Abstract
Behavioral inhibition (i.e. avoidance of unfamiliar) has been linked to significant differences in stress physiology and health. Developing an animal model of this common temperament provides a means to experimentally study the development and physiology of this trait as it relates to stress-related health processes. To elaborate such an animal model, we studied individual rat responses to two novel situations that mimic behavioral inhibition tests for humans (one non-social and one social). We measured individual consistency of behavioral responses across tests and time, and examined the relationship between behavior and glucocorticoid levels in outbred Sprague-Dawley male rats. Individuals were consistent in their behavioral responses to the same novel environment over time, but not in their responses across two different environments (i.e. non-social vs. social). A third of males were slow to approach novelty in both arenas (INHIBITED) and another third were fast to approach in both arenas (NON-INHIBITED). Behavioral inhibition was relatively stable across time and was associated with increased glucocorticoid production at baseline and in response to novelty but not during a post-novelty recovery period. Glucocorticoid levels were more closely related to their responses to the social novel arena than the non-social arena. Thus, behavioral inhibition is associated with acute and basal glucocorticoid over production and social inhibition is a more important predictor of adrenal activity than non-social inhibition. These preliminary observations provide strong support for an animal model of human behavioral inhibition and identify specific aspect of glucocorticoid production dynamics to examine in behaviorally inhibited children.
Keywords: behavioral inhibition, neophobia, exploration, glucocorticoid profiles, individual differences, stress
In the United States, approximately 20% of children tested show stable signs of behavioral inhibition or shyness - a behavioral predisposition that indicates fear of the unfamiliar and avoidance of novelty in different situations [1–2]. In children, this trait is relatively stable over time, with 60% of shy 1-year-olds remaining inhibited 4 years later [3]. These children are also more prone to certain behavioral and health problems, including increased risk for drug use in boys [4], problem behavior [5], anxiety disorders [2,6–8] and allergies [9]. One physiological trait associated with inhibition that may explain certain behavioral and health trajectories is a greater release of glucocorticoids from the hypothalamic-pituitary-adrenal (HPA) axis during unstimulated and stimulated periods [3,10–13]. Given the complex relationships between genetics, environment and development, an animal model of this trait would provide a beneficial tool to understand factors involved in development of the trait and associated health trajectories. Benefits of an animal model include: (a) longitudinal studies to monitor trait develop over the life span and to identify life-long costs and benefits of a trait, and (b) experimental studies to identify environmental conditions that alter this trait and physiological processes associated with it. In the current paper, we test the applicability of a rodent model of behavioral inhibition.
A trait similar to behavioral inhibition (e.g. ‘neophobia’, ‘emotionality’, ‘shyness’) can be found among many animal species including rats [14–18], suggesting this trait may be highly conserved across species. These results support the notion that the human equivalent of behavioral inhibition can be identified in other species, and therefore that an animal model of naturally occurring variance in this trait can be developed. As an animal model for behavioral inhibition, rats provide an ideal species in that they live relatively short lives, they have relatively complex social lives like ourselves, and there is a great deal of background literature on their behavior, development, and physiology [19]. In particular, outbred rat strains (e.g. Sprague-Dawley) allow enough behavioral variance within a population to mimic that seen in humans.
Elevated glucocorticoid levels in inhibited children suggests these children are more fearful than non-inhibited children, given the association between HPA axis activation and fear [20–21]. Glucocorticoid hormones, produced by the adrenal gland, help mobilize stored energy to help animals cope with challenging situations and are beneficial [22–23]. However, it has become increasingly clear that chronic elevations are associated with a host of health problems [24–26]. Given this dichotomy, this physiological tendency in shy individuals may be either beneficial or harmful to health, depending on the duration of glucocorticoid elevations. Thus, it is important to determine if inhibited individuals experience longer periods of glucocorticoid elevations than non-inhibited individuals, or if their elevated HPA axis activation is limited to greater acute glucocorticoid elevations immediately after a challenge. If shy/inhibited individuals experience long-term changes in their basal set point of glucocorticoid secretion or lengthened exposure after each challenging situation, they may be prone to a suite of negative health consequences associated with chronic glucocorticoid elevations [27–28]. Chronic glucocorticoid elevations may provide one mechanism dictating specific health risks and shortened life spans in inhibited individuals [e.g. 2,6–7,9,29–30]. Unfortunately, the temporal dynamics of this elevation is unknown, both in animal models and humans.
To develop an animal model of behavioral inhibition, individuals must be characterized with tests comparable to those with humans. In humans, behavioral inhibition is assessed as consistent inhibition in unfamiliar social and non-social situations [1]. In addition, slightly more emphasis is placed on behavioral responses to unfamiliar social partners than to unfamiliar physical objects (e.g. [3]). Thus, in developing an animal model, it is particularly important to include a test of inhibition in response to a novel non-threatening social partner [16]. In current animal models of anxiety/fear/emotionality, behavioral test batteries often include only non-social (and potentially dangerous) novelty tests and rarely include social and/or both social and non-social novelty tests [14,16,30–38]. In models that measure behavioral responses to non-social and social novelty, there are no associated glucocorticoid response measures. Thus, it is difficult to determine whether social or non-social inhibition, or a combination of the two, is most important in predicting glucocorticoid response dynamics in inhibited individuals.
In the current study, we characterize behavioral and glucocorticoid aspects of rat behavioral inhibition. We used Sprague-Dawley rats because they are outbred and less neophobic in non-social or social novelty tests than Wistar rats [36,38]. As has been done with children, we determined the proportion of rats inhibited across two different novel situations – one non-social and one social, and then assessed stability of individual responses over time. Second, we determined which, if either, of the two novel situations provided a reliable predictor of glucocorticoid production and if rats inhibited on both novel situations had greater glucocorticoid levels than consistently non-inhibited rats or those inhibited in only one of the two arenas. To determine the duration of elevated glucocorticoid levels in inhibited individuals, we collected repeat measures over time. We tested the following hypotheses. (1) If inhibition is a relatively stable trait in the rats (as is true in humans) we expected individual latency to approach novelty would be stable over time. (2) If behavioral inhibition is dependent on novelty context (non-social or social), we expected approach latencies across different situations will not necessarily be related. (3) If consistently slow approach latencies across different situations represent a generalized fear response, we expected such individuals to have higher glucocorticoid levels (either at baseline, response or recovery) than those slow to approach in only one or neither novel situation.
METHODS
Overall Design & Sample
Sixty young Sprague-Dawley male rats (60 days of age) ordered from Charles River Laboratories (Wilmington, MA) were individually housed in solid bottom plastic cages (43.5 × 23.5 × 20.5 cm). They were maintained on a 14L:10D lighting schedule (lights on at 1900h EST) with food and water available ad libitum and the colony room at 72°F with ~50% humidity. Cages were cleaned once a week by trained animal facility personnel. Rats were allowed to acclimate to the housing conditions for 2 weeks prior to testing, during which time they were handled and weighed daily. Methods detailed below were approved by the Pennsylvania State University Institute for Animal Care and Use Committee and adhered to methods specified in the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996).
