Abstract
FGF21 recently has been proposed as a missing link in the biology of fasting, raising the question of whether it directly reaches the brain. We used multiple time-regression analysis to quantify the influx rate of this polypeptide across the blood-brain barrier (BBB), size-exclusion chromatography to examine degradation, capillary depletion to differentiate entry into brain parenchyma from retention in the microvasculature, and measurement of efflux rate to determine a possible confounding effect on measurement of entry. FGF21 was 94 % intact in serum and 75 % in brain 10 min after intravenous bolus delivery. Its influx rate was 0.23 ± 0.12 μl/g-min, nearly 4-times faster than that of the vascular marker albumin. At 10 min, about 0.5 % of the administered FGF21 was present in a gram of brain tissue. Of this, 70 % reached the parenchyma of the brain. Co-injection of excess FGF21 failed to inhibit the influx, showing a lack of saturation. Efflux, which occurred at the same rate as the bulk reabsorption of cerebrospinal fluid, also was not saturable. In summary, FGF21 shows significant, non-saturable, unidirectional influx across the BBB.
Keywords: FGF21, blood-brain barrier, metabolism
1. Introduction
The fibroblast growth factor (FGF) polypeptides were initially named by their ability to stimulate fibroblast proliferation. FGF21 activates glucose uptake of adipocytes, reduces plasma glucose and triglyceride concentrations in ob/ob and db/db mice, protects against diet-induced obesity when overexpressed in transgenic mice, and improves lipoprotein profiles in diabetic rhesus monkeys [10;11;19].
FGF21 recently was described as a missing link in the biology of fasting, important in regulating the adjustment to food deprivation [1;2;18]. The brain senses fasting with neurobehavioral changes, leading to various adaptations such as torpor, which is involved in the conservation of energy. Reitman posed the unanswered question of whether FGF21 reaches the brain directly [18].
Although it is now recognized that many peptides and polypeptides cross the blood-brain barrier (BBB), permeability varies with the peptide. Several peptides/polypeptides that have a molecular weight similar to that of FGF21, or even smaller, do not cross the BBB any faster than albumin. These include obestatin [14], adiponectin [14], orexin B [3], melanin-concentrating hormone [5], interleukin-10 [8], transforming growth factor-β [6], glial cell line-derived neurotrophic factor [7], and platelet-derived growth factors AA and BB [4]. The pharmacokinetics of the interactions of FGF21 with the BBB could have direct therapeutic implications for this recently discovered peptide. Therefore, we quantified the influx of intact FGF21 into the brain and its possible efflux after intracerebroventricular administration.
2. Materials and Methods
2.1. Animals and labeling
Male CD1 mice 5–6 weeks of age, used in accordance with a protocol approved by the Institutional Animal Care and Use Committee, were anesthetized by intraperitoneal injection of urethane. Recombinant human His-tagged full-length FGF21, containing 195 amino acids with a molecular weight of 20.996 kDa, was purchased from Phoenix Pharmaceuticals (Burlingame, CA). Bovine serum albumin (BSA) and the chloramine-T reagent used for radioactive labeling (radiolabeling) were purchased from Sigma (St. Louis, MO). Chloramine-T was used to radiolabel FGF21 with 125I (Amersham, Piscataway, NJ), the reaction being stopped 1 min later with sodium metabisulfite. This material was purified on a Sephadex G-10 column. The specific activity of the 125I- FGF21 was about 44 Ci/g.
2.2. Degradation
The supernatants from brain homogenates of 6 mice obtained 0, 10, and 20 min after injection of 125I-FGF21 were pooled (2/sample) and run on size-exclusion chromatography. The time point 0 represents the processing control involving addition of 125I-FGF21 to homogenate and blood to determine the extent of degradation occurring after collection. Each mouse in the experimental groups received an intravenous (iv) injection of 3 μCi of 125I-FGF21 in 100 μl injected at time 0. At 10 and 20 min, arterial blood and brain were taken and processed on ice. Brain tissue was homogenized in 1 ml of lactated Ringer’s solution containing 1% bovine serum albumin (LR/BSA) and Complete Protease Inhibitor Cocktail (Sigma). For size-exclusion chromatography, 100 μl of lyophilized brain supernatant was added to a 9 × 6 cm column of polyacrylamide P10. Fractions of 100 μl were collected, as described previously [16].
