Introduction
It is established that the temporal and lineage specificity of V(D)J rearrangement is controlled at multiple levels [1,2]. These include germ-line transcription, chromatin remodeling, histone acetylation and DNA methylation. It is, however, unknown how a region spanning large genomic distances allows the assembly of antigen receptor genes. Recent data have suggested that an underlying structural order must exist that facilitates the association of DNA elements separated by large genomic distances.
B cell development and ordered immunoglobulin gene rearrangement
The developmental progression of B cells has been characterized on the basis of antigen receptor gene rearrangements and on the dynamic expression patterns of cell surface proteins, which have served as maturation markers [3]. The Igh locus is comprised of distinct DNA elements encoding the variable (V), diversity (D), joining (J), and constant (C) regions. Eight Igh constant regions encode for distinct isotypes that include Cμ, Cδ, Cγ1 Cγ2a, Cγ2b, Cγ3, Cα and Cε. Upstream of the IgM constant region are located twelve DH and four JH segments. Fifteen partially dispersed V region families are present that span approximately 2 Mbp of genomic sequence. The IgK and L light chain loci are also organized into distinct DNA elements, encoding VH, JH and CH segments. Ig VH, DH and JH elements are flanked by recombination signal sequences that interact with the RAG1 and RAG2 gene products.
In pro-B cells, Igh DHJH joining precedes that of VH(DH)JH gene rearrangement [4]. Once a V(D)J gene rearrangement leads to a productive joint, pre-BCR signaling acts to inhibit RAG1 and RAG2 gene expression and to promote the survival, expansion and developmental progression of large pre-B cells [5.6]. The expansion phase is followed by cell cycle arrest, during which RAG gene expression is reactivated to initiate the rearrangement of the Ig light chain genes. In the presence of self-reactivity, continued rearrangements will replace primary IgK VJ joints, generating receptors with novel specificities. Once a BCR is expressed which lacks auto-reactivity, tonic signaling mediated by the BCR will permanently suppress RAG1 and RAG2 gene expression. Tonic BCR signaling will also trigger the migration of B cells to the peripheral lymphoid organs where they, upon interacting with pathogens, will undergo class switching and somatic hypermutation.
T-lineage development and T cell receptor gene rearrangement
T-lineage cells also develop sequentially [7]. Two distinct T cell lineages develop from a common progenitor, named αβ and γδ T cells. The rearrangement of the TCRβ and γδ loci is initiated in a compartment that lacks the expression of the co-receptors, CD4 and CD8. This population is often referred to as the double-negative (DN) compartment. Once a productive TCRβ V(D)J gene rearrangement has been generated, a pre-TCR complex is formed and continued TCRβ rearrangement is suppressed by signals emanating from the pre-TCR. Committed αβ T cells that express a pre-TCR, will undergo extensive proliferation and ultimately will develop into cells that express both CD4 and CD8, also named double-positive (DP) cells. If developing thymocytes generate a γδ TCR prior to the expression of a pre-TCR, cells will become committed to the γδ T cell lineage. In the DP compartment, TCRα VJ rearrangement will be initiated and completed. Developmental progression at this stage depends on the ability of the αβ TCR to recognize with the appropriate affinity, peptides that are presented by the major histocompatibility complex gene products.
Regulatory factors that control B- and T-lineage specification and commitment
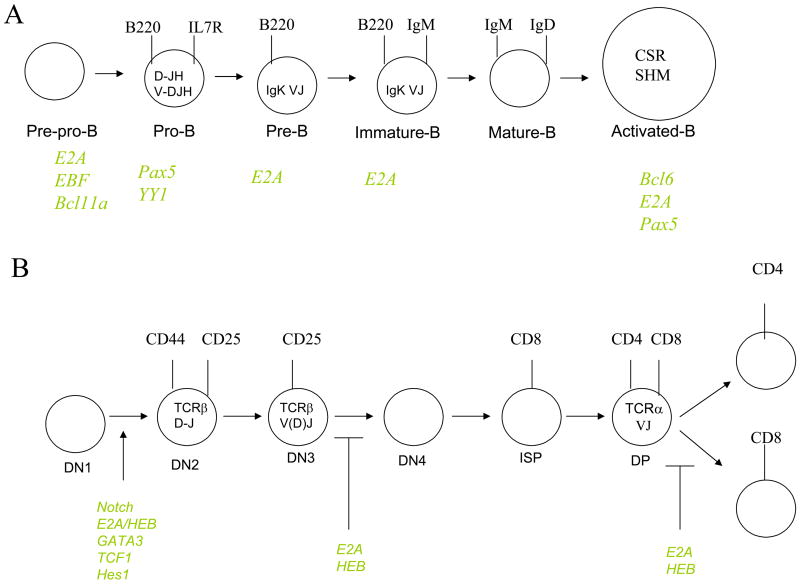
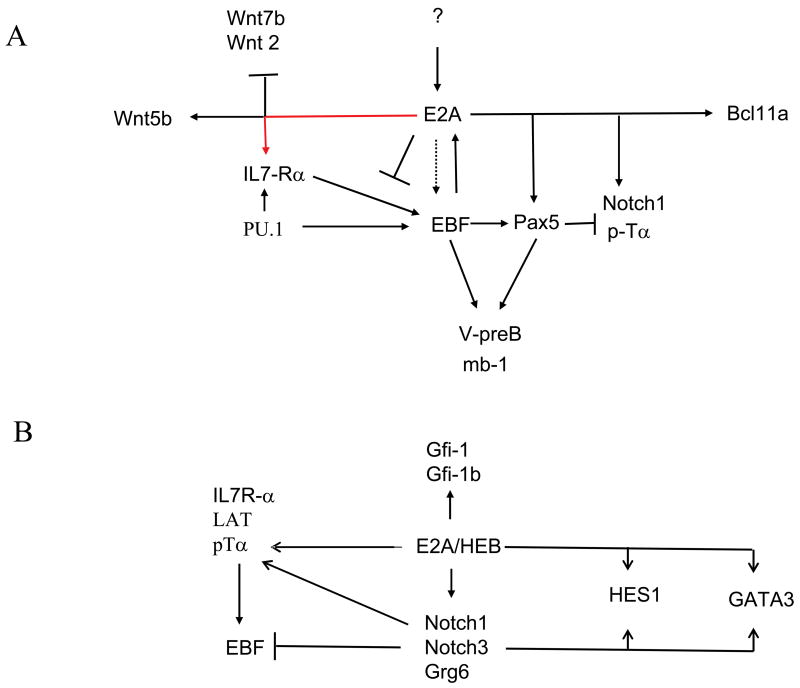
