Abstract
Norepinephrine (NE) has widespread projections throughout brain, and thus is ideally positioned to orchestrate neural functions based on arousal state. For example, NE can increase “signal/noise” ratio in the processing of sensory stimuli, and can enhance long-term memory consolidation in the amygdala and hippocampus through actions at α-1 and β adrenoceptors. Over the last 20 years, NE has also been shown to play a powerful role in regulating the working memory and attention functions of the prefrontal cortex (PFC). Moderate levels of NE released under control conditions strengthen prefrontal cortical functions via actions at post-synaptic α-2A adrenoceptors with high affinity for NE, while high levels of NE release during stress impair PFC cortical functions via α-1 and possibly β-1 receptors with lower affinity for NE. Thus, levels of NE determine whether prefrontal cortical or posterior cortical systems control our behavior and thought. Understanding these receptor mechanisms has led to new, intelligent treatments for neuropsychiatric disorders associated with PFC dysfunction.
Keywords: norepinephrine, adrenoceptor, frontal lobe, working memory, guanfacine, prazosin, clenbuterol, betaxolol, cAMP, protein kinase C
1. Introduction: Functions of the Prefrontal Cortex and Their Relevance to Mental Illness
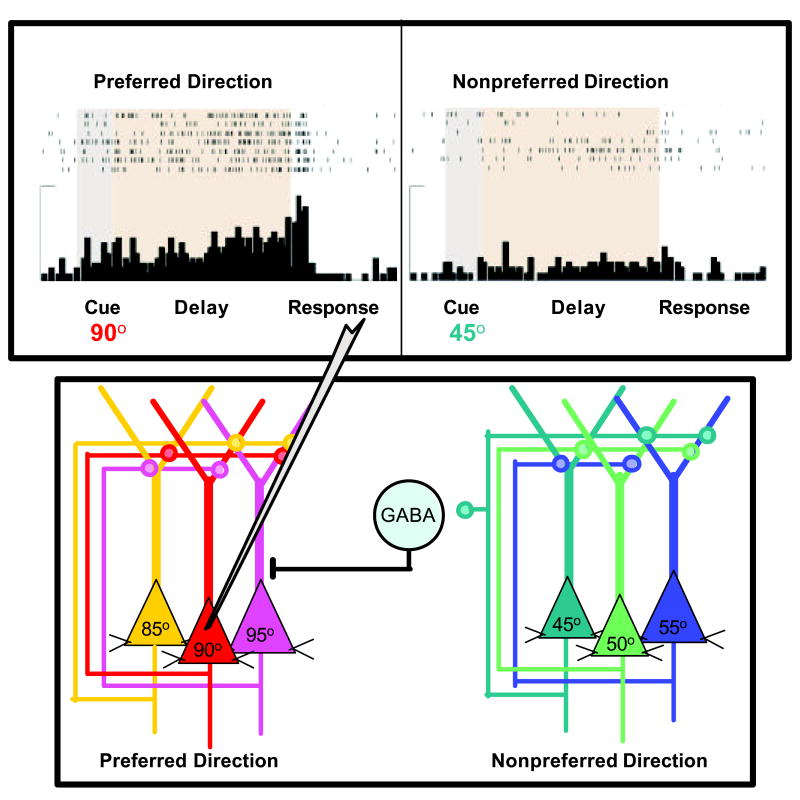
The cognitive functions of the prefrontal cortex (PFC) are arguably the most advanced in our cognitive repertoire, and likely the most vulnerable to disruption. PFC circuits have the unique ability to represent information that is no longer in the environment- even in the face of distraction and to use this “representational knowledge” to guide behavior, thought and affect. This process is often referred to as “working memory”. Working memory is thought to arise from networks of PFC pyramidal cells with shared properties engaged in recurrent excitation. These networks are thought maintain task relevant information during the delay period when stimuli are no longer present in the environment (Goldman-Rakic, 1995; see Figure 1). During this period that follows cue presentation, prefrontal neurons show increased firing rate in association with a specific location in the visual field where the cue was presented (i.e. 90° vs 45 °; Figure 1). The ability of PFC neuronal networks to keep task-relevant information ‘online’ in the form of delay-related firing is thought to represent the physiological basis of working memory. These firing patterns are tuned by GABAergic inputs, and by proper catecholamine modulation (Rao et al., 2000; Constantinidis et al., 2002). Optimal PFC network firing allows the regulation of attentional focus, the inhibition of inappropriate motor responses, and planning for the future.
Figure 1.
The cellular basis of spatial working memory. (A) A neuron with spatially tuned persistent activity during the delay period of a spatial working memory task. Data from Dr. Min Wang. (B) Schematic representation of PFC networks of pyramidal cells that represent the cellular basis of working memory. Networks with shared mnemonic properties (preferred direction) engage in recurrent excitation to maintain information (increase in firing rate) during the delay period in the absence of environmental stimuli. GABAergic interneurons activated by networks firing to non-preferred directions enhance spatial tuning by inhibiting firing to nonpreferred directions. Adapted from Goldman-Rakic.
Deficits in PFC function are evident in most neuropsychiatric disorders (indeed, the term “psychiatric” may be synonymous with PFC dysfunction), and they are amongst the most prominent cognitive problems with normal aging (Nielsen-Bohlman & Knight, 1995; Schacter et al., 1996; Albert, 1997; Chao & Knight, 1997). Even in young, so-called “normal” individuals, PFC cognitive abilities fluctuate, eroding when we are fatigued or when we are exposed to uncontrollable stress. Even mild uncontrollable stressors have been shown to impair PFC working memory functions in both humans and animals (reviewed in Arnsten, 2000a). Furthermore, stress can precipitate or exacerbate many neuropsychiatric disorders. For example, stress has been linked to the onset of schizophrenic symptoms (Breier et al., 1991; Dohrenwend et al., 1995), and to the precipitation of manic episodes in patients with bipolar disorder (Hammen & Gitlin, 1997). Chronic uncontrollable stress is used as a model of depression, and even an acute, traumatic stress can induce Post-Traumatic Stress Disorder (PTSD), a syndrome associated with overactive amygdala and impaired PFC function (Bremner, 2002). Thus, it is critical that we understand how the PFC is modulated, and how modulation changes with age and with stress. Many neurotransmitters (glutamate, GABA) and neuromodulators (e.g. dopamine, serotonin, acetylcholine) contribute to PFC cognitive functioning in critical ways (reviewed in Arnsten & Robbins, 2002). This review focuses on the mechanisms by which NE influences PFC functions, as the field has achieved a surprising consistency, and is directly relevant to the treatment of neuropsychiatric disorders.
2. Background on Norepinephrine
The noradrenergic neurons arise from the locus coeruleus (LC) within the brainstem and their terminals project to many different brain regions, including the PFC (Arikuni & Ban, 1978; Gerfen & Clavier, 1979; Morrison et al., 1979; Morrison et al., 1982; Porrino & Goldman-Rakic, 1982). There is a reciprocal relationship between the PFC and the LC, as the PFC provides one of the few higher cortical inputs back to the LC neurons (Arnsten & Goldman-Rakic, 1984; Sara & Herve-Minvielle, 1995; Jodo et al., 1998). Within the monkey PFC, noradrenergic fibers target both deep and superficial layers of the cortex (Lewis & Morrison, 1989). NE released by these fibers interacts with three families of adrenergic receptors: the α1, the α2 and the β receptors (1-3).
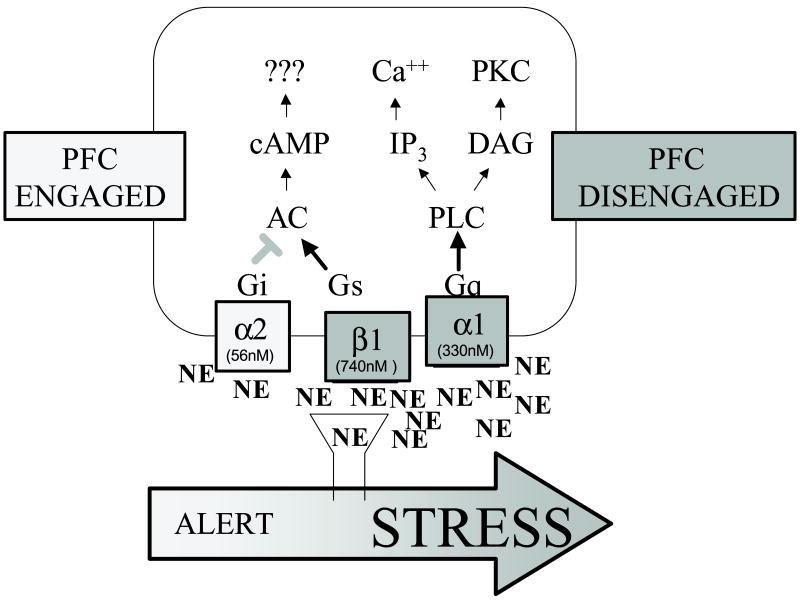
NE has highest affinity for the α2 receptors, which consist of three subtypes: the α2A, the α2B and the α2C. The α2A, and to a lesser extent the α2C, are found pre-synaptically on NE cells and terminals, while all three subtypes are found post-synaptically (MacDonald et al., 1997). The α2A receptor is the most common subtype found in the PFC, however, there are low levels of α2C receptors as well (Aoki et al., 1994; Aoki et al., 1998a). Binding studies suggest that α2 receptors are most concentrated in superficial layers in primate PFC (Goldman-Rakic et al., 1990), although electron microscopic (EM) analyses also see receptors in deep layers. These studies have found α2A receptors, among other cellular locations, over post-synaptic densities on dendritic spines in the primate PFC (Aoki et al., 1994; Aoki et al., 1998a). α2 receptors are generally coupled to Gi proteins (Duman & Nestler, 1995; Ramos et al., 2006a), which can reduce intracellular cyclic adenosine monophosphate (cAMP) production by inhibiting some adenylyl cyclase isoforms (Figure 2).
Figure 2.
Norepinephrine (NE) released in the PFC activates different intracellular signaling pathways through distinct adrenoceptors with varying affinities for NE. PFC cognitive function is enhanced by moderate levels of NE engaging post-synaptic α2A receptors with high affinity for NE, while high levels of NE impair PFC cognitive function by engaging α1 and β1 receptors with lower affinity for NE. AC, adenylyl cyclase; LC, locus coeruleus; PLC, phospholipase C; DAG, diacylglycerol.
NE has lower affinity for α1 adrenergic receptors, of which there are three subtypes: the α1A, the αB, and the α1D (Hieble et al., 1995). The α1A and α1D are most prominent in rodent PFC (Pieribone et al., 1994; Day et al., 1997). Receptor binding studies of primate PFC have shown that α1 receptors are concentrated in superficial layers (Goldman-Rakic et al., 1990), similar to α2 receptors. However, in contrast to the α2 subtype, the subcellular localization of these receptors is not yet known. α1 Receptor stimulation has been found to enhance excitatory processes in many brain regions, particularly in the somatosensory cortex (Waterhouse et al., 1981; Mouradian et al., 1991). α1 receptors are generally coupled to Gq proteins, and can thus activate phospholipase C and phosphotidyl inositol intracellular signaling, resulting in activation of protein kinase C (PKC) and the release of intracellular calcium via inositol 1, 4, 5-triphosphate (Duman & Nestler, 1995; Birnbaum et al., 2004; see Figure 2).
Finally, NE has lowest affinity for β adrenergic receptors. There are three subtypes of β receptors: β1 (localized in heart), β2 (localized in lung), and β3 (localized in stomach), and all are found in the central nervous system as well (Insel, 1993). These subtypes are differentially expressed in various regions of the brain, with β1 receptors in higher concentrations in the adult rat cortex compared to the other receptor subtypes (Rainbow et al., 1984; Nicholas et al., 1993; Summers et al., 1995). β Receptors are densest in the intermediate layers of the PFC (Goldman-Rakic et al., 1990), where thalamic inputs are concentrated. Moreover, these receptors have been identified in high concentration in the monkey PFC (Goldman-Rakic et al., 1990; Aoki et al., 1998b). EM studies of monkey PFC have localized β2 receptors on dendritic spines (presumably of PFC pyramidal neurons) and on GABAergic interneurons (Aoki et al., 1998b). Electrophysiological studies of somatosensory cortex have shown that β receptors can potentiate GABAergic processes (Waterhouse et al., 1980). As GABA has a key influence on tuning of PFC neuronal response (Rao et al., 2000; Constantinidis et al., 2002), similar effects in PFC would be of great interest. Finally, many β receptors are found on glia, where they have multiple actions including reducing the uptake of glutamate (Hansson & Ronnback, 1991) and regulating glucose availability (Fillenz & Lowry, 1998; Fillenz et al., 1999). In general, β receptors are coupled via Gs to adenylyl cyclase, increasing cAMP signaling (Ordway et al., 1987; Duman & Nestler, 1995; Ferry et al., 1999a; Zhang et al., 2005; see Figure 2).
