were injected into the cerebellum (3ul in PND4-10, 5ul into older animals) | Rapidly increased pERK beginning on PND8; Co-administration with E2 inhibited rapid E2 induced ERK1/2 activation. Dose dependent | PND4-PND19 and adults | Cyclodextrin encapsulated estradiol | |
| Effects on Brain Structure | |||||||||
| Kubo, 2001 [45] | Sex Diff. | Wistar Rat | F, M | Oral, Water | mating to weaning | 1,500* | Brain, Behavior. Loss of sexual dimorphism in behavior and in size of locus coeruleus | 6-12 weeks | |
| Kubo, 2003 [46] | Sex Diff., Prostate |
Rat | F, M | Oral, water | mating to weaning | 30*, 300* | Brain/Behavior. Reversal in the sexually dimorphic volume of the locus coeruleus, behavioral changes | postnatally, 6, 11-12 and 14 Weeks | DES, Resveratrol |
| Masuo, 2004 [49] | Behav.. | Wistar Rat | M | Intracisternal Injection, Oil 10 μl into pups | PND 5 | Approximately 2, 20*, 200*, 2,000*
2,000* |
Motor Hyperactivity (Spontaneous Motor Activity) Midbrain Gene Expression | PND 56 | Nonylphenol, Octylphenol, DEHP |
| Nakamura, 2006 [50] | ICR/Jcl Mice | F, M | sc injection, oil | GD 0- collection | 20* | Altered Neuronal Migration and Gene Expression | GD 10.5- GD 16.5 | ||
| Patisaul, 2006 [48] | Sex Diff. | Sprague Dawley Rat, CRL | F, M | SC injection in sesame oil into pups | PND1-2 | Approximately 75000* | Altered AVPV development Altered Sexual dimorphism of Dopamine (TH) neuron number and number of TH/ER expressing Neurons | PND 19 | |
| Rubin, 2006 [47] | CD-1 Mouse | F, M | Osmotic minipump | GD 8- PND 21 | 0.025, 0.250* | Dopamine neuron number in the AVPV reduced/ loss of expected sexual dimorphism | PND 24-27 | ||
| Effects on Behavior | |||||||||
| Adriani, 2003 [64] | Sprague Dawley Rat (Italy) | F, M | Oral, Oil | GD 0 – PND 26 | 40* | Behavioral Tests, M & F; Amphetamine Response, M | PND30-35, PND 70 | ||
| Aloisi, 2002 [56] | Sprague Dawley Rats, CRL | F, M | Oral, Oil Trained to suck BPA from pipette | Dams exposed thru pregnancy and lactation. 2 groups: placental exp suckling exp | 40* | Pain Behavior; formalin induced nociception | 22 weeks of age | ||
| Della Seta, 2005 [61] | Metab. | Rat | F | Oral, Oil | adult | 40* | Maternal Behavior | ||
| Della Seta, 2006 [67] | Testost. | Sprague Dawley (Italy) | M | Oral, Oil | PND 23- PND 30 | 40* | Decreased Serum Testosterone | PND 37, 45, >90 | Ethinylestradiol |
| Dessi-Fulgheri, 2002 [62] | Sprague Dawley Rat (Italy) | F, M | Oral, Oil | 40, GD 0- PND 21; 400, GD 14- PND 6 | 40*, 400*
40*, 400 40, 400* |
Play Behaviors | PND 35, 45, 55 | ||
| Farabollini, 2002 [51] | Sprague Dawley Rat (Italy) | F, M | Oral, Oil | GD 0- GD 22; PND1- PND21 | 40*
40* |
Aggression, Sexual Behavior | PND 100 | ||
| Ishido, 2004 [54] | Metab. | Wistar Rat | M | Intracistern al Injection, Oil 10 μl | PND5 | ≈3, ≈30, ≈300*, ≈3,000*
≈3, ≈30, ≈300, ≈3,000* (87fmol- 87nmol BPA/rat)- 0.02-20 ug/rat |
Hyperactivity Reduced gene expression of Dopamine Receptor and Dopamine Transporter, reduded midbrain TH- IR | 4-8 weeks | |
| Kawai, 2003 [52] | Testost. | CD-1 Mouse | M | Oral, Oil | GD 11-17 | 2*, 20* | Aggression, Testis Wt. | 8, 12, 16 weeks | |
| Laviola, 2005 [66] | Sex Diff. | CD-1 Mouse CRL | F, M | Oral, Oil Drink from syringe | GD 11-18 | 10* | Amphetamine- Induced Conditioned Place Preference | 60 days | Methoxychlor |
| Masuo, 2004 [53] | Rat | Intracistern al Injection | neonatal | ~20 μg/kg* | Motor Hyperactivity | 4-5 weeks | |||
| Mizuo, 2004 [55] | ddY mice | oral, feed | GD 0- ~PND 14 | 0.002, 0.5*, 2* mg/g in food | Enhanced Reward Effect and Hyperlocomotion in Response to Morphine | adult | |||
| Negishi, 2004 [59] | F344/N Rat | M | Oral, Oil (corn) gavage | GD3- PND20 | 100* | Behavioral Alterations Active Avoidance Test | 8 weeks- 24 weeks | Nonylphenol | |
| Nishizawa, 2005 [35, 36] | Mouse | F, M | Oral, Oil | GD 6 -13 GD 6-17 | 0.02*, 2*, 200*, 2,000* | Embryonic Brain and Gonad AhR Expression; RARα, RXRα Expression | |||
| Palanza, 2002 [60] | Metab. | CD-1 Mouse, CRL | F | Oral, Oil | GD 14-18 | 10* | Maternal Behaviors | In adulthood | |
| Porrini, 2005 [63] | Sprague Dawley Rat, CRL Italy | F, M | Oral, Peanut Oil, micropipette | daily from mating to parturition | 40* | Socio-Sexual Behaviors | 35,45,55 days | ||
| Razzoli, 2005 [122] | Gerbil | F | Oral, Oil | adult | 2*, 20
2*, 20*; 2*, 20; & 2, 20* |
Social Investigative Behavior Free Exploratory Tests | Ethinylestradiol | ||
| Ryan, 2006 [57] | Puberty, Metab. | C57Bl6 Mice | Ov × F | Oral, gavage needle into back of mouth | GD3- PND21 | 2, 200 | Accelerated puberty Altered Anxiety Related Behaviors | Adult , 6 weeks + | Ethinylestradiol |
| Suzuki, 2003 [65] | ddY Mouse | M | Oral, in Feed | GD 0- PND 21 | 0.002, 0.5, 2 mg/g feed ≈300*, ≈75,000*, ≈300,000* | Dopamine D1 Receptor-Mediated Enhanced Induced Abuse State | not specified | ||
| Effects on Sex Differences in the Brain | |||||||||
| Carr, 2003 [68] | Fischer 344 Rat | F, M | Oral, Oil gavage in pups | PND1- PND14 | 100, 250*
100*, 250 |
Morris Water Maze; Elimination of Sex Difference | Tests begin PND 34 | Estradiol | |
| Farabollini, 1999 [58] | Sprague Dawley Rat (Italy) | F, M | Oral, Oil | GD 0- PND 21 | 40*, 400* | Exploratory behavior, Activity | PND 85 | ||
| Fujimoto, 2006 [73] | Wistar rat | F, M | Oral, Drinking water | GD 0- PND 1 | 15* | Sex differences in open field and forced swimming behavioral tests | 7-9 weeks | ||
| Funabashi, 2004 [72] | Wistar Rat | F, M | Oral, Drinking water | Gestation and lactation, no GD identified | ≈2,500*,(2500) | Sex Differences in CRH Neurons in Bed Nucleus of the Stria Terminalis | 4-7 months | ||
| Imanishi, 2003 [70] | ICR Mouse | F, M | Oral, Oil | GD 6- GD 17 | 2* 2* | Placental Nuclear Receptor Gene Expression, 9 of 20 examined; Six Non-Nuclear Receptor Genes | GD 18 | ||
| Nishizawa, 2003 [71] | ICR Mouse | F, M | Oral, Oil | GD 6 - collection | 2* | Embryonic Brain and Gonad Retinoid Receptor Expression RARα, RXRα | GD 12, 14, 16, or 18 | ||
| Shibata, 2002 [69] | Metab. | Wistar Rat | M | Oral | 2 or 4 weeks adulthood | 1,500* | BPA and sex steroid metabolism in adult male | adult | DES |
| Effects on Puberty in Females | |||||||||
| Honma, 2002 [13] | Metab. | ICR/Jcl Mouse | F, M | sc Injection, Oil | GD 11- GD 17 | 2*, 20*
2*, 20 2, 20* |
BW, Estrous Cycle Length, Male AGD Vaginal Cytology; Female AGD Age of Vaginal Opening, First Estrus | up to PND 120 | DES |
| Howdeshell, 1999 [78] | Metab. | CF-1 Mouse | F, M | Oral, Oil | GD 11- GD 17 | 2.4* | Puberty, BW | puberty | |
| Vandenberg, 2007 [79] | Mamm. | CD-1 Mouse | F | Alzeet osmotic pump | GD 8 -18 | 0.25* | Mammary gland morphology | GD 18 | |
| Effects on the Mammary Gland | |||||||||
| Colerangle, 1997 [85] | Rat | F | Osmotic minipump | 4 weeks old administered 11 days | 100*, 54,000* | Mammary Gland differentiation | 5.5 weeks | DES | |
| Durando, 2006 [82] | Wistar Rat | F | Osmotic minipump | GD 7 -22 | 25* | Mammary Gland Cancer | PND 30, 50, 110, 180 | ||
| Markey, 2001 [80] | CD-1 Mouse | F | Osmotic minipump | GD 8- GD 19 | 25*, 250* | Mammary Gland | PND 10, 1 month, 6 months | ||
| Munoz-de-Toro, 2005 [81] | Mouse | F | Osmotic minipump | GD 8 - lactation | 0.025*, 0.250* | Mammary Gland Morphogenesis, Terminal End Bud Density | PND 30 , 120 | ||
| Murray, 2006 [83] | Wistar Rat | F | Osmotic minipump | 2.5*, 25*, 250*, 1000* | Mammary Gland Hyperplasia (carcinoma precursor) | PND 55, 95 | |||
| Effects on the Uterus and Vagina | |||||||||
| Markey, 2005 [88] | Mouse | F | Osmotic minipump | GD 9 - Lactation | 0.025*, 0.250* | Genital Tract Alterations; ERα and Progesterone Receptor Up- Regulation Developmentally | adult | ||
| Nagel, 2001 [173] | Mouse | F | Injection, Oil | adult | 25 (P<0.06), 791*, 25,000* | Uterus, gene expression | DES | ||
| Schönfelder, 2002 [87] | Rat | F | Oral | GD 6-21 | 100*, 50,000* | Vaginal ERα Expression Vaginal Histology | adult | Ethinylestradiol | |
| Schönfelder, 2004 [86] | Sprague- DawleyRat | F | Oral | GD 6-21 | 100*, 50,000*
50,000* 100*, 50,000* |
Uterine Epithelial Histology Uterine ERα Expression Uterine ERβ Expression | adult | Ethinylestradiol | |
| Suzuki, 2002 [14] | Mouse | F | Injection, Oil | GD 10-18 | 10,000*, 100,000* | Uterine and Vaginal Mitotic Indices Following Prenatal Exposure | PND 30 | DES | |
| Effects on the Ovary, Oocytes and Fertility | |||||||||
| Al-Hiyasat, 2004 [90] | Mouse | F | Oral, Water | Adult for 28 days | 5, 25*, 100*
5, 25, 100* |
Increased Resorptions, Uterine
Weight Ovarian Weight |
adult | ||
| Berger, 2007 [92] | Mouse | F | Injection s.c. | GD 1-4 | 3,375*, 10,125* | Implantation rate Fecundity | GD 5 PND 1 | ||
| Hunt, 2003 [89] | C57BL/6 Mouse | F | Oral, Oil | Peripubertal for 6-8 days | 20*, 40*, 100* | Disruption of Meiosis; Aneuploidy | ~30 days old | ||
| Susiarjo, 2007 [91] | C57BL/6 Mouse | F | Oral Injection | GD 11 - 18 | 20* | Disruption of chromosomes | GD 18 | ||
| Effects on Testosterone in Males | |||||||||
| Takao, 1999 [133] | Mouse | M | Oral, Drinking water | adult | ≈120, ≈12,000* | Plasma Free Testosterone | adult | ||
| Effects on the Prostate | |||||||||
| Gupta, 2000 [10] | Mouse | M | Oral, Oil | GD 14-18 | 50* | Prostate Wt., Prostate AR (see also In Vitro) | PND 3, 20, 60 | DES, Aroclor | |
| Ho, 2006 [29] | Sprague- Dawley Rat | M | Injection s.c. | PND 1-5 | 10* | Prostate Cancer (PIN lesions) | adult | Estradiol | |
| Nagel, 1997 [94] | Metab. | CF-1Mouse | M | Oral, Oil | GD11- GD17 | 2*, 20* | Prostate Wt. | 6 months | Octylphenol |
| Ramos, 2001 [97] | Wistar Rat | M | Osmotic Pump, DMSO | GD 7- GD 22 | 25*, 250* | Ventral Prostate | PND 30 | ||
| Stoker, 1999 [100] | Wistar Rat | M | sc injection, oil | PND 22- PND 32 | 50,000* | Serum Prolactin, Lateral Prostate Weight | PND 29, PND 120 | estradiol, pimozide | |
| Timms, 2005 [16] | Mouse | M | Oral, Oil | GD 14-18 | 10* | Fetal Development of Prostate and Urethra | GD 19 | Ethinylestradiol DES | |
| Effects on the Testes, Epididymis, Sperm and Seminal Vesicles | |||||||||
| Aikawa, 2004 [102] | SHN Mouse | M | sc Injection, Oil | PND 1- PND 5 | ≈175*, ≈17,500*
≈175, ≈17,500* |
Abnormal Sperm Decreased Motility | 10 weeks | Estradiol | |
| Al-Hiyasat, 2002 [129] | Metab. | Swiss Mouse | M | Oral | Adult For 1 month | 5*, 25* 100* | Reduced fertility, epididymidal sperm Body weight | adult | |
| Chitra, 2003 [131, 132] | Metab. | Wistar Rat | M | Oral, Oil | Adult For 45 days | 0.2*, 2*, 20*
0.2*, 2*, 20* 0.2*, 2*, 20* 0.2, 2*, 20* 0.2*, 2*, 20* 0.2*, 2*, 20* |
Decreased Testis, Epididymis Weight Increased Ventral Prostate Weight Reduced Sperm Motility, Sperm Count Oxidative Stress Enzymes Increased H2O2 | ||
| Chitra, 2003 [132] | Wistar Rat | M | Oral | adult | 0.2*, 2*, 20* | Decrease in sperm motility Decrease in oxidative stress enzymes | adult | ||
| Fisher, 1999 [104] | Wistar Rat | M | sc Injection, Oil | PND 2- PND 12 | 37,000* | Efferent duct epithelial height | PND 10, 18, 25, 35, and 75 | DES, Ethinylestradiol, Genistein, Octylphenol, Parabens | |
| Sakaue, 2001 [127] | Rat | M | Oral, Oil | adult | 0.2, 2, 20*, 200*, 2,000*, 200,000* | Sperm Production | |||
| Takahashi, 2003 [136] | Rat, Mouse | M | Injection, Propylene glycol | adult | 2,000, 20,000* | BPA Low-Dose Challenge After Extended High- Dose Exposure | |||
| Takao, 2003 [134] | Mouse | M | Oral, Drinking Water | adult | ≈200, ≈20,000* | Testicular ERα, ERβ | |||
| Thuillier, 2003 [188] | Sprague Dawley Rat, CRL | M | Gavage (BPA); sc Injection (DES) | GD 14- GD 22 | 100, 1,000*, 10,000*, 200,000* | Neonatal Testicular Gonocyte PDGFα, PDGFβ | GD 21-PND 3 | DES, Genistein, Coumestrol | |
| Tohei, 2001 [135] | Rat | M | Injection | adult | 3,000* | Testes & Serum Hormones | |||
| Toyama, 2004 [103] | ICR Mouse, Wistar Rat | M | sc Injection, Oil | PND 2- PND 12 | 71, 714*, 3,600*, 7,100*
180, 1,800*, 18,000* |
Spermatogenesis, Mouse Rat | 2 – 10 weeks | Estradiol, Estradiol benzoate | |
| Toyama, 2004 [130] | Mice | M | Injection | Adult For 6 days | 20*, 200* | Abnormal Sperm morphology | adult | ||
| vom Saal, 1998 [101] | CF-1 Mouse | M | Oral, Oil | GD 11- GD 17 | 2*, 20*
2, 20* |
Reproductive Organs, Sperm Production | 6 months | Octylphenol | |
| Wang, 2004 [105] | Sprague Dawley Rat, CRL | M | Oral, Oil | GD 14- GD 22 | 1,000, 10,000*, 200,000*
1,000*, 10,000, 200,000 |
Estrogen Receptor- Associated Protein Expr: Hsp90 p23 | GD 21, PND 3, PND 21 | DES, Genistein, Coumestrol | |
| Wistuba, 2003 [106] | Sprague Dawley Rat (Germany) | M | Oral gavage, Cornstarch suspension | GD 6- GD 21 | 100*, 50,000* | Sertoli Cell Number per Testis | PND 290- 370 | Ethinylestradiol | |
| Effects on Metabolism | |||||||||
| Alonso- Magdalena, 2006 [140] | OF1 Mice | M | Injection oil | adult | 10* | Insulin release Insulin sensitivity | Estradiol | ||
| Kabuto, 2003 [141] | ICR Mice | M | Injection i.p. for 5 days | adult | 25,000*
50,000* |
Antioxidant scavenging enzymes | |||
| Kabuto, 2004 [114] | Mouse | M | Oral, Drinking water | GD 1 - lactation | 2.5 - 5* | Testis Weight Brain Weight Kidney Weight Various Oxidation Markers | PND 28 | ||
| Lemmen, 2004 [189] | Mouse | F, M | Injection, Oil | GD 13.5 | 100, 1,000*, 10,000* | Transgenic Embryonic Gene Expression in Whole Embryo Lysates | DES, Estradiol propionate | ||
| Markey, 2003 [110] | Mouse | F | Osmotic Minipump | GD 8 -19 | 25*, 250*
25*, 250* 25*, 250* |
Estrus Cycle Alterations Blood-Filled Ovarian Bursae Mammary Gland Budding | adult | ||
| Nunez, 2001 [139] | Sprague Dawley Rat | F | Osmotic Pump | adult | ≈5,000, 18,000*, 23,000* | Body weight Blood and tissue levels of BPA | adult | ||
| Takai, 2000 [107] | B6C3F1 Mouse | F, M | Ex vivo in culture medium | GD 2-4 | 0.1, 0.3, 1*, 3*, 10, 100,000* nM (0.023, 0.068, 0.23*, 0.68*, 2.3, 23,000* ppb) | Increased rate of development at 1 and 3 nM, decreased rate of development at 100,000 nM | blastocyst | ||
| Takai, 2001 [108] | B6C3F1 Mouse | F, M | During IVF in culture medium | GD 0-2 | 1 nM (0.228 ppb)* | Postnatal weight gain increase | Weaning | ||
| Effects on the Immune System | |||||||||
| Lee, 2003 [145] | Mouse BALB/c | F | Injection (i.p.) | adult | 25,000 * | Increased IL-4 production, increased serum antigen-specific immunoglobulin E (IgE) levels | adult | nonylphenol | |
| Sawai, 2003 [142] | Mouse C57BL/6 NZB/WF1 | F, M | Oral, in Feed | PND 35-42 | 2.5* | Decreased IFNγ and IL-10 secretion by splenic mononuclear cells; Decreased IgG2a Production; Protection in Lupus-Prone Mice (see also In vitro) | adult | ||
| Sugita-Konishi, 2003 [147] | Mouse BALB/c | F | Injection (s.c.), Oil | adult | 5,000*
5,000* |
Immune Cells and Functions, Reduced IL-6 production and neutrophil phagocytic activity against bacterial infection | adult | ||
| Tian, 2003 [144] | Mouse BALB/cA | M | Oral, Oil | adult | ≈370, 1,800*, 9,000*
≈370, 1,800, 9,000* |
IL-10 production
IL-4 production |
adult | ||
| Yoshino, 2003 [143] | Mouse DBA/1J | F, M | Oral, Oil | adult | 3, 30, 300*, 3,000*
3, 30*, 300*, 3,000* |
Increased serum anti-HEL IgG2a Augmentation of cytokine response | adult | Estradiol | |
| Yoshino, 2004 [116] | Mouse DBA/1J | F, M | Oral, Oil | GD 0 -GD 17 | 3, 30*, 300*, 3,000*
3, 30, 300*, 3,000* |
Increased serum anti-HEL IgG2a
T-Helper Cytokines (both Th1 and Th2) increased |
adult | ||
| Yurino, 2004 [148] | BWF1 Mice | Silastic Capsule | 4 weeks | 25 ng/ml in blood* | IgM antibodies | 5 Months | Estradiol DES | ||
Timing of exposure
Exposures to endocrine disruptors have different effects depending on the life stage of the exposed animals. Effects resulting from adult exposure are generally reversible and are termed “activational”. Effects resulting from exposure during organ development (beginning during prenatal development and continuing in postnatal life through puberty) may result in persistent alterations of the affected systems, even in the absence of subsequent exposure; these effects are termed “organizational” [26]. Some organizational effects are measurable immediately upon exposure and persist throughout the life of the animal [16]. Other organizational effects are undetectable at the time of exposure, but they become apparent in subsequent adulthood [26, 27]. Windows of vulnerability, also known as critical periods, during which the developing system is most sensitive to exposure, are common features of organizational effects [28]. Exposures occurring outside the critical periods will not elicit organizational effects. There is evidence that organizational effects of estrogenic endocrine disruptors such as BPA are mediated by epigenetic alterations in DNA [29]. Organizational and activational effects on the same tissue often differ qualitatively as well as in duration and in the dose required to elicit effects. In this review, we will first discuss organizational effects of prenatal through pubertal BPA exposure, termed “developmental effects” and then activational effects of adult BPA exposure, termed “adult effects”.
