Abstract
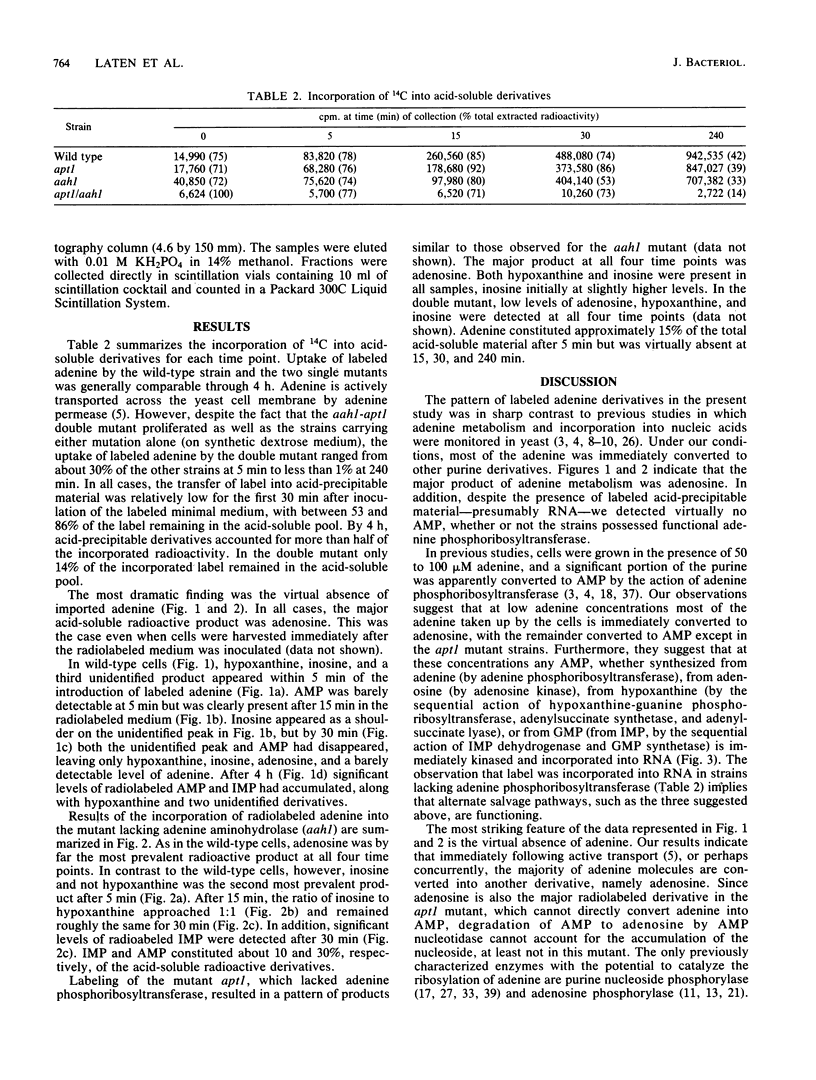
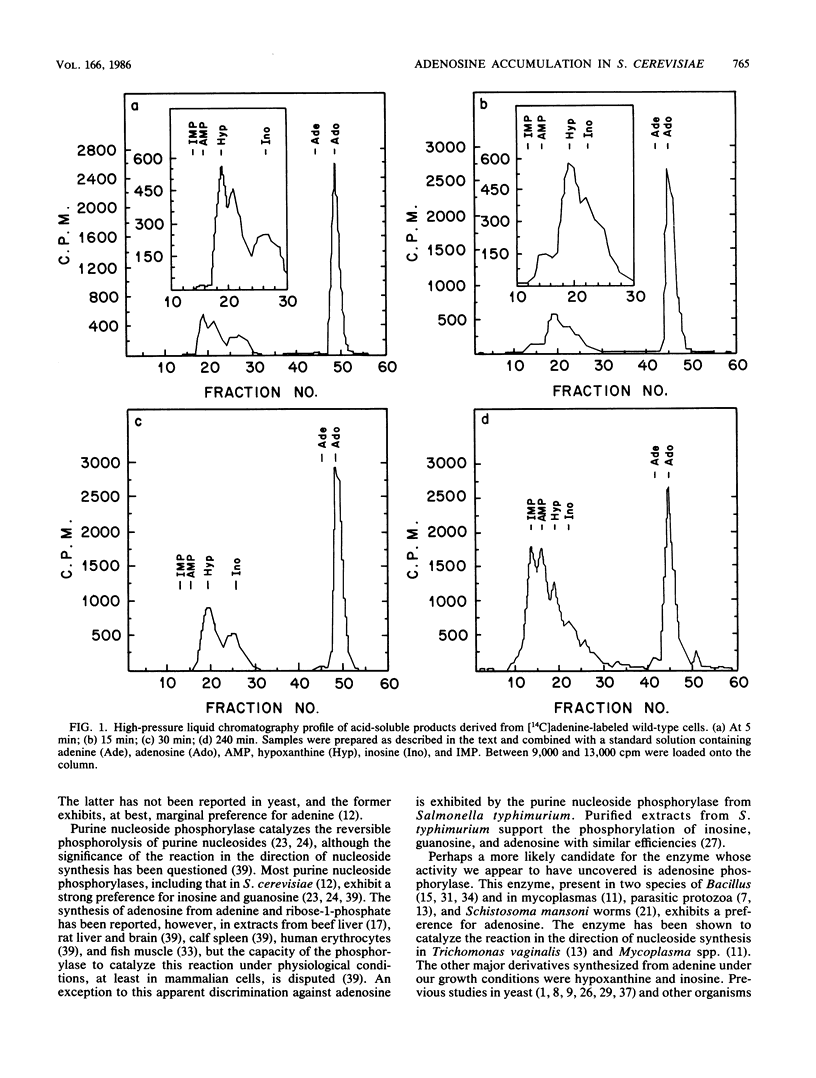
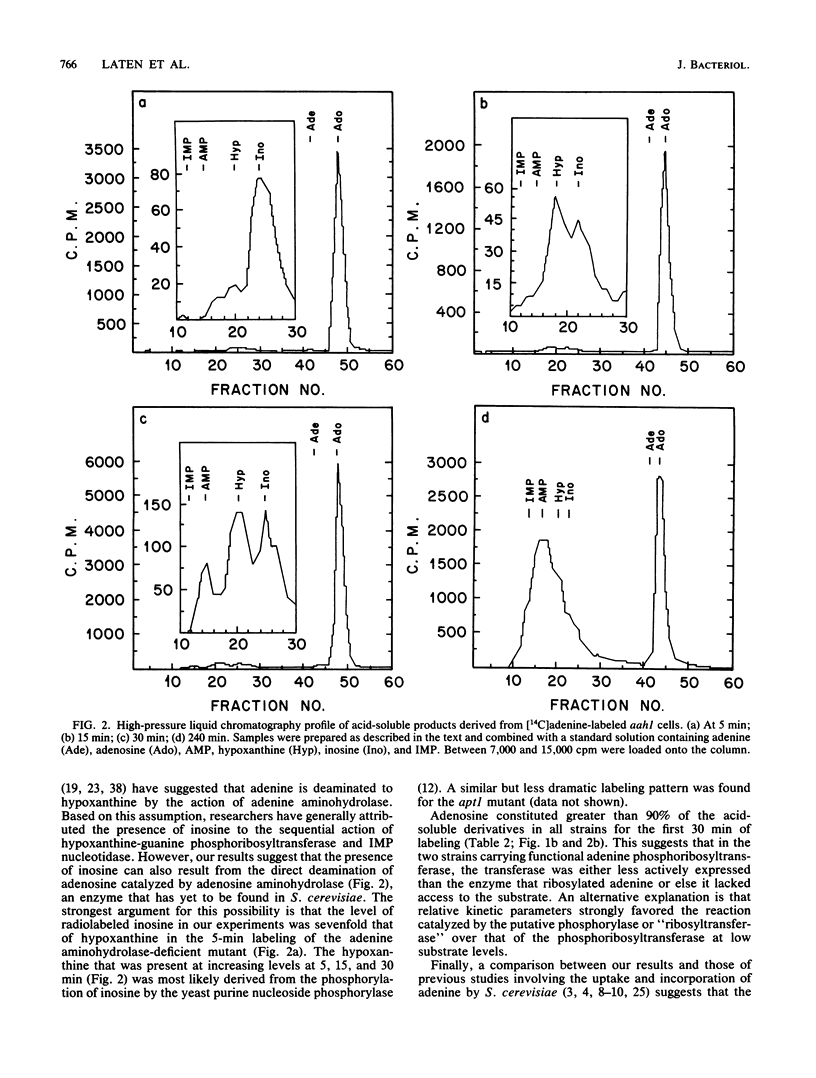
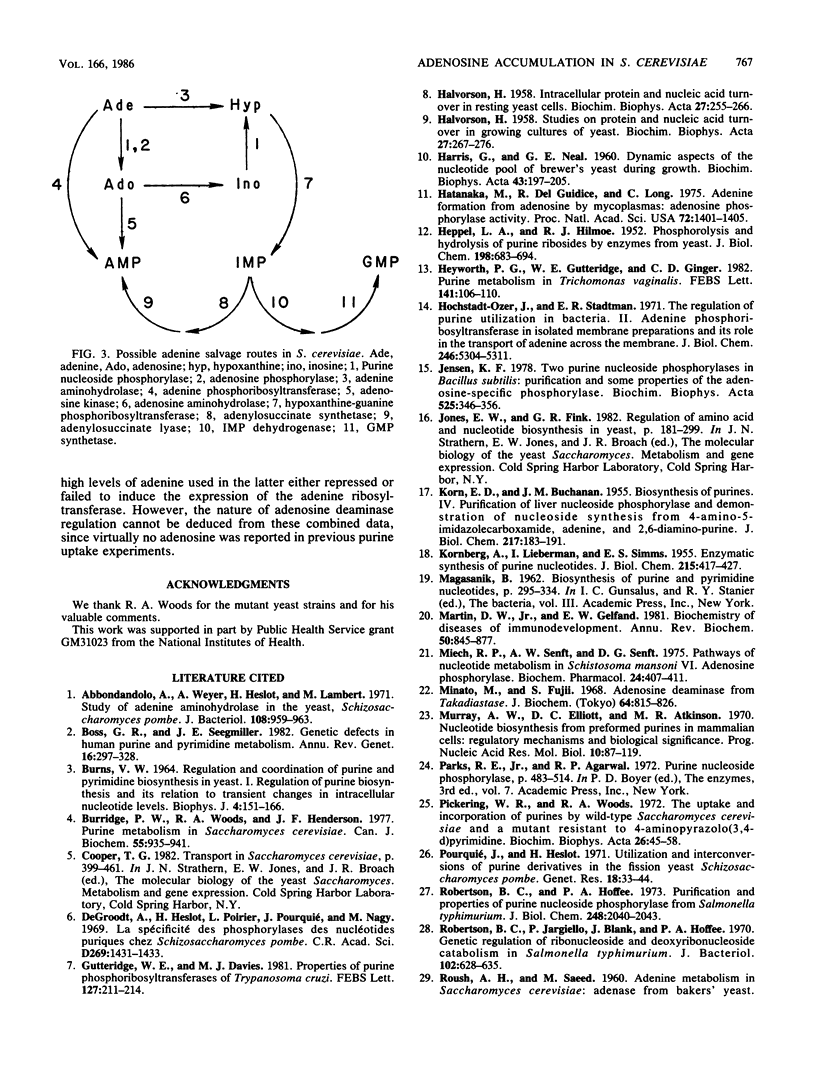
By monitoring the in vivo incorporation of low concentrations of radiolabeled adenine into acid-soluble compounds, we observed the unusual accumulation of two nucleosides in Saccharomyces cerevisiae that were previously considered products of nucleotide degradation. Under the culture conditions used in the present study, radiolabeled adenosine was the major acid-soluble intracellular derivative, and radiolabeled inosine was initially detected as the second most prevalent derivative in a mutant lacking adenine aminohydrolase. The use of yeast mutants defective in the conversion of adenine to hypoxanthine or to AMP renders very unlikely the possibility that the presence of adenosine and inosine is attributable to nucleotide degradation. These data can be explained by postulating the existence of two enzyme activities not previously reported in S. cerevisiae. The first of these activities transfers ribose to the purine ring and may be attributable to purine nucleoside phosphorylase (EC 2.4.2.1) or adenosine phosphorylase (EC 2.4.2.-). The second enzyme converts adenosine to inosine and in all likelihood is adenosine aminohydrolase (EC 3.5.4.4).