Rats were tested twice on each of two novel environments: a non-social arena that contained novel rat-sized objects, and a social arena that contained a caged, similar aged novel male rat. To determine if individual responses to both novel arenas was stable over time, males were tested on both arenas at two ages: 2.5–4 mos and 8 mos. Males were exposed to both the non-social and social test arenas at each test age. Half were tested on the non-social arena first and half tested on the social arena first. The order of rat testing within each test day was reversed for the two arenas (e.g. if a male was the first tested on the novel non-social arena within a given test day, he was then the last tested within a given day on the novel social arena). The order of novel arena tests (either non-social or social test first) was reversed from the first to the second test age so responses were not linked to test order. To determine if stimulated or unstimulated glucocorticoid production related to behavioral responses to novelty, we measured circulating corticosterone levels at multiple time points after novelty testing at 4mos of age.
Behavioral Response to Novelty
Behavioral testing was done in the middle of the rats’ active period (starting between 1300h and 1500h, 4–6h after lights off). Rats were tested in a non-colony room illuminated with two 25-watt red bulbs reflected off the walls of the room providing approximately 6 lux of light at the center of the novelty test arenas.
Novel Non-social Arena
This previously described arena [30], was designed to be minimally anxiety-provoking. The square test arena (120cm × 120cm) included 46cm-high white polypropylene walls and clear plastic cover. Three of the four corners contained a novel rat-sized object (a plastic tube, an inverted bowl, or a wire tunnel), placed 13cm from the arena walls. The floor was covered with clean corn cob bedding and sprinkled with some soiled bedding (feces removed) from all cages in the colony room to provide rat odors. During testing, males were placed into a clean ceramic bowl with 5cm high walls and lowered into the empty arena corner. Rats were videotaped for 5min in the arena, with a camera placed 1.5m directly above the arena. Males were removed immediately after testing, the ceramic bowl was rinsed with water and dried for the next subject. On the rare occasion that feces were left in the arena, they were removed, but the arena floor was not cleaned any further so as to maintain a conspecific odor for the next rats tested.
Novel Social Arena
The novel social arena was the same size and height as the non-social arena, but instead of novel physical objects, the arena contained two wire cages – an empty cage and one with an unfamiliar male rat of similar size and age as the test rat. To minimize the impact of the stimulus animal’s behavior on test animals, the stimulus remained in a cage. This is slightly different than the ‘social interaction’ test [39] and the test used to identify behavioral inhibition in young rats [40]. Test animals could come in contact with the novel stimulus animal, but the two rats could not injure one another through the cage walls. Rats to be tested were introduced to the arena and videotaped, and the start bowl cleaned in the same way as for the novel non-social arena.
To assess behavioral inhibition in a manner comparable to measures used with children [1,41], a trained coder who did not know the identity of each rat, timed each male’s latency to approach the first novel objects or social partner in the novel non-social and social arena. An approach was defined as coming within one centimeter of the novel object/rat with the nose facing the object. For comparison to earlier studies, we also scored male locomotion from video-recordings. For this coding, the video-image of the arena was superimposed with an 8×8 grid that divided the arena into 64 equally-sized squares. Each square was approximately equivalent to one rat’s width. Locomotion was quantified as the cumulative number of squares crossed on the grid.
Corticosterone: Response, Recovery and Baseline following Novelty
To determine if inhibited males experienced elevated glucocorticoid levels following a novel experience we collected repeat blood samples after novelty testing at 4mos. For half the males, this blood collection procedure occurred following the novel non-social arena, for the other half it occurred after the novel social test. Several novel experiences occurred for the rats prior to and during the blood collection protocol: (1) behavioral testing in the novel arena, (2) housing in a novel cage in a novel room, and (3) handling and tail clipping for blood collection. This complex series of novel experiences was meant to trigger maximal HPA responses in inhibited individuals [42] and to mimic the complex novel conditions experienced by children tested for behavioral inhibition (1). To control for the glucocorticoid circadian rhythm (e.g. [43–44]), we restricted blood sampling to between 1300–1500h (for 8-wk baseline samples, the sampling was restricted to between 1300–1400h).
After testing in the novel arena, each male was carried to a separate room and placed into a new cage comparable to their home cage, with access to water but not food. Ten minutes after first placement in the novel test arena, the rat was removed from this holding cage, placed on the experimenter’s lap, and a short segment (< 3mm) of the tail tip removed with a scalpel. Using gentle tail palpation, blood (200μl) was collected into EDTA microtainer tubes (Becton Dickinson and Company) within 3 minutes. After the first sample, the male was returned to the holding cage and repeat samples were collected at 40, 80 and 120min from the beginning of behavioral testing. Repeat blood samples were collected by removing the rat from the holding cage and gently palpating the tail to re-stimulate blood flow. Thirty min following the 120-min sample, rats were returned to their home cage in the colony room. Twenty-four hrs and 8 weeks following the initial blood sample, rats were transported back to the collection room in their home cage, removed from the cage and a blood sample collected within 3-min by either palpating or cutting the tail tip to re-stimulate blood flow. Within each sampling day, rats were kept in the collection room and not returned to the colony room until all animals had been sampled. For the 10min through 24hr samples, we tested 6–12 males on each of eight test days. For the 8-wk samples, we tested 17–21 males on each of three test days.
The 10- and 40-min blood samples were timed to capture initial reactivity and near-maximal corticosterone response to the complex challenge of novel-arena testing, placement into a novel holding cage, and having blood sampled from the tail tip [45]. The 80- and 120-min samples were collected to measure glucocorticoid recovery following novelty, an index of glucocorticoid negative feedback efficiency. And the 24-h and 8-wk samples were analyzed to measure baseline/unstimulated glucocorticoid production.
Blood samples were kept on ice for 40min until centrifuged and plasma collected. Plasma was frozen at −80°C until assayed, and corticosterone was measured using a commercial radioimmunoassay kit (Rat & Mice Corticosterone kit, MP Biomedicals, Solon, OH). All samples were run in duplicate across six assays, with males balanced across assays according to their locomotion scores on the novel non-social arena. Intra-assay variability for a low and high control was 11.8 and 7.3%; inter-assay variability for these controls was 14.0 and 11.5%.
Analyses
We used correlation analyses and t-tests to determine: (1) if male latency to approach in either the non-social or social novelty was stable over time (from 2.5–4 mos to 8 mos), and (2) if male latency to approach novelty was consistent across the social and non-social novel environments. To meet statistical requirements of normality, log-transformed latency values were used. For categorical analyses (t-tests and ANOVAs), all males were categorized as either FAST or SLOW on each of the novel arenas based on a median split of approach latencies. Males were further classified according to their responses across both arenas. Males slower than median on both arenas within a given test age were classified as INHIBITED, males faster than median on both arenas were NON-INHIBITED, and all others were categorized as MIXED. We used a chi-square analysis to determine if male inhibition categories were stable from 2.5–4 mos to 8 mos.