DEVELOPMENTAL EFFECTS OF BPA DUE TO EXPOSURE DURING GESTATION THROUGH PUBERTY
Many studies have examined the effects of prenatal, neonatal (shortly after birth) and lactational (birth through weaning) exposure to low doses of BPA. These experiments involved examining effects of exposure to low doses of BPA during “critical periods” in the development of different tissues. These critical periods continue through puberty, the period of physiological transition to fertility.
Effects on Neurotransmitters and their Receptors, Neuroendocrine Function and Hormone Receptors in the Brain in Males and Females
A large number of studies have involved examination of the effects of exposure to low doses of BPA during critical periods in brain development on subsequent brain structure, function and behavior.
Receptors
Increases in estrogen receptors α (ERα) and β (ERβ) have been observed in diverse areas of the brain in response to developmental BPA exposure. BPA was administered to Wistar rats via minipumps at doses of 25 and 250 μg/kg/day from gestation day (GD) 8 through parturition [30]. The male offspring of these mothers were examined, and BPA was found to permanently up-regulate estrogen receptor 2 (β) (Esr2) mRNA levels in the preoptic area at 25 μg/kg/day [30]. Female offspring were not examined in this study [30]. Neonatal [postnatal days (PND) 1-5] injection of Fischer 344 rat pups with a dose of approximately 15 mg BPA/kg/day resulted in increased estrogen receptor 1 (α) (Esr1) mRNA expression in the medial basal hypothalamus of females, and increased both Esr1 and Esr2 mRNA expression in the anterior pituitary in males [31]. In Sprague-Dawley rats (Harlan), fed 40 μg BPA/kg/day at early puberty, from PND 23-30, an increased number of ERα-labelled neurons was found at puberty (PND 37) in the arcuate nucleus (ARC) in males and females, and in the ventromedial nucleus (VMH) in females only. At maturity, more ERα-labelled neurons were found in the medial preoptic area (MPA) in females [32]. Esrl and Esr2 mRNA expression were also increased in the dorsal raphe nucleus (DRN) of male ICR (CD-1) mice prenatally exposed to 2 μg BPA/kg/day via oral administration to the pregnant female on GD 11 – 17 [33]. In this study, serotonin, serotonin transporter, and serum testosterone were not altered [33]. BPA induced changes in somatostatin receptors in the brains of offspring born to Sprague-Dawley rats that received an oral dose of 400 μg BPA/kg/day during pregnancy and lactation. Exposed offspring were examined on PND 10 and 23 [34]. BPA also induces increased mRNA expression of the ligand-activated transcription factors aryl hydrocarbon receptor (AhR), retinoic acid receptor (RAR) α, and retinoid X receptor (RXR) α in embryonic brain [35, 36].
Neuroendocrine Effects
BPA-induced changes in function of the hypothalamus-pituitary-gonad axis have been observed in both males and females. In females, disruption of LH and estrous cyclicity have been reported. The female offspring of Sprague-Dawley rats exposed during gestation and lactation to approximately 1.2 mg/kg/day of BPA in their drinking water exhibited disrupted, prolonged estrous cycles and decreased hypersecretion of LH in response to ovariectomy, suggesting lasting neuroendocrine effects of early BPA treatment [37]. Disruption of adult estrous cycles seen as an increase in the length of the estrus phase was also reported to occur in female offspring of pregnant Sprague-Dawley rats administered BPA orally at a dose of 100 μg/kg/day on GD 6 to 21 [38], and prolonged estrous cycles were observed in female offspring of CD-1 mice injected with a dose of 500 μg/kg/day BPA on GD 15 to 18 [15]. Exposure via i.m. injection of prepubertal female Poll Dorset lambs to DES (0.005 mg/kg/day) or BPA (1 mg/kg/day) for 7 weeks beginning at 4 weeks of age (prior to puberty) had significant effects on LH secretion (a decrease in LH pulse frequency) that were similar, with the dose of DES being 200-fold lower than BPA [39]. Treatment of pregnant Suffolk Ewes with 5 mg/kg/day of BPA dissolved in cottonseed oil via daily subcutaneous injections from GD30-GD60 resulted in marked effects in the female offspring. Levels of LH were increased during the first two months of postnatal life, breeding season was extended, and the magnitude of the LH surge was dampened [40].
In males, effects on LH, prolactin, and brain aromatase activity have been observed. Exposure of weanling males to 2.4 μg/kg/day BPA for 15 days resulted in decreased serum LH and testosterone levels due to alterations in LH synthesis and secretion at the pituitary level; neuroendocrine effects in this study were observed following exposure of the weanling mice to BPA, but not following gestational and lactational exposure [41]. Injecting approximately 15 mg/kg/day of BPA into neonatal Fisher 344 rat pups from postnatal days 1-5 resulted in an increase in serum prolactin levels (hyperprolactinemia) in both males and females on PND 30 [31]. Male offspring of pregnant and lactating rats exposed to 20 μg/kg/day BPA in drinking water showed an increase in estrogen synthesis in hippocampal neurons due to an increase in aromatase activity [42].
BPA exposure also induces alterations in the hypothalamus-pituitary-thyroid axis. Dietary exposure to doses of 1, 10 or 50 mg/kg/day of BPA in Sprague-Dawley rats from GD 6 through weaning caused a significant increase in serum total T4 in pups on PND 15, but serum thyroid-stimulating hormone was not different from controls. The expression of the thyroid hormone-responsive gene RC3/Neurogranin, measured by in situ hybridization, was significantly up-regulated by all BPA doses in the dentate gyrus. These findings suggest that BPA acts as an antagonist of TRβ, which mediates the negative feedback effect of thyroid hormone on the pituitary gland, but that BPA is less effective at antagonizing TRα, leaving TRα-mediated events to respond to elevated plasma thyroid hormone levels [9].
Rapid Signaling Effects
BPA has effects similar to E2 (similar potency and efficacy) in stimulating rapid-response ERK1/2 activity in the cerebellar cortex of Sprague-Dawley rat pups at PND 5-19; treatment consisted of a 6-min intracerebellar injection followed by rapid fixation for immunohistochemistical analysis for phosphorylation of ERK1/2. Significant effects of both BPA and E2 were observed at the lowest dose examined of 1 pM (0.228 ppt). Interestingly, in addition to acting as an estrogen, when co-administered in conjunction with E2, BPA was able to inhibit the rapid E2-induced ERK activity in the developing cerebellum, thus disrupting the action of E2 in a dose-dependent manner [43]. There is another report that BPA can act as an estrogen antagonist in the hippocampus when BPA is co-administered with E2 [44].
Brain Structure
Developmental exposure to BPA resulted in a significant change in the locus coeruleus, where BPA at oral doses of 30 μg/kg/day (in drinking water) and above reversed the normal sex differences in this brain structure and eliminated sex differences in behavior [45, 46]. Pregnant and lactating CD-1 mice were exposed to very low doses of BPA, 0.025 and 0.250 μg/kg/day BPA, via Alzet minipumps, and the brain of the offspring were examined at PND 24-27. The expected robust sex difference in the number of tyrosine hydroxylase (TH) positive neurons in the sexually dimorphic anteroventral periventricular preoptic area (AVPV) was apparent in the control offspring. However, in the offspring exposed to BPA, the sex difference in TH neuron number was undetectable due to a decline in TH neuron number in BPA exposed females. No significant difference in TH neuron number was noted in the males [47]. It is interesting to note that injection of much higher doses of BPA (approximately 300,000-fold higher then the highest daily dose administered to pregnant and lactating females in the study discussed above, 500 μg/day/pup or approximately 75 mg/kg/day) directly into rat pups on PND 1 - 2 also altered TH neuron number in the AVPV; however, the alteration was in a different direction from that reported with very low doses of BPA. With this much higher dose of BPA, the male offspring showed differences from controls, and the male AVPV resembled the female AVPV, whereas the females showed no significant effect of the BPA injection. These data suggest that at this high dose, BPA may have acted as an antiestrogen and interfered with the masculinization of TH neuron number [48]. The importance of dose is discussed in more detail below.
In a study with male Wistar rats that received a single intracisternal injection of approximately 20 μg BPA/kg dose on PND 5, BPA reduced the number of dopamine-containing neurons and resulted in changes in gene expression [49]. A 20 μg/kg/day injection of BPA into pregnant ICR (CD-1) mice disrupted normal neocortical development in fetuses by accelerating neuronal differentiation/migration [50].
Behavioral Effects
Aggression
BPA resulted in an increase in defensive aggression in male Sprague-Dawley rat offspring prenatally exposed to BPA (administered orally to mothers throughout gestation) at a dose of 40 μg/kg/day; no effect was found in the offspring of mothers treated during lactation [51]. In addition, increased aggressiveness (using a composite score of aggression) in male CD-1 mouse offspring occurred as a result of oral administration of 2 μg/kg/day of BPA to pregnant females on GD 11 - 17 [52].
Activity level and reactivity to stimuli
Treatment of male Wistar rat pups at PND 5 with an intracisternal injection of 0.87 nmol (approximately 20 μg/kg) BPA resulted in hyperactivity at 4 weeks of age [49, 53]. Ishido et al. reported that single intracisternal injections of approximately 15 μg BPA/kg or 150 μg BPA/kg at PND 5 increased spontaneous motor activity at 4 weeks of age [54]. Prenatal and lactational exposure to BPA in the feed (approximately 3 – 300 mg/kg/day) also resulted in an increase in activity with dose and altered the response to morphine [55]. BPA increased reactivity to painful or fear-provoking stimuli in the offspring of Sprague-Dawley rats fed 40 μg/kg/day throughout pregnancy and lactation: a hyperalgesic effect was found following prenatal, but not postnatal exposure [56]. When pregnant and lactating C57BL/6 mice were administered an oral BPA dose of 2 or 200 μg/kg/day, BPA increased anxious behavior in a dose-dependent fashion when females were examined in adulthood. Significant effects were observed in animals exposed to 200 μg/kg/day of BPA and to 5 μg/kg/day of ethinylestradiol, which was used as a positive control for the study [57]. In contrast, Farabollini et al. reported that maternal exposure to 40 μg/kg/day of BPA throughout gestation and lactation resulted in reduced anxiety in adult male offspring, and lowered motivation to explore in both male and female adult offspring [58].
Learning
BPA impaired learning of both passive and active avoidance tasks in offspring of Fisher 344 rats fed 100 μg/kg/day of BPA during pregnancy and lactation [59].
Maternal behavior
Feeding pregnant CD-1 mice 10 μg/kg/day decreased subsequent maternal behavior in female offspring, similar to behavioral effects seen with adult exposure [60, 61].
Social interactions and response to addictive drugs
Prenatal and lactational oral exposure of Sprague-Dawley rats to 40 μg/kg/day altered adult play and other socio-sexual behaviors in both males and females [62, 63]. Prenatal and lactational exposure to BPA in the feed (approximately 3 – 300 mg/kg/day) resulted in an altered response to morphine [55]. The behavioral response to amphetamine was enhanced in rats and mice at 40 - 300 μg/kg/day [64, 65]. However, at a prenatal dose of 10 μg/kg/day of BPA, exposed adult female CD-1 mice lost their responsiveness to the reward pathways normally stimulated by amphetamine, possibly through effects on the dopamine system; in this study there was no effect on male amphetamine responsiveness, and there was no effect on activity levels [66].
Sexual behavior
An impairment in the timing of copulatory sequence was found in Sprague-Dawley male rats, perinatally exposed to BPA (40 μg/kg/day fed to the mothers through gestation or lactation) [51] or treated during early puberty [67]. In animals exposed via oral administration perinatally, a reduced performance in terms of latency and frequency of intromission was observed [51]. Effects in the same direction were found with prepubertal exposure to BPA; this was in agreement with results obtained with the positive control ethinylestradiol [67].
Sex Differences
One of the most interesting findings concerning organizational effects of BPA on the brain and behavior and other aspects of physiology is the loss of sex differences that are typically observed in control males and females. Also intriguing is the effect of BPA on one sex but not the other [45, 46, 48, 58, 66, 68-73]. The mechanisms responsible for different effects of BPA in males and females are not clear, although it is known that BPA metabolism is influenced by testosterone [74, 75], and BPA modifies the metabolism of testosterone [41, 76, 77]. Thus, one possibility is that the sex-specific effects of BPA in the brain and other tissues are due to an interaction with background levels of gonadal steroids. The effects of BPA on metabolism are discussed in more detail in a companion paper in this issue (see: L. Vandenberg et al.).
Developmental Effects on the Female Reproductive Tract
Timing of Puberty
Early onset of sexual maturation in females occurred at maternal doses between 2.4 – 500 μg/kg/day in a number of different mouse strains [13, 15, 57, 78]. Puberty in female rodents can be assayed by age at vaginal opening or by age at first ovulation, signaling the onset of fertility. The age at first ovulation can be detected by assessing the age at which the vaginal epithelium is first cornified, indicating that the female is in estrus, or by pairing prepubertal females with experienced males and monitoring the females’ age at first parturition. Timing of puberty is linked to postnatal growth, since puberty is dependent on age, body size, and energy stores. A study with CF-1 mice showed that the response to prenatal exposure (via oral dosing of the mother) to a very low dose of BPA (2.4 μg/kg/day) from GD 11-17 was influenced by the relative position of the fetus with respect to its female and male siblings in the uterus. The response to BPA was greatest in the females that were located in utero between female siblings (2F females) in terms of stimulating an increase in postnatal growth and accelerating the age at first vaginal estrus; postnatal growth was also stimulated in males that were located in utero between female siblings (2F males) but not in male offspring that were located in utero between male siblings (2M males). This finding is consistent with the observation that a very low maternal dose of BPA stimulated a change in the developing mammary gland in 2F female but not 2M female CD-1 mouse fetuses [79]. Taken together, these findings suggest that the endogenous concentrations of E2 and/or testosterone are significant factors is determining the response to BPA during fetal life in mice.