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbondandolo A., Weyer A., Heslot H., Lambert M. Study of adenine aminohydrolase in the yeast, Schizosaccharomyces pombe. J Bacteriol. 1971 Dec;108(3):959–963. doi: 10.1128/jb.108.3.959-963.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNS V. W. REGULATION AND COORDINATION OF PURINE AND PYRIMIDINE BIOSYNTHESES IN YEAST. I. REGULATION OF PURINE BIOSYNTHESIS AND ITS RELATION TO TRANSIENT CHANGES IN INTRACELLULAR NUCLEOTIDE LEVELS. Biophys J. 1964 May;4:151–166. doi: 10.1016/s0006-3495(64)86775-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boss G. R., Seegmiller J. E. Genetic defects in human purine and pyrimidine metabolism. Annu Rev Genet. 1982;16:297–328. doi: 10.1146/annurev.ge.16.120182.001501. [DOI] [PubMed] [Google Scholar]
- Burridge P. W., Woods R. A., Henderson J. F. Purine metabolism in Saccharomyces cerevisiae. Can J Biochem. 1977 Sep;55(9):935–941. doi: 10.1139/o77-140. [DOI] [PubMed] [Google Scholar]
- Gutteridge W. E., Davies M. J. Enzymes of purine salvage in Trypanosoma cruzi. FEBS Lett. 1981 May 18;127(2):211–214. doi: 10.1016/0014-5793(81)80207-4. [DOI] [PubMed] [Google Scholar]
- HALVORSON H. Intracellular protein and nucleic acid turnover in resting yeast cells. Biochim Biophys Acta. 1958 Feb;27(2):255–266. doi: 10.1016/0006-3002(58)90332-9. [DOI] [PubMed] [Google Scholar]
- HALVORSON H. Studies on protein and nucleic acid turnover in growing cultures of yeast. Biochim Biophys Acta. 1958 Feb;27(2):267–276. doi: 10.1016/0006-3002(58)90333-0. [DOI] [PubMed] [Google Scholar]
- HARRIS G., NEAL G. E. Dynamic aspects of the nucleotide pool of Brewer's yeast during growth. Biochim Biophys Acta. 1960 Sep 23;43:197–205. doi: 10.1016/0006-3002(60)90430-3. [DOI] [PubMed] [Google Scholar]
- HEPPEL L. A., HILMOE R. J. [Phosphorolysis and hydrolysis of purine ribosides by enzymes from yeast]. J Biol Chem. 1952 Oct;198(2):683–694. [PubMed] [Google Scholar]
- Hatanaka M., Del Giudice R., Long C. Adenine formation from adenosine by mycoplasmas: adenosine phosphorylase activity. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1401–1405. doi: 10.1073/pnas.72.4.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyworth P. G., Gutteridge W. E., Ginger C. D. Purine metabolism in Trichomonas vaginalis. FEBS Lett. 1982 May 3;141(1):106–110. doi: 10.1016/0014-5793(82)80026-4. [DOI] [PubMed] [Google Scholar]
- Hochstadt-Ozer J., Stadtman E. R. The regulation of purine utilization in bacteria. II. Adenine phosphoribosyltransferase in isolated membrane preparations and its role in transport of adenine across the membrane. J Biol Chem. 1971 Sep 10;246(17):5304–5311. [PubMed] [Google Scholar]
- Jensen K. F. Two purine nucleoside phosphorylases in Bacillus subtilis. Purification and some properties of the adenosine-specific phosphorylase. Biochim Biophys Acta. 1978 Aug 7;525(2):346–356. doi: 10.1016/0005-2744(78)90229-2. [DOI] [PubMed] [Google Scholar]
- KORNBERG A., LIEBERMAN I., SIMMS E. S. Enzymatic synthesis of purine nucleotides. J Biol Chem. 1955 Jul;215(1):417–427. [PubMed] [Google Scholar]
- KORN E. D., BUCHANAN J. M. Biosynthesis of the purines. VI. Purification of liver nucleoside phosphorylase and demonstration of nucleoside synthesis from 4-amino-5-imidazolecarboxamide, adenine, and 2, 6-diaminopurine. J Biol Chem. 1955 Nov;217(1):183–191. [PubMed] [Google Scholar]