To determine if approach latencies on the non-social and/or social novelty arenas related to glucocorticoid profiles, we compared corticosterone levels between FAST vs. SLOW males in each arena (using median split described above). Repeated measures ANOVAs were used for comparison of glucocorticoid levels at 10-min, 40-min, 80-min, 120-min, 24-h, and 8-wks following novelty. To determine if males with generalized behavioral inhibition (i.e. long approach latencies in both novel arenas) had greater glucocorticoid levels than less inhibited males, we conducted a similar repeated measures ANOVA comparing males grouped in the three categories described above (INHIBITED, NON-INHIBITED, and MIXED) based on latencies in both the non-social and social arenas.
Prior to conducting the above analyses, we determined whether the following factors had a significant effect on behavioral or glucocorticoid responses: (1) time of day, and (2) order of testing across the two arenas (i.e. first tested on the novel non-social or on the novel social arena). (Time of day was expressed as minutes into the day, with midnight equal to 0min and 10am lights off equal to 600min.) These analyses were done with regression analyses (time of day) or t-tests (order tested on the two arenas). When these variables had a significant impact on behavior or corticosterone levels, we used regression analyses residuals to account for these secondary variables (see Results section). Because corticosterone and latency to approach were not normally-distributed (according to Kolmogorov-Smirnov test), we used log-transformed values in all statistical tests to satisfy parametric statistical test distribution requirements.
Some missing data were involved. One male was sacrificed prior to the second behavioral tests at 8 months, and behavioral data were lost for an additional 9 males at this test age, due to technical difficulties with recording equipment. Thus, analyses including behavior data at 8 mos included 50 males. In corticosterone analyses, one male had a value at 40-min more than 6 standard deviations higher than the mean and three times higher than the next highest male’s values. This male was eliminated as an outlier. Samples from two other males were mis-processed and could not be recovered, and recovery samples (80- and 120-min) could not be collected from two other males. Thus, for the repeated measures ANOVA of corticosterone values, there were 55 males with a complete set of repeat measures (0min, 10min, 40min, 80min, 120min and 24h). The set included 15 ‘NON-INHIBITED’ males, 16 ‘INHIBITED’ males, and 24 ‘MIXED’ males.
RESULTS
Behavioral Response to each Novel Arena
Males varied considerably in how quickly they approached novelty in the two test arenas. They consistently approached novel non-social objects faster than a novel social partner (2.5–4mos: paired t59 = 2.51, p < .05; 8mos: paired t49 = 2.58, p < .05). Approach latencies on the non-social tests ranged from 1–161s (mean = 35s, median = 26s) and 2–300s (mean = 59s, median = 23s) at the first and second test ages, whereas they ranged from 3–300s (mean = 57s, median = 33s) and 5–300s (mean = 69s, median = 40s) on the social arena. Approach latencies, within each arena, were stable for greater than 4-mos (from 2.5–4 to 8 mos). Latencies in the non-social test were linearly associated across test ages (r57 = 0.415, p < .001). Latencies in the social arena were not linearly associated across time (r57 = 0.102, ns), but FAST males at 2.5–4 mos were significantly faster to approach at 8 mos, compared to SLOW males at 2.5–4 mos (t57 = 3.21, p < .01). Approach latency was negatively associated with locomotion (Non-Social: 2.5–4mos, r58 = −0.477, p < .0001, 8mos, r48 = −0.740, p < .0001; Social: 2.5-mos, r58 = −0.382, p < .01, 8mos, r48 = −0.456, p < .001). Latencies were not affected by the order in which the two tests were run within each age (Non-Social: 2.5–4mos, t58 = 0.12, ns, 8mos, t48 = 0.21, ns; Social: 2.5–4mos, t58 = 1.14, ns, 8 mos, t48 = 0.27, ns).
Behavioral Response – Inhibited vs. Non-Inhibited Males
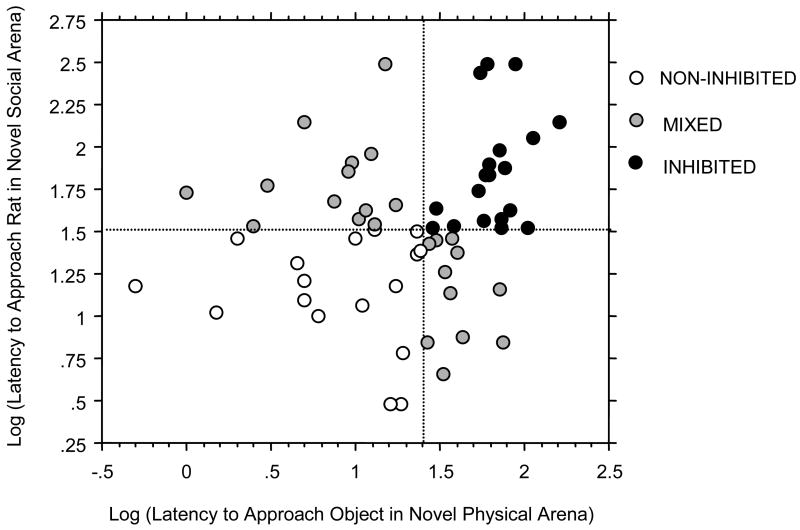
A male’s approach latency in one arena (e.g. non-social) was unrelated to their latency in the other (e.g. social; Figure 1: r58 = 0.194, ns; t57 = 1.21, ns). Thirty percent of males (18 of 60) took longer than median time to approach novelty in both test arenas (upper right quadrant of Figure 1, solid circles, ‘INHIBITED’). Another 28% of males (17 of 60) took less than median time to approach on both arenas (lower left quadrant in Figure 1, open circles, ‘NON-INHIBITED’). Of the remaining 25 males (‘MIXED’), half were inhibited in the non-social but not in the social arena (n=12) and the other half inhibited in the social but not the non-social arena (n=13). At 8 mos, one third of males were ‘INHIBITED’ on both arenas (17 of 50), 30% were ‘NON-INHIBITED’ on both (15 of 50) and 36% ‘MIXED’ (18 of 50). Males across the three behavioral categories were tested at the same time of day in each of the two novel environments (2.5–4mos: Non-Social F 2,56 = 0.89, ns; Social F 2,56 = 0.69, ns; 8mos: Non-Social F 2,47 = 1.54, ns; Social F 2,47 = 0.59, ns).
Figure 1.
Comparison of individual rat’s latency to approach novelty (logged) in the novel non-social and social arenas. Each point represents an individual male’s log-transformed latency to approach in the two arenas. Dashed lines indicate median response in each arena. Males were classified as falling into one of the four quadrants; color of each data point indicates whether a male was INHIBITED in both arenas (dark circles), NON-INHIBITED in both arenas (open circles), or inhibited in only one of the two arenas (MIXED, grey circles).
Male responses across the two novel arenas were relatively stable from 2.5–4 to 8 mos (chi-square=5.33, P < .05; Table 1). A majority of males INHIBITED on both tests at 2.5–4mos were again INHIBITED at 8 mos (chi-square=5.00, P < .05). The other classifications, NON-INHIBITED and MIXED at 2.5–4 mos, also remained relatively stable over time (NON-INHIBITED: chi-square=6.14, p<.05; MIXED: chi-square=5.43, p=.066) although for MIXED males results were not statistically different from chance.
Table 1.