Mammary Gland
Stimulation of mammary gland development in CD-1 mice was observed in the offspring of dams exposed to the very low maternal dose of 0.025 and 0.250 μg/kg/day delivered tonically by an Alzet pump [80]. A significant increase in the percentage of ducts, terminal ducts, terminal end buds, and alveolar buds was observed in female offspring of BPA-treated dams at 6 months of age, collected during proestrus. At 6 months of age, the authors identified terminal end buds as appearing much less bulbous than terminal end buds observed during puberty. The treatment-induced changes in histo-architecture, coupled with an increased presence of secretory product within alveoli observed in the BPA-exposed offspring, resembled those of early pregnancy. Examination of BPA-exposed females (using the same paradigm) at 30 days of age [81] revealed an increase in mammary gland area and number of terminal end buds, as well as an increase of progesterone receptor-positive ductal epithelial cells that were localized in clusters, suggesting future branching points. These sites may be involved in the increase in lateral branching noted in the mammary glands of offspring born to BPA-exposed dams [80].
The female offspring of pregnant Wistar rats implanted throughout pregnancy with an Alzet minipump that released 25 μg/kg/day of BPA were examined at various times up to 6 months of age [82]. At puberty, animals exposed prenatally to BPA showed an increased mammary gland proliferation/apoptosis ratio both in the epithelial and stromal compartments. BPA exposed animals showed an increased number of hyperplastic ducts with signs of desmoplasia, suggesting a heightened risk of neoplastic transformation. Administration of a sub-carcinogenic dose of N-nitroso-N-methylurea (NMU) to the female rats exposed prenatally to BPA increased the percentage of hyperplastic ducts and induced the development of neoplastic lesions. In a related report Wistar-Furth offspring born to mothers implanted with osmotic minipumps that released BPA at a dose of 2.5 μg/kg/day during gestation and lactation revealed evidence of ductal hyperplasias at PND 50 and 95 that included increased expression of Ki67 and Esr1. Some of the ductal lesions were identified as carcinoma in situ [83].
The effect of exposure to 0.25 μg/kg/day delivered to CD-1 mice by Alzet minipump from the evening of gestational day 8 throughout pregnancy was examined in female fetuses on GD 18 [79]. In unexposed fetuses, the mammary gland ductal tree was more developed in 2M females than in 2F females [79]; this result is counterintuitive, since prior research has shown that 2M female fetuses have elevated serum testosterone and lower serum E2 relative to the 2F female siblings [84]. BPA exposure increased mammary gland ductal area and ductal extension and eliminated intrauterine position differences; this was due to BPA stimulating a significant change in 2F females but not 2M females [79]. In the stroma, BPA exposure promoted maturation of the fat pad and altered the localization of collagen. Within the epithelium, BPA exposure led to a decrease in cell size and delayed lumen formation [79]. Pubertal exposure to 100 μg BPA/kg/day also increased differentiation of mammary gland structures and increased proliferation of epithelial cells [85].
Uterus and vagina
Oral administration of 100 μg/kg/day or 50 mg/kg/day of BPA to pregnant Sprague-Dawley rats resulted in a significantly decreased uterine ERβ protein expression in BPA-treated animals at both doses (relative to controls) when measured during the estrous phase of the cycle. Interestingly, a high dose of 200 μg/kg/day ethinylestradiol caused a similar effect [86]. These same doses of BPA and ethinylestradiol caused striking changes in vaginal morphology during estrus, including a decrease in the thickness of the epithelial layer [87]. In addition, Western Blot analysis indicated that following exposure to either dose of BPA, the full-length variant (64 kDa) of Esr1 was not expressed in the vagina of female offspring during estrus, whereas during the diestrus stage, Erα protein expression did not differ from the control group [87].
Studies in the CD-1 mouse have also revealed alterations in the uterus and vagina in offspring born to mothers treated with 0.025 and 0.25 μg BPA/kg/day via Alzet minipumps [88]. These alterations include decreased vaginal wet weight, decreased volume of the endometrial lamina propria and increased protein expression of both ERα and progesterone receptor in the luminal epithelium and subepithelial stroma of the uterus. Consistent with these effects of BPA, studies have shown that exposure during early development (via injection on PND 1-5) to very low doses of DES (0.01 μg/kg/day) alters ERα receptor levels and morphology of the uterus in CD-1 mice [18].
Ovary, Oocytes and Fertility
Significant disruption of the alignment of chromosomes during meiosis was observed in developing oocytes due to leaching of BPA from polycarbonate drinking bottles at doses between 15 - 70 μg/kg/day; this finding leads to the prediction that exposure to BPA during the time that meiosis is reinitiated by the mid-cycle surge in luteinizing hormone (LH) can result in an increase in aneuploidy, which is one of the major causes of spontaneous abortion in humans [89]. As predicted by this finding, there was an increase in mortality of embryos that occurred at a maternal dose of 25 μg/kg/day [90]. Subsequently, a defect in meiosis was induced in C57BL/6 mouse embryos by maternal oral or injected BPA at 20 μg/kg/day by Hunt et al., and the effect was similar to the defect seen in untreated Esr2-knockout mice [91]. The impact of this effect would not be directly on the in utero exposed female, but on the embryos produced from her oocytes (F2 generation effect). Implantation of embryos is not affected at low BPA maternal doses (as low as 10 μg/kg/day), and is significantly decreased only at a maternal dose of approximately 70 mg/kg/day, which is just above the low-dose range [92].
Developmental Effects on the Male Reproductive Tract
Serum Testosterone Levels
Male Long-Evans rats born to mothers exposed to 2.4 μg/kg/day of BPA via feeding, from GD 12 thorough lactation had decreased levels of testicular testosterone, which was not due to a decline in serum LH levels but was consistent with a decline in the steroidogenic capacity of Leydig cells in the BPA exposed males [41]. Decreased serum testosterone in male CD-1 mice also occurred at a maternal dose of 2 μg/kg/day given on GD 11 - 17 [52]. In Sprague-Dawley (Harlan) male rats, alterations in circulating testosterone levels were observed after BPA treatment with 40 μg/kg/day at early puberty (PND 23-30): in this study testosterone was reduced in the juveniles (PND 37), and this decrement persisted in the adults [67].
Prostate
Development of the prostate gland from the urogenital sinus is dependent on systemic testosterone, which is metabolized to 5α-dihydrotestosterone in the urogenital sinus mesenchyme. The mesenchyme directs the development of the epithelium into the glandular structure of the prostate [93]. An increase in adult prostate size in male offspring occurred when pregnant females were fed BPA at 2 or 20 μg/kg/day on GD 11 - 17 in CF-1 mice [94], at 10 μg/kg/day on GD 14 - 18 in CD-1 mice [16], and 50 μg/kg/day on GD 16-18 in CD-1 mice [10]; in the experiments conducted by Timms et al. [16] and Gupta [10], DES caused the same effects as BPA at a maternal oral dose of 0.1 μg DES/kg/day. Gupta [10] also showed that the effect of BPA on the prostate was directly on prostate tissue by removing the prostatic region of the fetal urogenital sinus and examining the effect of BPA in organ culture; the lowest effective concentration (LoEC) of BPA that stimulated an increase in prostate growth was 50 pg/ml (ppt). DES also stimulated prostate growth in primary culture at a dose of 0.5 pg/ml (ppt) [11]. Richter et al. removed the prostatic region of the fetal urogenital sinus and examined the prostatic mesenchyme cells, which contain the androgen and estrogen receptors, in primary culture. With a constant physiological concentration of 5α-dihydrotestosterone, E2 stimulated androgen receptor mRNA levels at 1 pM (0.27 pg/ml), while BPA caused the same effect at 1 nM (0.23 ng/ml), which is within the range of BPA detected in human fetal umbilical cord blood [23]. Maternal exposure to DES (0.1 μg/kg/day, p.o.), an increase in free serum E2 (of 0.1 pg/ml, via Silastic implant) and BPA (50 μg/kg/day, p.o.) all caused a permanent increase in prostatic androgen receptors in mice in addition to an increase in adult prosate weight, relative to negative controls [10, 16, 17].
In addition to effects on prostate size and androgen responsiveness, prenatal exposure to BPA may affect the development of prostate cancer in later life. Timms et al. [16] observed in GD 19 male CD-1 mouse fetuses that a maternal oral dose of 10 μg BPA/kg/day on GD 14 - 18 stimulated an increase in the number of primary prostatic ducts as well as proliferation of basal cells (the progenitor cells thought to be responsible for the development of prostate cancer) in the dorsolateral, but not ventral, primary ducts. This is of interest in that a similar dose of BPA administered via injection to neonatal rats resulted in 100% of the subsequent adult males exhibiting prostate interepithelial neoplasia (PIN) lesions, which are pre-tumorous prostate cancer lesions [29]. Epigenetic changes occurring during development may be the basis for the adult altered susceptibility to disease [95]. BPA is also implicated as a factor in the disruption of therapy for human prostate cancer, since BPA can bind and activate a mutant form of the androgen receptor, T877A, found in some human prostate cancers, and thus stimulate proliferation of human prostate cancer cells in the absence of androgen [96].
The prostate in rodents is composed of distinct lobes that respond differently to endocrine disruptors. Differences in cell proliferation and differentiation markers in stromal cells were observed in ventral prostate following prenatal exposure to 25 μg/kg/day of BPA in prepubertal Wistar rats [30, 97]. This finding is different from those reported by Timms et al. [98], in which male Sprague-Dawley rats exposed to the highest natural serum levels of E2 (due to developing between female fetuses [99]) showed enlargement of the dorsolateral prostate but not the ventral prostate. In another study, prepubertal male Wistar rats were injected with 50 mg BPA/kg/day between PND 22-32. BPA induced a transient surge in prolactin (implicated in the regulation of prostate growth) and a subsequent increase in adult prostate size in later adulthood [100]. On PND 70, prostate weight was significantly increased and daily sperm production was decreased in Sprague-Dawley rats exposed during gestation via a maternal oral dose of 100 μg/kg/day of BPA [38]. In contrast to these findings, DES (6.5 μg/kg/day) or BPA (30 and 300 μg/kg/day) administered via drinking water to pregnant and lactating Wistar rats did not induce significant effects on the ventral prostate (the only region examined) [46].
Testes, Epididymis, Sperm and Seminal Vesicles
In contrast to development of the prostate from the urogenital sinus, development of the epididymis, vas deferens and seminal vesicles from the Wolffian ducts is dependent on diffusion of testosterone from the testis rather than systemic testosterone, and does not involve metabolism of testosterone to 5α-dihydrotestosterone. Thus, drugs and environmental chemicals can have quite different impacts on differentiation of the Wolffian duct vs. the urogenital sinus [93]. Adult CF-1 male offspring that had been exposed prenatally to a maternal oral BPA dose of 2 μg/kg/day showed decreased weights of the epididymis and seminal vesicles, but increased weights of the prostate and preputial glands; a 20 μg/kg/day dose of BPA resulted in a decrease in daily sperm production per gram testis [101]. Injection (s.c.) of BPA (50 μg/animal, about 15-20 mg/kg/day) for the first 5 days after birth resulted in a decrease in the percentage of moving sperm, an increase in the incidence of malformed sperm, and an increase in the number of ERα-positive cells in the epididymides of SHN strain mice at 10 weeks of age [102]. Abnormalities in the acrosomal granule and nucleus of step 2-3 spermatids were also observed in neonatal ICR (CD-1) mice and Wistar rats injected with 300 μg BPA/kg/day or 1 μg E2/kg/day [103]. Ectoplasmic specialization between the Sertoli cell and spermatids was also affected, and some specializations were partially or totally deleted [103]. Decreased epithelial height in the efferent ducts of the testes was observed in prepubertal Wistar rats exposed prenatally to 37 mg BPA/kg/day [104].
Alterations in heat shock proteins in response to BPA were examined in testicular germ cells, since they are involved in the response to environmental stress and have also been implicated in developmental events. Hsp90 protein levels were significantly increased at maternal oral doses of 1 mg/kg/day of BPA and 0.01 μg/kg/day of DES [105]. A finding that contrasts with those described above occurred when pregnant Sprague-Dawley rats were fed BPA at 0.1 or 50 mg/kg/day. Spermatogenesis was qualitatively normal in all groups, but both doses of BPA increased testicular weight and Sertoli cell number per organ [106].
Developmental Effects on Metabolism
The earliest reported exposure regime is direct dosing of pre-implantation mouse embryos at the two-cell stage [107]. In this experiment, development to the eight-cell stage and to the blastocyst stage was accelerated by exposure to 1 to 3 nM (0.23 – 0.69 ng/ml (ppb)) BPA, and was delayed by exposure to 100 μM (23 μg/ml) BPA. Both the acceleration and the delay of development to blastocyst were inhibited by co-treatment with 100 nM tamoxifen, which seems unexpected since that dose is 1000-fold less than the high BPA concentration. Also, the effects on embryo development were not seen with E2 over a dose range of 10 fM to 1 μM [107]. When embryos treated with 1 nM BPA were implanted in a female and allowed to develop, the resulting pup weights at weaning were significantly increased compared to control pups treated with solvent only (0.1 % ethanol) as embryos [108].
Increased postnatal growth in both male and female rats and mice occurred at maternal doses between 2.4 – 500 μg/kg/day [15, 37, 41, 78, 107, 109, 110]. Evidence is accumulating that during critical periods in development, estrogenic chemicals can have unexpected effects on the differentiation of adipocytes as well as postnatal growth [111]. Newbold et al. reported that neonatal exposure to a low dose (1 μg/kg/day) of DES stimulated a subsequent increase in body weight and an increase in body fat in mice [18]. In a related study a high 100 μM dose of BPA stimulated an increase in the glucose transporter GLUT4 and glucose uptake into 3T3-F442A adipocytes in cell culture [112]. In a separate study of transgenic mice over expressing the GLUT4 gene, increased basal and insulin-induced glucose uptake was observed in whole body and in isolated adipocytes [113]. Whether the mouse 3T3 cell lines, which are relatively insensitive to estrogen, are an appropriate model to study the effects of BPA in vitro remains to be determined.
Some studies have found decreased body weight in response to developmental BPA exposures [13, 38, 94], and some have found no effects on body weight [54, 57, 114]. Recent research on the effect of the type of animal feed used in an experiment on postnatal growth suggests that whether or not an increase or decrease in body weight occurs may be related to the type of feed used [115]. In addition, the impact of exposure to BPA during pregnancy and/or lactation on the maternal behavior and lactational efficiency of mothers cannot be ignored [60, 61]. For example, an experiment by Howdeshell et al., which reported increased body weight in response to prenatal BPA exposure [78], differed in design from an experiment conducted by Nagel et al., which reported decreased body weight [94]. Howdeshell cesarean delivered the pups and fostered them to untreated dams. In contrast, Nagel allowed prenatally treated mice to nurse their own offspring, leading to an opposite effect on adult body weight. These two studies used the same low (2 μg/kg/day) maternal dose of BPA, the same CF-1 strain of mice, and the same type of feed.
Developmental Effects on the Immune System
There has been only one study of the developmental effects of BPA on immune function. Prenatal BPA exposure appeared to increase all tested immune responses to soluble antigen in exposed offspring [116]. Following feeding of DBA/1J mice a 30 μg BPA/kg/day dose from day 0 through day 17 of gestation, adult male offspring produced increased antigen-specific IgG2a antibody. A higher prenatal dose of 300 μg/kg/day increased adult production of both T helper 1 interferon gamma (IFN-γ) and T helper 2 IL-4 cytokines in exposed male and female offspring [116]. The two cytokines tested have wide-ranging and distinct effects on immune function; dysregulation of cytokine production could have implications for inflammation or allergic responses.
EFFECTS OF BPA EXPOSURE DURING ADULTHOOD
BPA exposure at low doses has diverse activational effects. Some of these effects are predicted due to the affinity of BPA for ERα and ERβ, while other effects diverge from those observed in response to activation of estrogen receptors.
Adult Effects on the Brain and Behavior
Funabashi et al. [117-119] reported that a single injection of adult ovariectomized female Wistar rats with BPA at 400 μg/kg - 40 mg/kg increased progesterone receptor protein in the preoptic area, ventromedial hypothalamus and frontal cortex, as well as progesterone receptor mRNA in the preoptic area. Specifically, estrogen acts to induce progesterone receptor in the hypothalamus, and Northern blot analysis revealed an increase in progesterone receptor mRNA in the preoptic area and anterior pituitary of adult female rats acutely dosed with approximately 35 mg BPA/kg [117]. In this study, BPA caused responses similar to estrogen in the preoptic area and anterior pituitary, but not in the mediobasal hypothalamus [117]. A single dose of approximately 35 mg BPA/kg in adult female rats induced progesterone receptors in the frontal cortex, but repressed progesterone receptor expression in the temporal cortex [119].
Activational effects of BPA in the brain include an increase in ERα-expressing cells in the medial preoptic area of pregnant and lactating adult females and non-pregnant, cycling adult female Sprague-Dawley rats at a BPA dose of 40 mg/kg/day administered orally for a period of 42 days, corresponding to the time from mating to weaning in the pregnant and lactating group. In contrast, a decrease in ERα-expressing cells was observed in the arcuate nucleus of pregnant and lactating females but not non-pregnant, cycling females in response to this treatment [120].