2.3. Quantification of blood-to-brain influx
Multiple time-regression analysis was used as previously detailed [9;14]. 125I-FGF21 and 131I-albumin (about 30,000 cpm/μl for each mouse) were delivered in the same injectate of 100 μl of freshly made LR/BSA. The injection was made into the isolated left jugular vein of anesthetized mice, each mouse representing a specific time point. At various times after iv injection, blood was collected by transection of the right carotid artery and the mice were decapitated immediately afterwards. Since degradation assays showed that substantial degradation had occurred at 20 min in the brain (see Results section), only the mice studied within 10 min after iv injection were used to calculate the influx rate from blood to the brain.
To test whether there was saturation of FGF21 permeation across the BBB, two groups of mice were studied simultaneously. The control group received only radioisotopes (n = 7/group), whereas the inhibition group received co-injection of 2 μg/mouse of unlabeled FGF21 in addition to 125I-FGF21 and 131I-albumin (n = 8/group). At the end of the study, radioactivity in brain and serum was measured, and the brain/serum ratio of 125I-FGF21 in each g of brain was measured. The serum values were also used in the calculation of exposure time, a theoretical steady-state value with correction for the decrease in blood concentration of 125I-FGF21 after iv bolus injection. This represents the theoretical value correlated with each experimental time t as if the blood concentration of 125I-FGF21 were constant from time 0 to time t. It is obtained by division of the integral of serum radioactivity over time by the serum radioactivity at each experimental time [9]. The influx rate and volume of distribution of 125I-FGF21 is determined from the linear regression correlation between the tissue/serum ratio of radioactivity and exposure time, as described previously [16]. The unidirectional influx rate Ki is the slope of the linear regression line, and the initial volume of distribution Vi is the intercept. Differences in the regression lines between groups were compared by the least square method with the GraphPad program.
2.4. Compartmental distribution by capillary depletion
Two groups of mice (n = 4/group) were used to determine whether the injected radioactive FGF21 actually reached brain tissue rather than remaining trapped in the cerebral vasculature. One group of mice was perfused intracardially to remove loosely adherent radiotracers in the brain vasculature whereas the other group did not receive intracardial perfusion for vascular washout. In both groups, each mouse received 125I-FGF21 and 131I-albumin in 100 μl of LR/BSA buffer by bolus injection into the left jugular vein. Ten min later, blood was collected by transection of the abdominal aorta. Either with or without vascular washout afterwards, the mouse was decapitated to obtain the cerebral cortex. The cortex was homogenized in 0.7 ml of capillary buffer and mixed thoroughly with 1.7 ml of 26 % dextran (about 67 kD). This mixture was centrifuged (5400 g, 15 min, 4 °C) with a swing bucket rotor to obtain the parenchymal and capillary fractions. After measurement with a dual-channel γ-counter, the ratios of tissue/serum radioactivity for 125I-FGF21 were calculated. Group means are shown with their standard errors. Significant differences among the groups were determined by ANOVA followed by Tukey’s post-hoc comparison test.