Male responses to two different novel arenas – one non-social the other social – at two test ages (2.5–4mos to 8 mos). Males were classified as being either SLOW or FAST (below or above median approach latency) on each arena, and then further classified as INHIBITED, MIXED or NON-INHIBITED based on responses to both the non-social and social arenas at each test age (INHIBITED males were SLOW on both the non-social and social arenas, MIXED males were SLOW on one arena and FAST on the other arena, NON-INHIBITED males were FAST on both arenas). Behavioral categories were relatively stable across the two time points (χ2=5.33, P < .05). There were 50 males tested at both ages.
| Behavioral category (8 mos) | |||
|---|---|---|---|
| Behavioral category (2.5–4 mos) | INHIBITED | MIXED | NON-INHIBITED |
| INHIBITED | 10 | 4 | 1 |
| MIXED | 4 | 12 | 5 |
| NON-INHIBITED | 3 | 2 | 9 |
Similar results were seen with male locomotion in the two arenas. Locomotion was stable within each arena across time (Non-Social: r48 = 0.337, p < .05; Social: r57 = 0.357, p < .05), but individual locomotion across the two novel arenas was not related (r58 = −0.102, ns). Males fell into similar categories of INHIBITED, NON-INHIBITED and MIXED whether categorized according to latency or locomotion scores.
Corticosterone Response to Novelty - General
Male peak corticosterone levels following novelty (at 40-min) were more than twice as high as basal levels measured the next day and 8 weeks later (40-min vs. 24-hr, paired t54= 12.9, p<.0001; 40-min vs. 8-wks, paired t54= 12.6, p<.0001). By 120-min after novelty, corticosterone levels were very close to levels the next day (120-min vs. 24-hr, paired t54= 1.34, ns), and levels at 24-h after novelty were identical to those 8-wks later (paired t58 = 0.20, ns). The kind of novelty test (non-social vs. social) prior to blood sampling did not influence glucocorticoid levels (repeated measures ANOVA: F1,53 =0.61, ns).
All blood samples within each sampling interval (e.g. 10-min, 40-min, etc.) were collected within a 2-hr window. Even within this limited time window, there was a significant effect of time-of-day on glucocorticoid levels. Plasma corticosterone concentrations steadily decreased in later samples, and this was true for the 40-, 80- and 120-min samples and the 24-h samples (mean R2 = 0.125, mean stand. coeff. = −0.353, mean F1,55 = 7.91, mean p = 0.009). Blood samples at 8-wks following novelty were collected within a 1-hr window and time of day did not have a significant impact on corticosterone levels (R2 = 0.007, stand. coeff. = 0.155, F1,57 = 1.40, ns). The influence of time-of-day on corticosterone levels was included in regression analyses by using time-of-day as an independent variable; this effect was included in the repeated measures ANOVA by using residual corticosterone values regressed on time of day. The order in which an animal was tested within a day, and the specific novel arena experienced just prior to blood collection did not significantly influence corticosterone concentrations.
Corticosterone Response relative to Behavioral Response in each Novel Arena
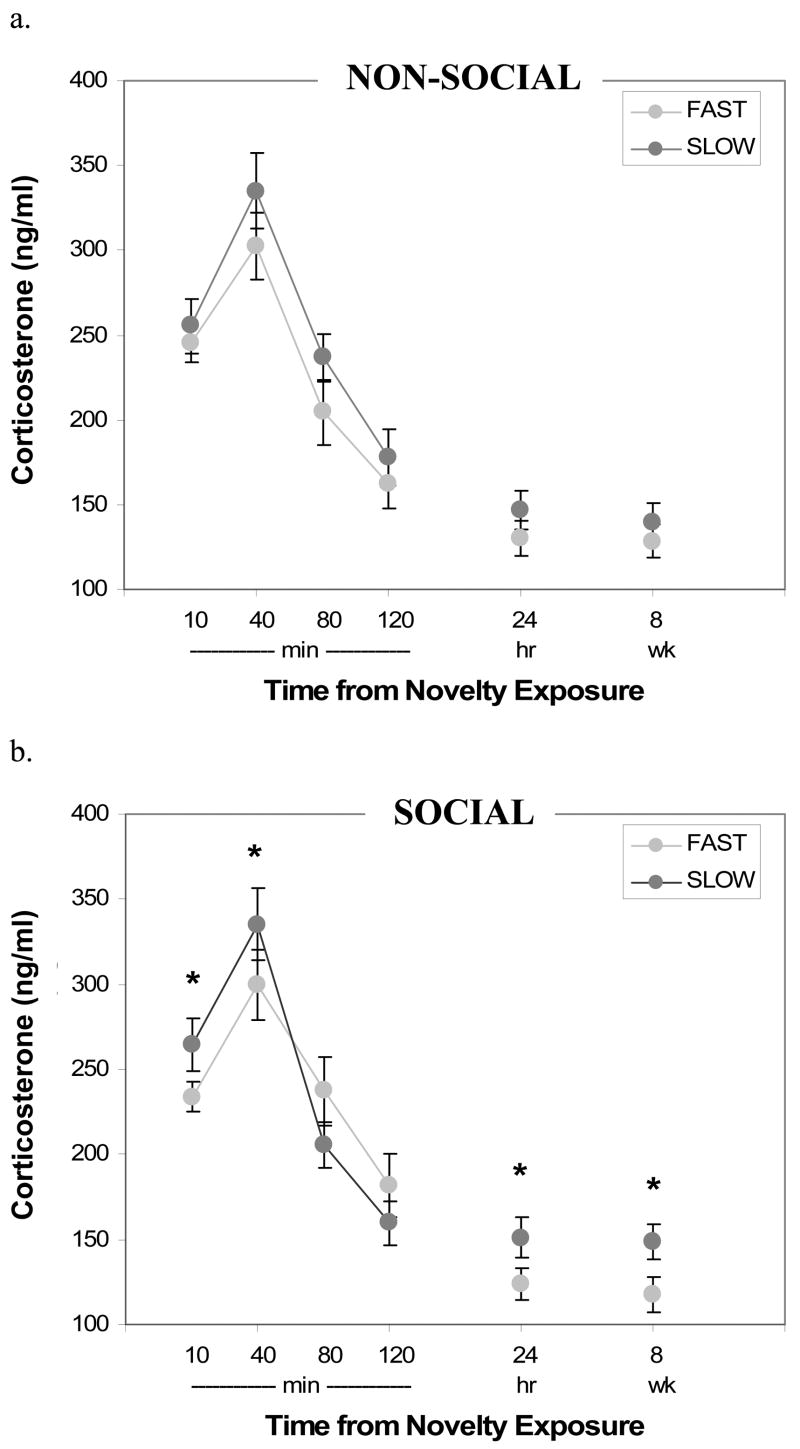
Males with longer approach latencies (SLOW males) in the first novel non-social arena (at 2.5–4mos) had moderately higher reactivity, recovery and baseline corticosterone levels than males with shorter latencies (FAST males; Figure 2a). This effect was not quite statistically significant (repeated measures ANOVA: F1,53 =3.64, p=.062). On average, SLOW males’ glucocorticoid levels were approximately 12% higher than FAST males’ levels at all time points. Glucocorticoid levels at all time points were not linearly associated with approach latencies in this arena (range of r55: 0.04–0.22, ns).