3. Activity Patterns of the Locus Coeruleus
LC neurons fire in accordance to arousal state, and the attentional interest of the animal (Foote et al., 1980). LC cells are silent during REM sleep, and increase their firing rate with increasing state of arousal. During alert waking, LC cells are in a so-called “phasic” state, with low levels of spontaneous firing and bursts of firing to stimuli that are of interest to the animal (Rajkowski et al., 1998; Aston-Jones et al., 1999). In contrast, when animals are stressed or anxious the cells enter a “tonic” state, with very high levels of spontaneous activity and less phasic activity to stimuli (ibid). The interaction between arousal state and information processing was observed in monkeys performing a continuous performance task where they had to distinguish the correct target stimuli and ignore distracting stimuli (ibid). When the monkey was alert and attentive, LC cells were in a “phasic” state, and fired to the targets but not the distractors. In contrast, when the animal was either drowsy or stressed it made errors, and the LC cells responded to the distractors with less response to the targets. It is tempting to speculate that the PFC regulates appropriate LC responding during the phasic, alert state, as it would be one of the few brain regions that contacts the LC with higher order information. The importance of NE and LC firing to attention regulation has been appreciated for a long time. Depletion of forebrain NE in rats leads to distractibility and attentional deficits in a variety of paradigms (Carli et al., 1983; Cole & Robbins, 1992).
4. The Effects of Norepinephrine on Prefrontal Cortex Function
4.1. Catecholamine depletion of the PFC dramatically impairs function
The pioneering study of Brozoski and colleagues was the first to demonstrate that catecholamines play a critical role in the modulation of the spatial working memory functions of the PFC (Brozoski et al., 1979). Dopamine (DA) and NE depletion in the PFC was produced by infusion of the catecholamine neurotoxin, 6-hydroxy-dopamine (6-OHDA), into the dorsolateral PFC of monkeys. Animals with large catecholamine depletion of the PFC were as impaired similar to those with PFC ablations, highlighting the critical role of catecholamine modulatory influences. The effect of catecholamine depletion was specific to the cognitive functions of the PFC, since animals were not impaired on performance of a non-PFC task, visual pattern discrimination. Although the Brozoski study focused on the importance of DA to PFC function, as monkeys with large NE depletion and small DA depletion did not show deficits, it is now appreciated that both catecholamines are important to PFC function. Hence, it is likely that both must be substantially depleted to produce marked impairment in chronic studies. The Brozoski study has been replicated in rats with 6-OHDA lesions of the medial PFC (Simon, 1981), and in marmosets with 6-OHDA lesions to the dorsolateral PFC (Roberts et al., 1994; Collins et al., 1998). More recently, studies in marmosets have shown that 6-OHDA lesions of the dorsolateral PFC impair the ability to acquire an attentional set, a related function of this brain region (Crofts et al., 2001).
Spatial working memory deficits with preserved visual discrimination function have also been observed with global catecholamine depletion. For example, systemic, chronic reserpine treatment depletes monoamines and impairs spatial working memory performance without altering visual discrimination performance in young adult monkeys (Cai et al., 1993). Furthermore, aged monkeys and rats with naturally-occurring catecholamine depletion exhibit prominent spatial working memory deficits and generally spared performance on discrimination tasks (Bartus et al., 1978; Luine et al., 1990). In both young (Sahakian et al., 1985) and aged (Luine et al., 1990) rats, cognitive performance correlates with levels of PFC catecholamines. Pharmacological studies in aged animals and young, depleted animals indicate that PFC working memory function can be restored by administering compounds that mimic NE actions at α2 adrenergic receptors (Arnsten & Goldman-Rakic, 1985; Cai et al., 1993).
4.2. α2 Improves PFC function
4.2.1. α2 Agonists restore PFC functions in catecholamine-depleted animals: evidence for post-synaptic actions
Studies in rodents, monkeys and humans have all shown that NE has an important beneficial influence on spatial working memory performance through its actions at α2 adrenergic receptors. Young monkeys with working memory impairment induced by local PFC (Arnsten & Goldman-Rakic, 1985) or global (Cai et al., 1993) catecholamine depletion are greatly improved by systemic treatment with α2 agonists such as clonidine and guanfacine. The beneficial effects of α2 agonists have also been observed in aged monkeys (Arnsten & Goldman-Rakic, 1985; Arnsten et al., 1988; Rama et al., 1996) and aged rats (Carlson et al., 1992; Ramos et al., 2006a) with naturally-occurring catecholamine loss. α2 Agonists also improve working memory performance in intact, young monkeys, but at higher doses than those needed to improve aged or depleted animals (Franowicz & Arnsten, 1998). Improvements with α2 agonists can be reversed with α2, but not α1, antagonists, and α2 antagonists by themselves impair PFC function, consistent with an α2 receptor mechanism (Arnsten & Goldman-Rakic, 1985). α2 Agonists have been shown to improve working memory for both visuo-spatial (Arnsten et al., 1988) and visuo-feature (Jackson & Buccafusco, 1991) cues, suggesting enhancement of both dorsolateral and ventrolateral PFC function.
Most studies of NE modulation of PFC function have used working memory tasks, but a few animal studies have shown effects on PFC tasks that challenge attention regulation and behavioral inhibition. For example, α2 agonists are particularly effective at enhancing working memory during distracting conditions (Jackson & Buccafusco, 1991;Arnsten & Contant, 1992), a finding consistent with earlier studies showing that forebrain NE depletion increases distractibility (Carli et al., 1983). Clonidine and guanfacine have been shown to improve attentional regulation, behavioral inhibition and planning in humans as well (see below). A recent study by Chamberlain et al. suggested that NE is more sensitive in modulating lateral compared to orbital PFC function (Chamberlain et al., 2006). In this study, atomoxetine, a NE reuptake blocker, enhanced response inhibition, but had no significant effect on a probabilistic learning task dependent on the orbital PFC, a region regulated more by serotonin (Clark et al., 2005; Chamberlain et al., 2006). The latter study may help explain why higher doses of guanfacine are required to improve performance of an object reversal task associated with orbital PFC function (Steere & Arnsten, 1997).
4.2.2. α2 Agonists improve via the A-subtype
The greater the loss of NE, the lower the dose of α2 agonist needed to improve PFC performance (Franowicz & Arnsten, 1999), a pattern consistent with drug actions at supersensitive, post-synaptic receptors. Pharmacological profiles further indicate that the α2A receptor subtype may be the most critical for cognitive enhancement (see (Arnsten et al., 1996) for review). For example, the α2A selective agonist, guanfacine, is the most effective compound in enhancing working memory without side effects (Arnsten et al., 1988; Rama et al., 1996). Guanfacine is about ten times weaker than clonidine in inhibiting firing of the NE cell bodies in the LC or in decreasing NE release (Engberg & Eriksson, 1991), but is 10-100 times more potent in improving working memory in aged monkeys (Arnsten et al., 1988). Studies in genetically-altered mice emphasize the importance of the α2A receptor subtype, as mice with a mutation of the α2A subtype no longer show beneficial effects on working memory after guanfacine treatment (Franowicz et al., 1998), while knockout of the α2C subtype has no effect on the response to another alpha-2 adrenergic agonist, dexmedetomidine (Tanila et al., 1999). Although knockout of the α2C receptor had no effect on working memory, it does seem to be related to the modulation of other NE-associated behaviors. Interestingly, knockout of the α2C subtype diminished the response to stress in classic tests of depression (i.e. forced swim test), suggesting that α2C receptor blockade may be useful for treating stress-related psychiatric disorders such as depression (Sallinen et al., 1999).
4.2.3. α2 Adrenergic agonists improve PFC, but not nonPFC, cognitive functions
Although α2 agonists improve the performance of tasks that challenge the PFC, they often have little beneficial effect under conditions that do not challenge the PFC. For example, these drugs have no effect or impair the spatial reference memory functions of the hippocampus (Sirviö et al., 1991), the visual feature discrimination memory functions of the inferior temporal cortex (Arnsten & Goldman-Rakic, 1985; Steere & Arnsten, 1997),the visual feature recognition memory functions of the perirhinal cortex (Arnsten & Goldman-Rakic, 1990), and the covert visual-spatial attention shifting functions of the parietal cortex (Witte & Marrocco, 1997). Thus, the beneficial effects of α2 agonists are relatively specific to PFC functions.
4.2.4. Evidence for actions in the PFC
A number of studies in rodents and monkeys demonstrate that α2 compounds act directly in the PFC to alter working memory function. As with systemic injection, direct infusion of α2 antagonists, but not α1 or β antagonists, into the dorsolateral PFC produces a delay-related impairment in spatial working memory (Li & Mei, 1994), demonstrating that endogenous NE stimulation of α2 receptors in the PFC is critical to working memory performance. Infusions of the α2 antagonist, yohimbine, into dorsolateral PFC also impaired impulse control (Ma et al., 2003) and induced locomotor hyperactivity (Ma et al., 2005), thus producing a profile of behavioral disinhibition. Conversely, intra-PFC infusion of α2 agonists improved working memory performance in either young (Mao et al., 1999) or aged monkeys (Arnsten, 1997), or aged rats (Tanila et al., 1996; Ramos et al., 2006a).
Imaging studies in monkeys are also consistent with α2 adrenergic mechanisms enhancing PFC function. We have observed increased regional cerebral blood flow (rCBF) in the dorsolateral PFC of monkeys treated with guanfacine prior to performing a spatial working memory task (Avery et al., 2000). Guanfacine improved working memory performance and increased rCBF in the PFC surrounding the principal sulcus, the same region essential for spatial working memory function (Goldman & Rosvold, 1970). In contrast, guanfacine had no effect on rCBF in the auditory association cortex (superior temporal cortex), a region not involved in task performance (Avery et al., 2000). These results are consistent with imaging studies in humans treated with guanfacine (see below).
4.2.5. Effects on PFC neurons
Electrophysiological studies have observed parallel findings at the cellular level. Iontophoresis of the α2 antagonist, yohimbine, reduced delay-related activity in PFC neurons of monkeys performing spatial working memory tasks (Sawaguchi, 1998; Li et al., 1999). Conversely, systemic clonidine administration enhances delay-related firing in PFC cells, and this effect is reversed by iontophoretic application of yohimbine (Li et al., 1999). Similar results have recently been observed with guanfacine (Wang et al., 2006). Clonidine and guanfacine increase delay-related firing for the preferred direction, but have little effect on firing for nonpreferred spatial directions, thus enhancing spatial tuning during the delay period (ibid). Thus, NE actions at α2A receptors in PFC have powerful effects on delay-related firing, the presumed neuronal substrate of working memory function (see introduction above). It is possible that the beneficial effects of α2 receptor stimulation occur on dendritic spines, as α2A receptors have been localized on the post-synaptic membranes of spines in monkey PFC (Aoki et al., 1994; Arnsten et al., 1996).
4.2.6. Second messenger actions
α2 adrenergic receptors are commonly coupled to Gi proteins which inhibit adenylyl cyclase/cAMP pathways (Duman & Nestler, 1995; see Figure 2). As activation of the adenylyl cyclase/cAMP pathway seems to impair PFC function (Taylor et al., 1999; Ramos et al., 2003), inhibition of this intracellular signaling pathway may contribute to the beneficial effects of α2 agonists on PFC cognitive function. Indeed, recent evidence demonstrated that treatments that increase cAMP signaling block guanfacine’s beneficial effects in both aging rats and monkeys (Ramos et al., 2006a). For example, in rats, a dose of Sp-cAMPS (a compound that mimics cAMP) that has no effect on its own completely reversed guanfacine’s enhancing effects. Similar effects were observed in monkeys using rolipram, a drug that inhibits the phosphodiesterases that normally catabolize cAMP, thus leading to an increase in endogenous levels of cAMP. The enhancing effects of guanfacine were significantly reduced by co-administration of rolipram, using a low dose that had no effect on task performance on its own (ibid). Thus, guanfacine’s beneficial effects on working mediated appear to be mediated via inhibition of cAMP signaling in the PFC. Recent electrophysiological studies in monkeys are consistent with this hypothesis (Wang et al., 2006).