2.5. Quantification of brain-to-blood efflux
A slow rate of influx might reflect increased efflux. Therefore, one group of mice (n = 9) received only 125I-FGF21 and 131I-albumin (40,000 and 30,000 cpm, respectively) while a second group received 100-fold excess unlabeled FGF21 (40 ng/mouse) in addition to these two radioactively labeled compounds. A perfusion pump with a 5 μl Hamilton syringe connected to a PE10 cannula was used to deliver these solutions into the right lateral ventricle 1.0 mm lateral and 0.2 mm posterior to the bregma and 2.2 mm below the skull. The perfusion rate was 12 μl/min and the duration of delivery was 5 sec; the cannula remained in place for another 5 sec before being slowly withdrawn. The mice were decapitated 2, 5, 10, 15, 20, 30, 45, and 60 min later. The control time 0 was determined after euthanasia of the mice by an overdose of urethane before injection, and the mice were decapitated immediately. The half-time disappearance of 125I-FGF21 and 131I-albumin was determined from the linear regression correlation between the brain radioactivity and time. Statistical significance between groups was determined with the Prism GraphPad program.
3. Results
3.1. Degradation of 125I-FGF21
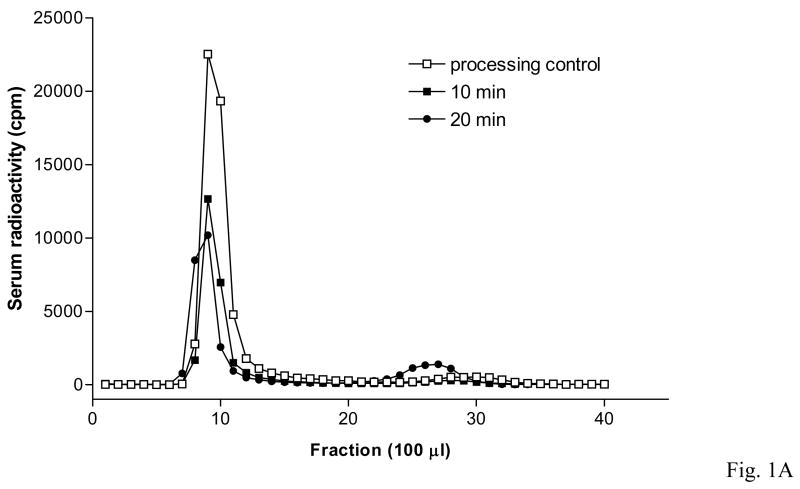
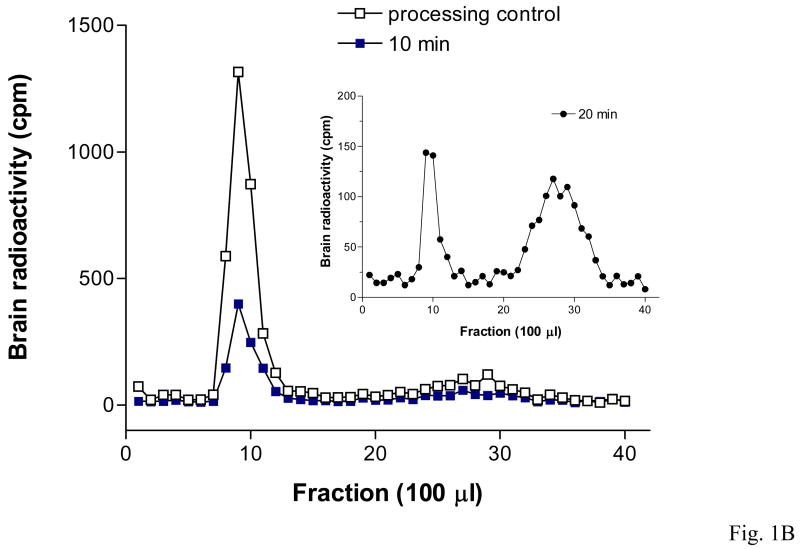
Size-exclusion chromatography confirmed that 125I-FGF21 was mainly intact in both serum and brain 10 min after injection. After correction for degradation during ex-vivo processing determined from the controls, almost 100 % of the radioactivity in serum and 88 % in brain represented intact 125I-FGF21. However, 20 min after iv delivery of 125I-FGF21 there was substantial degradation in brain (41 % intact) and serum (83 % intact). These results are shown in Table 1 and figures 1A and 1B.