Figure 2.
Corticosterone levels following 5-min novelty testing, blood sampling, and housing in novel cage. Corticosterone levels are shown for males that were SLOW (dark grey) and FAST (light grey) to approach novelty in a: (a) non-social arena (n=27 and 28), and (b) social arena (n=29 and 26). Error bars indicate S.E.M. (Significantly different values between groups are indicated at each time point with *, p<.05.)
Males SLOW to approach in the first novel social arena had higher reactivity and baseline glucocorticoid levels than the FAST males, but lower levels during the recovery period (overall repeated measures ANOVA: F1,53=5.42, p<.05; time × behavioral latency category: F5,265=1.99, p=.081; Figure 2b). On average, SLOW males’ immediate glucocorticoid responses to novelty were 13% higher and recovery levels 15% lower than FAST males. At baseline (24-hrs and 8-wks following novelty), SLOW males’ levels were 22–26% higher than FAST males. Glucocorticoid reactivity and baseline levels were linearly associated with approach latencies in the social arena (10-min: r55=0.37, p<.01; 40-min: r55=0.32, p<.05; 24-hr: r55=0.28, p<.05; 8-wk: r55=0.27, p<.05). Recovery values were not linearly associated with approach latencies (80- & 120-min: r55=−0.11, ns). Similar results were seen when comparing locomotion in the two novel arenas to glucocorticoid levels at baseline, reactivity and recovery periods.
Corticosterone Response - Inhibited vs. Non-Inhibited Males
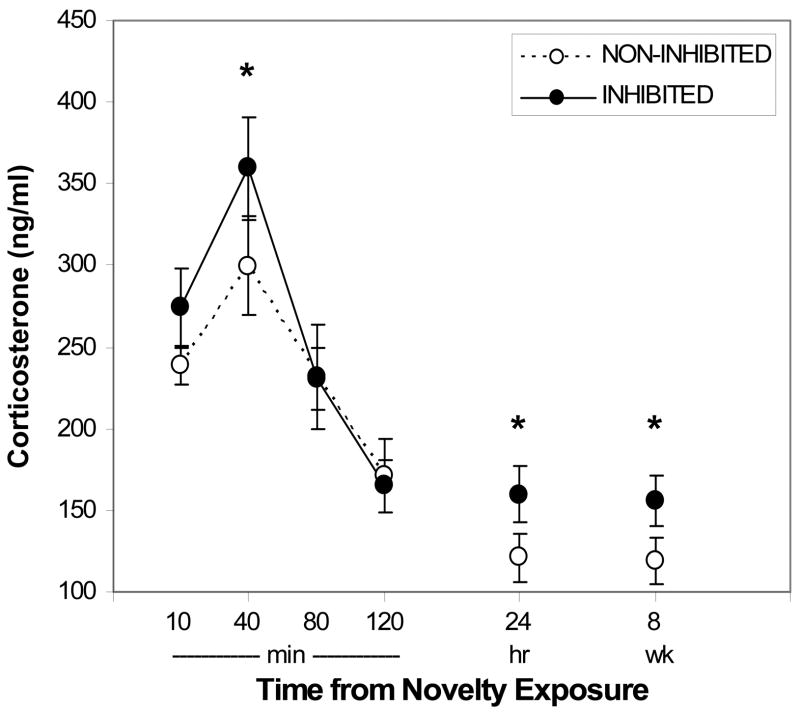
Males that were slow to approach novelty in both arenas (INHIBITED) had significantly higher reactivity and baseline corticosterone levels compared to less inhibited males (NON-INHIBITED and MIXED; repeated measures ANOVA: F2,52=5.31, p<.01; Figure 3). INHIBITED males’ immediate corticosterone responses to novelty (10- and 40-min post-novelty) were 17% greater than other males. This elevation was not present during the recovery period (80- and 120-min post-novelty), but was evident again at baseline (24-hr and 8 wks post-novelty), with 30% greater corticosterone production by INHIBITED males compared to the others. For MIXED males, corticosterone levels were no different if they were inhibited only in the novel non-social situation or only in the novel social situation (F1,22=0.16, ns). Similar glucocorticoid results were found when males were categorized according to their locomotion scores on the two arenas (i.e. consistently above or below median locomotion scores).
Figure 3.
Corticosterone production in INHIBITED and NON-INHIBITED males (n=16 and 15) following 5-min novel arena test, blood sampling, and housing in novel cage. Error bars indicate S.E.M. (Significantly different values between groups are indicated at each time point with *, p<.05.)
DISCUSSION
In summary, using relatively low anxiety-provoking novelty tests that mimic those used with children, we identified a large range of behavioral responses to novelty in a rodent model (male Sprague-Dawley rats), and found many similarities with humans. Thirty percent of the study population was consistently inhibited in both a novel non-social and a novel social situation, and inhibition was relatively stable from young adulthood to early middle age [1,3]. Comparable to consistently inhibited children, inhibited males, compared to non-inhibited, had significantly elevated glucocorticoid levels, both following novelty and during an unstimulated period [3,10–11]. Finally, social inhibition was a better predictor of glucocorticoid reactivity and basal levels than was non-social inhibition. Because stable individual differences in responses to novelty can be identified at weaning in these rats [30], an animal model may provide an interesting system to study the development and physiology of this trait to understand mechanisms underlying specific health trajectories in inhibited children.
An animal model allows for experimental studies on: (1) the influence of early environment (e.g. prenatal and postnatal) on the development of behavioral inhibition, (2) identifying predictable differences in the life-long development of this trait, and (3) specific biological mechanisms (i.e. neuronal, metabolic, endocrine, etc.) underlying differential health trajectories of inhibited vs. non-inhibited individuals. For such studies to occur, further validation of the current animal model is required. For example, further study of the physiological correlates of rodent inhibition is required to determine if they are comparable to those seen in human behavioral inhibition (e.g. elevated sympathetic activation, elevated limbic activity, etc.). Initial support for rodent models comes from studies showing that rats and mice defined as ‘neophobic/non-exploratory’ die earlier than those identified as ‘neophilic/exploratory’ which is comparable to results in elderly individuals identified as ‘cautious’ vs. ‘curious’ [19,29–30,46]. Results from the current and previous reports provide support for further validation of these animal models.
Complex Test of Behavioral Inhibition
Male responses to each of two different novel situations were relatively stable over a 4-month test-retest interval, suggesting the novelty tests stimulated trait-like responses. However, responses across the two tests were not highly related, suggesting each test accessed different underlying response motives. Only 30% of males had slower than median approach latencies in both test arenas (‘INHIBITED’ males). This proportion of the population is comparable to, although slightly higher than, that identified in children tested across multiple novel conditions [1,3]. This difference in rat and human proportions may reflect the smaller array of novelty tests, particularly social tests, used to identify inhibition in rats vs. humans. Inconsistent responses to two different novel experiences support the use of multiple tests to assess a complex behavioral trait such as behavioral inhibition [34,47–49]. Male response latencies to social novelty were better predictors of short-term glucocorticoid reactivity and basal levels. Given this and the bias of human behavioral inhibition tests toward measures of social inhibition, rodent testing batteries should include either a variety of novel social tests and/or frequent repeat testing to identify individuals that do not habituate to novel social partners across situations or time. Lastly, of males inhibited at 2.5–4mos, 67% of them remained inhibited at 8 mos of age. This level of stability in this trait is similar to that identified in children [3].