4.2.7. Clinical relevance
Recent studies with α2 agonists in healthy humans and patients with PFC disorders have found surprising similarity to basic studies in animals. Earlier studies had focused on clonidine, a suboptimal drug due to its potent inhibition of LC firing, prominent side effect profile, and high affinity for imidazoline I1 receptors. Clonidine usually has mixed effects on PFC functions in healthy young adults, presumably due to competing pre- vs. post-synaptic effects and dose limitations from sedative and hypotensive side effects (Coull, 1994; Jakala et al., 1999a). However, improvement with clonidine has been observed in patient groups with PFC deficits and likely alterations in PFC catecholamines. For example, clonidine improved performance of memory recall and the Stroop interference task in patients with Korsakoff’s amnesia, and was most effective in those with the greatest signs of NE loss (Mair & McEntree, 1986). Clonidine has also been shown to improve memory recall and performance of the Trails B behavioral inhibition task in schizophrenic patients (Fields et al., 1988). Clonidine improves spatial working memory in Parkinson’s patients with presumed catecholamine depletion in the PFC (Riekkinen & Riekkinen, 1999a). One recent study has shown that clonidine can even improve working memory in patients with Alzheimer’s Disease (Riekkinen & Riekkinen, 1999b), although previous studies have not shown benefit (Mohr et al., 1989).
Imaging studies in humans generally support findings from animal research. Studies using low doses of clonidine in normal adults generally show a picture of impaired attention and emerging sedation associated with imaging changes in thalamus and parietal cortex (Coull et al., 1997). These findings are consistent with an older study showing clonidine impaired attentional orienting (Clark et al., 1987), a function dependent on parietal lobe function (Posner et al., 1984). These findings reinforce the notion that posterior cortex and most subcortical structures are impaired by α2 receptor stimulation. However, a different picture emerges when higher doses of clonidine are given to patients with presumed NE loss, or when guanfacine is used. For example, administration of higher doses of clonidine to Korsakoff’s patients increased regional cerebral blood flow in frontal lobe, and the increased blood flow in the left PFC correlated with improved verbal fluency performance (Moffoot et al., 1994). A more sophisticated analysis also suggests that clonidine can have important modulatory effects on higher cortical function. Clonidine has also been shown to increase the effective connectivity between the LC, parietal cortex and PFC during an attentional task, while decreasing connectivity during rest (Coull et al., 1999). More recently, guanfacine has been shown to increase regional cerebral blood flow in the frontal lobe of healthy adults as measured by PET imaging (Swartz et al., 2000). These studies reinforce the idea that α2 receptor stimulation often impairs the functioning of most brain regions, and that the PFC is exceptional in its beneficial influence from α2A receptor stimulation.
A superior profile has been observed with the more selective α2A agonist, guanfacine. Guanfacine has been shown to improve working memory, planning and paired associates learning tasks in healthy young adults (Jakala et al., 1999a;Jakala et al.,1999b), although this effect was not replicated in a study of younger, healthy subjects (Muller et al., 2005). However, guanfacine has been shown to be very effective in patients with PFC dysfunction.
Much research with α2 agonists has focused on patients with attention-deficit hyperactivity disorder (ADHD), a disorder with prominent PFC dysfunction. Early studies demonstrated that clonidine can improve symptoms of ADHD (Hunt et al., 1985), but serious hypotensive and sedative side effects have limited its use. Current studies have turned to the more selective α2A agonist, guanfacine. Guanfacine has been shown to be effective in three open label trials (Chappell et al., 1995; Horrigan & Barnhill, 1995; Hunt et al., 1995; Boon-yasidhi et al., 2005). In addition, two placebo controlled trials have come to similar results (trial in ADHD adults, guanfacine favorable to dexedrine, Taylor & Russo, 2001; trial in ADHD children with tics, guanfacine improved tics and ADHD symptoms, Scahill et al., 2001). In addition to having therapeutic effects on standard rating scales, guanfacine has been shown to improve performance of PFC tasks such as the Stroop (Taylor & Russo, 2001, guanfacine superior to dexedrine) and the Connors CPT which assesses vigilance, working memory and behavioral inhibition (Scahill et al., 2001). These published findings are consistent with clinical reports that guanfacine reduces impulsivity (ibid), a sign of improved PFC function. Interestingly, susceptibility to ADHD has been associated with a Taq IA polymorphism of the dopamine beta hydroxylase (DBH) gene (Smith et al., 2003; Tang et al., 2006). DBH catalyzes the conversion of dopamine to norepinephrine, and this polymorphism results in lower DBH activity. Thus, patients with ADHD may have less endogenous activation of α2 receptors and benefit from treatment with an α2 agonist such as guanfacine.
Guanfacine may also be useful in treating PFC dysfunction in other types of patients. It has been tested in patients with schizophrenia (Friedman et al., 1999), and more recently in subjects with schizotypal disorder where it normalized cognitive performance on a Connors CPT (McClure, 2006). Guanfacine is also being tried in patients with mild traumatic injury to the PFC (McAllister et al., 2004), and in PTSD (Horrigan, 1996). Thus, this is a rare instance where basic research has led to a treatment for neuropsychiatric dysfunction.
4.3. α1 Impairs PFC function
4.3.1. High levels of NE impair working memory function via α1 receptor stimulation
New evidence indicates that high concentrations of NE impair PFC function through activation of α1 adrenergic receptors. NE has higher affinity for α2A than α1 receptors (α2A receptors: 56 nM, O’Rourke et al., 1994; α1 receptors: 330 nM, Mohell et al., 1983). Thus, it is likely that low levels of NE (e.g. under basal or nonstress conditions) preferentially engage α2 receptors and improve PFC function, while during conditions of high NE release, α1 receptors would become engaged and override the effects of α2 receptor stimulation. It is well established that high levels of NE are released in the PFC during stress exposure (e.g. Finlay et al., 1995; Goldstein et al., 1996), and recent evidence suggests that these high NE levels stimulate α1 receptors and impair PFC function (Birnbaum et al., 1999). Thus, stress-induced cognitive deficits were blocked by infusion of the α1 receptor antagonist, urapidil, into the PFC prior to cognitive testing (Birnbaum et al., 1999). Infusions of urapidil had no effect under nonstress conditions (ibid), presumably due to little endogenous NE α1 receptor stimulation during nonstressful conditions.
4.3.2. Evidence for actions in the PFC
The effects of stress on working memory performance can be mimicked by infusion of an α1 adrenergic receptor agonist into the PFC. Infusions of the α1 agonist, phenylephrine, into the PFC in rats markedly impaired working memory performance (Arnsten et al., 1999). This impairment was reversed by co-infusion of the α1 receptor, antagonist, urapidil, (ibid), consistent with actions at α1 receptors. Similar effects have been observed in monkeys, where infusions of phenylephrine into the dorsolateral PFC produced a marked, delay-related impairment in working memory performance (Mao et al., 1999). Infusions were most effective in the caudal two-thirds of the principal sulcal cortex (ibid), the cortical region most tightly associated with spatial working memory performance in monkeys (Goldman & Rosvold, 1970). Thus, high levels of NE release in the PFC may engage α1 receptors and impair PFC working memory function. Interestingly, most effective antipsychotic medications, including the new “atypical” neuroleptics, have potent α1 blocking properties (Baldessarini et al., 1992). Although most previous attention has focused on the sedating effects of these α1 blocking properties, the current data suggest that α1 blcockade may have therapeutic effects as well.
4.3.3. Effects on PFC neurons
Iontophoresis of the α1 agonist, phenylephrine, onto PFC neurons suppressed delay-related firing in monkeys performing a spatial working memory task (Birnbaum 2004). The suppression in firing was most evident for the preferred direction, thus resulting in an erosion of spatial mnemonic tuning. These results are the opposite of what was observed with α2 agonists, consistent with the behavioral findings.
4.3.4. Second messenger actions
α1 Adrenergic receptors are commonly coupled to the phosphotidyl inositol/PKC intracellular pathway via Gq proteins (Duman & Nestler, 1995; see Figure 2). Evidence to date suggests that α1 receptor stimulation impairs PFC function through activation of this second messenger pathway. For example, both the α1 adrenergic agonist, phenylephrine, and pharmacological stressor, FG7142 (increases catecholamine release), can increase PKC enzymatic activity in the membrane fraction of prefrontal cortical slices (Birnbaum et al., 2004). The cognitive impairment induced by phenylephrine infusions into the rat PFC can be completely reversed by pretreatment with a dose of lithium known to suppress phosphotidyl inositol turnover (Arnsten et al., 1999). However, lithium can alter other second messenger pathways. Therefore, more recent studies in animals have focused on agents which selectively target molecules in the phosphotidyl inositol/PKC cascade. For example, intra-PFC infusion of the PKC inhibitors, chelerythrine or NPC15437, blocked the detrimental effects of α1 agonists and/or stress (Birnbaum et al., 1999). Similar results have been reported by Dash and colleagues, whereby performance of an aversive working memory task was improved by infusion of PKC inhibitors into the rat PFC (Runyan et al., 2005). Systemic administration of the PKC inhibitor, chelerythrine, also protects working memory in monkeys (Birnbaum et al., 2004). Interestingly, electrophysiological recordings showed that the suppression of delay-related firing induced by iontophoresis of phenylephrine was prevented by co-iontophoresis of chelerythrine (ibid). These results are consistent with activation of the phosphotidyl inositol/PKC pathway underlying α1 receptor-mediated impairment of PFC cognitive function at a behavioral, biochemical, and physiological level.
4.3.5. Clinical relevance
This basic research is highly relevant to PTSD, and may also have direct relevance to bipolar disorder and schizophrenia.
In humans as well as animals, traumatic stressors likely lead to excessive NE release and α1 adrenergic receptor engagement. The animal research suggests that high levels of α1 receptor stimulation should weaken PFC inhibitory functions (see above) and strengt’hen amygdala function (Ferry et al., 1999b), the profile observed in PTSD. Thus it has been very compelling that α1 receptor blockade with prazosin has been shown to lessen symptoms of PTSD in patients with combat (Raskind et al., 2000; Raskind et al., 2003) or civilian (Taylor & Raskind, 2002; Taylor et al., 2006) PTSD. It has also been helpful in treating elderly patients with longstanding PTSD (Peskind et al., 2003). Together, these studies suggest that α1 receptor blockade strengthens the inhibitory functions of the PFC function and thus lessens intrusive thoughts and flashbacks that are cardinal symptoms of this disorder.
The finding that excessive activation of PKC impairs PFC function also has relevance to bipolar disorder and schizophrenia. Bipolar disorder is associated with excessive levels and overactivity of PKC, and most effective treatments for mania (e.g. lithium, valproic acid) reduce the activity of the phosphotidyl inositol/PKC cascade (Manji & Lenox, 1999). The antiestrogen, tamoxifen, has PKC-blocking properties at high doses, and proof of concept studies suggest that this compound can be helpful in bipolar disorder (Bebchuk et al., 2000; Kulkarni et al., 2006). The successful use of atypical antipsychotics is likely related to the α1 and 5HT2A-blocking properties of these compounds, as both of these receptors activate the PKC signaling pathway. Recent gene array and genetic studies suggest that overactive PKC may also contribute to schizophrenia. RGS4 is a molecule that inhibits PKC signaling, and its levels are greatly reduced in the PFC of patients with schizophrenia, and may be genetically linked to schizophrenia and bipolar disorder (Mirnics et al., 2001; Prasad et al., 2004; Erdely et al., 2006), although see (Lipska et al., 2006; Talkowski et al., 2006). Thus, disinhibited PKC signaling may contribute to these psychotic disorders, and may worsen during stress exposure. It is hoped that more selective PKC inhibitors may have more rapid and powerful effects in treating psychotic disorders.