Injection of ovariectomized E2-treated female Sprague-Dawley rats with 40 – 300 μg BPA/kg/day resulted in a monotonic dose-related inhibition of E2-stimulated increase in hippocampal synapses, with all doses producing significant effects [44]. Thus, in the hippocampus, BPA acted to antagonize the action of E2 on synaptogenesis.
BPA induced a significant increase in serum prolactin levels in 8-10 week old ovariectomized Fisher 344 rats when administered at a low dose of 40-45 μg/kg/day of BPA for a period of 3 days via Silastic capsules. In contrast, Sprague-Dawley rats were relatively insensitive to both BPA and E2 in this experiment, which was the first report that the model animal was an important factor in BPA studies [121].
Exposure of adult female Sprague-Dawley rats from the day of mating through lactation to 40 μg/kg/day of BPA via feeding resulted in alterations in maternal behavior towards their young; specifically, grooming was reduced in BPA-exposed dams [61]. In Mongolian gerbils (Charles River, Italy), exposure to pairs of adult males and females via feeding (in oil) 2 or 20 μg BPA/kg/day for 3 weeks increased their social interactions and reduced their exploratory behavior; similar effects were observed in response to 0.04 μg/kg/day of ethinylestradiol [122].
Adult Effects on the Female Reproductive Tract
Acute exposure of C57BL/6 female mice to 20 through 100 μg/kg/day of BPA resulted in a significant increase in meiotic abnormalities in the oocytes when exposure occurred during the peri-pubertal period, suggesting that BPA exposure would lead to aneuploidy; this abnormality was also observed in mice that were housed in polycarbonate cages and that were provided water in polycarbonate bottles that had been damaged by exposure to a harsh detergent during washing [89]. The effect of BPA on aneuploidy has also been examined in cell culture [123-126].
Adult Effects on the Male Reproductive Tract
A decrease in daily sperm production and fertility in male Sprague-Dawley rats was reported at oral doses between 20 – 200,000 μg/kg/day due to adult exposure, and maximum suppression of sperm production occurred at 20 μg/kg/day. At doses below 20 μg/kg/day, daily sperm production was not significantly different from controls [127]. This suggests that there is a sub-population of cells that are impacted by BPA, and an approximately 40% decrease in daily sperm production is the maximum that occurs, regardless of the dose of BPA administered above 20 μg/kg/day. This finding is similar to data reported for prenatal exposure to ethinylestradiol, where maximum suppression of daily sperm production occurred at 2 ng/kg/day, with no further suppression occurring at higher doses [128].
Similar to the findings in rats, oral exposure of adult Swiss mice for one month to 25 and 100 μg/kg/day resulted in a decrease in daily sperm production and epididymidal sperm concentration, which was associated with a decrease in fertility. A dose of 5 μg/kg/day also resulted in a decrease in the weight of the testes and seminal vesicles [129] (Note that the doses were incorrectly reported in the paper as ng/kg/day instead of μg/kg/day.) A dose of 20 μg/kg body weight of BPA was injected (s.c.) to adult ICR (CD-1) mice and Wistar rats for 6 days, and abnormalities were observed in the spermatids: acrosomal vesicles, acrosomal caps, acrosomes and nuclei of the spermatids were severely deformed. The ectoplasmic specialization between the Sertoli cell and spermatids was also affected: incomplete specialization, redundant ectopic specialization, and aplasia were observed [130]. A significant decrease in testis and epididymidal weight was also reported to occur in adult male Wistar rats exposed orally for 45 days to 0.2, 2 and 20 μg/kg/day, while an increase in ventral prostate weight occurred at all doses [131, 132].
Successive i.p. administration of BPA to adolescent male C57BL/6 mice at a dose of 20 mg/kg/day for 4 weeks decreased the prostate and seminal vesicle weights (but not testis or epididymis weights) and also decreased serum testosterone and both liver and kidney weights [133].
BPA at concentrations of approximately 0.2 and 20 mg/kg/day was administered in the drinking water to young male C57BL/6 mice for eight weeks beginning at 3 weeks of age (before the onset of puberty), and the number of ERβ-containing cells and Esr2 mRNA per testis were significantly decreased in the 20 mg BPA/kg/day treated group compared with controls. In contrast, ERα-immunopositive cells and Esr1 mRNA per testis were markedly increased in these males relative to controls [134].
An s.c. injection for two weeks of 3 mg/kg/day dose of BPA significantly reduced testicular testosterone content, and serum testosterone, while plasma LH showed an increase in adult male Wistar rats [135]. Injection (i.p.) of BPA at a dose of 20 mg/kg/day for 4 weeks decreased the prostate and seminal vesicle weights but not the testis or epididymis weights, and also decreased serum testosterone and both liver and kidney weight in Wistar rats [136]. Plasma free testosterone levels were dramatically decreased following 8 weeks of 12 mg/kg/day of BPA treatment of adult male C57BL/6 male mice compared with control group, and morphologically abnormal multinucleated giant cells having greater than three nuclei were found in seminiferous tubules in the testis following 8-week BPA treatment at 120 μg/kg/day, while no controls showed this [133].
Adult Effects on Metabolism
There is considerable experimental evidence that in adult mice E2 acts via ERα to have an inhibitory effect on adipocyte number and lipogenesis, and removal of estrogen by ovariectomy or ERα via a genetic mutation also causes impaired glucose tolerance and insulin resistance in addition to increased fat mass [137, 138]. Estrogen has central effects on food consumption and energy expenditure that also contribute to its overall inhibitory effects on adipose deposition in adults. BPA has also been reported to decrease body weight in adults [129, 139]. These findings thus contrast numerous reports that low-dose BPA or DES exposure during early development stimulates postnatal growth.
Very low doses of BPA stimulated rapid secretion of insulin in mouse pancreatic β cells in primary culture through a non-classical, non-genomic rapid estrogen-response system. In the same study prolonged exposure to a low oral dose of BPA (10 μg/kg/day) resulted in stimulation of insulin secretion in adult mice that was mediated by the classical nuclear estrogen receptors; the prolonged hypersecretion of insulin was followed by insulin resistance [140].
Other effects on metabolic pathways have also been observed in response to BPA. A decrease in antioxidant enzymes occurred at the very low dose of 0.2 μg/kg/day of BPA in adult Wistar rat males [131], and at a higher dose of 50 mg/kg/day in ICR (CD-1) male mice [141]. In adult Wistar rats, oral administration of 1 mg/kg/day of BPA reduced in adult males but not females the expression of UGT2B1 and other UGT isoforms that mediate the glucuronidation of BPA and sex hormones [69].
Adult Effects on the Immune System
BPA has been reported to modulate immune function at doses between 2.5 - 30 μg/kg/day [142, 143], including patterns of cytokine and antibody production, response to infection, and autoimmune disease progression. T helper lymphocytes are a source of cytokine families that stimulate inflammatory responses and resistance to intracellular infections (Th1 cytokines), or that shift the response to antibody production, resistance to extracellular organisms, and allergy (Th2 cytokines). Antigen-specific IFN-γ (Th1) secretion was increased in adult DBA1/J mice given 30 μg/kg/day BPA p.o. for 20 days and sacrificed 24 hr later [143]. In contrast, a shorter term oral dose of 2.5 μg/kg/day BPA given PND 35-42 to C57BL/6 and autoimmune-prone NZB/WF1 mice significantly decreased the production of IFN-γ by splenocytes stimulated with 4 μg/mL of the T lymphocyte mitogen ConA, up to five weeks post treatment [142]. A single oral dose of approximately 9 mg BPA/kg just prior to infection with the parasite Trichinella spiralis increased production of both IL-4 and IL-10 by lymph node cells stimulated with parasite antigen [144]. Addition of BPA at doses ranging from 0.1 to 30 μM to cultures of lymphocytes from Leishmania major-infected BALB/c or C57BL/6 mice did not increase IFN-γ production [144]. Other studies also report an increase in the Th2-associated cytokine, IL-4, following BPA exposure. Injection (i.p.) of 25 mg/kg/day BPA every other day for one week in adult KLH-primed BALB/c mice resulted in significant increase of IL-4 production in CD4+ T cells and of serum antigen-specific immunoglobulin E (IgE) levels [145]. Antigen specific spleen cells of adult BPA-exposed DBA1/J mice (300 μg/kg/day p.o. for 20 days) produced an elevated IL-4 response [143]. Thus, most models indicate that BPA may enhance or shift the pattern of cytokine production following antigen stimulation. Skewing of the Th1/Th2 cytokine profile by EDCs has been associated with allergy and asthma [146].
Exposure to BPA has also been associated with modulation of innate immune system cell function. For example, administration of 5 mg/kg/day s.c. to adult BALB/c mice for 5 days decreased innate host defense to bacterial infection. Upon challenge with i.p. E. coli injection, both neutrophil phagocytosis and IL-6 production were significantly reduced [147].
Autoimmune diseases, particularly those mediated by an antibody response to host tissues, are more common in females; estrogens and prolactin are believed to act as drivers for differential disease development and progression in individuals with an underlying genetic susceptibility to autoimmunity. However, BPA exposure modulates the course of glomerulonephritis in the NZB/WF1 mouse model of systemic lupus erythematosus. An oral dose of 2.5 μg/kg/day given on PND 35-42 to female NZB/WF1 mice decreased the production of IgG2a antibody by splenocytes and delayed the onset of glomerulonephritis as measured by albuminuria [142]. Yurino et al. used Silastic implants to administer BPA, E2, or DES to ovariectomized, 4 week old, female NZB/WF1 mice [148]. Serum concentrations of 30 ng/ml BPA were measured 4 months after implantation. At five months of age, both DES- and E2-treated mice showed an increase in IgG anti-DNA antibody and prominent deposition of immune complexes in the glomeruli, both indicators of disease. In contrast, although BPA, DES, and E2 increased IgM-class autoantibodies, there was no evidence of glomerular immune complex deposition and no increase in serum anti-DNA IgG antibody in the BPA-exposed mice.
In summary, while it is well accepted that estrogen alters immune function, the interaction is complex [149]. Estrogen has been reported to both decrease and increase immune function, depending on dose, including conflicting reports on the production of the T helper 1 cytokine, IFN-γ [150, 151]. The effects of BPA exposure on the immune system may be critically dependent on the timing of BPA exposure. Estrogen receptor expression by lymphocytes is dependent upon the age and strain of the animal; in addition, recent evidence suggests the spleen undergoes significant molecular remodeling during puberty, resulting in both age and gender-dependent differences in immune gene expression [152]. Nevertheless, studies conducted by Yoshino et al. [116, 143] indicate similar dose-associated, gender-independent immune system effects in 8 week old offspring of BPA-exposed dams and animals exposed as adults. These results suggest quantitative, rather than qualitative, differences in lifestage-dependent immune system sensitivity to BPA.
COMPARISON OF FINDINGS OF SIGNIFICANT EFFECTS AND NO-SIGNIFICANT EFFECTS IN LOW-DOSE BPA STUDIES
As of the end of October 2006 we are aware of 27 in vivo studies reporting no significant effects in response to low doses of BPA. The variables that account for most of the studies that find no significant effects have recently been reviewed [5, 153]. Below we discuss a number of these variables.
Strain Differences
The major factor that accounts for 13 studies that draw the conclusion of no effect of low doses of BPA is the use of a strain of rat sold by Charles River Laboratories, referred to as the CD-SD rat [Crl:CD(SD)] (Table 2). According to Charles River [154], rats were purchased by Charles River from Sprague-Dawley in 1950. This colony was continuously subjected to selective breeding for rapid postnatal growth and large litter size for over 40 years. The Crl:CD(SD) rat strain is insensitive to BPA, and 0 of 13 studies report low dose effects [155-167]. In addition, this rat strain is insensitive to the drug ethinylestradiol at doses (~0.5 μg/kg/day) used in oral contraceptives [163, 167]. As shown above, a large number of studies show that significant low-dose effects of BPA have been observed in the original Sprague-Dawley rat. However, a number of studies have reported that the inbred Fisher 344 rat is more sensitive to low dose effects of BPA relative to the original Sprague-Dawley rat [121, 168, 169]. Wistar rats also appear to be more sensitive than Sprague-Dawley rats to the uterotrophic effects of BPA [168, 170]. One assessment of the uterotrophic response of immature Sprague-Dawley rats revealed a response following 3 days of exposure to 300 mg/kg/day of BPA. In the same study, a significant response to ethinylestradiol was noted at 0.3 ug/kg/day [171]. Why the Sprague-Dawley rat does not respond to low doses of BPA in the uterotrophic bioassay or in the vaginal stimulation bioassay has been investigated, and the lack of responsiveness appears to be due to factors after binding to estrogen receptors [169].
The importance of the strain of animal used in low-dose research was acknowledged in the 2001 Low Dose National Toxicology Program (NTP) report. The NTP panel emphasized the need to test for the sensitivity of any animal model by including a positive control, such as the well characterized estrogenic drugs DES or ethinylestradiol, and stated [172] p vii): “Because of clear species and strain differences in sensitivity, animal model selection should be based on responsiveness to endocrine active agents of concern (i.e., responsive to positive controls), not on convenience and familiarity.”
BPA as a SERM: Responses in Different Tissues and in Different Animal Models
A difference in the response of tissues to low doses of BPA is shown in articles that have been published reporting that low doses of BPA do not stimulate a typical uterotrophic (increase in uterine weight) response in mice and rats, while low-dose effects are observed in many other endpoints. For example, effects on uterine weight have been observed at relatively high doses of BPA (25 to 40 mg/kg/day), while changes in uterine gene activity occurred at lower doses of 10 mg/kg/day in B6C3F1 mice and 0.8 mg/kg in transgenic ERIN mice [173-175]. In prepubertal CD-1 mice, a dose of 100 mg/kg/day injected s.c. for 3 days was required to stimulate an increase in uterine weight [176]; however, 5 mg/kg/day was sufficient to cause a significant increase in the height of uterine epithelial cells in this strain, Similar findings regarding the inability of low doses of BPA to stimulate a uterotrophic response in prepubertal CD-1 mice were reported in another study [177], which, in comparison to the B6C3F1 mouse data, provides further evidence for strain differences in sensitivity to BPA.
The prepubertal uterotrophic assay is thus a relatively insensitive endpoint for studies of BPA, since the dose required to stimulate a uterotrophic response (one of the most commonly used bioassays in toxicology to assess estrogenic activity), is markedly higher relative to doses that cause significant effects in CD-1 mice in the prostate [10, 16], testes [52], mammary glands [79-81, 88], and the brain and behavior [47, 52, 60, 66]. All of these studies report significant effects in response to developmental exposure of CD-1 mice to doses of BPA at and far below the reference dose of 50 μg/kg/day (there are over 20 low-dose BPA studies published reporting significant effects of BPA in CD-1 mice). As a specific example, when the magnitude of the response to BPA in mammary gland and uterus are compared, the mammary gland appears to be a more sensitive target for BPA action, but clearly, in utero exposure to low doses of BPA does have lasting effects on the uterus in CD-1 mice [88].
Batch-to-Batch Variability in Feed: Impact on Low-Dose Endocrine Disruptor Research
The other major variable that has been implicated in contributing to discrepancies in the outcome of experiments on low-dose effects of BPA is the type of feed used. Two workshops on this issue were held in 2005 and 2006 to address this issue, and a manuscript reviewing the conclusions from these workshops is being prepared. The consensus was that there is a critical need for researchers to better understand the potential for components of commercial feed to impact research outcomes. Importantly, no one type of feed was deemed appropriate for the many different types of research conducted with laboratory rats and mice, and both soy-based and non-soy-based feeds were reported to contain variable amounts of estrogenic contaminants that were not soy phytoestrogens [178]. The major concern was that feed manufacturers should make every effort to reduce the batch-to-batch variability in feed components that can lead to phenotypic variation in control animals.
Findings also presented at the workshops emphasized the critical importance of including appropriate positive controls in experimental studies. With regard to BPA for example, the inclusion of an appropriate positive control (based on experimental objectives and techniques, such as route of administration) allows one to determine whether or not the test system has been rendered insensitive to any estrogenic chemical or drug when drawing conclusions concerning the potential safety of estrogenic chemicals such as BPA. A specific example was provided by the finding of Thigpen et al. that a commonly used feed in toxicological studies, PMI 5002, showed significant batch-to-batch variability in phytoestrogens that related to an inability to detect significant effects of the potent estrogenic drug diethylstilbestrol (DES) with some batches of this feed [19]. Some studies that reported finding no significant effects at low doses of BPA [166, 179] used PMI 5002 feed, raising concerns that effects of DES would not have been found with the batches of this feed that were used. In fact, 0.1 μg/kg/day DES (administered orally to pregnant CF-1 mice) was used as a positive control in one study [179], and DES-exposed animals did not differ from negative controls on any outcome.
The Use of Positive Controls
The NTP panel commented in its 2001 report on the issue regarding: “a study in which the positive control does not produce the expected positive response. The prudent course of action in such cases may be to declare the study inadequate and repeat it, regardless of the experimental outcome in the test groups” [172 p. 5-10]. The NTP panel went on to note that: “For those studies that included DES exposure groups, those that showed an effect with BPA showed a similar low-dose effect with DES (e.g. prostate and uterus enlargement in mice), while those that showed no effect with BPA also found no effect with DES.” In many studies in which no statistically significant effect of low doses of BPA were reported, no positive control was included [155, 156, 159, 160, 166, 180-185]. In particular, a positive control is the only way to distinguish whether an experiment has been compromised by the presence of an estrogenic contaminant in animal feed, water, or housing.