Behavioral Inhibition and Glucocorticoid Dynamics
Rat behavioral inhibition was related to glucocorticoid production in a similar way as seen in humans (e.g. [3]). Inhibited Sprague-Dawley rats had significantly greater glucocorticoid levels immediately after novelty and at baseline, as compared to non- or less inhibited males. The magnitude of this hormonal difference (30% at baseline and 17% immediately after novelty) is similar to the difference seen in children at baseline, although the difference following novelty was significantly smaller than that seen between inhibited and non-inhibited children during an experimental protocol/challenge [3]. This smaller difference may result from familiar cages and lack of continued novel social stimulation after novelty testing and during blood sampling. For children, the largest difference between inhibited and non-inhibited glucocorticoid levels occurs during completely novel conditions - e.g. an extended laboratory visit including novel social stimulation. In a prior study with neophobic and neophilic male rats identified by their response to only one novel situation (non-social), we found a significant difference in glucocorticoid reactivity levels but no difference in baseline glucocorticoid levels between inhibited and non-inhibited males [30]. With the additional social novelty test in the current study, we identified significant differences in baseline glucocorticoid levels between inhibited and non-inhibited or slightly inhibited males. Again, with multiple novelty tests to identify inhibition, we may more reliably identify individuals that experience generalized fear of novelty, as opposed to inhibition in only specific novel settings [1,37]. Alternatively, it may be that the additional novel social test provides a more accurate measure of behavioral inhibition in male rats than their response to a novel non-social arena. And in fact, multiple and repeat novel social tests may prove the best predictor of glucocorticoid reactivity, recovery and baseline levels.
Although inhibited males’ had elevated glucocorticoid levels immediately after novelty and during unstimulated periods, their glucocorticoid levels during a recovery period (80–120min after novelty) were no different from non-inhibited males. Thus, young individuals inhibited in both non-social and social situations experience greater short-term adrenal reactivity to novelty, but not longer elevations following novelty. However, with continued novelty exposure, these glucocorticoid recovery profiles may be quite different if inhibited individuals fail to habituate to novelty. The temporal dynamics of inhibited individual’s glucocorticoid responses is an area that requires further study. Total glucocorticoid exposure and/or duration of glucocorticoid elevations are a key component in understanding the influence of these steroids on other physiological systems [23,50]. If glucocorticoid elevations in inhibited individuals can be kept short by keeping exposure to novelty to short periods, these differential glucocorticoid responses may have minimal negative influences on inhibited individuals’ health trajectories [28]. However, we must consider another aspect of glucocorticoid production: inhibited individuals maintained elevated baseline levels. These males may experience elevated glucocorticoid production during unstimulated periods, altered glucocorticoid circadian rhythms, and/or have a more sensitive HPA axis leading to more frequent or larger low-grade glucocorticoid spikes during seemingly benign conditions. In any of these cases, elevated baseline levels suggest there may be a chance of HPA axis dysregulation in inhibited individuals; this requires further exploration.
Several factors may lead to HPA axis dysregulation. Chronic stress and/or glucocorticoid elevations are associated with glucocorticoid receptor down-regulation in the hippocampus [24,51]. Consequences may include HPA dysregulation or circadian rhythm alterations that have been associated with certain mood disorders (e.g. depression [52], PTSD: [53–54]). Whether this down-regulation and/or disruption can occur as a result of natural variance in glucocorticoid production dynamics has yet to be shown. However, the current results suggest it would be beneficial to track glucocorticoid dynamics in inhibited vs. non-inhibited children over time to determine if young children with elevated basal and/or reactivity glucocorticoid levels experience HPA dysregulation over time, associated with increased risk for anxiety or other disorders in adulthood. Specifically, it would be beneficial to track basal circadian rhythms and short- and long-term glucocorticoid responses to novelty over time. Further work must examine glucocorticoid receptor density/distributions and/or altered glucocorticoid rhythms in inhibited individuals. Physiological consequences of HPA axis dysregulation may be one mechanism underlying altered health trajectories and life spans in inhibited individuals [2,6–7,29–30,46]. Further examination of HPA dynamics and could provide information for preventative interventions.
It must not be forgotten that behavioral inhibition and differential stress response profiles may provide certain advantages [55]. For example, behavioral inhibition and elevated glucocorticoid production can be advantageous in real world risky situations that require increased energy expenditure and/or vigilance for daily survival [56]. From an evolutionary perspective, variations in behavioral temperament may be adaptive in social species where role division is possible and in animals that experience frequently changing environmental conditions [17,57–58]. In demanding environments (low resources, high predation, social instability), behavioral inhibition may decrease one’s exposure to dangerous situations (unnecessary use of resources, predation, aggression), whereas favorable environments may favor non-inhibited individuals. Rapidly changing environmental conditions will favor the success of both inhibited and non-inhibited individuals, particularly in social groups [17].
There are other rat models of anxiety/emotionality, with both inbred and outbred animals [16,31–34,37]. As with any animal model, necessary limitations exist when interpreting results from rodents to humans. We can never fully assess the extent of analogous behavioral and physiological traits among species. In addition, minimally variant laboratory conditions may diminish environmental factors that lead to specific traits. On the other hand, rodent models allow for systematic manipulation of environmental conditions to understand their influence on the development of such traits and to provide novel insights for further examination in humans. The unique aspect of the current study is that rodent behavioral and glucocorticoid responses were compared across both non-social and social situations, as is done with children. Rats slow to approach in two different novel situations (one non-social and one social) had the greatest glucocorticoid responses and the most elevated glucocorticoid levels at baseline – again, similar to glucocorticoid profiles seen in children. These results suggest that social novelty test be used with other rodent models of anxiety to determine if traits identified in these models involve a component of social inhibition as is true for inhibited children. In addition, this study highlights the importance of documenting individual differences in glucocorticoid temporal dynamics. It is important to identify whether certain temperaments are associated with alteration of glucocorticoid reactivity, recovery or baseline production and/or circadian rhythm production. Differences in temporal dynamics may have different implications for health and survival.