4.4. β Receptors
4.4.1. Opposing effects of β1 and β2?
β Adrenergic receptor stimulation has been known to play a critical role in long-term memory consolidation in the amygdala and hippocampus. For example, infusion of β adrenergic antagonists into the amygdala impairs, whereas infusion of a β adrenergic agonist improves memory consolidation (reviewed in (Cahill & McGaugh, 1996). Similarly, β receptor stimulation in the dentate gyrus can induce long-term potentiation (Lacaille & Harley, 1985; Chaulk & Harley, 1998) and is involved in the late phase of memory consolidation in the hippocampus (Roullet & Sara, 1998; Sara et al., 1999). In contrast, previous studies had observed no effect on the working memory functions of the PFC when β1 and β2 receptors were blocked with the mixed β1/β2 antagonist, propanolol. For example, neither microinjection of propanolol into the PFC (Li & Mei, 1994), nor systemic administration of propanolol (Arnsten & Goldman-Rakic, 1985) altered PFC function in monkeys. This lack of effect is surprising as β receptors have been identified in high concentration in the monkey PFC (Goldman-Rakic et al., 1990; Aoki et al., 1998b). However, different results are obtained when selective β adrenergic drugs are used that target specific receptor subtypes. Indeed, a recent study showed that endogenous activation of β1 receptors, as can occur with stress, impairs PFC cognitive function (Ramos et al., 2005). In this study, infusion of the β1 adrenergic antagonist, betaxolol, improved working memory performance in both rats and monkeys (ibid). The effect of betaxolol seen in the latter study contrasts with the effects described above in the amygdala where infusion of a β adrenergic antagonist impairs memory consolidation.
In contrast, recent evidence from our lab suggests that β2 receptors have beneficial effects on working memory. Stimulation of β2 receptors with clenbuterol significantly, yet modestly, enhances working memory function in aging animals (Ramos et al., 2006b). The enhancement with clenbuterol is similar to its effects in the amygdala and hippocampus. For example, McGaugh and colleagues have demonstrated that β2 receptor stimulation within the amygdala immediately after training enhances performance in an inhibitory avoidance task (Introini-Collison et al., 1991; Ferry & McGaugh, 1999). Thus, clenbuterol could be one of the few agents to produce global cognitive improvement. β2 receptor stimulation could produce generalized improvement by enhancing glucose availability through influences on astrocytes.
Finally, the influence of β3 receptor stimulation on PFC function has yet to be studied. There are high levels of β3 receptor mRNA in rat cortex (Summers et al., 1995), indicating that this should be a direction for future research.
4.4.2. Second messenger actions
In contrast to α2 adrenergic receptors, β receptors can be coupled to Gs which can lead to an increase in cAMP levels (Figure 2). Previous research has shown that increasing cAMP levels impairs PFC function in both young and aged animals (Taylor et al., 1999; Ramos et al., 2003). Thus, β1 receptors, like DA D1 receptors, may impair PFC cognitive function via this intracellular pathway during conditions of high catecholamine release (i.e. stress). Similar to β1 receptors, β2 receptors can also couple to Gs proteins. Indeed, activation of β2 receptors in the cortex increases levels of cAMP (Ordway et al., 1987). Moreover, this increase in cAMP is significantly reduced by the β2 antagonist, ICI 118551 (ibid). Similarly, in the amygdala, β2 receptors modulate memory storage by a direct coupling to adenylyl cyclase and influencing cAMP formation (Ferry et al., 1999a). A recent study by O’Donnell and colleagues suggests that clenbuterol’s antidepressant effects are also via increased cAMP signaling in cortical neurons (Zhang et al., 2005). Taken together, clenbuterol’s effects on working memory performance may be via activation of this pathway. The fact that both β receptors can couple to the same pathway, but have opposite effects suggests different sites of action. Future studies should compare β1 vs β2 receptors’ subcellular localization within the PFC and examine whether or not activation of a cAMP-dependent signaling mediates the effects of these receptors in the PFC.
4.4.3. Clinical relevance
β Adrenergic receptor blockers such as propranolol are being tested immediately post-trauma in the hopes of alleviating the development of PTSD (Vaiva et al., 2003). Studies in animals suggest that this drug treatment may prevent amygdala-induced enhancement of the traumatic memories, but may not strengthen PFC inhibitory abilities. Specific blockade of the β1 receptor subtype may provide a more powerful therapeutic effect because this strategy strengthens the PFC and could weaken amygdala function. Thus, unlike propanolol, betaxolol may prove useful in treating established PTSD as has been suggested previously (Ramos et al., 2005).
Previous evidence suggests that the effects of clenbuterol could result from β receptors’ ability to enhance the breakdown of glycogen and the export of glucose from astrocytes to increase local cerebral blood flow (Fillenz et al., 1999).Since glucose metabolism has been found to be decreased in the frontal and temporal lobes of aged humans and monkeys (De Santi et al., 1995; Eberling et al., 1997; Noda et al., 2002), the effects of clenbuterol could be important for the elderly population with prefrontal cortical deficits. Interestingly, age-related memory impairment can be reversed by administration of glucose or epinephrine, which binds preferentially to β receptors (Korol & Gold, 1998). Thus, β2 receptor activation may be particularly relevant in the aged population where reduced glucose metabolism may be contributing to prefrontal cortical deficits. However, to our knowledge, no study has examined the effects of β2 receptors in human cognition and thus it is hard to speculate as to clenbuterol’s clinical efficacy. Despite the initial positive findings with β adrenergic agonists in rats and monkeys, it is still too early to have this basic research translated into the clinic.
5. Comparison with Other Brain Regions: Why the Qualitative Differences?
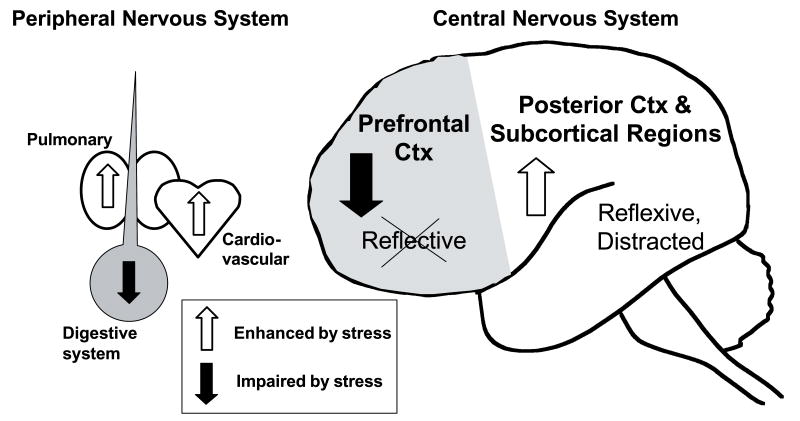
The PFC successfully guides behavior under nonstressful conditions when we feel in control. However, there is abundant evidence that the PFC goes ‘off-line’ during stress. Our emerging picture suggests that the PFC may be modulated differently than other than brain regions, and that the neurochemical conditions that are optimal for PFC are suboptimal for posterior cortical and subcortical regions, and vice versa. NE seems to play an important role in this ‘neurochemical switch’ (reviewed in Arnsten, 2000b; Arnsten, 2000a). NE regulation of the PFC is “upside down and backwards” from much of the rest of the brain. Thus, NE stimulation of α2 adrenergic receptors enhances PFC functions but impairs many posterior cortical functions, while stimulation of α1 and/or β receptors enhances posterior cortical functions but impairs or has no effect on PFC function. As NE has higher affinity for α2 than α1 or β receptors (see above), lower levels of NE release during non-stressed conditions may preferentially engage α2 receptors and facilitate PFC regulation of behavior, while high levels of NE release during stress may engage α1 and β1 receptors, taking the PFC “off-line” but providing areas such as the amygdala, hippocampus, sensory/motor cortices and cerebellum with a more optimal neurochemical environment. This may have survival value, allowing more habitual or reflexive mechanisms to control behavior during dangerous conditions (Figure 3). However, this differential neurochemical regulation may render the PFC particularly vulnerable to dysfunction in our daily lives, and in a wide variety of neuropsychiatric disorders, particularly under conditions of repeated or uncontrollable stress. Finally, the enhancement of amygdala or hippocampal function could be important to improve the recollection of memories with strong survival value and thus help to be better equipped for similar, future experiences. However, as is the case of PTSD, overactivation of these traumatic memories can have very deleterious effects in our daily lives.
Figure 3.
Differential effects of the adrenergic system on peripheral versus central nervous systems. Epinephrine is released into blood by the adrenal gland in response to stress. NE is released by the sympathetic nervous system and throughout most of brain. Turning off PFC control of behavior during stress may have survival value under conditions of danger by switching control of our behavior from a slow, “reflective” region to more reflexive and instinctual brain areas. However, shutting off the PFC may make us more vulnerable to neuropsychiatric illness.
6. Noradrenergic biology: Peripheral versus Central Effects
A parallel to this process can be observed in the peripheral nervous system. Noradrenergic neurons contribute to the stress response in the periphery via the sympathetic nervous system, and epinephrine is released by the adrenal medulla. As can be seen in Figure 3, increases in epinephrine or norepinephrine can increase heart rate (via β1 receptors), enhance pulmonary function (via β2 receptors), and increase blood pressure (via α1 and β receptors) to increase the amount of oxygenated blood being delivered to striated muscle for the fight and flight response. At the same time, norepinephrine and epinephrine reduce digestive function via β3 receptors, as one can “stop digesting lunch in order to not become someone else’s lunch”. Thus, in the periphery, norepinephrine orchestrates physiological functions to switch us from a nonstress to a stressful state. A similar orchestration appears to occur in the central nervous system, where the PFC is the thoughtful, “digestive” organ that is strengthened by moderate levels of NE and shut off by stressful levels of NE release, while the sensor-motor and affective regions of brain are enhanced by higher levels of NE.
7. Conclusion
Understanding adrenergic pharmacology of PFC helps to develop therapeutic agents for mental illness. Indeed, symptoms such as poor concentration, impulsivity, working memory impairment, and inappropriate behaviors are common in mental illness and are thought to reflect PFC dysfunction. Furthermore, psychiatric disorders are influenced by stress, which, as has described above, impairs PFC cognitive function. For example, schizophrenia and affective disorder are often exacerbated or precipitated by stress exposure (reviewed in (Mazure, 1995). The current review described how stress can increase the release of catecholamines in both peripheral and central nervous systems, with a focus on NE’s effects on the PFC via its different adrenergic receptor subtypes (α1, α2, and β). α2 Receptors regulate working memory in a beneficial way during non-stressful moments, whereas α1 or β1 are engaged during stress to impair prefrontal cortical function. Moreover, many psychiatric disorders such as schizophrenia (Baldessarini et al., 1992), anxiety disorders such as PTSD (Krystal et al., 1996; Bremner et al., 1997; Southwick et al., 1997a; Southwick et al., 1997b), bipolar disorder (Post et al., 1973; Schildkraut, 1974; Young et al., 1994), and dementia (Gottfries et al., 1983; Lawlor et al., 1995; Elrod et al., 1997) are associated with high levels of NE turnover. Currently, adrenergic receptors, particularly the α1 and α2 receptor subtypes, are targets for the treatment of PTSD and ADHD, respectively. With further research, the β receptors may become part of the treatment repertoire for diseases with PFC dysfunction. Hence, greater understanding of the effects of norepinephrine on PFC function will greatly benefit the field of neuropsychiatry and could lead to better treatments for various mental illnesses.
Acknowledgments
This work was supported by the MERIT Award AG06036 to AFTA.
Abbreviations
- PFC
prefrontal cortex
- NE
norepinephrine
- LC
locus coeruleus
- EM
electron microscopic
- cAMP
cyclic adenosine monophosphate
- PKC
protein kinase C
- DA
dopamine
- 6-OHDA
6-hydroxy-dopamine
- rCBF
regional cerebral blood flow
- ADHD
attention-deficit hyperreactivity disorder
- DBH
dopamine beta hydroxylase
- PTSD
post-traumatic stress disorder
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication.As a service to our customers we are providing this early version of the manuscript.The manuscript will undergo copyediting, typesetting, and review of the resulting proofbefore it is published in its final citable form. Please note that during the productionprocess errorsmaybe discovered which could affect the content, and all legal disclaimersthat apply to the journal pertain.