Three studies that concluded that there was no effect of low doses of BPA on the male [179, 186] or on both the male and female [109] reproductive system in mice suffered from a lack of statistically significant effect of the positive control, DES. One study did report some effects at a relatively high (15 μg/kg/day) dose of DES and no significant effects at any dose of BPA [161]. As discussed previously, this high dose of DES is not a valid positive control in experiments that fail to show effects of low doses of BPA. In summary, experiments reporting only the absence of statistically significant effects of low doses of BPA that do not include a positive control, or that fail to show significant effects of the positive control, cannot be interpreted. Without positive control data obtained concurrently with the BPA data, there is no way to determine the reason that significant effects were not observed for any test chemical.
There are now numerous studies that have been published showing similar effects of low doses of BPA and DES, the most commonly used positive control for examining estrogenic effects of BPA. For many outcomes, BPA has been reported to have the same efficacy but a 100-1000 – fold lower potency relative to DES [10, 13-16, 46, 69, 105, 148, 187-189].
There are some studies showing that, particularly for rapid responses mediated by receptors associated with the cell membrane [190-192], BPA and DES (as well as E2) have equal potency. This subject is covered in detail in the report from the panel concerned with molecular mechanisms. As discussed above, there is evidence that BPA is a SERM, and thus, while there are studies showing similar effects of BPA and endogenous E2 or estrogenic drugs such as DES, there are also studies showing qualitative differences in the responses of BPA and other estrogens.
The Importance of Dose
There have been many published in vivo and in vitro mechanistic studies providing examples of effects of BPA that are observed at a low dose, but are not observed at higher doses. This type of dose-response relationship is known as an inverted-U-shaped curve. In toxicology, it was long assumed that the only valid dose-related effect is a monotonic dose-response relationship. However, as toxicologists have investigated more sub-lethal endpoints and more extensive dose ranges, non-monotonic dose-response relationships have been encountered with increasing frequency. The molecular basis for such findings is beginning to be understood in terms of the stimulation and inhibition of unique sets of genes as one moves across the dose-response curve [193, 194]. These effects are likely to be mediated by different receptors [for estrogens, ERα, ERβ, ERRγ and membrane-associated receptors [43, 195, 196], and both positive and negative feedback effects on estrogen receptors and other receptors [6, 7]]. Several examples of unique effects of low doses of BPA have now been reported and are available for review [45, 46].
CONCLUSIONS AND LEVELS OF CONFIDENCE FOR DIFFERENT OUTCOMES
1. Based on existing evidence, we are confident of the following
The criterion for an outcome being assessed as achieving this level (we are confident) is that multiple independent studies have shown the same or similar outcomes.
Developmental effects on the brain and behavior
There is extensive evidence for effects of exposure during critical periods to low doses of BPA on subsequent brain structure and chemistry, receptors for hormones and neurotransmitters, and behavior. We can thus state with confidence that low doses of BPA during development have persistent effects on brain structure, function and behavior in rats and mice.
It is likely that due to species and strain differences, effects of BPA on specific brain structures, functions and behaviors may show differences. However, some effects appear consistent. For example, a number of studies have shown that expected differences between males and females are not observed in animals exposed to BPA during development. In some experiments this outcome has been due to males showing a more feminine phenotype and females showing a more masculine phenotype [47, 73], while in other experiments, the loss of the sex difference was due to a measurable change in only one sex [197]. Further research may reveal whether estrogenic effects dominate when low doses of BPA are administered and whether other effects of BPA such as antiestrogenic or antiandrogenic effects are likely to be observed in the presence of higher doses of BPA.
Developmental effects on the male reproductive tract
There is extensive evidence that BPA impacts the reproductive system in male rats and mice, although there appear to be species and strain differences in the sensitivity of specific outcomes to BPA. The evidence supports an effect on the testes, with subsequent changes in testosterone secretion and sperm production. Impacts on other reproductive structures have been reported in a number of independent studies, including the epididymis and epididymal sperm, prostate, and seminal vesicles. These findings are consistent with effects of low doses of positive control chemicals, such as DES and ethinylestradiol.
Developmental effects on enzyme activity, growth and metabolism
There is extensive evidence for “programming” effects of BPA on subsequent activity of enzymes in tissues and thus metabolic processes. An increase in postnatal growth rate due to developmental exposure to low doses of BPA has been shown in many studies, and this finding is consistent with the effect of developmental exposure to a low, but not high, dose of DES.
Adult effects on the male reproductive tract
There is a significant amount of evidence that adult exposure to BPA has adverse consequences for testicular function in male rats and mice. This is not surprising as estrogen, while essential for normal epididymis function, has inhibitory effects on the brain-pituitary-gonadal axis in males, and it is well documented that elevated E2 inhibits spermatogenesis and testicular testosterone secretion [1].
2. We consider the following to be likely but requiring confirmation
The criterion for achieving this level is that significant effects have been reported, but the number of independent replications is limited. However, confidence in the findings is increased by the plausibility of the results, based on mechanistic information available from other related studies.
Developmental effects on the female reproductive tract
There is extensive evidence for effects of BPA on development of the mammary gland. Studies in both rats and mice have shown effects of developmental exposure to BPA on mammary gland morphology that may predispose animals to develop cancer. These findings have not yet been repeated in multiple independent laboratories.
Adult effects on the brain and behavior
There are a number of studies that have found a variety of significant outcomes of adult exposure to BPA. Given the more extensive literature showing developmental effects, there is no reason to expect that adult effects will not also occur. However, effects in the adult may require higher doses of BPA exposure or longer periods of BPA exposure.
An important unanswered question and research need is whether long-term adult exposure to BPA may have adverse consequences, as has been recently found to be the case for hormone replacement therapy (HRT), which is now thought to increase the risk for a number of diseases [198]. The finding that BPA can stimulate proliferation of human cancer cells in the absence of androgen is also cause for concern.
Adult effects on the female reproductive tract
While a number of studies have found significant effects of BPA on the female reproductive system, not as much research has been conducted as for the male reproductive system. For example, effects on meiosis in oocytes need to be confirmed by additional studies. The recent report that maternal exposure to a very low dose of BPA also disrupts meiosis in the embryonic oocyte during formation of the primary follicles adds to our concern.
Adult effects on the immune system
There is extensive evidence that BPA modulates both T helper 1 and T helper 2 cytokine production and alters antibody production.
Acknowledgments
This review was prepared in conjunction with the Bisphenol A Conference, Chapel Hill, NC, November 28-29, 2006. Support was provided by the National Institute of Environmental Health Sciences and the National Institute of Dental and Craniofacial Research, NIH, DHHS, the W.M. Keck Center for Behavioral Biology at NC State University, and from Commonweal.
Financial Support: This review was prepared in conjunction with the Bisphenol A Conference, Chapel Hill, NC, November 28-29, 2006. Support was provided by the National Institute of Environmental Health Sciences, the National Institute of Dental and Craniofacial Research, the W.M. Keck Center for Behavioral Biology at NC State University and from Commonweal.
Abbreviations used
- 2F
located in utero between female siblings
- 2M
located in utero between male siblings
- ADI
acceptable daily intake
- AhR
aryl hydrocarbon receptor
- ARC
arcuate nucleus
- AVPV
anteroventral periventricular preoptic area
- BPA
bisphenol A
- Crl:CD(SD)
Charles River Laboratories Sprague-Dawley rat strain
- DES
diethylstilbestrol
- DRN
dorsal raphe nucleus
- EDC
endocrine disrupting chemical
- E2
17β-estradiol
- ERα
estrogen receptor protein α
- ERβ
estrogen receptor protein β
- Esr1
estrogen receptor gene 1 (α)
- Esr2
estrogen receptor gene 2 (β)
- GD
gestation day
- HRT
hormone replacement therapy
- IFN
interferon
- IgE
immunoglobulin E
- i.m.
intramuscular
- i.p.
intraperitoneal
- LD50
lethal dose, 50 % of population
- LH
luteinizing hormone
- LoEC
lowest effective concentration
- LOAEL
lowest observed adverse effect level
- MPA
medial preoptic area
- NMU
N-nitroso-N-methylurea
- NTP
National Toxicology Program
- PND
postnatal day
- PIN
prostate interepithelial neoplasia
- p.o.
oral adminisration
- RAR
retinoic acid receptor
- RXR
retinoid X receptor
- SERM
selective estrogen receptor modulator
- s.c.
subcutaneous
- Th1
T helper lymphocyte 1
- Th2
T helper lymphocyte 2
- TH
tyrosine hydroxylase
- VMH
ventromedial nucleus
Footnotes
The information in this document has been subjected to review by the National Health and Environmental Effects Research Laboratory, U.S. Environmental Protection Agency, and approved for publication. Approval does not signify that the contents reflect the views of the Agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Staples CA, Dome PB, Klecka GM, Oblock ST, Harris LR. A review of the environmental fate, effects, and exposures of bisphenol A. Chemosphere. 1998;36(10):2149–73. doi: 10.1016/s0045-6535(97)10133-3. [DOI] [PubMed] [Google Scholar]
- 2.Burridge E. Bisphenol A: Product Profile. Eur Chem News. 2003 April 14-20;:17. [Google Scholar]
- 3.Kawagoshi Y, Fujita Y, Kishi I, Fukunaga I. Estrogenic chemicals and estrogenic activity in leachate from municipal waste landfill determined by yeast two-hybrid assay. J Environ Monitoring. 2003;5(2):269–74. doi: 10.1039/b210962j. [DOI] [PubMed] [Google Scholar]
- 4.Coors A, Jones PD, Giesy JP, Ratte HT. Removal of estrogenic activity from municipal waste landfill leachate assessed with a bioassay based on reporter gene expression. Environ Sci Technol. 2003;37(15):3430–4. doi: 10.1021/es0300158. [DOI] [PubMed] [Google Scholar]
- 5.vom Saal FS, Hughes C. An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment. Environ Health Perspect. 2005;113:926–33. doi: 10.1289/ehp.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Welshons WV, Thayer KA, Judy BM, Taylor JA, Curran EM, vom Saal FS. Large effects from small exposures. I. Mechanisms for endocrine-disrupting chemicals with estrogenic activity. Environ Health Perspect. 2003;111(8):994–1006. doi: 10.1289/ehp.5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Welshons WV, Nagel SC, vom Saal FS. Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology. 2006;147(6 Suppl):S56–S69. doi: 10.1210/en.2005-1159. [DOI] [PubMed] [Google Scholar]
- 8.Lee HJ, Chattopadhyay S, Gong EY, Ahn RS, Lee K. Antiandrogenic effects of bisphenol A and nonylphenol on the function of androgen receptor. Toxicol Sci. 2003;75(1):40–6. doi: 10.1093/toxsci/kfg150. [DOI] [PubMed] [Google Scholar]
- 9.Zoeller RT, Bansal R, Parris C. Bisphenol-A, an environmental contaminant that acts as a thyroid hormone receptor antagonist in vitro, increases serum thyroxine, and alters RC3/neurogranin expression in the developing rat brain. Endocrinology. 2005;146(2):607–12. doi: 10.1210/en.2004-1018. [DOI] [PubMed] [Google Scholar]
- 10.Gupta C. Reproductive malformation of the male offspring following maternal exposure to estrogenic chemicals. Proc Soc Exp Biol Med. 2000;224(2):61–8. doi: 10.1046/j.1525-1373.2000.22402.x. [DOI] [PubMed] [Google Scholar]
- 11.Gupta C. The role of estrogen receptor, androgen receptor and growth factors in diethylstilbestrol-induced programming of prostate differentiation. Urol Res. 2000;28(4):223–9. doi: 10.1007/s002400000107. [DOI] [PubMed] [Google Scholar]
- 12.Richter CA, Taylor JA, Ruhlen RR, Welshons WV, vom Saal FS. Estradiol and bisphenol A stimulate androgen receptor and estrogen receptor gene expression in fetal mouse prostate mesenchyme cells. Environ Health Perspect. 2007;115:902–8. doi: 10.1289/ehp.9804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Honma S, Suzuki A, Buchanan DL, Katsu Y, Watanabe H, Iguchi T. Low dose effect of in utero exposure to bisphenol A and diethylstilbestrol on female mouse reproduction. Reprod Toxicol. 2002;16:117–22. doi: 10.1016/s0890-6238(02)00006-0. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki A, Sugihara A, Uchida K, Sato T, Ohta Y, Katsu Y, et al. Developmental effects of perinatal exposure to bisphenol A and diethylstilbestrol on reproductive organs in female mice. Reprod Toxicol. 2002;16(2):107–16. doi: 10.1016/s0890-6238(02)00005-9. [DOI] [PubMed] [Google Scholar]
- 15.Nikaido Y, Yoshizawa K, Danbara N, Tsujita-Kyutoku M, Yuri T, Uehara N, et al. Effects of maternal xenoestrogen exposure on development of the reproductive tract and mammary gland in female CD-1 mouse offspring. Reprod Toxicol. 2004;18(6):803–11. doi: 10.1016/j.reprotox.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Timms BG, Howdeshell KL, Barton L, Bradley S, Richter CA, vom Saal FS. Estrogenic chemicals in plastic and oral contraceptives disrupt development of the mouse prostate and urethra. Proc Natl Acad Sci U S A. 2005;102(19):7014–9. doi: 10.1073/pnas.0502544102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.vom Saal FS, Timms BG, Montano MM, Palanza P, Thayer KA, Nagel SC, et al. Prostate enlargement in mice due to fetal exposure to low doses of estradiol or diethylstilbestrol and opposite effects at high doses. Proc Natl Acad Sci U S A. 1997;94(5):2056–61. doi: 10.1073/pnas.94.5.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newbold RR, Jefferson WN, Padilla-Banks E, Haseman J. Developmental exposure to diethylstilbestrol (DES) alters uterine response to estrogens in prepubescent mice: low versus high dose effects. Reprod Toxicol. 2004;18(3):399–406. doi: 10.1016/j.reprotox.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Thigpen JE, Haseman JK, Saunders HE, Setchell KDR, Grant MG, Forsythe DB. Dietary phytoestrogens accelerate the time of vaginal opening in immature CD-1 mice. Comp Med. 2003;53:477–85. [PubMed] [Google Scholar]
- 20.Howdeshell KL, Peterman PH, Judy BM, Taylor JA, Orazio CE, Ruhlen RL, et al. Bisphenol A is released from used polycarbonate animal cages into water at room temperature. Environ Health Perspect. 2003;111:1180–7. doi: 10.1289/ehp.5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pottenger LH, Domoradzki JY, Markham DA, Hansen SC, Cagen SZ, Waechter JM. The relative bioavailability and metabolism of bisphenol A in rats is dependent upon the route of administration. Toxicol Sci. 2000;54(1):3–18. doi: 10.1093/toxsci/54.1.3. [DOI] [PubMed] [Google Scholar]
- 22.Wilson NK, Chuang JC, Morgan MK, Lordo RA, Sheldon LS. An observational study of the potential exposures of preschool children to pentachlorophenol, bisphenol-A, and nonylphenol at home and daycare. Environ Res. 2007;103(1):9–20. doi: 10.1016/j.envres.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 23.Schönfelder G, Wittfoht W, Hopp H, Talsness CE, Paul M, Chahoud I. Parent bisphenol A accumulation in human maternal-fetal-placental unit. Environ Health Perspect. 2002;110:A703–A7. doi: 10.1289/ehp.110-1241091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, et al. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005;146(4):1650–73. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- 25.IRIS. Bisphenol A. (CASRN 80-05-7) US-EPA Integrated Risk Information System Substance file. 1988 http://www.epa.gov/iris/subst/0356.htm.