Table 1.
125I-FGF21 degradation assay after intravenous injection shown by size-exclusion chromatography
| Serum | Serum | Brain | Brain | |
|---|---|---|---|---|
| Raw data | corrected | Raw data | corrected | |
| Control (0 min) | 94.2% | 100% | 86.3% | 100% |
| 10 min | 94.1% | 99.9% | 75.8% | 87.8% |
| 20 min | 78.1% | 82.9% | 35.2% | 40.7% |
Fig. 1.
Intact 125I-FGF21 found by polyacrylamide size exclusion chromatography in samples of serum (1A) and brain (1B) 10 min after iv delivery. More degradation occurred at 20 min.
3.2. Blood-to-brain influx of 125I-FGF21
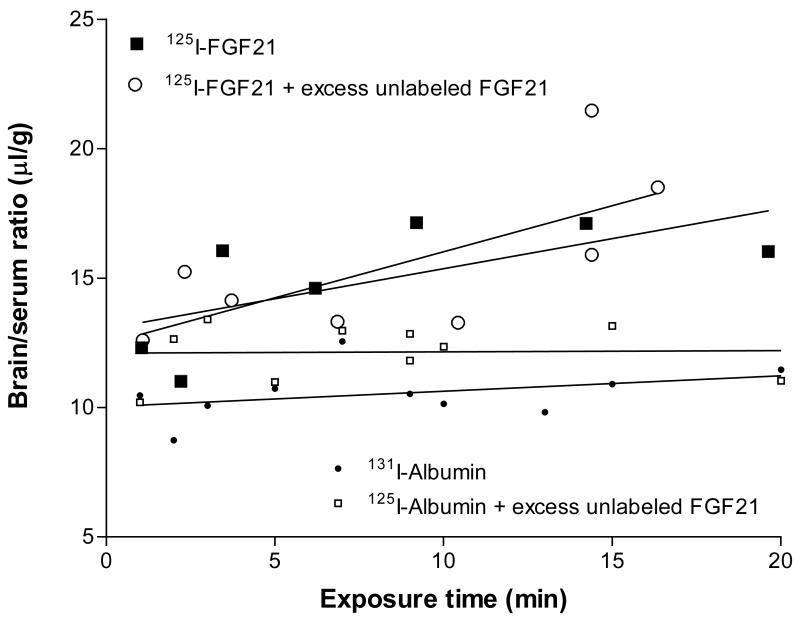
The Ki of 125I-FGF21 was 0.23 ± 0.12 μl/g-min and the initial volume of distribution was 13.04 ± 1.19 μl/g. This indicates a brain uptake of about 0.5 %/g brain at 10 min. With 1.36 μCi injected at a specific activity of 44 Ci/g, this shows that about 179 pg of 125I-FGF21-1 entered one g of brain after a single bolus injection at this time. After addition of 2 μg/mouse of unlabeled FGF21 (about 65-fold excess), the resulting Ki (0.36 ± 0.15 μl/g-min) was not significantly different, indicating that the influx of FGF21 into brain was not inhibited at this dose (Fig. 2).
Fig. 2.
The blood-to-brain influx rate of 125I-FGF21 after iv bolus injection was about 4-times higher than that of the vascular control, 131I-albumin. Excess unlabeled FGF21 did not affect the influx of either 125I-FGF21 or 131I-albumin.