References
- Albert MS. The ageing brain: normal and abnormal memory. Phil Trans R Soc Lond B. 1997;352:1703–1709. doi: 10.1098/rstb.1997.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki C, Go CG, Venkatesan C, Kurose H. Perikaryal and synaptic localization of alpha-2A-adrenergic receptor-like immunoreactivity. Brain Res. 1994;650:181–204. doi: 10.1016/0006-8993(94)91782-5. [DOI] [PubMed] [Google Scholar]
- Aoki C, Venkatesan C, Go CG, Forman R, Kurose H. Cellular and subcellular sites for noradrenergic action in the monkey dorsolateral prefrontal cortex as revealed by the immunocytochemical localization of noradrenergic receptors and axons. Cerebral Cortex. 1998a;8:269–277. doi: 10.1093/cercor/8.3.269. [DOI] [PubMed] [Google Scholar]
- Aoki C, Venkatesan C, Kurose H. Noradrenergic modulation of the prefrontal cortex as revealed by electron microscopic immunocytochemistry. Adv Pharmacol. 1998b;42:777–780. doi: 10.1016/s1054-3589(08)60862-5. [DOI] [PubMed] [Google Scholar]
- Arikuni T, Ban T. Subcortical afferents to the prefrontal cortex in rabbits. Exp Brain Res. 1978;32:69–75. doi: 10.1007/BF00237391. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Steere JC, Hunt RD. The contribution of alpha 2-noradrenergic mechanisms of prefrontal cortical cognitive function. Potential significance for attention-deficit hyperactivity disorder. Arch Gen Psychiatry. 1996;53:448–455. doi: 10.1001/archpsyc.1996.01830050084013. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT. Catecholamine regulation of the prefrontal cortex. J Psychopharmacology. 1997;11:151–162. doi: 10.1177/026988119701100208. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT. Stress impairs PFC function in rats and monkeys: Role of dopamine D1 and norepinephrine alpha-1 receptor mechanisms. Prog Brain Res. 2000a;126:183–192. doi: 10.1016/S0079-6123(00)26014-7. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT. Through the looking glass: Differential noradrenergic modulation of prefrontal cortical function. Neural Plasticity. 2000b;7:133–146. doi: 10.1155/NP.2000.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AFT, Cai JX, Goldman-Rakic PS. The alpha-2 adrenergic agonist guanfacine improves memory in aged monkeys without sedative or hypotensive side effects. J Neurosci. 1988;8:4287–4298. doi: 10.1523/JNEUROSCI.08-11-04287.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AFT, Contant TA. Alpha-2 adrenergic agonists decrease distractability in aged monkeys performing a delayed response task. Psychopharmacology. 1992;108:159–169. doi: 10.1007/BF02245302. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT, Goldman-Rakic PS. Selective prefrontal cortical projections to the region of the locus coeruleus and raphe nuclei in the rhesus monkey. Brain Res. 1984;306:9–18. doi: 10.1016/0006-8993(84)90351-2. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT, Goldman-Rakic PS. Alpha-2 adrenergic mechanisms in prefrontal cortex associated with cognitive decline in aged nonhuman primates. Science. 1985;230:1273–1276. doi: 10.1126/science.2999977. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT, Goldman-Rakic PS. Analysis of alpha-2 adrenergic agonist effects on the delayed nonmatch-to-sample performance of aged rhesus monkeys. Neurobiol Aging. 1990;11:583–590. doi: 10.1016/0197-4580(90)90021-q. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT, Mathew R, Ubriani R, Taylor JR, Li BM. Alpha-1 noradrenergic receptor stimulation impairs prefrontal cortical cognitive function. Biol Psychiatry. 1999;45:26–31. doi: 10.1016/s0006-3223(98)00296-0. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT, Robbins TW. Neurochemical modulation of prefrontal cortical function in humans and animals. In: Stuss DT, Knight RT, editors. Principles of Frontal Lobe Function. New York: Oxford University Press; 2002. pp. 51–84. [Google Scholar]
- Aston-Jones G, Rajkowski J, Cohen J. Role of locus coeruleus in attention and behavioral flexibility. Biological Psychiatry. 1999;46:1309–1320. doi: 10.1016/s0006-3223(99)00140-7. [DOI] [PubMed] [Google Scholar]
- Avery RA, Franowicz JS, Studholme C, van Dyck CH, Arnsten AFT. The alpha-2A-adenoceptor agonist, guanfacine, increases regional cerebral blood flow in dorsolateral prefrontal cortex of monkeys performing a spatial working memory task. Neuropsychopharmacology. 2000;23:240–249. doi: 10.1016/S0893-133X(00)00111-1. [DOI] [PubMed] [Google Scholar]
- Baldessarini RJ, Huston-Lyons D, Campbell A, Marsh E, Cohen BM. Do central antiadrenergic actions contribute to the atypical properties of clozapine? Br J Psychiatry. 1992;160(S17):12–16. [PubMed] [Google Scholar]
- Bartus RT, Fleming D, Johnson HR. Aging in the rhesus monkey: Debilitating effects on short-term memory. J Gerontol. 1978;33:858–871. doi: 10.1093/geronj/33.6.858. [DOI] [PubMed] [Google Scholar]
- Bebchuk JM, Arfken CL, Dolan-Manji S, Murphy J, Manji HK. A preliminary investigation of a protein kinase C inhibitor (Tamoxifen) in the treatment of acute mania. Arch Gen Psychiatry. 2000;57:95–97. doi: 10.1001/archpsyc.57.1.95. [DOI] [PubMed] [Google Scholar]
- Birnbaum SB, Yuan P, Bloom A, Davis D, Gobeske K, Sweatt D, et al. Protein kinase C overactivity impairs prefrontal cortical regulation of working memory. Science. 2004;306:882–884. doi: 10.1126/science.1100021. [DOI] [PubMed] [Google Scholar]
- Birnbaum SG, Gobeske KT, Auerbach J, Taylor JR, Arnsten AFT. A role for norepinephrine in stress-induced cognitive deficits: Alpha-1-adrenoceptor mediation in prefrontal cortex. Biol Psychiatry. 1999;46:1266–1274. doi: 10.1016/s0006-3223(99)00138-9. [DOI] [PubMed] [Google Scholar]
- Boon-yasidhi V, Kim YS, Scahill L. An open-label, prospective study of guanfacine in children with ADHD and tic disorders. J Med Assoc Thai. 2005;88:S156–162. [PubMed] [Google Scholar]
- Breier A, Wolkowitz O, Pickar D. Stress and schizophrenia: Advances in neuropsychiatry and psychopharmacology. In: Tamminga C, Schult S, editors. Schizophrenia Research. New York: Raven Press, Ltd; 1991. [Google Scholar]
- Bremner JD. Neuroimaging Studies in Post-traumatic Stress Disorder. Curr Psychiatry Rep. 2002;4:254–263. doi: 10.1007/s11920-996-0044-9. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Innis RB, Ng CK, Staib LH, Salomon RM, Bronen RA, et al. Positron emission tomography measurement of cerebral metabolic correlates of yohimbine administration in combat-related posttraumatic stress disorder. Arch Gen Psychiatry. 1997;54:246–254. doi: 10.1001/archpsyc.1997.01830150070011. [DOI] [PubMed] [Google Scholar]
- Brozoski T, Brown RM, Rosvold HE, Goldman PS. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science. 1979;205:929–931. doi: 10.1126/science.112679. [DOI] [PubMed] [Google Scholar]
- Cahill L, McGaugh JL. Modulation of memory storage. Current Opinion Neurobiol. 1996;6:237–242. doi: 10.1016/s0959-4388(96)80078-x. [DOI] [PubMed] [Google Scholar]
- Cai JX, Ma Y, Xu L, Hu X. Reserpine impairs spatial working memory performance in monkeys: Reversal by the alpha-2 adrenergic agonist clonidine. Brain Res. 1993;614:191–196. doi: 10.1016/0006-8993(93)91034-p. [DOI] [PubMed] [Google Scholar]
- Carli M, Robbins TW, Evenden JL, Everitt BJ. Effects of lesions to ascending noradrenergic neurons on performance of a 5-choice serial reaction task in rats; implications for theories of dorsal noradrenergic bundle function based on selective attention and arousal. Behav Brain Res. 1983;9:361–380. doi: 10.1016/0166-4328(83)90138-9. [DOI] [PubMed] [Google Scholar]
- Carlson S, Tanila H, Rama P, Mecke E, Pertovaara A. Effects of medetomidine, an alpha-2 adrenoceptor agonist, and atipamezole, an alpha-2 antagonist, on spatial memory performance in adult and aged rats. Behav Neural Biol. 1992;58:113–119. doi: 10.1016/0163-1047(92)90327-z. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Muller U, Blackwell AD, Clark L, Robbins TW, Sahakian BJ. Neurochemical modulation of response inhibition and probabilistic learning in humans. Science. 2006;311:861–863. doi: 10.1126/science.1121218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao LL, Knight RT. Age-related prefrontal alterations during auditory memory. Neurobiol Aging. 1997;18:87–95. doi: 10.1016/s0197-4580(96)00161-3. [DOI] [PubMed] [Google Scholar]
- Chappell PB, Riddle MA, Scahill L, Lynch KA, Schultz R, Arnsten A, et al. Guanfacine treatment of comorbid attention deficit hyperactivity disorder and Tourette’s Syndrome: Preliminary clinical experience. J Amer Acad Child Adol Psychiat. 1995;34:1140–1146. doi: 10.1097/00004583-199509000-00010. [DOI] [PubMed] [Google Scholar]
- Chaulk PC, Harley CW. Intracerebroventricular norepinephrine potentiation of the perforant path-evoked potential in dentate gyrus of anesthetized and awake rats: A role for both alpha- and beta-adrenoceptor activation. Brain Res. 1998;787:59–70. doi: 10.1016/s0006-8993(97)01460-1. [DOI] [PubMed] [Google Scholar]
- Clark CR, Geffen GM, Geffen LB. Catecholamines and attention II: Pharmacological studies in normal humans. Neurosci Biobehav Reviews. 1987;11:353–364. doi: 10.1016/s0149-7634(87)80007-6. [DOI] [PubMed] [Google Scholar]
- Clark L, Roiser JP, Cools R, Rubinsztein DC, Sahakian BJ, Robbins TW. Stop signal response inhibition is not modulated by tryptophan depletion or the serotonin transporter polymorphism in healthy volunteers: implications for the 5-HT theory of impulsivity. Psychopharmacology (Berl) 2005;182:570–578. doi: 10.1007/s00213-005-0104-6. [DOI] [PubMed] [Google Scholar]
- Cole BJ, Robbins TW. Forebrain norepinephrine: role in controlled information processing in the rat. Neuropsychopharmacology. 1992;7:129–142. [PubMed] [Google Scholar]
- Collins P, Roberts AC, Dias R, Everitt BJ, Robbins TW. Perseveration and strategy in a novel spatial self-ordered sequencing task for nonhuman primates: effects of excitotoxic lesions and dopamine depletions of the prefrontal cortex. J Cognitive Neuroscience. 1998;10:332–354. doi: 10.1162/089892998562771. [DOI] [PubMed] [Google Scholar]
- Constantinidis C, Williams GV, Goldman-Rakic PS. A role for inhibition in shaping the temporal flow of information in prefrontal cortex. Nat Neurosci. 2002;5:175–180. doi: 10.1038/nn799. [DOI] [PubMed] [Google Scholar]
- Coull JT. Pharmacological manipulations of the a-2 noradrenergic system: Effects on cognition. Drugs and Aging. 1994;5:116–126. doi: 10.2165/00002512-199405020-00005. [DOI] [PubMed] [Google Scholar]