- 26.Young WC, Goy RW, Phoenix CH. Hormones and Sexual Behavior. Science. 1964;143(3603):212–8. doi: 10.1126/science.143.3603.212. [DOI] [PubMed] [Google Scholar]
- 27.Birnbaum LS, Fenton SE. Cancer and developmental exposure to endocrine disruptors. Environ Health Perspect. 2003;111(4):389–94. doi: 10.1289/ehp.5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Selevan SG, Kimmel CA, Mendola P. Identifying critical windows of exposure for children’s health. Environ Health Perspect. 2000;108(Suppl 3):451–5. doi: 10.1289/ehp.00108s3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ho SM, Tang WY, Belmonte de Frausto JB, Prins GS. Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res. 2006;66(11):5624–32. doi: 10.1158/0008-5472.CAN-06-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramos JG, Varayoud J, Kass L, Rodriguez H, Costabel L, Munoz-De-Toro M, et al. Bisphenol A induces both transient and permanent histofunctional alterations of the hypothalamic-pituitary-gonadal axis in prenatally exposed male rats. Endocrinology. 2003;144(7):3206–15. doi: 10.1210/en.2002-0198. [DOI] [PubMed] [Google Scholar]
- 31.Khurana S, Ranmal S, Ben-Jonathan N. Exposure of newborn male and female rats to environmental estrogens: delayed and sustained hyperprolactinemia and alterations in estrogen receptor expression. Endocrinology. 2000;141(12):4512–7. doi: 10.1210/endo.141.12.7823. [DOI] [PubMed] [Google Scholar]
- 32.Ceccarelli I, Della Seta D, Fiorenzani P, Farabollini F, Aloisi AM. Estrogenic chemicals at puberty change ERα in the hypothalamus of male and female rats. Neurotoxicol Teratol. 2007;29(1):108–15. doi: 10.1016/j.ntt.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 33.Kawai K, Murakami S, Senba E, Yamanaka T, Fujiwara Y, Arimura C, et al. Changes in estrogen receptors α and β expression in the brain of mice exposed prenatally to bisphenol. A Regul Toxicol Pharmacol. 2007;47(2):166–70. doi: 10.1016/j.yrtph.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 34.Facciolo RM, Alo R, Madeo M, Canonaco M, Dessi-Fulgheri F. Early cerebral activities of the environmental estrogen bisphenol A appear to act via the somatostatin receptor subtype sst2. Environ Health Perspect. 2002;110(Suppl 3):397–402. doi: 10.1289/ehp.02110s3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishizawa H, Morita M, Sugimoto M, Imanishi S, Manabe N. Effects of in utero exposure to bisphenol A on mRNA expression of arylhydrocarbon and retinoid receptors in murine embryos. J Reprod Dev. 2005;51(3):315–24. doi: 10.1262/jrd.16008. [DOI] [PubMed] [Google Scholar]
- 36.Nishizawa H, Imanishi S, Manabe N. Effects of exposure in utero to bisphenol A on the expression of aryl hydrocarbon receptor, related factors, and xenobiotic metabolizing enzymes in murine embryos. J Reprod Dev. 2005;51(5):593–605. doi: 10.1262/jrd.17026. [DOI] [PubMed] [Google Scholar]
- 37.Rubin BS, Murray MK, Bamassa DA, King JC, Soto AM. Perinatal exposure to low doses of bisphenol A affects body weight, patterns of estrous cyclicity, and plasma LH levels. Environ Health Perspect. 2001;109(7):675–80. doi: 10.1289/ehp.01109675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Talsness C, Fialkowski O, Gericke C, Merker H-J, Chahoud I. The effects of low and high doses of bisphenol A on the reproductive system of female and male rat offspring. Congenital Anomalies. 2000;40:S94–S107. [Google Scholar]
- 39.Evans NP, North T, Dye S, Sweeney T. Differential effects of the endocrine-disrupting compounds bisphenol-A and octylphenol on gonadotropin secretion, in prepubertal ewe lambs. Domest Anim Endocrinol. 2004;26(1):61–73. doi: 10.1016/j.domaniend.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 40.Savabieasfahani M, Kannan K, Astapova O, Evans NP, Padmanabhan V. Developmental programming: Differential effects of prenatal exposure to bisphenol-A or methoxychlor on reproductive function. Endocrinology. 2006;147:5956–66. doi: 10.1210/en.2006-0805. [DOI] [PubMed] [Google Scholar]
- 41.Akingbemi BT, Sottas CM, Koulova AI, Klinefelter GR, Hardy MP. Inhibition of testicular steroidogenesis by the xenoestrogen bisphenol A is associated with reduced pituitary luteinizing hormone secretion and decreased steroidogenic enzyme gene expression in rat Leydig cells. Endocrinology. 2004;145(2):592–603. doi: 10.1210/en.2003-1174. [DOI] [PubMed] [Google Scholar]
- 42.Kawato S. Endocrine disrupters as disrupters of brain function: a neurosteroid viewpoint. Environ Sci. 2004;11(1):1–14. [PubMed] [Google Scholar]
- 43.Zsarnovszky A, Le HH, Wang HS, Belcher SM. Ontogeny of rapid estrogen-mediated extracellular signal-regulated kinase signaling in the rat cerebellar cortex: potent nongenomic agonist and endocrine disrupting activity of the xenoestrogen bisphenol A. Endocrinology. 2005;146(12):5388–96. doi: 10.1210/en.2005-0565. [DOI] [PubMed] [Google Scholar]
- 44.MacLusky NJ, Hajszan T, Leranth C. The environmental estrogen bisphenol A inhibits estrogen-induced hippocampal synaptogenesis. Environ Health Perspect. 2005;113:675–9. doi: 10.1289/ehp.7633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kubo K, Arai O, Ogata R, Omura M, Hori T, Aou S. Exposure to bisphenol A during the fetal and suckling periods disrupts sexual differentiation of the locus coeruleus and of behaviour in the rat. Neurosci Lett. 2001;304(12):73–6. doi: 10.1016/s0304-3940(01)01760-8. [DOI] [PubMed] [Google Scholar]
- 46.Kubo K, Arai O, Omura M, Watanabe R, Ogata R, Aou S. Low dose effects of bisphenol A on sexual differentiation of the brain and behavior in rats. Neurosci Res. 2003;45(3):345–56. doi: 10.1016/s0168-0102(02)00251-1. [DOI] [PubMed] [Google Scholar]
- 47.Rubin BS, Lenkowski JR, Schaeberle CM, Vandenberg LN, Ronsheim PM, Soto AM. Evidence of altered brain sexual differentiation in mice exposed perinatally to low, environmentally relevant levels of bisphenol A. Endocrinology. 2006;147(8):3681–91. doi: 10.1210/en.2006-0189. [DOI] [PubMed] [Google Scholar]
- 48.Patisaul HB, Fortino AE, Polston EK. Neonatal genistein or bisphenol-A exposure alters sexual differentiation of the AVPV. Neurotoxicol Teratol. 2006;28(1):111–8. doi: 10.1016/j.ntt.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 49.Masuo Y, Ishido M, Morita M, Oka S. Effects of neonatal treatment with 6-hydroxydopamine and endocrine disruptors on motor activity and gene expression in rats. Neural Plas. 2004;11(12):59–76. doi: 10.1155/NP.2004.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakamura K, Itoh K, Yaoi T, Fujiwara Y, Sugimoto T, Fushiki S. Murine neocortical histogenesis is perturbed by prenatal exposure to low doses of bisphenol A. J Neurosci Res. 2006;84(6):1197–205. doi: 10.1002/jnr.21020. [DOI] [PubMed] [Google Scholar]
- 51.Farabollini F, Porrini S, Della Seta D, Bianchi F, Dessi-Fulgheri F. Effects of perinatal exposure to bisphenol A on sociosexual behavior of female and male rats. Environ Health Perspect. 2002;110(Suppl 3):409–14. doi: 10.1289/ehp.02110s3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kawai K, Takehiro N, Nishikata H, Aou S, Takii M, Kubo C. Aggressive behavior and serum testosterone concentration during the maturation process of male mice: The effects of fetal exposure to bisphenol A. Environ Health Perspect. 2003;111:175–8. doi: 10.1289/ehp.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Masuo Y, Morita M, Oka S, Ishido M. Motor hyperactivity caused by a deficit in dopaminergic neurons and the effects of endocrine disruptors: a study inspired by the physiological roles of PACAP in the brain. Regul Pept. 2004;123(13):225–34. doi: 10.1016/j.regpep.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 54.Ishido M, Masuo Y, Kunimoto M, Oka S, Morita M. Bisphenol A causes hyperactivity in the rat concomitantly with impairment of tyrosine hydroxylase immunoreactivity. J Neurosci Res. 2004;76(3):423–33. doi: 10.1002/jnr.20050. [DOI] [PubMed] [Google Scholar]
- 55.Mizuo K, Narita M, Miyagawa K, Okuno E, Suzuki T. Prenatal and neonatal exposure to bisphenol-A affects the morphine-induced rewarding effect and hyperlocomotion in mice. Neurosci Lett. 2004;356(2):95–8. doi: 10.1016/j.neulet.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 56.Aloisi AM, Della Seta D, Rendo C, Ceccarelli I, Scaramuzzino A, Farabollini F. Exposure to the estrogenic pollutant bisphenol A affects pain behavior induced by subcutaneous formalin injection in male and female rats. Brain Res. 2002;937(12):1–7. doi: 10.1016/s0006-8993(02)02446-0. [DOI] [PubMed] [Google Scholar]
- 57.Ryan BC, Vandenbergh JG. Developmental exposure to environmental estrogens alters anxiety and spatial memory in female mice. Horm Behav. 2006;50(1):85–93. doi: 10.1016/j.yhbeh.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 58.Farabollini F, Porrini S, Dessi-Fulgheri F. Perinatal exposure to the estrogenic pollutant bisphenol A affects behavior in male and female rats. Pharmacol Biochem Behav. 1999;64:687–94. doi: 10.1016/s0091-3057(99)00136-7. [DOI] [PubMed] [Google Scholar]
- 59.Negishi T, Kawasaki K, Suzaki S, Maeda H, Ishii Y, Kyuwa S, et al. Behavioral alterations in response to fear-provoking stimuli and tranylcypromine induced by perinatal exposure to bisphenol A and nonylphenol in male rats. Environ Health Perspect. 2004;112(11):1159–64. doi: 10.1289/ehp.6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Palanza P, Howdeshell KL, Parmigiani S, vom Saal FS. Exposure to a low dose of bisphenol A during fetal life or in adulthood alters maternal behavior in mice. Environ Health Perspect. 2002;110(Suppl 3):415–22. doi: 10.1289/ehp.02110s3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Della Seta D, Minder I, Dessi-Fulgheri F, Farabollini F. Bisphenol-A exposure during pregnancy and lactation affects maternal behavior in rats. Brain Res Bull. 2005;65(3):255–60. doi: 10.1016/j.brainresbull.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 62.Dessi-Fulgheri F, Porrini S, Farabollini F. Effects of perinatal exposure to bisphenol A on play behavior of female and male juvenile rats. Environ Health Perspect. 2002;110(Suppl 3):403–7. doi: 10.1289/ehp.110-1241190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Porrini S, Belloni V, Della Seta D, Farabollini F, Giannelli G, Dessi-Fulgheri F. Early exposure to a low dose of bisphenol A affects socio-sexual behavior of juvenile female rats. Brain Res Bull. 2005;65(3):261–6. doi: 10.1016/j.brainresbull.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 64.Adriani W, Della Seta D, Dessi-Fulgheri F, Farabollini F, Laviola G. Altered profiles of spontaneous novelty seeking, impulsive behavior, and response to D-amphetamine in rats perinatally exposed to bisphenol A. Environ Health Perspect. 2003;111:395–401. doi: 10.1289/ehp.5856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Suzuki T, Mizuo K, Nakazawa H, Funae Y, Fushiki S, Fukushima S, et al. Prenatal and neonatal exposure to bisphenol-A enhances the central dopamine D1 receptor-mediated action in mice: enhancement of the methamphetamine-induced abuse state. Neuroscience. 2003;117(3):639–44. doi: 10.1016/s0306-4522(02)00935-1. [DOI] [PubMed] [Google Scholar]
- 66.Laviola G, Gioiosa L, Adriania W, Palanza P. D-Amphetamine-related reinforcing effects are reduced in mice exposed prenatally to estrogenic endocrine disruptors. Brain Res Bull. 2005;65:235–40. doi: 10.1016/j.brainresbull.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 67.Della Seta D, Minder I, Belloni V, Aloisi AM, Dessi-Fulgheri F, Farabollini F. Pubertal exposure to estrogenic chemicals affects behavior in juvenile and adult male rats. Horm Behav. 2006;50(2):301–7. doi: 10.1016/j.yhbeh.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 68.Carr R, Bertasi F, Betancourt A, Bowers S, Gandy BS, Ryan P, et al. Effect of neonatal rat bisphenol A exposure on performance in the Morris water maze. J Toxicol Environ Health A. 2003;66(21):2077–88. doi: 10.1080/713853983. [DOI] [PubMed] [Google Scholar]
- 69.Shibata N, Matsumoto J, Nakada K, Yuasa A, Yokota H. Male-specific suppression of hepatic microsomal UDP-glucuronosyl transferase activities toward sex hormones in the adult male rat administered bisphenol A. Biochem J. 2002;368(Pt 3):783–8. doi: 10.1042/BJ20020804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Imanishi S, Manabe N, Nishizawa H, Morita M, Sugimoto M, Iwahori M, et al. Effects of oral exposure of bisphenol A on mRNA expression of nuclear receptors in murine placentae assessed by DNA microarray. J Reprod Dev. 2003;49:329–36. doi: 10.1262/jrd.49.329. [DOI] [PubMed] [Google Scholar]
- 71.Nishizawa H, Manabe N, Morita M, Sugimoto M, Imanishi S, Miyamoto H. Effects of in utero exposure to bisphenol A on expression of RARα and RXRβ mRNAs in murine embryos. J Reprod Dev. 2003;49(6):539–45. doi: 10.1262/jrd.49.539. [DOI] [PubMed] [Google Scholar]
- 72.Funabashi T, Kawaguchi M, Furuta M, Fukushima A, Kimura F. Exposure to bisphenol A during gestation and lactation causes loss of sex difference in corticotropin-releasing hormone-immunoreactive neurons in the bed nucleus of the stria terminalis of rats. Psychoneuroendocrinology. 2004;29(4):475–85. doi: 10.1016/s0306-4530(03)00055-6. [DOI] [PubMed] [Google Scholar]
- 73.Fujimoto T, Kubo K, Aou S. Prenatal exposure to bisphenol A impairs sexual differentiation of exploratory behavior and increases depression-like behavior in rats. Brain Res. 2006;1068(1):49–55. doi: 10.1016/j.brainres.2005.11.028. [DOI] [PubMed] [Google Scholar]
- 74.Takeuchi T, Tsutsumi O. Serum bisphenol A concentrations showed gender differences, possibly linked to androgen levels. Biochem Biophys Res Commun. 2002;291(1):76–8. doi: 10.1006/bbrc.2002.6407. [DOI] [PubMed] [Google Scholar]
- 75.Takeuchi T, Tsutsumi O, Ikezuki Y, Kamei Y, Osuga Y, Fujiwara T, et al. Elevated serum bisphenol A levels under hyperandrogenic conditions may be caused by decreased UDP-glucuronosyltransferase activity. Endocr J. 2006;53(4):485–91. doi: 10.1507/endocrj.k06-032. [DOI] [PubMed] [Google Scholar]
- 76.Hanioka N, Jinno H, Tanaka-Kagawa T, Nishimura T, Ando M. Interaction of bisphenol A with rat hepatic cytochrome P450 enzymes. Chemosphere. 2000;41(7):973–8. doi: 10.1016/s0045-6535(99)00529-9. [DOI] [PubMed] [Google Scholar]
- 77.Matsumoto J, Yokota H, Yuasa A. Developmental increases in rat hepatic microsomal UDP-glucuronosyltransferase activities toward xenoestrogens and decreases during pregnancy. Environ Health Perspect. 2002;110(2):193–6. doi: 10.1289/ehp.02110193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Howdeshell KL, Hotchkiss AK, Thayer KA, Vandenbergh JG, vom Saal FS. Exposure to bisphenol A advances puberty. Nature. 1999;401(6755):763–4. doi: 10.1038/44517. [DOI] [PubMed] [Google Scholar]
- 79.Vandenberg LN, Maffini MV, Wadia PR, Sonnenschein C, Rubin BS, Soto AM. Exposure to environmentally relevant doses of the xenoestrogen bisphenol-A alters development of the fetal mouse mammary gland. Endocrinology. 2007;148(1):116–27. doi: 10.1210/en.2006-0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Markey CM, Luque EH, Munoz De Toro M, Sonnenschein C, Soto AM. In utero exposure to bisphenol A alters the development and tissue organization of the mouse mammary gland. Biol Reprod. 2001;65(4):1215–23. doi: 10.1093/biolreprod/65.4.1215. [DOI] [PubMed] [Google Scholar]
- 81.Munoz-de-Toro M, Markey CM, Wadia PR, Luque EH, Rubin BS, Sonnenschein C, et al. Perinatal exposure to bisphenol-A alters peripubertal mammary gland development in mice. Endocrinology. 2005;146(9):4138–47. doi: 10.1210/en.2005-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Durando M, Kass L, Piva J, Sonnenschein C, Soto AM, Luque E, et al. Prenatal bisphenol A exposure induces preneoplastic lesions in the mammary gland in Wistar rats. Environ Health Perspect. 2007;115(1):80–6. doi: 10.1289/ehp.9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Murray TJ, Maffini MV, Ucci AA, Sonnenschein C, Soto AM. Induction of mammary gland ductal hyperplasias and carcinoma in situ following fetal bisphenol A exposure. Reprod Toxicol. 2006 doi: 10.1016/j.reprotox.2006.10.002. In Press, Available online 24 October 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.vom Saal FS. Sexual differentiation in litter-bearing mammals: influence of sex of adjacent fetuses in utero. J Anim Sci. 1989;67(7):1824–40. doi: 10.2527/jas1989.6771824x. [DOI] [PubMed] [Google Scholar]