3.3. Uptake of 125I-FGF21 by muscle and liver
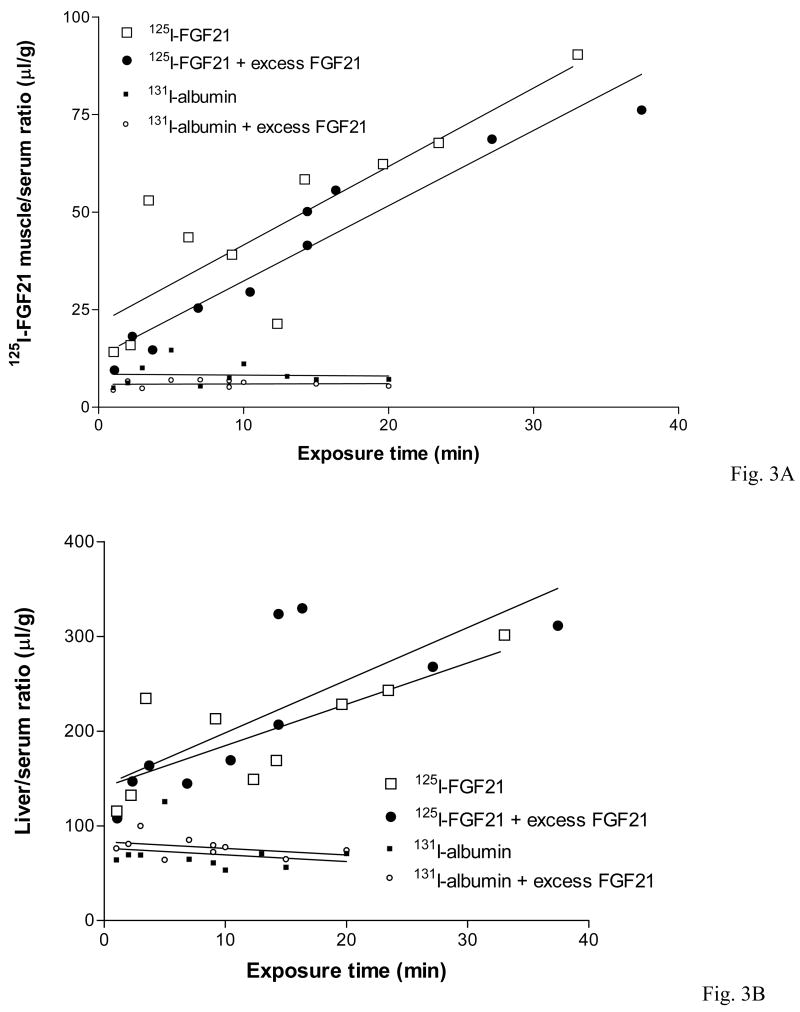
Two peripheral organs, muscle and liver, were used as controls for the uptake of FGF21 into brain, an organ which is protected by the BBB consisting of less permeable microvessels reinforced by tight junctions. In the gluteus major muscle, 125I-FGF21 had an influx rate 10-times higher than that in the brain. This is also significantly higher than that of albumin. Excess unlabeled FGF21 had no effect on the muscle uptake of either substance (Fig. 3A). Although FGF21 is produced by the liver, the influx of 125I-FGF21 from blood to liver was even higher than that to the muscle, but this also did not show saturability in the presence of excess unlabeled FGF21 (Fig. 3B).
Fig. 3.
The influx of 125I-FGF21 into muscle (3A) and liver (3B) was significantly higher than into brain and was also higher than that of 131I-albumin. There was no significant change caused by excess unlabeled FGF21. The influx rate of 125I-FGF21 into the liver was at least twice as fast as that into muscle.