- Coull JT, Buchel C, Friston KJ, Frith CD. Noradrenergically mediated plasticity in a human attentional neuronal network. Neuroimage. 1999;10:705–715. doi: 10.1006/nimg.1999.0513. [DOI] [PubMed] [Google Scholar]
- Coull JT, Frith CD, Dolan RJ, Frackowiak RS, Grasby PM. The neural correlates of the noradrenergic modulation of human attention, arousal and learning. Eur J Neurosci. 1997;9:589–598. doi: 10.1111/j.1460-9568.1997.tb01635.x. [DOI] [PubMed] [Google Scholar]
- Crofts HS, Dalley JW, Collins P, van Denderen JCM, Everitt BJ, Robbins TW, et al. Differential effects of 6-OHDA lesions of the prefrontal cortex and caudate nucleus on the ability to acquire an attentional set. Cerebral Cortex. 2001;11:1015–1026. doi: 10.1093/cercor/11.11.1015. [DOI] [PubMed] [Google Scholar]
- Day HE, Campeau S, Watson SJJ, Akil H. Distribution of alpha 1a-, alpha 1b- and alpha 1d-adrenergic receptor mRNA in the rat brain and spinal cord. J Chem Neuroanat. 1997;13:115–139. doi: 10.1016/s0891-0618(97)00042-2. [DOI] [PubMed] [Google Scholar]
- De Santi S, de Leon MJ, Convit A, Tarshish C, Rusinek H, Tsui WH, et al. Age-related changes in brain: II. Positron emission tomography of frontal and temporal lobe glucose metabolism in normal subjects. Psychiatr Q. 1995;66:357–370. doi: 10.1007/BF02238755. [DOI] [PubMed] [Google Scholar]
- Dohrenwend BP, Shrout PE, Link BG, Skodol AE, Stueve A. Life events and other possible risk factors for episodes of schizophrenia and major depression: a case-control study. In: Mazure CM, editor. Does stress cause psychiatric illness? Washington DC; American Psychiatric Press: 1995. pp. 43–65. [Google Scholar]
- Duman RS, Nestler EJ. Signal transduction pathways for catecholamine receptors. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: The Fourth Generation of Progress. N.Y: Raven Press; 1995. pp. 303–320. [Google Scholar]
- Eberling JL, Roberts JA, Rapp PR, Tuszynski MH, Jagust WJ. Cerebral glucose metabolism and memory in aged rhesus macaques. Neurobiol Aging. 1997;18:437–443. doi: 10.1016/s0197-4580(97)00040-7. [DOI] [PubMed] [Google Scholar]
- Elrod R, Peskind ER, DiGiacomo L, Brodkin KI, Veith RC, Raskind MA. Effects of Alzheimer’s disease severity on cerebrospinal fluid norepinephrine concentration. Am J Psychiat. 1997;154:25–30. doi: 10.1176/ajp.154.1.25. [DOI] [PubMed] [Google Scholar]
- Engberg G, Eriksson E. Effects of alpha-2-adrenoceptor agonists on locus coeruleus firing rate and brain noradrenaline turnover in EEDQ-treated rats. Naunyn-Schmiedebergs Arch Pharmacol. 1991;343:472–477. doi: 10.1007/BF00169548. [DOI] [PubMed] [Google Scholar]
- Erdely HA, Tamminga CA, Roberts RC, Vogel MW. Regional alterations in RGS4 protein in schizophrenia. Synapse. 2006;59:472–479. doi: 10.1002/syn.20265. [DOI] [PubMed] [Google Scholar]
- Ferry B, McGaugh JL. Clenbuterol administration into the basolateral amygdala post-training enhances retention in an inhibitory avoidance task. Neurobiol Learn Mem. 1999;72:8–12. doi: 10.1006/nlme.1998.3904. [DOI] [PubMed] [Google Scholar]
- Ferry B, Roozendaal B, McGaugh JL. Basolateral amygdala noradrenergic influences on memory storage are mediated by an interaction between beta- and alpha-1-adrenoceptors. J Neurosci. 1999a;19:5119–5123. doi: 10.1523/JNEUROSCI.19-12-05119.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry B, Roozendaal B, McGaugh JL. Involvement of alpha-1-adrenoceptors in the basolateral amygdala in modulation of memory storage. Eur J Pharmacol. 1999b;372:9–16. doi: 10.1016/s0014-2999(99)00169-7. [DOI] [PubMed] [Google Scholar]
- Fields RB, Van Kammen DP, Peters JL, Rosen J, Van Kammen WB, Nugent A, et al. Clonidine improves memory function in schizophrenia independently from change in psychosis. Schiz Res. 1988;1:417–423. doi: 10.1016/0920-9964(88)90024-2. [DOI] [PubMed] [Google Scholar]
- Fillenz M, Lowry JP. Studies of the source of glucose in the extracellular compartment of the rat brain. Dev Neurosci. 1998;20:365–368. doi: 10.1159/000017332. [DOI] [PubMed] [Google Scholar]
- Fillenz M, Lowry JP, Boutelle MG, Fray AE. The role of astrocytes and noradrenaline in neuronal glucose metabolism. Acta Physiol Scand. 1999;167:275–284. doi: 10.1046/j.1365-201x.1999.00578.x. [DOI] [PubMed] [Google Scholar]
- Finlay JM, Zigmond MJ, Abercrombie ED. Increased dopamine and norepinephrine release in medial prefrontal cortex induced by acute and chronic stress: effects of diazepam. Neuroscience. 1995;64:619–628. doi: 10.1016/0306-4522(94)00331-x. [DOI] [PubMed] [Google Scholar]
- Foote SL, Aston-Jones G, Bloom FE. Impulse activity of locus coeruleus neurons in awake rats and monkeys is a function of sensory stimulation and arousal. PNAS. 1980;77:3033–3037. doi: 10.1073/pnas.77.5.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franowicz JCS, Arnsten AFT. The alpha-2A noradrenergic agonist, guanfacine, improves delayed response performance in young adult rhesus monkeys. Psychopharmacol. 1998;136:8–14. doi: 10.1007/s002130050533. [DOI] [PubMed] [Google Scholar]
- Franowicz JS, Arnsten AFT. Treatment with the noradrenergic alpha-2 agonist clonidine, but not diazepam, improves spatial working memory in normal young rhesus monkeys. Neuropsychopharmacology. 1999;21:611–621. doi: 10.1016/S0893-133X(99)00060-3. [DOI] [PubMed] [Google Scholar]
- Franowicz JS, Kessler L, Morgan C, Arnsten AFT. The alpha-2 noradrenergic agonist, guanfacine, improves spatial working memory in wild type mice but not mice with point mutations of the gene for the alpha-2A receptor. Soc Neurosci Abstracts. 1998;24:711. [Google Scholar]
- Friedman JI, Adler DN, Davis KL. The role of norepinephrine in the pathophysiology of cognitive disorders: potential applications to the treatment of cognitive dysfunction in schizophrenia and Alzheimer’s disease. Biological Psychiatry. 1999;46:1243–1252. doi: 10.1016/s0006-3223(99)00232-2. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Clavier RM. Neural inputs to the prefrontal agranular insular cortex in the rat: horseradish peroxidase study. Brain Res Bull. 1979;4:347–353. doi: 10.1016/s0361-9230(79)80012-x. [DOI] [PubMed] [Google Scholar]
- Goldman PS, Rosvold HE. Localization of function within the dorsolateral prefrontal cortex of the rhesus monkey. Exp Neurol. 1970;27:291–304. doi: 10.1016/0014-4886(70)90222-0. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Lidow MS, Gallager DW. Overlap of dopaminergic, adrenergic, and serotonergic receptors and complementarity of their subtypes in primate prefrontal cortex. J Neurosci. 1990;10:2125–2138. doi: 10.1523/JNEUROSCI.10-07-02125.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein LE, Rasmusson AM, Bunney SB, Roth RH. Role of the amygdala in the coordination of behavioral, neuroendocrine and prefrontal cortical monoamine responses to psychological stress in the rat. J Neurosci. 1996;16:4787–4798. doi: 10.1523/JNEUROSCI.16-15-04787.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfries CG, Adolfsson R, Aquilonius SM, Carlsson A, Eckernas SA, Nordberg A, et al. Biochemical changes in dementia disorders of Alzheimer’s Type (AD/SDAT) Neurobiol Aging. 1983;4:261–271. doi: 10.1016/0197-4580(83)90002-7. [DOI] [PubMed] [Google Scholar]
- Hammen C, Gitlin M. Stress reactivity in bipolar patients and its relation to prior history of disorder. American Journal of Psychiatry. 1997;154:856–857. doi: 10.1176/ajp.154.6.856. [DOI] [PubMed] [Google Scholar]
- Hansson E, Ronnback L. Receptor regulation of the glutamate, GABA and taurine high-affinity uptake into astrocytes in primary culture. Brain Res. 1991;548:215–221. doi: 10.1016/0006-8993(91)91124-j. [DOI] [PubMed] [Google Scholar]
- Hieble JP, Bylund DB, Clarke DE, Eikenburg DC, Langer SZ, Lefkowitz RJ, et al. International Union of Pharmacology. X. Recommendation for nomenclature of alpha 1-adrenoceptors: consensus update. Pharmacol Rev. 1995;47:267–270. [PubMed] [Google Scholar]
- Horrigan JP. Guanfacine for PTSD nightmares. J Amer Acad Child Adol Psychiatry. 1996;35:975–976. doi: 10.1097/00004583-199608000-00006. [DOI] [PubMed] [Google Scholar]
- Horrigan JP, Barnhill LJ. Guanfacine for treatment of Attention-Deficit-Hyperactivity Disorder in boys. J Child Adolescent Psychopharmacol. 1995;5:215–223. [Google Scholar]
- Hunt RD, Arnsten AFT, Asbell MD. An open trial of guanfacine in the treatment of attention deficit hyperactivity disorder. J Amer Acad Child Adoles Psychiatry. 1995;34:50–54. doi: 10.1097/00004583-199501000-00013. [DOI] [PubMed] [Google Scholar]
- Hunt RD, Mindera RB, Cohen DJ. Clonidine benefits children with Attention Deficit Disorder and Hyperactivity: Reports of a double-blind placebo-crossover therapeutic trial. J Amer Acad Child Psychiatry. 1985;24:617–629. doi: 10.1016/s0002-7138(09)60065-0. [DOI] [PubMed] [Google Scholar]
- Insel PA. Adrenergic receptors, G proteins, and cell regulation: implications for aging research. Exp Gerontol. 1993;28:341–348. doi: 10.1016/0531-5565(93)90061-h. [DOI] [PubMed] [Google Scholar]
- Introini-Collison IB, Miyazaki B, McGaugh JL. Involvement of the amygdala in the memory-enhancing effects of clenbuterol. Psychopharmacology. 1991;104:541–544. doi: 10.1007/BF02245663. [DOI] [PubMed] [Google Scholar]
- Jackson WJ, Buccafusco JJ. Clonidine enhances delayed matching-to-sample performance by young and aged monkeys. Pharmacol Biochem Behav. 1991;39:79–84. doi: 10.1016/0091-3057(91)90400-v. [DOI] [PubMed] [Google Scholar]
- Jakala P, Riekkinen M, Sirvio J, Koivisto E, Kejonen K, Vanhanen M, et al. Guanfacine, but not clonidine, improves planning and working memory performance in humans. Neuropsychopharmacology. 1999a;20:460–470. doi: 10.1016/S0893-133X(98)00127-4. [DOI] [PubMed] [Google Scholar]
- Jakala P, Sirvio J, Riekkinen M, Koivisto E, Kejonen K, Vanhanen M, et al. Guanfacine and clonidine, alpha-2 agonists, improve paired associates learning, but not delayed matching to sample, in humans. Neuropsychopharmacol. 1999b;20:119–130. doi: 10.1016/S0893-133X(98)00055-4. [DOI] [PubMed] [Google Scholar]
- Jodo E, Chiang C, Aston-Jones G. Potent excitatory influence of prefrontal cortex activity on noradrenergic locus coeruleus neurons. Neuroscience. 1998;83:63–79. doi: 10.1016/s0306-4522(97)00372-2. [DOI] [PubMed] [Google Scholar]
- Korol DL, Gold PE. Glucose, memory, and aging. Am J Clin Nutr. 1998;67:764–771. doi: 10.1093/ajcn/67.4.764S. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Deutsch DN, Charney DS. The biological basis of panic disorder. Clin Psychiatry. 1996;57(S10):23–31. [PubMed] [Google Scholar]