- 85.Colerangle JB, Roy D. Profound effects of the weak environmental estrogen-like chemical bisphenol A on the growth of the mammary gland of Noble rats. J Steroid Biochem Mol Biol. 1997;60(12):153–60. doi: 10.1016/s0960-0760(96)00130-6. [DOI] [PubMed] [Google Scholar]
- 86.Schönfelder G, Friedrich K, Paul M, Chahoud I. Developmental effects of prenatal exposure to bisphenol A on the uterus of rat offspring. Neoplasia. 2004;6:584–94. doi: 10.1593/neo.04217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schönfelder G, Flick B, Mayr L, Talsness C, Paul M, Chahoud I. In utero exposure to low doses of bisphenol A lead to long-term deleterious effects in the vagina. Neoplasia. 2002;4:98–102. doi: 10.1038/sj.neo.7900212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Markey CM, Wadia PR, Rubin BS, Sonnenschein C, Soto AM. Long-term effects of fetal exposure to low doses of the xenoestrogen bisphenol-A in the female mouse genital tract. Biol Reprod. 2005;72(6):1344–51. doi: 10.1095/biolreprod.104.036301. [DOI] [PubMed] [Google Scholar]
- 89.Hunt PA, Koehler KE, Susiarjo M, Hodges CA, Hagan A, Voigt RC, et al. Bisphenol A causes meiotic aneuploidy in the female mouse. Curr Biol. 2003;13:546–53. doi: 10.1016/s0960-9822(03)00189-1. [DOI] [PubMed] [Google Scholar]
- 90.Al-Hiyasat AS, Darmani H, Elbetieha AM. Leached components from dental composites and their effects on fertility of female mice. Eur J Oral Sci. 2004;112:267–72. doi: 10.1111/j.1600-0722.2004.00136.x. [DOI] [PubMed] [Google Scholar]
- 91.Susiarjo M, Hassold TJ, Freeman E, Hunt PA. Bisphenol A exposure in utero disrupts early oogenesis in the mouse. PLoS Genetics. 2007;3(1):e5. doi: 10.1371/journal.pgen.0030005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Berger RG, Hancock T, Decatanzaro D. Influence of oral and subcutaneous bisphenol-A on intrauterine implantation of fertilized ova in inseminated female mice. Reprod Toxicol. 2007;23(2):138–44. doi: 10.1016/j.reprotox.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 93.Richter CA, Timms BG, vom Saal FS. Prostate Development: Mechanisms for opposite effects of low and high doses of estrogenic chemicals. In: Naz RK, editor. Endocrine Disruptors: Effects on Male and Female Reproductive Systems. 2. Boca Raton, FL: CRC Press; 2005. pp. 379–410. [Google Scholar]
- 94.Nagel SC, vom Saal FS, Thayer KA, Dhar MG, Boechler M, Welshons WV. Relative binding affinity-serum modified access (RBA-SMA) assay predicts the relative in vivo bioactivity of the xenoestrogens bisphenol A and octylphenol. Environ Health Perspect. 1997;105(1):70–6. doi: 10.1289/ehp.9710570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Prins GS, Birch L, Tang W-Y, Ho S-M. Developmental estrogen exposures predispose to prostate carcinogenesis with aging. Reprod Toxicol. 2007;23(3):374–82. doi: 10.1016/j.reprotox.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wetherill YB, Hess-Wilson JK, Comstock CES, Shah SA, Buncher CR, Sallans L, et al. Bisphenol A facilitates bypass of androgen ablation therapy in prostate cancer. Mol Cancer Therapeutics. 2006;5(12):3181–90. doi: 10.1158/1535-7163.MCT-06-0272. [DOI] [PubMed] [Google Scholar]
- 97.Ramos JG, Varayoud J, Sonnenschein C, Soto AM, Munoz De Toro M, Luque EH. Prenatal exposure to low doses of bisphenol A alters the periductal stroma and glandular cell function in the rat ventral prostate. Biol Reprod. 2001;65(4):1271–7. doi: 10.1095/biolreprod65.4.1271. [DOI] [PubMed] [Google Scholar]
- 98.Timms BG, Petersen SL, vom Saal FS. Prostate gland growth during development is stimulated in both male and female rat fetuses by intrauterine proximity to female fetuses. J Urol. 1999;161:1694–701. [PubMed] [Google Scholar]
- 99.Timms BG, Peterson RE, vom Saal FS. 2,3,7,8-Tetrachlorodibenzo-p-dioxin interacts with endogenous estradiol to disrupt prostate gland morphogenesis in male rat fetuses. Toxicol Sci. 2002;67:264–74. doi: 10.1093/toxsci/67.2.264. [DOI] [PubMed] [Google Scholar]
- 100.Stoker TE, Robinette CL, Britt BH, Laws SC, Cooper RL. Prepubertal exposure to compounds that increase prolactin secretion in the male rat: effects on the adult prostate. Biol Reprod. 1999;61(6):1636–43. doi: 10.1095/biolreprod61.6.1636. [DOI] [PubMed] [Google Scholar]
- 101.vom Saal FS, Cooke PS, Buchanan DL, Palanza P, Thayer KA, Nagel SC, et al. A physiologically based approach to the study of bisphenol A and other estrogenic chemicals on the size of reproductive organs, daily sperm production, and behavior. Toxicol Ind Health. 1998;14(12):239–60. doi: 10.1177/074823379801400115. [DOI] [PubMed] [Google Scholar]
- 102.Aikawa H, Koyama S, Matsuda M, Nakahashi K, Akazome Y, Mori T. Relief effect of vitamin A on the decreased motility of sperm and the increased incidence of malformed sperm in mice exposed neonatally to bisphenol A. Cell Tissue Res. 2004;315(1):119–24. doi: 10.1007/s00441-003-0806-1. [DOI] [PubMed] [Google Scholar]
- 103.Toyama Y, Yuasa S. Effects of neonatal administration of 17β-estradiol, β-estradiol 3-benzoate, or bisphenol A on mouse and rat spermatogenesis. Reprod Toxicol. 2004;19(2):181–8. doi: 10.1016/j.reprotox.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 104.Fisher JS, Turner KJ, Brown D, Sharpe RM. Effect of neonatal exposure to estrogenic compounds on development of the excurrent ducts of the rat testis through puberty to adulthood. Environ Health Perspect. 1999;107(5):397–405. doi: 10.1289/ehp.99107397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang Y, Thuillier R, Culty M. Prenatal estrogen exposure differentially affects estrogen receptor-associated proteins in rat testis gonocytes. Biol Reprod. 2004;71(5):1652–64. doi: 10.1095/biolreprod.104.030205. [DOI] [PubMed] [Google Scholar]
- 106.Wistuba J, Brinkworth MH, Schlatt S, Chahoud I, Nieschlag E. Intrauterine bisphenol A exposure leads to stimulatory effects on Sertoli cell number in rats. Environ Res. 2003;91(2):95–103. doi: 10.1016/s0013-9351(02)00019-1. [DOI] [PubMed] [Google Scholar]
- 107.Takai Y, Tsutsumi O, Ikezuki Y, Hiroi H, Osuga Y, Momoeda M, et al. Estrogen receptor-mediated effects of a xenoestrogen, bisphenol A, on preimplantation mouse embryos. Biochem Biophys Res Commun. 2000;270(3):918–21. doi: 10.1006/bbrc.2000.2548. [DOI] [PubMed] [Google Scholar]
- 108.Takai Y, Tsutsumi O, Ikezuki Y, Kamei Y, Osuga Y, Yano T, et al. Preimplantation exposure to bisphenol A advances postnatal development. Reprod Toxicol. 2001;15(1):71–4. doi: 10.1016/s0890-6238(00)00119-2. [DOI] [PubMed] [Google Scholar]
- 109.Ashby J, Tinwell H, Haseman J. Lack of effects for low dose levels of bisphenol A (BPA) and diethylstilbestrol (DES) on the prostate gland of CF1 mice exposed in utero. Regul Toxicol Pharmacol. 1999;30:156–66. doi: 10.1006/rtph.1999.1317. [DOI] [PubMed] [Google Scholar]
- 110.Markey CM, Coombs MA, Sonnenschein C, Soto AM. Mammalian development in a changing environment: exposure to endocrine disruptors reveals the developmental plasticity of steroid-hormone target organs. Evol Dev. 2003;5(1):67–75. doi: 10.1046/j.1525-142x.2003.03011.x. [DOI] [PubMed] [Google Scholar]
- 111.Cooke PS, Naaz A. Role of estrogens in adipocyte development and function. Exp Biol Med. 2004;229(11):1127–35. doi: 10.1177/153537020422901107. [DOI] [PubMed] [Google Scholar]
- 112.Sakurai K, Kawazuma M, Adachi T, Harigaya T, Saito Y, Hashimoto N, et al. Bisphenol A affects glucose transport in mouse 3T3-F442A adipocytes. Br J Pharmacol. 2004;141(2):209–14. doi: 10.1038/sj.bjp.0705520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Deems RO, Evans JL, Deacon RW, Honer CM, Chu DT, Burki K, et al. Expression of human GLUT4 in mice results in increased insulin action. Diabetologia. 1994;37(11):1097–104. doi: 10.1007/BF00418373. [DOI] [PubMed] [Google Scholar]
- 114.Kabuto H, Amakawa M, Shishibori T. Exposure to bisphenol A during embryonic/fetal life and infancy increases oxidative injury and causes underdevelopment of the brain and testis in mice. Life Sci. 2004;74(24):2931–40. doi: 10.1016/j.lfs.2003.07.060. [DOI] [PubMed] [Google Scholar]
- 115.vom Saal FS, Richter CA, Mao J, Welshons WV. Commercial animal feed: variability in estrogenic activity and effects on body weight in mice. Birth Defects Res A Clin Mol Teratol. 2005;73(7):474–5. doi: 10.1002/bdra.20149. [DOI] [PubMed] [Google Scholar]
- 116.Yoshino S, Yamaki K, Li X, Sai T, Yanagisawa R, Takano H, et al. Prenatal exposure to bisphenol A up-regulates immune responses, including T helper 1 and T helper 2 responses, in mice. Immunology. 2004;112(3):489–95. doi: 10.1111/j.1365-2567.2004.01900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Funabashi T, Kawaguchi M, Kimura F. The endocrine disrupters butyl benzyl phthalate and bisphenol A increase the expression of progesterone receptor messenger ribonucleic acid in the preoptic area of adult ovariectomized rats. Neuroendocrinology. 2001;74:77–81. doi: 10.1159/000054672. [DOI] [PubMed] [Google Scholar]
- 118.Funabashi T, Sano A, Mitsushima D, Kimura F. Bisphenol A increases progesterone receptor immunoreactivity in the hypothalamus in a dose-dependent manner and affects sexual behaviour in adult ovariectomized rats. J Neuroendocrinol. 2003;15(2):134–40. doi: 10.1046/j.1365-2826.2003.00872.x. [DOI] [PubMed] [Google Scholar]
- 119.Funabashi T, Nakamura TJ, Kimura F. p-Nonylphenol, 4-tert-octylphenol and bisphenol A increase the expression of progesterone receptor mRNA in the frontal cortex of adult ovariectomized rats. J Neuroendocrinol. 2004;16(2):99–104. doi: 10.1111/j.0953-8194.2004.01136.x. [DOI] [PubMed] [Google Scholar]
- 120.Aloisi AM, Della Seta D, Ceccarelli I, Farabollini F. Bisphenol-A differently affects estrogen receptors-α in estrous-cycling and lactating female rats. Neurosci Lett. 2001;310(1):49–52. doi: 10.1016/s0304-3940(01)02092-4. [DOI] [PubMed] [Google Scholar]
- 121.Steinmetz R, Brown NG, Allen DL, Bigsby RM, Ben-Jonathan N. The environmental estrogen bisphenol A stimulates prolactin release in vitro and in vivo. Endocrinology. 1997;138(5):1780–6. doi: 10.1210/endo.138.5.5132. [DOI] [PubMed] [Google Scholar]
- 122.Razzoli M, Valsecchi P, Palanza P. Chronic exposure to low doses bisphenol A interferes with pair-bonding and exploration in female Mongolian gerbils. Brain Res Bull. 2005;65(3):249–54. doi: 10.1016/j.brainresbull.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 123.Tsutsui T, Tamura Y, Suzuki A, Hirose Y, Kobayashi M, Nishimura H, et al. Mammalian cell transformation and aneuploidy induced by five bisphenols. Int J Cancer. 2000;86(2):151–4. doi: 10.1002/(sici)1097-0215(20000415)86:2<151::aid-ijc1>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 124.Tsutsui T, Tamura Y, Yagi E, Hasegawa K, Takahashi M, Maizumi N, et al. Bisphenol-A induces cellular transformation, aneuploidy and DNA adduct formation in cultured Syrian hamster embryo cells. Int J Cancer. 1998;75(2):290–4. doi: 10.1002/(sici)1097-0215(19980119)75:2<290::aid-ijc19>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 125.Parry EM, Parry JM, Corso C, Doherty A, Haddad F, Hermine TF, et al. Detection and characterization of mechanisms of action of aneugenic chemicals. Mutagenesis. 2002;17(6):509–21. doi: 10.1093/mutage/17.6.509. [DOI] [PubMed] [Google Scholar]
- 126.Eichenlaub-Ritter U, Sun F, Betzendahl I. Meiotic progression, spindle formation and chromosome segregation in in vitro maturing mouse oocytes exposed to bisphenol A. Environ Res. 2005;98(3):405–6. [Google Scholar]
- 127.Sakaue M, Ohsako S, Ishimura R, Kurosawa S, Kurohmaru M, Hayashi Y, et al. Bisphenol A affects spermatogenesis in the adult rat even at a low dose. J Occup Health. 2001;43:185–90. [Google Scholar]
- 128.Thayer KA, Ruhlen RL, Howdeshell KL, Buchanan DL, Cooke PS, Preziosi D, et al. Altered prostate growth and daily sperm production in male mice exposed prenatally to subclinical doses of 17a-ethinyl oestradiol. Hum Reprod. 2001;16(5):988–96. doi: 10.1093/humrep/16.5.988. [DOI] [PubMed] [Google Scholar]
- 129.Al-Hiyasat AS, Darmani H, Elbetieha AM. Effects of bisphenol A on adult male mouse fertility. Eur J Oral Sci. 2002;110(2):163–7. doi: 10.1034/j.1600-0722.2002.11201.x. [DOI] [PubMed] [Google Scholar]
- 130.Toyama Y, Suzuki-Toyota F, Maekawa M, Ito C, Toshimori K. Adverse effects of bisphenol A to spermiogenesis in mice and rats. Arch Histol Cytol. 2004;67(4):373–81. doi: 10.1679/aohc.67.373. [DOI] [PubMed] [Google Scholar]
- 131.Chitra KC, Latchoumycandane C, Mathur PP. Induction of oxidative stress by bisphenol A in the epididymal sperm of rats. Toxicology. 2003;185(12):119–27. doi: 10.1016/s0300-483x(02)00597-8. [DOI] [PubMed] [Google Scholar]
- 132.Chitra KC, Rao KR, Mathur PP. Effect of bisphenol A and co-administration of bisphenol A and vitamin C on epididymis of adult rats: A histological and biochemical study. Asian J Androl. 2003;5(3):203–8. [PubMed] [Google Scholar]
- 133.Takao T, Nanamiya W, Nagano I, Asaba K, Kawabata K, Hashimoto K. Exposure with the environmental estrogen bisphenol A disrupts the male reproductive tract in young mice. Life Sci. 1999;65(22):2351–7. doi: 10.1016/s0024-3205(99)00502-0. [DOI] [PubMed] [Google Scholar]
- 134.Takao T, Nanamiya W, Nazarloo HP, Matsumoto R, Asaba K, Hashimoto K. Exposure to the environmental estrogen bisphenol A differentially modulated estrogen receptor-α and -β immunoreactivity and mRNA in male mouse testis. Life Sci. 2003;72(10):1159–69. doi: 10.1016/s0024-3205(02)02364-0. [DOI] [PubMed] [Google Scholar]
- 135.Tohei A, Suda S, Taya K, Hashimoto T, Kogo H. Bisphenol A inhibits testicular functions and increases luteinizing hormone secretion in adult male rats. Exp Biol Med. 2001;226:216–21. doi: 10.1177/153537020122600309. [DOI] [PubMed] [Google Scholar]
- 136.Takahashi O, Oishi S. Testicular toxicity of dietarily or parenterally administered bisphenol A in rats and mice. Food Chem Toxicol. 2003;41(7):1035–44. doi: 10.1016/s0278-6915(03)00031-0. [DOI] [PubMed] [Google Scholar]
- 137.Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-α knockout mice. Proc Natl Acad Sci U S A. 2000;97(23):12729–34. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cooke PS, Heine PA, Taylor JA, Lubahn DB. The role of estrogen and estrogen receptor-alpha in male adipose tissue. Mol Cell Endocrinol. 2001;178(12):147–54. doi: 10.1016/s0303-7207(01)00414-2. [DOI] [PubMed] [Google Scholar]
- 139.Nunez AA, Kannan K, Giesy JP, Fang J, Clemens LG. Effects of bisphenol A on energy balance and accumulation in brown adipose tissue in rats. Chemosphere. 2001;42:917–22. doi: 10.1016/s0045-6535(00)00196-x. [DOI] [PubMed] [Google Scholar]
- 140.Alonso-Magdalena P, Morimoto S, Ripoll C, Fuentes E, Nadal A. The estrogenic effect of bisphenol A disrupts pancreatic β-cell function in vivo and induces insulin resistance. Environ Health Perspect. 2006;114(1):106–12. doi: 10.1289/ehp.8451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kabuto H, Hasuike S, Minagawa N, Shishibori T. Effects of bisphenol A on the metabolisms of active oxygen species in mouse tissues. Environ Res. 2003;93(1):31–5. doi: 10.1016/s0013-9351(03)00062-8. [DOI] [PubMed] [Google Scholar]
- 142.Sawai C, Anderson K, Walser-Kuntz D. Effect of bisphenol A on murine immune function: Modificattion of interferon-γ, IgG2a, and disease symptoms in NZB × NZW F1 mice. Environ Health Perspect. 2003;111(16):1883–7. doi: 10.1289/ehp.6359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yoshino S, Yamaki K, Yanagisawa R, Takano H, Hayashi H, Mori Y. Effects of bisphenol A on antigen-specific antibody production, proliferative responses of lymphoid cells, and TH1 and TH2 immune responses in mice. Br J Pharmacol. 2003;138(7):1271–6. doi: 10.1038/sj.bjp.0705166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tian X, Takamoto M, Sugane K. Bisphenol A promotes IL-4 production by Th2 cells. Int Arch Allergy Immunol. 2003;132:240–7. doi: 10.1159/000074305. [DOI] [PubMed] [Google Scholar]