3.4. Compartmental distribution 125I-FGF21 in the brain
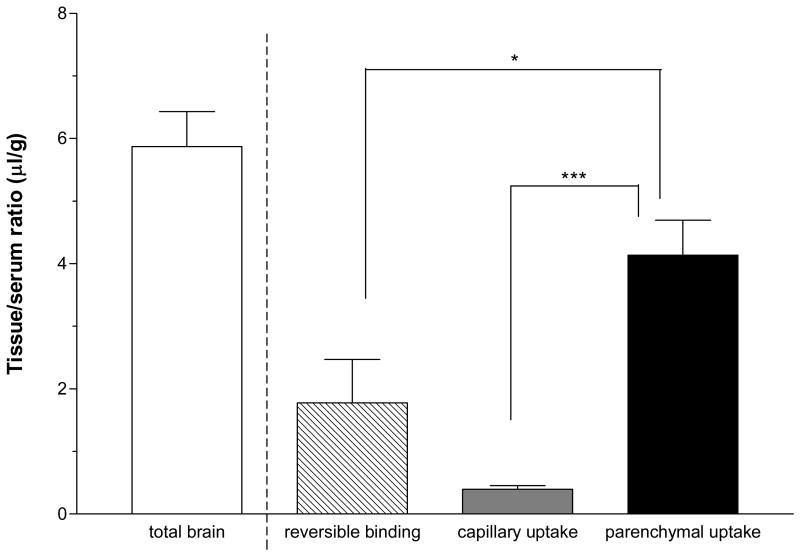
Ten min after iv injection of 125I-FGF21, 5.87 ± 0.56 μl/g was present in the cortex. Of this total radioactivity, about 70 % (4.14 ± 0.56 μl/g) reached the brain parenchyma. This was significantly higher than that retained in the capillaries (0.40 ± 0.06 μl/g) or remaining loosely bound in the vessel lumen (1.78 ± 0.70 μl/g). This reversible binding accounted for about 30 % of the total apparent brain uptake (Fig. 4).
Fig. 4.
Capillary depletion showed that most (70 %) of the 125I-FGF21 in the total brain had reached the parenchyma 10 min after iv delivery. *: p < 0.05; **: p < 0.01.
3.5. Brain-to-blood efflux of 125I-FGF21
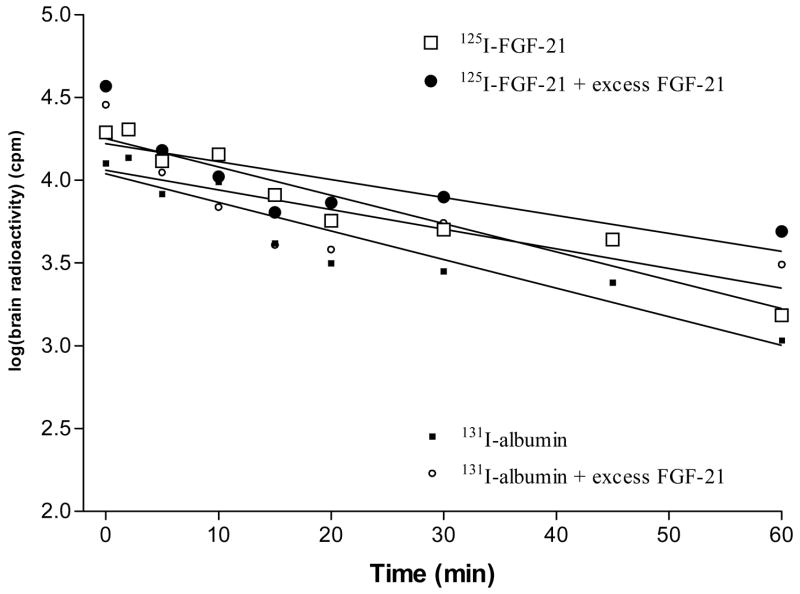
The efflux rate of 125I-FGF21 from brain was similar to that 131I-albumin, their half-time disappearances being 17.6 and 17.4 min, respectively. These values were not significantly altered by inclusion of excess unlabeled FGF21 in the injectate (Fig. 5).
Fig. 5.
The efflux of 125I-FGF21 from brain was similar to that of 131I-albumin. Excess unlabeled FGF21-1 showed no significant effect on the slow efflux of either substance.