- Kulkarni J, Garland KA, Scaffidi A, Headey B, Anderson R, de Castella A, et al. A pilot study of hormone modulation as a new treatment for mania in women with bipolar affective disorder. Psychoneuroendocrinology. 2006;31:543–547. doi: 10.1016/j.psyneuen.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Lacaille JC, Harley CW. The action of norepinephrine in the dentate gyrus: beta-mediated facilitation of evoked potentials in vitro. Brain Res. 1985;358:210–220. doi: 10.1016/0006-8993(85)90965-5. [DOI] [PubMed] [Google Scholar]
- Lawlor BA, Bierer LM, Ryan TM, Schmeidler J, Knott PJ, Williams LL, et al. Plasma MHPG and clinical symptoms in Alzheimer’s disease. Biol Psychiatry. 1995;38:185–188. doi: 10.1016/0006-3223(94)00259-6. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Morrison JH. Noradrenergic innervation of monkey prefrontal cortex: a dopamine-beta-hydroxylase immunohistochemical study. J Comp Neurol. 1989;282:317–330. doi: 10.1002/cne.902820302. [DOI] [PubMed] [Google Scholar]
- Li BM, Mao ZM, Wang M, Mei ZT. Alpha-2 adrenergic modulation of prefrontal cortical neuronal activity related to spatial working memory in monkeys. Neuropsychopharmacol. 1999;21:601–610. doi: 10.1016/S0893-133X(99)00070-6. [DOI] [PubMed] [Google Scholar]
- Li BM, Mei ZT. Delayed response deficit induced by local injection of the alpha-2 adrenergic antagonist yohimbine into the dorsolateral prefrontal cortex in young adult monkeys. Behav Neural Biol. 1994;62:134–139. doi: 10.1016/s0163-1047(05)80034-2. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Mitkus S, Caruso M, Hyde TM, Chen JG, Vakkalanka R, et al. RGS4 mRNA Expression in Postmortem Human Cortex Is Associated with COMT Val158Met Genotype and COMT Enzyme Activity. Hum Mol Gent Epub. 2006 doi: 10.1093/hmg/ddl222. [DOI] [PubMed] [Google Scholar]
- Luine V, Bowling D, Hearns M. Spatial memory deficits in aged rats: contributions of monoaminergic systems. Brain Res. 1990;537:271–278. doi: 10.1016/0006-8993(90)90368-l. [DOI] [PubMed] [Google Scholar]
- Ma CL, Qi XL, Peng JY, Li BM. Selective deficit in no-go performance induced by blockade of prefrontal cortical alpha 2-adrenoceptors in monkeys. Neuroreport. 2003;14:1013–6. doi: 10.1097/01.wnr.0000070831.57864.7b. [DOI] [PubMed] [Google Scholar]
- Ma CL, Arnsten AF, Li BM. Locomotor hyperactivity induced by blockade of prefrontal cortical alpha2-adrenoceptors in monkeys. Biol Psychiatry. 2005;57:192–5. doi: 10.1016/j.biopsych.2004.11.004. [DOI] [PubMed] [Google Scholar]
- MacDonald E, Kobilka BK, Scheinin M. Gene targeting--homing in on alpha 2-adrenoceptor-subtype function. Trends Pharmacol Sci. 1997;18:211–219. doi: 10.1016/s0165-6147(97)01063-8. [DOI] [PubMed] [Google Scholar]
- Mair RG, McEntree WJ. Cognitive enhancement in Korsakoff’s psychosis by clonidine: A comparison with 1-dopa and ephedrine. Psychopharmacology. 1986;88:374–380. doi: 10.1007/BF00180841. [DOI] [PubMed] [Google Scholar]
- Manji HK, Lenox RH. Protein kinase C signaling in the brain: Molecular transduction of mood stabilization in the treatment of manic-depressive illness. Biological Psychiatry. 1999;46:1328–1351. doi: 10.1016/s0006-3223(99)00235-8. [DOI] [PubMed] [Google Scholar]
- Mao ZM, Arnsten AFT, Li BM. Local infusion of alpha-1 adrenergic agonist into the prefrontal cortex impairs spatial working memory performance in monkeys. Biol Psychiatry. 1999;46:1259–1265. doi: 10.1016/s0006-3223(99)00139-0. [DOI] [PubMed] [Google Scholar]
- Mazure CM, editor. Does stress cause psychiatric illness? Washington, DC: American Psychiatric Press; 1995. [Google Scholar]
- McAllister TW, Flashman LA, Sparling MB, Saykin AJ. Working memory deficits after traumatic brain injury: catecholaminergic mechanisms and prospects for treatment -- a review. Brain Inj. 2004;18:331–350. doi: 10.1080/02699050310001617370. [DOI] [PubMed] [Google Scholar]
- Mirnics K, Middleton FA, Stanwood GD, Lewis DA, Levitt P. Disease-specific changes in regulator of G-protein signaling 4 (RGS4) expression in schizophrenia. Mol Psychiatry. 2001;6:293–301. doi: 10.1038/sj.mp.4000866. [DOI] [PubMed] [Google Scholar]
- Moffoot A, O’Carroll RE, Murray C, Dougall N, Ebmeier K, Goodwin GM. Clonidine infusion increases uptake of Tc-exametazime in anterior cingulate cortex in Korsakoff’s psychosis. Psycholog Med. 1994;24:53–61. doi: 10.1017/s0033291700026829. [DOI] [PubMed] [Google Scholar]
- Mohell N, Svartengren J, Cannon B. Identification of [3H]prazosin binding sites in crude membranes and isolated cells of brown adipose tissue as alpha-1 adrenergic receptors. Eur J Pharmacology. 1983;92:15–25. doi: 10.1016/0014-2999(83)90103-6. [DOI] [PubMed] [Google Scholar]
- Mohr E, Schlegel J, Fabbrini G, Williams J, Mouradian M, Mann UM, et al. Clonidine treatment of Alzheimer’s Disease. Arch Neurol. 1989;46:376–378. doi: 10.1001/archneur.1989.00520400030015. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Foote SL, O’Connor D, Bloom FE. Laminar, tangential and regional organization of the noradrenergic innervation of monkey cortex: dopamine-beta-hydroxylase immunohistochemistry. Brain Res Bull. 1982;9:309–319. doi: 10.1016/0361-9230(82)90144-7. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Molliver ME, Grzanna R. Noradrenergic innervation of cerebral cortex: widespread effects of local cortical lesions. Science. 1979;205:313–316. doi: 10.1126/science.451605. [DOI] [PubMed] [Google Scholar]
- Mouradian RD, Seller FM, Waterhouse BD. Noradrenergic potentiation of excitatory transmitter action in cerebrocortical slices: evidence of mediation by an alpha1-receptor-linked second messenger pathway. Brain Res. 1991;546:83–95. doi: 10.1016/0006-8993(91)91162-t. [DOI] [PubMed] [Google Scholar]
- Muller U, Clark L, Lam ML, Moore RM, Murphy CL, Richmond NK, et al. Lack of effects of guanfacine on executive and memory functions in healthy male volunteers. Psychopharmacology (Berl) 2005;182:205–213. doi: 10.1007/s00213-005-0078-4. [DOI] [PubMed] [Google Scholar]
- Nicholas AP, Pieribone VA, Hokfelt T. Cellular localization of messenger RNA for beta-1 and beta-2 adrenergic receptors in rat brain: an in situ hybridization study. Neuroscience. 1993;56:1023–1039. doi: 10.1016/0306-4522(93)90148-9. [DOI] [PubMed] [Google Scholar]
- Nielsen-Bohlman L, Knight RT. Prefrontal alterations during memory processing in aging. Cerebral Cortex. 1995;5:541–549. doi: 10.1093/cercor/5.6.541. [DOI] [PubMed] [Google Scholar]
- Noda A, Ohba H, Kakiuchi T, Futatsubashi M, Tsukada H, Nishimura S. Age-related changes in cerebral blood flow and glucose metabolism in conscious rhesus monkeys. Brain Res. 2002;936:76–81. doi: 10.1016/s0006-8993(02)02558-1. [DOI] [PubMed] [Google Scholar]
- O’Rourke MF, Blaxall HS, Iversen LJ, Bylund DB. Characterization of [3H]RX821002 binding to alpha-2 adrenergic receptor subtypes. J Pharmacol Exp Ther. 1994;268:1362–1367. [PubMed] [Google Scholar]
- Ordway GA, O’Donnell JM, Frazer A. Effects of clenbuterol on Beta-1 and Beta-2 adrenergic receptors of the rat. J of Pharm and Exp Ther. 1987;241:187–195. [PubMed] [Google Scholar]
- Peskind ER, Bonner LT, Hoff DJ, Raskind MA. Prazosin reduces trauma-related nightmares in older men with chronic posttraumatic stress disorder. J Geriatr Psychiatry Neurol. 2003;16:165–171. doi: 10.1177/0891988703256050. [DOI] [PubMed] [Google Scholar]
- Pieribone VA, Nicholas AP, Dagerlind A, Hokfelt T. Distribution of alpha1 adrenoceptors in rat brain revealed by in situ hybridization experiments utilizing subtype-specific probes. J Neurosci. 1994;14:4252–4268. doi: 10.1523/JNEUROSCI.14-07-04252.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrino LJ, Goldman-Rakic PS. Brainstem innervation of prefrontal and anterior cingulate cortex in the rhesus monkey revealed by retrograde transport of HRP. J Comp Neurol. 1982;205:63–76. doi: 10.1002/cne.902050107. [DOI] [PubMed] [Google Scholar]
- Posner MI, Walker JA, Friedrich FJ, Rafal RD. Effects of parietal injury on covert orienting of visual attention. J Neurosci. 1984;4:1863–1874. doi: 10.1523/JNEUROSCI.04-07-01863.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post RM, Gordon EK, Goodwin FK, Bunney WE. Central norepinephrine metabolism in affective illness: MHPG in the cerebrospinal fluid. Science. 1973;179:1002–1003. doi: 10.1126/science.179.4077.1002. [DOI] [PubMed] [Google Scholar]
- Prasad KM, Chowdari KV, Nimgaonkar VL, Talkowski ME, Lewis DA, Keshavan MS. Genetic polymorphisms of the RGS4 and dorsolateral prefrontal cortex morphometry among first episode schizophrenia patients. Mol Psychiatry. 2004 doi: 10.1038/sj.mp.4001562. Sep 21 Epub. [DOI] [PubMed] [Google Scholar]
- Rainbow TC, Parsons B, Wolfe BB. Quantitative autoradiography of beta1- and beta2-adrenergic receptors in rat brain. Proc Natl Acad Sci USA. 1984;81:1585–1589. doi: 10.1073/pnas.81.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowski J, Kubiak P, Inanova S, Aston-Jones G. State-related activity, reactivity of locus coeruleus neurons in behaving monkeys. Advances in Pharmacol. 1998;42:740–744. doi: 10.1016/s1054-3589(08)60854-6. [DOI] [PubMed] [Google Scholar]
- Rama P, Linnankoski I, Tanila H, Pertovaara A, Carlson S. Medetomidine, atipamezole, and guanfacine in delayed response performance of aged monkeys. Pharmacol Biochem Behav. 1996;54:1–7. doi: 10.1016/s0091-3057(96)00111-6. [DOI] [PubMed] [Google Scholar]
- Ramos B, Birnbaum SB, Lindenmayer I, Newton SS, Duman R, Arnsten AFT. Dysregulation of protein kinase A signaling in the aged prefrontal cortex: New strategy for treating age-related cognitive decline. Neuron. 2003;40:835–845. doi: 10.1016/s0896-6273(03)00694-9. [DOI] [PubMed] [Google Scholar]
- Ramos B, Colgan L, Nou E, Arnsten AFT. The b1 adrenergic antagonist, betaxolol, improves working memory performance in rats and monkeys. Biological Psychiatry (Online) 2005 doi: 10.1016/j.biopsych.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Ramos BP, Stark D, Verduzco L, van Dyck CH, Arnsten AFT. 2A-Adrenoceptor Stimulation Improves Prefrontal Cortical Regulation of Behavior Through Inhibition of cAMP Signaling in Aging Animals. Learning and Memory. 2006a doi: 10.1101/lm.298006. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos BP, Colgan LA, Nou E, Arnsten AFT. The β2 Adrenergic Agonist, Clenbuterol, Can Enhance Working Memory Performance in Aging Rats and Monkeys. 2006b doi: 10.1016/j.neurobiolaging.2007.02.003. In submission. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SG, Williams GV, Goldman-Rakic PS. Destruction and creation of spatial tuning by disinhibition: GABA(A) blockade of prefrontal cortical neurons engaged by working memory. J Neurosci. 2000;20:485–494. doi: 10.1523/JNEUROSCI.20-01-00485.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskind MA, Dobie DJ, Kanter ED, Petrie EC, Thompson CE, Peskind ER. The alpha1-adrenergic antagonist prazosin ameliorates combat trauma nightmares in veterans with posttraumatic stress disorder: a report of 4 cases. J Clin Psychiatry. 2000;61:129–133. doi: 10.4088/jcp.v61n0208. [DOI] [PubMed] [Google Scholar]