Acknowledgments
We thank I. Fassasi, K. Haskins, C. Matteo, J. McGovern, J. Patel, and R. Smull for their expert assistance with data collection. We thank L. Francis, J. Graham, J.M. Mateo and R.W. Schrauf and three anonymous reviewers for their helpful feedback on the manuscript. This research was supported by NIMH R03 MH071406 (to SAC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Garcia-Coll C, Kagan J, Reznick JS. Behavioral inhibition in young children. Child Dev. 1984;55:1005–1019. [Google Scholar]
- 2.Kagan J, Snidman N. Early childhood predictors of adult anxiety disorders. Biol Psychiatry. 1999;46:1536–1541. doi: 10.1016/s0006-3223(99)00137-7. [DOI] [PubMed] [Google Scholar]
- 3.Kagan J, Reznick JS, Snidman N. The physiology and psychology of behavioral inhibition in children. Child Dev. 1987;58:1459–1473. [PubMed] [Google Scholar]
- 4.Ensminger ME, Juon HS, Fothergill KE. Childhood and adolescent antecedants of substance use in adulthood. Addiction. 2002;97:833–844. doi: 10.1046/j.1360-0443.2002.00138.x. [DOI] [PubMed] [Google Scholar]
- 5.Stevenson-Hinde J, Glover A. Shy girls and boys: a new look. J Child Psychol Psychiatry. 1996;37:181–187. doi: 10.1111/j.1469-7610.1996.tb01389.x. [DOI] [PubMed] [Google Scholar]
- 6.Hirshfeld DR, Rosenbaum JF, Biederman J, Bolduc EA, Faraone SV, Snidman N, Reznick JS, Kagan J. Stable behavioral inhibition and its association with anxiety disorder. J Am Acad Child Adolesc Psychiatry. 1992;31:103–111. doi: 10.1097/00004583-199201000-00016. [DOI] [PubMed] [Google Scholar]
- 7.Turner SM, Beiderl DC, Wolff PL. Is behavioral inhibition related to the anxiety disorders? Clin Psychol Rev. 1996;16:157–172. [Google Scholar]
- 8.Cox BJ, McPherson PSR, Enns MW. Psychiatric correlates of childhood shyness in a nationally representative sample. Behav Res Ther. 2005;43:1019–1027. doi: 10.1016/j.brat.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 9.Kagan J, Snidman N. Infant predictors of inhibited and uninhibited profiles. Psychol Sci. 1991;2:40–44. [Google Scholar]
- 10.Tennes K, Downey K, Vernadakis A. Urinary Cortisol Excretion rates and anxiety in normal 1-year-old infants. Psychosom Med. 1977;39:178–187. doi: 10.1097/00006842-197705000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Nachmias M, Gunnar M, Mangelsdorf S, Parritz RH, Buss K. Behavioral inhibition and stress reactivity: the moderating role of attachment security. Child Dev. 1996;67:508–522. [PubMed] [Google Scholar]
- 12.Schmidt LA, Fox NA, Rubin KH, Sternberg EM, Gold PW, Smith CC, Schulkin J. Behavioral and neuroendocrine responses in shy children. Dev Psychobiol. 1997;30:127–140. doi: 10.1002/(sici)1098-2302(199703)30:2<127::aid-dev4>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 13.Dettling AC, Parker SW, Lane S, Sebanc A, Gunnar MR. Quality of care and temperament determine changes in cortisol concentrations over the day for young children in childcare. Psychoneuroendocrinology. 2000;25:819–836. doi: 10.1016/s0306-4530(00)00028-7. [DOI] [PubMed] [Google Scholar]
- 14.Einon DF, Morgan M. Habituation of object contact in socially-reared and isolated rats (rattus norvegicus) Anim Behav. 1976;24:415–420. [Google Scholar]
- 15.Buss AH, Plomin R. Temperament: Early Developing Personality Traits Hillsdale. N.J: Lawrence Erlbaum Associates; 1984. [Google Scholar]
- 16.Takahashi LK. Organizing action of corticosterone on the development of behavioral inhibition in the preweanling rat. Develop Brain Res. 1994;81:121–127. doi: 10.1016/0165-3806(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 17.Wilson DS, Clark AB, Coleman K, Dearstyne T. Shyness and boldness in humans and other animals. Trends Ecol Evol. 1994;9:442–446. doi: 10.1016/0169-5347(94)90134-1. [DOI] [PubMed] [Google Scholar]
- 18.Gosling SD, John OP. Personality dimensions in non-human animals: a cross-species review. Curr Dir Psychol Sci. 1999;8:69–75. [Google Scholar]
- 19.Cavigelli SA. Animal Personality and Health. Behaviour. 2005;142:1223–1244. [Google Scholar]
- 20.Kalin NH, Larson C, Shelton SE, Davidson RJ. Asymetric frontal brain activity, cortisol, and behavior associated with fearful temperaments in rhesus monkeys. Behav Neurosci. 1998;112:286–292. doi: 10.1037//0735-7044.112.2.286. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi LK. Role of CRF1 and CRF2 receptors in fear and anxiety. Neurosci. Biobehav. Rev. 2001;25:627–636. doi: 10.1016/s0149-7634(01)00046-x. [DOI] [PubMed] [Google Scholar]
- 22.McEwen BS, Albeck D, Cameron H, Chao HM, Gould E, Hastings N, Kuroda Y, Luine V, Magarinos AM, McKittrick CR, Orchinik M, Pavlides C, Vaher P, Watanabe Y, Weiland N. Stress and the brain: A paradoxical role for adrenal steroids. In: Parker KL, Schimmer BP, editors. Vitamins and hormones. Vol. 51. New York: Academic Press, Inc; 1995. pp. 371–402. [DOI] [PubMed] [Google Scholar]
- 23.Dhabhar FS, McEwen BS. Stress-induced enhancement of antigen-specific cell-mediated immunity. J Immunol. 1996;156:2608–2615. [PubMed] [Google Scholar]
- 24.Sapolsky RM. Glucocorticoid toxicity in the hippocampus - temporal aspects of neuronal vulnerability. Brain Res. 1985;359:300–305. doi: 10.1016/0006-8993(85)91440-4. [DOI] [PubMed] [Google Scholar]
- 25.Shively CA, Laber-Laird K, Anton RF. Behavior and physiology of social stress and depression in female cynomolgus monkeys. Biol Psychiatry. 1997;41:871–882. doi: 10.1016/S0006-3223(96)00185-0. [DOI] [PubMed] [Google Scholar]
- 26.Dhabhar FS. Enhancing versus suppressive effects of stress hormones on skin immune function. Proc Natl Acad Sci USA. 1999;96:1059–1064. doi: 10.1073/pnas.96.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sterling P, Eyer J. Allostasis: A new paradigm to explain arousal pathology. In: Fisher S, Reason J, editors. Handbook of Life Stress, Cognition and Health. London: Wiley & Sons; 1988. pp. 629–649. [Google Scholar]
- 28.McEwen B. Stress, Adaptation, and Disease: Allostasis and Allostatic Load. Ann NY Acad Sci. 1991:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- 29.Swan GE, Carmelli D. Curiosity and mortality in aging adults: A 5-year follow-up of the Western Collaborative Group Study. Psychol Aging. 1996;11:449–453. doi: 10.1037//0882-7974.11.3.449. [DOI] [PubMed] [Google Scholar]