4. Discussion
FGF21 was originally isolated from mouse embryos. Northern blotting analysis showed that it had the highest expression in liver and lower levels in the thymus, but was undetectable in several other peripheral tissues. Human and mouse FGF21 have a high degree of homology (75 % of the amino acids) [13]. This justified the use of His-tagged human FGF21 in our mouse studies. FGF21 belongs to the FGF19 subfamily of metabolic regulators that also includes FGF15 and FGF23. They bind to the isoforms of FGFR1–4 that have intrinsic tyrosine kinase activity and regulate glucose and lipid homeostasis.
Overexpression (transgenic mice) and subcutaneous treatment of genetically obese and diabetic animals (ob/ob and db/db mice, Zucker diabetic fatty rats) can induce a reduction of blood glucose and triglyceride [10]. FGF21 and other members of the FGF19 family of ligands have a low level of binding to heparin/heparan sulfate. This enables them to circulate as hormones rather than being trapped by the extracellular matrix, but this also raises the necessity of cofactors for receptor activation, such as the transmembrane protein beta-Klotho [12]. Since FGF23 is produced by the brain, predominantly in the ventrolateral thalamus [20], it is not known whether FGF21, which is elevated by fasting, can cross the BBB to act on the FGF receptors in the brain. The permeability of the BBB to FGF21 can be best quantified by multiple-time regression analysis [9]. This revealed slow but significant blood-to-brain penetration.
The results showed that intact FGF21 crossed the BBB at a rate comparable to that of polypeptides of similar size [15;17]. Size-gel chromatography showed that it crossed in intact form, and capillary depletion showed that it mainly entered the brain parenchyma rather than being trapped in the microvasculature that composes the BBB. We also ruled out the presence of an efflux transport system.
There was no saturation of the influx of FGF21 into liver or muscle. In doses of 125 or 750 μg/kg/d with once daily subcutaneous injection for a week, FGF21 administration does not cause mitogenicity or hypoglycemia, indicating that the influx to other organs does not induce metabolic dysfunction at large doses in rodents [10]. A similar lack of systemic toxicity for FGF21 has also been shown in studies in monkeys [11].
Thus, intact FGF21 crossed the BBB by simple diffusion. There was no saturable influx or efflux transport system. The small amount of FGF21 entering the CNS (about 0.5 %/g of brain tissue) could be physiologically important in fasting. To the question raised by the editorial asking whether FGF21 in the periphery can reach the brain directly [18], the answer is a definite yes.
Acknowledgments
The study was supported by NIH (NS45751, NS46528 and DK54880). Editorial assistance was provided by Ms. Loula Burton.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Badman M, Pissios P, Kennedy A, Koukos G, Flier J, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPAR alpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metabolism. 2007;5:426–37. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 2.Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, et al. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metabolism. 2007;5:415–25. doi: 10.1016/j.cmet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Kastin AJ, Akerstrom V. Orexin A but not orexin B rapidly enters brain from blood by simple diffusion. J Pharmacol Exp Ther. 1999;289:219–23. [PubMed] [Google Scholar]
- 4.Kastin AJ, Akerstrom V, Hackler L, Pan W. Different mechanisms influencing permation of PDGF-AA and PDGF-BB across the blood-brain barrier. JNeurochem. 2003;87:7–12. doi: 10.1046/j.1471-4159.2003.01933.x. [DOI] [PubMed] [Google Scholar]