- Raskind MA, Peskind ER, Kanter ED, Petrie EC, Radant A, Thompson C, et al. Prazosin reduces nightmares and other PTSD symptoms in combat veterans: A placebo-controlled study. Am J Psychiatry. 2003;160:371–373. doi: 10.1176/appi.ajp.160.2.371. [DOI] [PubMed] [Google Scholar]
- Riekkinen M, Riekkinen PJ. Alpha2-adrenergic agonist clonidine for improving spatial working memory in Parkinson’s disease. J Clin Psychopharmacol. 1999a;19:444–449. doi: 10.1097/00004714-199910000-00008. [DOI] [PubMed] [Google Scholar]
- Riekkinen P, Riekkinen M. THA improves word priming and clonidine enhances fluency and working memory in Alzheimer’s Disease. Neuropsychopharmacol. 1999b;20:357–364. doi: 10.1016/S0893-133X(98)00093-1. [DOI] [PubMed] [Google Scholar]
- Roberts AC, Salvia MA, Wilkinson LS, Collins P, Muir JL, Everitt BJ, et al. 6-Hydroxydopamine lesions of the prefrontal cortex in monkeys enhance performance on an analog of the Wisconsin Card Sort Test: Possible interactions with subcortical dopamine. Neurosci. 1994;14:2531–2544. doi: 10.1523/JNEUROSCI.14-05-02531.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roullet P, Sara S. Consolidation of memory after its reactivation: involvement of beta noradrenergic receptors in the late phase. Neural Plast. 1998;6:63–68. doi: 10.1155/NP.1998.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runyan JD, Moore AN, Dash PK. A role for prefrontal calcium-sensitive protein phosphatase and kinase activities in working memory. Learning and Memory. 2005;12:103–110. doi: 10.1101/lm.89405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahakian BJ, Sarna GS, Kantamaneni BD, Jackson A, Hutson PH, Curzon G. Association between learning and cortical catecholamines in non-drug-treated rats. Psychopharmacol. 1985;86:339–343. doi: 10.1007/BF00432225. [DOI] [PubMed] [Google Scholar]
- Sallinen J, Haapalinna A, MacDonald E, Viitamaa T, Lahdesmaki J, Rybnikova E, et al. Genetic alteration of the alpha2-adrenoceptor subtype c in mice affects the development of behavioral despair and stress-induced increases in plasma corticosterone levels. Molecular Psychiatry. 1999;4:443–452. doi: 10.1038/sj.mp.4000543. [DOI] [PubMed] [Google Scholar]
- Sara SJ, Herve-Minvielle A. Inhibitory influence of frontal cortex on locus coeruleus. Proc Nat Acad Sci USA. 1995;92:6032–6036. doi: 10.1073/pnas.92.13.6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara SJ, Roullet P, Przybyslawski J. Consolidation of memory for odor-reward association: beta-adrenergic receptor involvement in the late phase. Learn Mem. 1999;6:88–96. [PMC free article] [PubMed] [Google Scholar]
- Sawaguchi T. Attenuation of delay-period activity of monkey prefrontal cortical neurons by an alpha-2 adrenergic antagonist during an oculomotor delayed-response task. J Neurophysiology. 1998;80:2200–2205. doi: 10.1152/jn.1998.80.4.2200. [DOI] [PubMed] [Google Scholar]
- Scahill L, Chappell PB, Kim YS, Schultz RT, Katsovich L, Shepherd E, et al. Guanfacine in the treatment of children with tic disorders and ADHD: A placebo-controlled study. Amer J Psychiatry. 2001;158:1067–1074. doi: 10.1176/appi.ajp.158.7.1067. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Savage CR, Alpert NM, Rauch SL, Albert MS. The role of hippocampus and frontal cortex in age-related memory changes: a PET study. Neuroreport. 1996;7:1165–1169. doi: 10.1097/00001756-199604260-00014. [DOI] [PubMed] [Google Scholar]
- Schildkraut JJ. Biogenic amines and affective disorders. Annu Rev Med. 1974;25:333–348. doi: 10.1146/annurev.me.25.020174.002001. [DOI] [PubMed] [Google Scholar]
- Simon H. Dopaminergic A10 neurons and the frontal system. J de Physiol. 1981;77:81–95. [PubMed] [Google Scholar]
- Sirviö J, Riekkinen P, Vajanto I, Koivisto E, Riekkinnen PJ. The effects of guanfacine, alpha-2 agonist, on the performance of young and aged rats in spatial navigation task. Behav Neural Biol. 1991;56:101–107. doi: 10.1016/0163-1047(91)90327-m. [DOI] [PubMed] [Google Scholar]
- Smith KM, Daly M, Fischer M, Yiannoutsos CT, Bauer L, Barkley R, et al. Association of the dopamine beta hydroxylase gene with attention deficit hyperactivity disorder: genetic analysis of the Milwaukee longitudinal study. Am J Med Genet B Neuropsychiatr Genet. 2003;119:77–85. doi: 10.1002/ajmg.b.20005. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Krystal JH, Bremner JD, Morgan CAr, Nicolaou AL, Nagy LM, et al. Noradrenergic and serotonergic function in posttraumatic stress disorder. Arch Gen Psychiatry. 1997a;54:749–758. doi: 10.1001/archpsyc.1997.01830200083012. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Morgan CAr, Bremner JD, Grillon CG, Krystal JH, Nagy LM, et al. Noradrenergic alterations in posttraumatic stress disorder. Ann NY Acad Sci. 1997b;821:125–141. doi: 10.1111/j.1749-6632.1997.tb48274.x. [DOI] [PubMed] [Google Scholar]
- Steere JC, Arnsten AFT. The alpha-2A noradrenergic agonist, guanfacine, improves visual object discrimination reversal performance in rhesus monkeys. Behav Neurosci. 1997;111:1–9. doi: 10.1037//0735-7044.111.5.883. [DOI] [PubMed] [Google Scholar]
- Summers RJ, Papaioannou M, Harris S, Evans BA. Expression of beta 3-adrenoceptor mRNA in rat brain. Br J Pharmacol. 1995;116:2547–2548. doi: 10.1111/j.1476-5381.1995.tb17205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz BE, Kovalik E, Thomas K, Torgersen D, Mandelkern MA. The effects of an alpha-2 adrenergic agonist, guanfacine, on rCBF in human cortex in normal controls and subjects with focal epilepsy. Neuropsychopharmacology. 2000;23:263–275. doi: 10.1016/S0893-133X(00)00101-9. [DOI] [PubMed] [Google Scholar]
- Talkowski ME, Seltman H, Bassett AS, Brzustowicz LM, Chen X, Chowdari KV, et al. Evaluation of a susceptibility gene for schizophrenia: genotype based meta-analysis of RGS4 polymorphisms from thirteen independent samples. Biol Psychiatry. 2006;60:152–162. doi: 10.1016/j.biopsych.2006.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Buxbaum SG, Waldman I, Anderson GM, Zabetian CP, Kohnke MD, et al. A Single Nucleotide Polymorphism at DBH, Possibly Associated with Attention-Deficit/Hyperactivity Disorder, Associates with Lower Plasma Dopamine beta-Hydroxylase Activity and is in Linkage Disequilibrium with Two Putative Functional Single Nucleotide Polymorphisms. Biol Psychiatry. 2006 doi: 10.1016/j.biopsych.2006.02.017. Epub. [DOI] [PubMed] [Google Scholar]
- Tanila H, Mustonen K, Sallinen J, Scheinin M, Riekkinen P. Role of alpha-2C-adrenoceptor subtype in spatial working memory as revealed by mice with targeted disruption of the alpha-2C-adrenoceptor gene. Eur J Neurosci. 1999;11:599–603. doi: 10.1046/j.1460-9568.1999.00464.x. [DOI] [PubMed] [Google Scholar]
- Tanila H, Rama P, Carlson S. The effects of prefrontal intracortical microinjections of an alpha-2 agonist, alpha-2 antagonist and lidocaine on the delayed alternation performance of aged rats. Brain Res Bull. 1996;40:117–119. doi: 10.1016/0361-9230(96)00026-3. [DOI] [PubMed] [Google Scholar]
- Taylor F, Lowe K, Thompson C, McFall MM, Peskind ER, Kanter ED, et al. Daytime prazosin reduces psychological distress to trauma specific cues in civilian trauma posttraumatic stress disorder. Biol Psychiatry. 2006;59:577–581. doi: 10.1016/j.biopsych.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Taylor F, Raskind MA. The alpha1-adrenergic antagonist prazosin improves sleep and nightmares in civilian trauma posttraumatic stress disorder. J Clin Psychopharmacol. 2002;22:82–85. doi: 10.1097/00004714-200202000-00013. [DOI] [PubMed] [Google Scholar]
- Taylor FB, Russo J. Comparing guanfacine and dextroamphetamine for the treatment of adult Attention Deficit-Hyperactivity Disorder. J Clin Psychopharm. 2001;21:223–228. doi: 10.1097/00004714-200104000-00015. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Birnbaum SG, Ubriani R, Arnsten AFT. Activation of protein kinase A in prefrontal cortex impairs working memory performance. J Neuroscience. 1999;19:RC23. doi: 10.1523/JNEUROSCI.19-18-j0001.1999. (Online) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaiva G, Ducrocq F, Jezequel K, Averland B, Lestavel P, Brunet A, et al. Immediate treatment with propranolol decreases posttraumatic stress disorder two months after trauma. Biol Psychiatry. 2003;54:947–949. doi: 10.1016/s0006-3223(03)00412-8. [DOI] [PubMed] [Google Scholar]
- Wang M, Ramos BP, Paspalas CD, Shu Y-S, Simen AA, Duque A, Vijayraghavan S, Brennan A, Dudley A, Nou E, Mazer JA, McCormick DA, Arnsten AFT. α2A-Adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397–410. doi: 10.1016/j.cell.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Waterhouse BD, Moises HC, Woodward DJ. Noradrenergic modulation of somatosensory cortical neuronal responses to iontophoretically applied putative transmitters. Exp Neurol. 1980;69:30–49. doi: 10.1016/0014-4886(80)90141-7. [DOI] [PubMed] [Google Scholar]
- Waterhouse BD, Moises HC, Woodward DJ. Alpha-receptor-mediated facilitation of somatosensory cortical neuronal responses to excitatory synaptic inputs and iontophoretically applied acetylcholine. Neuropharmacology. 1981;20:907–920. doi: 10.1016/0028-3908(81)90020-4. [DOI] [PubMed] [Google Scholar]
- Witte EA, Marrocco RT. Alterations of brain noradrenergic activity in rhesus monkeys affects the alerting component of covert orienting. Psychopharmacol. 1997;132:315–323. doi: 10.1007/s002130050351. [DOI] [PubMed] [Google Scholar]
- Young LT, Walsh JJ, Kish SJ, Shannek K, Hornykeiwicz O. Reduced brain 5-HT and elevated NE turnover and metabolites in bipolar affective disorder. Biol Psychiatry. 1994;35:121–127. doi: 10.1016/0006-3223(94)91201-7. [DOI] [PubMed] [Google Scholar]
- Zhang HT, Huang Y, Mishler K, Roerig SC, O’Donnell JM. Interaction between the antidepressant-like behavioral effects of beta adrenergic agonists and the cyclic AMP PDE inhibitor rolipram in rats. Psychopharmacology (Berl) 2005;182:104–115. doi: 10.1007/s00213-005-0055-y. [DOI] [PubMed] [Google Scholar]