- Martin D. W., Jr, Gelfand E. W. Biochemistry of diseases of immunodevelopment. Annu Rev Biochem. 1981;50:845–877. doi: 10.1146/annurev.bi.50.070181.004213. [DOI] [PubMed] [Google Scholar]
- Miech F. P., Senft A. W., Senft D. G. Pathways of nucleotide metabolism in Schistosoma mansoni--VI adenosine phosphorylase. Biochem Pharmacol. 1975 Feb 1;24(3):407–411. doi: 10.1016/0006-2952(75)90226-9. [DOI] [PubMed] [Google Scholar]
- Minato S. Adenosine deaminase from takadiastase. V. Subunits of enzyme and interaction with adenosine analogues. J Biochem. 1968 Dec;64(6):815–826. doi: 10.1093/oxfordjournals.jbchem.a128964. [DOI] [PubMed] [Google Scholar]
- Murray A. W., Elliott D. C., Atkinson M. R. Nucleotide biosynthesis from preformed purines in mammalian cells: regulatory mechanisms and biological significance. Prog Nucleic Acid Res Mol Biol. 1970;10:87–119. doi: 10.1016/s0079-6603(08)60562-0. [DOI] [PubMed] [Google Scholar]
- Pickering W. R., Woods R. A. The uptake and incorporation of purines by wild-type Saccharomyces cerevisiae and a mutant resistant to 4-aminopyrazolo (3,4-d) pyrimidine. Biochim Biophys Acta. 1972 Mar 30;264(1):45–58. doi: 10.1016/0304-4165(72)90115-8. [DOI] [PubMed] [Google Scholar]
- Robertson B. C., Hoffee P. A. Purification and properties of purine nucleoside phosphorylase from Salmonella typhimurium. J Biol Chem. 1973 Mar 25;248(6):2040–2043. [PubMed] [Google Scholar]
- Robertson B. C., Jargiello P., Blank J., Hoffee P. A. Genetic regulation of ribonucleoside and deoxyribonucleoside catabolism in Salmonella typhimurium. J Bacteriol. 1970 Jun;102(3):628–635. doi: 10.1128/jb.102.3.628-635.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimandle C. M., Tanigoshi L., Mole L. A., Sherman I. W. Purine nucleoside phosphorylase of the malarial parasite, Plasmodium lophurae. J Biol Chem. 1985 Apr 10;260(7):4455–4460. [PubMed] [Google Scholar]
- Senesi S., Falcone G., Mura U., Sgarrella F., Ipata P. L. A specific adenosine phosphorylase, distinct from purine nucleoside phosphorylase. FEBS Lett. 1976 May 1;64(2):353–357. doi: 10.1016/0014-5793(76)80327-4. [DOI] [PubMed] [Google Scholar]
- TARR H. L. Lingcod muscle purine nucleoside phosphorylase. Can J Biochem Physiol. 1958 Jun;36(6):517–530. [PubMed] [Google Scholar]
- Tozzi M. G., Sgarrella F., Ipata P. L. Induction and repression of enzymes involved in exogenous purine compound utilization of Bacillus cereus. Biochim Biophys Acta. 1981 Dec 18;678(3):460–466. doi: 10.1016/0304-4165(81)90127-6. [DOI] [PubMed] [Google Scholar]
- Wolfenden R., Sharpless T. K., Allan R. Substrate binding by adenosine deaminase. Specificity, pH dependence, and competition by mercurials. J Biol Chem. 1967 Mar 10;242(5):977–983. [PubMed] [Google Scholar]
- Woods R. A., Roberts D. G., Friedman T., Jolly D., Filpula D. Hypoxanthine: guanine phosphoribosyltransferase mutants in Saccharomyces cerevisiae. Mol Gen Genet. 1983;191(3):407–412. doi: 10.1007/BF00425755. [DOI] [PubMed] [Google Scholar]
- Woods R. A., Roberts D. G., Stein D. S., Filpula D. Adenine phosphoribosyltransferase mutants in Saccharomyces cerevisiae. J Gen Microbiol. 1984 Oct;130(10):2629–2637. doi: 10.1099/00221287-130-10-2629. [DOI] [PubMed] [Google Scholar]
- Zimmerman T. P., Gersten N. B., Ross A. F., Miech R. P. Adenine as substrate for purine nucleoside phosphorylase. Can J Biochem. 1971 Sep;49(9):1050–1054. doi: 10.1139/o71-153. [DOI] [PubMed] [Google Scholar]