- 5.Kastin AJ, Akerstrom V, Hackler L, Zadina JE. Phe13,Tyr19-Melanin-concentrating hormone and the blood-brain barrier: role of protein binding. J Neurochem. 2000;74:385–91. doi: 10.1046/j.1471-4159.2000.0740385.x. [DOI] [PubMed] [Google Scholar]
- 6.Kastin AJ, Akerstrom V, Pan W. Circulating TGF beta 1 does not cross the intact blood-brain barrier. J Molec Neurosci. 2003;21:43–8. doi: 10.1385/JMN:21:1:43. [DOI] [PubMed] [Google Scholar]
- 7.Kastin AJ, Akerstrom V, Pan W. Glial cell line-derived neurotrophic factor does not enter normal mouse brain. Neurosci Lett. 2003;340:239–41. doi: 10.1016/s0304-3940(03)00007-7. [DOI] [PubMed] [Google Scholar]
- 8.Kastin AJ, Akerstrom V, Pan W. Interleukin-10 as a CNS therapeutic: the obstacle of the blood-brain/blood-spinal cord barrier. Mol Brain Res. 2003;114:168–71. doi: 10.1016/s0169-328x(03)00167-0. [DOI] [PubMed] [Google Scholar]
- 9.Kastin AJ, Akerstrom V, Pan W. Validity of multiple-time regression analysis in measurement of tritiated and iodinated leptin crossing the blood-brain barrier: meaningful controls. Peptides. 2001;22:2127–36. doi: 10.1016/s0196-9781(01)00569-1. [DOI] [PubMed] [Google Scholar]
- 10.Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, et al. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005;115:1627–35. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kharitonenkov A, Wroblewski VJ, Koester A, Chen YF, Clutinger CK, Tigno XT, et al. The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology. 2007;148:774–81. doi: 10.1210/en.2006-1168. [DOI] [PubMed] [Google Scholar]
- 12.Kurosu H, Choi M, Ogawa Y, Dickson AS, Goetz R, Eliseenkova AV, et al. Tissue-specific expression of beta KLOTHO and fibroblast growth factor receptor isoforms determines metabolic activity of FGF19 and FGF21. J Biol Chem. 2007 doi: 10.1074/jbc.M704165200. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishimura T, Nakatake Y, Konishi M, Itoh N. Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochim Biophys Acta. 2000;1492:203–6. doi: 10.1016/s0167-4781(00)00067-1. [DOI] [PubMed] [Google Scholar]
- 14.Pan W, Tu H, Kastin AJ. Differential BBB interactions of three ingestive peptides: obestatin, ghrelin, and adiponectin. Peptides. 2006;27:911–6. doi: 10.1016/j.peptides.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 15.Pan W, Xiang S, Tu H, Kastin AJ. Cytokines interact with the blood-brain barrier. In: Dermietzel R, Spray DC, Nedergaard M, editors. Blood-Brain Barrier Interfaces: From Ontogeny to Artificial Barriers. Weinheim, Germany: Wiley-VCH; 2006. pp. 247–64. [Google Scholar]
- 16.Pan W, Tu H, Yu C, Hsuchou H, Yang Y, Kastin AJ. Differential role of TNF receptors in cellular trafficking of intact TNF. Cell Phyiosiol Biochem. 2007;20:559–68. doi: 10.1159/000107539. [DOI] [PubMed] [Google Scholar]
- 17.Pan W, Vallance K, Kastin AJ. TGFα and the blood-brain barrier: accumulation in cerebral vasculature. Exp Neurol. 1999;160:454–9. doi: 10.1006/exnr.1999.7215. [DOI] [PubMed] [Google Scholar]
- 18.Reitman M. FGF21: A missing link in the biology of fasting. Cell Metabolism. 2007;5:405–6. doi: 10.1016/j.cmet.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 19.Wente W, Efanov AM, Brenner M, Kharitonenkov A, Koster A, Sandusky GE, et al. Fibroblast growth factor-21 improves pancreatic beta-cell function and survival by activation of extracellular signal-regulated kinase 1/2 and Akt signaling pathways. Diabetes. 2006;55:2470–8. doi: 10.2337/db05-1435. [DOI] [PubMed] [Google Scholar]
- 20.Yamashita T, Yoshioka M, Itoh N. Identification of a novel fibroblast growth factor, FGF-23, preferentially expressed in the ventrolateral thalamic nucleus of the brain. Biochem Biophys Res Commun. 2000;277:494–8. doi: 10.1006/bbrc.2000.3696. [DOI] [PubMed] [Google Scholar]