- 145.Lee MH, Chung SW, Kang BY, Park J, Lee CH, Hwang SY, et al. Enhanced interleukin-4 production in CD4+ T cells and elevated immunoglobulin E levels in antigen-primed mice by bisphenol A and nonylphenol, endocrine disruptors: involvement of nuclear factor-AT and Ca2+ Immunology. 2003;109(1):76–86. doi: 10.1046/j.1365-2567.2003.01631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chalubinski M, Kowalski ML. Endocrine disruptors - potential modulators of the immune system and allergic response. Allergy. 2006;61:1326–35. doi: 10.1111/j.1398-9995.2006.01135.x. [DOI] [PubMed] [Google Scholar]
- 147.Sugita-Konishi Y, Shimura S, Nishikawa T, Sunaga F, Naito H, Suzuki Y. Effect of Bisphenol A on non-specific immunodefenses against non-pathogenic Escherichia coli. Toxicol Lett. 2003;136(3):217–27. doi: 10.1016/s0378-4274(02)00388-0. [DOI] [PubMed] [Google Scholar]
- 148.Yurino H, Ishikawa S, Sato T, Akadegawa K, Ito T, Ueha S, et al. Endocrine disruptors (environmental estrogens) enhance autoantibody production by B1 cells. Toxicol Sci. 2004;81(1):139–47. doi: 10.1093/toxsci/kfh179. [DOI] [PubMed] [Google Scholar]
- 149.Beagley KW, Gockel CM. Regulation of innate and adaptive immunity by the female sex hormones oestradiol and progesterone. FEMS Immunol Med Microbiol. 2003;38(1):13–22. doi: 10.1016/S0928-8244(03)00202-5. [DOI] [PubMed] [Google Scholar]
- 150.Liu H-b, Loo KK, Palaszynski K, Ashouri J, Lubahn DB, Voskuhl RR. Estrogen receptor α mediates estrogen’s immune protection in autoimmune disease. J Immunol. 2003;171:6936–40. doi: 10.4049/jimmunol.171.12.6936. [DOI] [PubMed] [Google Scholar]
- 151.Karpuzoglu-Sahin E, Hissong BD, Ansar Ahmed S. Interferon-gamma levels are upregulated by 17-beta-estradiol and diethylstilbestrol. J Reprod Immunol. 2001;52(12):113–27. doi: 10.1016/s0165-0378(01)00117-6. [DOI] [PubMed] [Google Scholar]
- 152.Lamason R, Zhao P, Rawat R, Davis A, Hall J, Chae J, et al. Sexual dimorphism in immune response genes as a function of puberty. BMC Immunology. 2006;7(2):1472. doi: 10.1186/1471-2172-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.vom Saal FS, Welshons WV. Large effects from small exposures. II. The importance of positive controls in low-dose research on bisphenol A. Environ Res. 2006;100:50–76. doi: 10.1016/j.envres.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 154.White WJ, Lee CS. The development and maintanence of the Crl:CD®(SD)IGS BR rat breeding system. Charles River Laboratories Research Models and Services. 1998;1998:8–14. [Google Scholar]
- 155.Elswick BA, Welsch F, Janszen DB. Effect of different sampling designs on outcome of endocrine disruptor studies. Reprod Toxicol. 2000;14:359–67. doi: 10.1016/s0890-6238(00)00092-7. [DOI] [PubMed] [Google Scholar]
- 156.Ema M, Fujii S, Furukawa M, Kiguchi M, Ikka T, Harazono A. Rat two-generation reproductive toxicity study of bisphenol A. Reprod Toxicol. 2001;15(5):505–23. doi: 10.1016/s0890-6238(01)00160-5. [DOI] [PubMed] [Google Scholar]
- 157.Kamata R, Koda T, Morohoshi K, Umezu T, Morita M. RNA constitution and estrogen-responsive gene expression in the ovariectomized rat uterus. Anal Biochem. 2005;341(1):131–40. doi: 10.1016/j.ab.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 158.Kato H, Furuhashi T, Tanaka M, Katsu Y, Watanabe H, Ohta Y, et al. Effects of bisphenol A given neonatally on reproductive functions of male rats. Reprod Toxicol. 2006;22(1):20–9. doi: 10.1016/j.reprotox.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 159.Kobayashi K, Miyagawa M, Wang RS, Sekiguchi S, Suda M, Honma T. Effects of in utero and lactational exposure to bisphenol A on somatic growth and anogenital distance in F1 rat offspring. Ind Health. 2002;40(4):375–81. doi: 10.2486/indhealth.40.375. [DOI] [PubMed] [Google Scholar]
- 160.Kobayashi K, Miyagawa M, Wang RS, Suda M, Sekiguchi S, Honma T. Effects of in utero and lactational exposure to bisphenol A on thyroid status in F1 rat offspring. Ind Health. 2005;43(4):685–90. doi: 10.2486/indhealth.43.685. [DOI] [PubMed] [Google Scholar]
- 161.Kwon S, Stedman DB, Elswick BA, Cattley RC, Welsch F. Pubertal development and reproductive functions of Crl:CD BR Sprague-Dawley rats exposed to bisphenol A during prenatal and postnatal development. Toxicol Sci. 2000;55(2):399–406. doi: 10.1093/toxsci/55.2.399. [DOI] [PubMed] [Google Scholar]
- 162.Kim HS, Han SY, Kim TS, Kwack SJ, Lee RD, Kim IY, et al. No androgenic/antiandrogenic effects of bisphenol-A in Hershberger assay using immature castrated rats. Toxicol Lett. 2002;135:111–23. doi: 10.1016/s0378-4274(02)00130-3. [DOI] [PubMed] [Google Scholar]
- 163.Masutomi N, Shibutani M, Takagi H, Uneyama C, Lee KY, Hirose M. Alteration of pituitary hormone-immunoreactive cell populations in rat offspring after maternal dietary exposure to endocrine-active chemicals. Arch Toxicol. 2004;78(4):232–40. doi: 10.1007/s00204-003-0528-x. [DOI] [PubMed] [Google Scholar]
- 164.Nagao T, Saito Y, Usumi K, Kuwagata M, Imai K. Reproductive function in rats exposed neonatally to bisphenol A and estradiol benzoate. Reprod Toxicol. 1999;13(4):303–11. doi: 10.1016/s0890-6238(99)00017-9. [DOI] [PubMed] [Google Scholar]
- 165.Takagi H, Shibutani M, Masutomi N, Uneyama C, Takahashi N, Mitsumori K, et al. Lack of maternal dietary exposure effects of bisphenol A and nonylphenol during the critical period for brain sexual differentiation on the reproductive/endocrine systems in later life. Arch Toxicol. 2004;78(2):97–105. doi: 10.1007/s00204-003-0517-0. [DOI] [PubMed] [Google Scholar]
- 166.Tyl RW, Myers CB, Marr MC, Thomas BF, Keimowitz AR, Brine DR, et al. Three-generation reproductive toxicity study of dietary bisphenol A in CD Sprague-Dawley rats. Toxicol Sci. 2002;68(1):121–46. doi: 10.1093/toxsci/68.1.121. [DOI] [PubMed] [Google Scholar]
- 167.Yamasaki K, Sawaki M, Noda S, Inmatanaka N, Takatsuki M. Subacute oral toxicity study of ethinylestradiol and bisphenol A, based on the draft protocol for the ‘Enhanced OECD Test Guideline no. 407’. Arch Toxicol. 2002;76:65–74. doi: 10.1007/s00204-001-0319-1. [DOI] [PubMed] [Google Scholar]
- 168.Steinmetz R, Mitchner NA, Grant A, Allen DL, Bigsby RM, Ben-Jonathan N. The xenoestrogen bisphenol A induces growth, differentiation, and c-fos gene expression in the female reproductive tract. Endocrinology. 1998;139(6):2741–7. doi: 10.1210/endo.139.6.6027. [DOI] [PubMed] [Google Scholar]
- 169.Long X, Steinmetz R, Ben-Jonathan N, caperell-Grant A, Young PCM, Nephew KP, et al. Strain differences in vaginal responses to the xenoestrogen bisphenol A. Environ Health Perspect. 2000;108:243–7. doi: 10.1289/ehp.00108243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Goloubkova T, Ribeiro MFM, Rodrigues LP, Cecconello AL, Spritzer PM. Effects of xenoestrogen bisphenol A on uterine and pituitary weight, serum prolactin levels and immunoreactive prolactin cells in ovariectomized Wistar rats. Arch Toxicol. 2000;74:92–8. doi: 10.1007/s002040050658. [DOI] [PubMed] [Google Scholar]
- 171.Kim HS, Kang TS, Kang IH, Kim TS, Moon HJ, Kim IY, et al. Validation study of OECD rodent uterotrophic assay for the assessment of estrogenic activity in Sprague-Dawley immature female rats. J Toxicol Environ Health A. 2005;68(2324):2249–62. doi: 10.1080/15287390500182354. [DOI] [PubMed] [Google Scholar]
- 172.National Toxicology Program (NTP) Endocrine Disruptors Low Dose Peer Review. Raleigh, NC: 2001. Final Report of the Endocrine Disruptors Low Dose Peer Review Panel. http://ntp.niehs.nih.gov/index.cfm?objectid=06F5CE98-E82F-8182-7FA81C02D3690D47.; 2001. p. Requests for hard copies of the NTP report and inquiries about the Endocrine Disruptors Low-Dose Peer Review can be made to the NTP Liaison and Scientific Review Office NIEHS, P.O. Box 12233, Research Triangle Park, NC 27709; t: 919-541-0530; f: 919-541-0295; liaison@starbase.niehs.nih.gov).
- 173.Nagel SC, Hagelbarger JL, McDonnell DP. Development of an ER action indicator mouse for the study of estrogens, selective ER modulators (SERMs), and xenobiotics. Endocrinology. 2001;142(11):4721–8. doi: 10.1210/endo.142.11.8471. [DOI] [PubMed] [Google Scholar]
- 174.Papaconstantinou AD, Fisher BR, Umbreit TH, Goering PL, Lappas NT, Brown KM. Effects of β-estradiol and bisphenol A on heat shock protein levels and localization in the mouse uterus are antagonized by the antiestrogen ICI 182,780. Toxicol Sci. 2001;63(2):173–80. doi: 10.1093/toxsci/63.2.173. [DOI] [PubMed] [Google Scholar]
- 175.Papaconstantinou AD, Umbreit TH, Fisher BR, Goering PL, Lappas NT, Brown KM. Bisphenol A-induced increase in uterine weight and alterations in uterine morphology in ovariectomized B6C3F1 mice: Role of the estrogen receptor. Toxicol Sci. 2000;56:332–9. doi: 10.1093/toxsci/56.2.332. [DOI] [PubMed] [Google Scholar]
- 176.Markey CM, Michaelson CL, Veson EC, Sonnenschein C, Soto AM. The mouse uterotrophic assay: a reevaluation of its validity in assessing the estrogenicity of bisphenol A. Environ Health Perspect. 2001;109(1):55–60. doi: 10.1289/ehp.0110955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mehmood Z, Smith AG, Tucker MJ, Chuzel F, Carmichael NG. The development of methods for assessing the in vivo oestrogen-like effects of xenobiotics in CD-1 mice. Food Chem Toxicol. 2000;38(6):493–501. doi: 10.1016/s0278-6915(00)00022-3. [DOI] [PubMed] [Google Scholar]
- 178.Kato H, Iwata T, Katsu Y, Watanabe H, Ohta Y, Iguchi T. Evaluation of estrogenic activity in diets for experimental animals using in vitro assay. J Agric Food Chem. 2004;52(5):1410–4. doi: 10.1021/jf034896d. [DOI] [PubMed] [Google Scholar]
- 179.Cagen SZ, Waechter JM, Jr, Dimond SS, Breslin WJ, Butala JH, Jekat FW, et al. Normal reproductive organ development in CF-1 mice following prenatal exposure to bisphenol A. Toxicol Sci. 1999;11:15–29. doi: 10.1093/toxsci/50.1.36. [DOI] [PubMed] [Google Scholar]
- 180.Ashby J, Odum J. Gene expression changes in the immature rat uterus: Effects of uterotrophic and sub-uterotrophic doses of bisphenol A. Toxicol Sci. 2004;82:458–67. doi: 10.1093/toxsci/kfh283. [DOI] [PubMed] [Google Scholar]
- 181.Ashby J, Tinwell H, Lefevre PA, Joiner R, Haseman J. The effect on sperm production in adult Sprague-Dawley rats exposed by gavage to bisphenol A between postnatal days 91-97. Toxicol Sci. 2003;74(1):129–38. doi: 10.1093/toxsci/kfg093. [DOI] [PubMed] [Google Scholar]
- 182.Nagao T, Saito Y, Usumi K, Yoshimura S, Ono H. Low-dose bisphenol A does not affect reproductive organs in estrogen-sensitive C57BL/6N mice exposed at the sexually mature, juvenile, or embryonic stage. Reprod Toxicol. 2002;16(2):123–30. doi: 10.1016/s0890-6238(02)00003-5. [DOI] [PubMed] [Google Scholar]
- 183.Tinwell H, Joiner R, Pate I, Soames A, Foster J, Ashby J. Uterotrophic activity of bisphenol A in the immature mouse. Regul Toxicol Pharmacol. 2000;32(1):118–26. doi: 10.1006/rtph.2000.1412. [DOI] [PubMed] [Google Scholar]
- 184.Yoshida M, Shimomoto T, Katashima S, Watanabe G, Taya K, Maekawa A. Maternal exposure to low doses of bisphenol A has no effects on development of female reproductive tract and uterine carcinogenesis in Donryu rats. J Reprod Dev. 2004;50(3):349–60. doi: 10.1262/jrd.50.349. [DOI] [PubMed] [Google Scholar]
- 185.Yoshino H, Ichihara T, Kawabe M, Imai N, Hagiwara A, Asamoto M, et al. Lack of significant alteration in the prostate or testis of F344 rat offspring after transplacental and lactational exposure to bisphenol A. J Toxicol Sci. 2002;27(5):433–9. doi: 10.2131/jts.27.433. [DOI] [PubMed] [Google Scholar]
- 186.Cagen SZ, Waechter JM, Jr, Dimond SS, Breslin WJ, Butala JH, Jekat FW, et al. Normal reproductive organ development in Wistar rats exposed to bisphenol A in the drinking water. Regul Toxicol Pharmacol. 1999;30(2 Pt 1):130–9. doi: 10.1006/rtph.1999.1340. [DOI] [PubMed] [Google Scholar]
- 187.Atanassova N, McKinnell C, Turner KJ, Walker M, Fisher JS, Morley M, et al. Comparative effects of neonatal exposure of male rats to potent and weak (environmental) estrogens on spermatogenesis at puberty and the relationship to adult testis size and fertility: evidence for stimulatory effects of low estrogen levels. Endocrinology. 2000;141:3898–907. doi: 10.1210/endo.141.10.7723. [DOI] [PubMed] [Google Scholar]
- 188.Thuillier R, Wang Y, Culty M. Prenatal exposure to estrogenic compounds alters the expression pattern of platelet-derived growth factor receptors α and β in neonatal rat testis: Identification of gonocytes as targets of estrogen exposure. Biol Reprod. 2003;68(3):867–80. doi: 10.1095/biolreprod.102.009605. [DOI] [PubMed] [Google Scholar]
- 189.Lemmen JG, Arends RJ, van der Saag PT, van der Burg B. In vivo imaging of activated estrogen receptors in utero by estrogens and bisphenol A. Environ Health Perspect. 2004;112(15):1544–9. doi: 10.1289/ehp.7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Alonso-Magdalena P, Laribi O, Ropero AB, Fuentes E, Ripoll C, Soria B, et al. Low doses of bisphenol A and diethylstilbestrol impair Ca2+ signals in pancreatic α-cells through a nonclassical membrane estrogen receptor within intact islets of Langerhans. Environ Health Perspect. 2005;113(8):969–77. doi: 10.1289/ehp.8002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Quesada I, Fuentes E, Viso-León MC, Soria B, Ripoll C, Nadal A. Low doses of the endocrine disruptor bisphenol-A and the native hormone 17β-estradiol rapidly activate transcription factor CREB. FASEB J. 2002;16(12):1671–3. doi: 10.1096/fj.02-0313fje. [DOI] [PubMed] [Google Scholar]
- 192.Walsh DE, Dockery P, Doolan CM. Estrogen receptor independent rapid non-genomic effects of environmental estrogens on [Ca2+]i in human breast cancer cells. Mol Cell Endocrinol. 2005;230(12):23–30. doi: 10.1016/j.mce.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 193.Coser KR, Chesnes J, Hur J, Ray S, Isselbacher KJ, Shioda T. Global analysis of ligand sensitivity of estrogen inducible and suppressible genes in MCF7/BUS breast cancer cells by DNA microarray. Proc Natl Acad Sci U S A. 2003;100:13994–9. doi: 10.1073/pnas.2235866100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Shioda T, Chesnes J, Coser KR, Zou LH, Hur J, Dean KL, et al. Importance of dosage standardization for interpreting transcriptomal signature profiles: Evidence from studies of xenoestrogens. Proc Natl Acad Sci U S A. 2006;103(32):12033–8. doi: 10.1073/pnas.0605341103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Belcher SM, Le HH, Spurling L, Wong JK. Rapid estrogenic regulation of extracellular signal- regulated kinase 1/2 signaling in cerebellar granule cells involves a G protein- and protein kinase A-dependent mechanism and intracellular activation of protein phosphatase 2A. Endocrinology. 2005;146(12):5397–406. doi: 10.1210/en.2005-0564. [DOI] [PubMed] [Google Scholar]
- 196.Takayanagi S, Tokunaga T, Liu X, Okada H, Matsushima A, Shimohigashi Y. Endocrine disruptor bisphenol A strongly binds to human estrogen-related receptor γ (ERRγ) with high constitutive activity. Toxicol Lett. 2006;167(2):95–105. doi: 10.1016/j.toxlet.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 197.Ewing LL, Desjardins C, Irby DC, Robaire B. Synergistic interaction of testosterone and oestradiol inhibits spermatogenesis in rats. Nature. 1977;269(5627):409–11. doi: 10.1038/269409a0. [DOI] [PubMed] [Google Scholar]
- 198.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 199.Morrissey RE, George JD, Price CJ, Tyl RW, Marr MC, Kimmel CA. The developmental toxicity of bisphenol A in rats and mice. Fundam Appl Toxicol. 1987;8(4):571–82. doi: 10.1016/0272-0590(87)90142-4. [DOI] [PubMed] [Google Scholar]