- 30.Cavigelli SA, McClintock MK. Fear of novelty in infant rats predicts adult corticosterone dynamics and early death. Proc Natl Acad Sci USA. 2003;100:16131–16136. doi: 10.1073/pnas.2535721100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Broadhurst PL. The Maudsley reactive and nonreactive strains of rats: A survey. Behav Genet. 1975;5:299–319. doi: 10.1007/BF01073201. [DOI] [PubMed] [Google Scholar]
- 32.Landgraf R. HAB/LAB rats: an animal model of extremes in trait anxiety and depression. Clin Neurosci Res. 2003;3:239–244. [Google Scholar]
- 33.Márquez C, Nadal R, Amario A. Responsiveness of the hypothalamic-pituitary-adrenal axis to different novel environments is a consistent individual trait in adult male outbred rats. Psychoneuroendocrinology. 2005;30:179–187. doi: 10.1016/j.psyneuen.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 34.Steimer T, Driscoll P. Inter-individual vs. line/strain differences in psychogenetically selected Roman High-(RHA) and Low-(RLA) Avoidance rats: neuroendocrine and behavioural aspects. Neurosci Biobehav Rev. 2005;29:99–112. doi: 10.1016/j.neubiorev.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 35.Haemisch A. Coping with social conflict, and short-term changes in plasma cortisol titers in familiar and unfamiliar environments. Physiol Behav. 1990;47:1265–1270. doi: 10.1016/0031-9384(90)90381-d. [DOI] [PubMed] [Google Scholar]
- 36.Rex A, Voigt JP, Gustedt C, Beckett S, Fink H. Anxiolytic-like profile in Wistar, but not Sprague-Dawley rats in the social interaction test. Psychopharmacology. 2004;177:23–34. doi: 10.1007/s00213-004-1914-7. [DOI] [PubMed] [Google Scholar]
- 37.Taylor GT, Yuede CM. Behaviorally timid rats respond differently to conventional and atypical neuroleptics. Pharmacol Biochem Behav. 2005;81:478–484. doi: 10.1016/j.pbb.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 38.Mällo T, Alttoa A, Kõiv K, Tõnissaar M, Eller M, Harro J. Rats with persistently low or high exploratory activity: Behaviour in tests of anxiety and depression, and extracellular levels of dopamine. Behav Brain Res. 2007;177:269–281. doi: 10.1016/j.bbr.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 39.File SE, Seth P. A review of 25 years of the social interaction test. Eur J Pharmacol. 2003;463:35–53. doi: 10.1016/s0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- 40.Takahashi LK. Ontogeny of behavioral inhibition induced by unfamiliar adult male conspecifics in preweanling rats. Physiol Behav. 1992;52:493–8. doi: 10.1016/0031-9384(92)90336-z. [DOI] [PubMed] [Google Scholar]
- 41.Calkins SD, Fox NA. The relations among infant temperament, security of attachment, and behavioral inhibition at twenty-four months. Child Dev. 1992;63:1456–1472. [PubMed] [Google Scholar]
- 42.Fluttert M, Dalm S, Oitzl MS. A refined method for sequential blood sampling by tail incision in rats. Lab Anim. 2000;34:372–8. doi: 10.1258/002367700780387714. [DOI] [PubMed] [Google Scholar]
- 43.Krieger DT. Effect of ocular enucleation and altered lighting regimens at various ages on the circadian periodicity of plasma corticosteroid levels in the rat. Endocrinology. 1973;93:1077–1091. doi: 10.1210/endo-93-5-1077. [DOI] [PubMed] [Google Scholar]
- 44.Atkinson HC, Waddell BJ. Circadian variation in basal plasma corticosterone and adrenocorticotrophin in the rat: sexual dimorphism and changes across the estrous cycle. Endocrinology. 1997;138:3842–3848. doi: 10.1210/endo.138.9.5395. [DOI] [PubMed] [Google Scholar]
- 45.Hinson JP, Vinson GP, Whitehouse BJ, Price G. Control of zona glomerulosa function in the isolated perfused rat adrenal gland in situ. J. Endocrinol. 1985;104:387–395. doi: 10.1677/joe.0.1040387. [DOI] [PubMed] [Google Scholar]
- 46.Péréz-Álvarez L, Baeza I, Arranz L, Marco EM, Borcel E, Guaza C, Viveros MP, de la Fuente M. Behavioral, endocrine, and immunological characteristics of a murine model of premature aging. Dev Comp Immunol. 2005;11:965–976. doi: 10.1016/j.dci.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 47.Fiske DW. Consistency of the factorial structures of personality ratings from different sources. J Abnorm Soc Psychol. 1949;44:329–344. doi: 10.1037/h0057198. [DOI] [PubMed] [Google Scholar]
- 48.Funder DC, Colvin CR. Explorations in behavioral consistency: properties of persons, situations, and behaviors. J Pers Soc Psychol. 1991;60:773–794. doi: 10.1037//0022-3514.60.5.773. [DOI] [PubMed] [Google Scholar]
- 49.Coleman K, Wilson DS. Shyness and blodness in pumpkinseed sunfish: individual differences are context-specific. Anim Behav. 1998;56:927–936. doi: 10.1006/anbe.1998.0852. [DOI] [PubMed] [Google Scholar]
- 50.Sapolsky RM. Glucocorticoids, stress, and their adverse neurological effects: relevance to aging. Exp Gerontol. 1999;34:721–732. doi: 10.1016/s0531-5565(99)00047-9. [DOI] [PubMed] [Google Scholar]
- 51.Paskitti ME, McCreary BJ, Herman JP. Stress regulation of adrenocorticosteroid receptor gene transcription and mRNA expression in rat hippocampus: Time-course analysis. Mol Brain Res. 2000;80:142–152. doi: 10.1016/s0169-328x(00)00121-2. [DOI] [PubMed] [Google Scholar]
- 52.Peeters F, Nicolson NA, Berkhof J. Levels of variability of daily life cortisol secretion in major depression. Psychiatry Res. 2004;126:1–13. doi: 10.1016/j.psychres.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 53.Kanter ED, Wilkinson CW, Radant AD, Petrie EC, Dobie DJ, McFall ME, Peskind Er, Raskind MA. Glucocorticoid feedback sensitivity and adrenocortical responsiveness in posttraumatic stress disorder. Biol Psychiatry. 2001;50:238–245. doi: 10.1016/s0006-3223(01)01158-1. [DOI] [PubMed] [Google Scholar]
- 54.Yehuda R. Advances in understanding neuroendocrine alterations in PTSD and their therapeutic implications. Ann NY Acad Sci. 2006;1071:137–166. doi: 10.1196/annals.1364.012. [DOI] [PubMed] [Google Scholar]
- 55.Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Dev Psychopathol. 2005;17:271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- 56.Cote J, Clobert J, Meylan S, Fitze PS. Experimental effects of corticosterone levels positively affects subsequent male survival. Horm Behav. 2006;49:320–327. doi: 10.1016/j.yhbeh.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 57.Sih A, Bell A, Johnson JC, Ziemba RE. Behavioural syndromes: an integrative overview. Q Rev Biol. 2004;79:241–277. doi: 10.1086/422893. [DOI] [PubMed] [Google Scholar]
- 58.Dingemanse NJ, Both C, Drent PJ, Tinbergen JM. Fitness consequences of avian personalities in a fluctuating environment. Proc R Soc Lond B Biol Sci. 2004;271:847–852. doi: 10.1098/rspb.2004.2680. [DOI] [PMC free article] [PubMed] [Google Scholar]