B cell commitment and developmental progression is regulated by the combined activities of distinct classes of transcriptional regulators, which include PU.1, E2A, EBF, and Pax5 (Figure 1A). Recent data have provided a regulatory network in which these proteins act to induce B- and T-lineage specific programs of gene expression [8,9]. In the common lymphoid compartment, PU1, E2A, EBF and Pax5 act together to promote a B cell fate. The gene encoding the IL7Rα chain is a critical target for PU.1 and E2A [8,10]. IL7Rα̃mediated signaling then acts in concert with E2A and PU.1 to activate EBF transcription, which in turn induces the expression of Pax5 [11,12]. Once activated, E2A, EBF and Pax5 then act together to induce B-lineage specification and commitment (Figure 2) [8,9].
Figure 1.
(A) Diagram depicting B cell development. Ig DHJH and VHDHJH gene rearrangements are indicated. Transcriptional regulators controlling distinct checkpoints during B-lineage maturation are shown. (B) Diagram depicting T-lineage development. TCR gene rearrangements are indicated. Notch signaling and transcriptional regulators controlling distinct checkpoints during T-lineage maturation are shown.
Figure 2.
(A) Regulatory network controlling B-lineage specification and commitment is shown. (B) Regulatory network controlling T-lineage development is indicated.
T-lineage development is controlled by the combined activities of E2A, HEB, GATA-3, TCF1 and Notch-mediated signaling (Figure 1B) [8,13]. The E-proteins, E2A and HEB, form heterodimers to induce the expression of genes involved in Notch- and pre-TCR mediated signaling [8]. Once the Notch signaling pathway is activated, T cells become committed to the T cell lineage, in part, through the induction of Hes1, GATA3 and TCF1 transcription (Figure 2) [9,13,14]. Notch signaling and the E-proteins also act to induce TCRβ V(D)J gene rearrangement [15,16]. Upon formation of a productive TCRβ V(D)J gene rearrangement, pre-TCR signaling inhibits E-protein activity to suppress continued rearrangement, to promote developmental progression and cellular expansion [17].
Developmental-specific regulation of antigen receptor gene assembly
It is established that the rearrangement of antigen receptor loci is dependent on lineage- and developmental-specific modulation of chromatin structure. For example, pro-B cells express high levels of the V(D)J recombinase, RAG1 and RAG2, but only the Igh locus will undergo V(D)J gene rearrangement. IgL VJ joining occurs in pre-B cells, but Igh V(D)J rearrangement is permanently suppressed beyond the pro-B cell stage. TCRβ V(D)J joints are generated in the DN compartment but not in the DP compartment.
How is lineage- and stage-specific antigen receptor gene assembly regulated? In vitro studies have indicated that the IgK, but not the TCRβ locus, in pre-B cells is accessible to the V(D)J recombinase, indicating that chromatin structure determines accessibility [18]. Chromatin accessibility, in part, is regulated by the activities of transcriptional regulators. Ig V(D)J gene rearrangement is controlled by STAT5, Pax5 and YY1, whereas IgL VJ gene rearrangement is regulated by the E2A proteins [10,19,20,21,22,23]. The E2A proteins interact directly with binding sites present in the IgK light chain gene enhancer to modulate IgK VJ gene rearrangement [24]. Furthermore, in the presence of self-reactivity they promote receptor editing [25]. Expression of RAG1 and RAG2 in non-lymphoid cells does not promote IgH and IgL chain gene rearrangement. However, upon enforced expression of E2A, EBF and Pax5, IgH and IgL chain gene rearrangements can be readily induced [22,23,26]. Collectively, these data indicate that the targeting of the V(D)J recombinase is regulated in a developmental- and lineage-specific manner by the combinatorial activities of transcriptional regulators. How is chromatin accessibility regulated by the activities of these transcriptional regulators? Various mechanisms have been demonstrated to play critical roles in modulating chromatin accessibility including, germ-line transcription, histone modification, DNA methylation, nuclear localization, chromatin compaction and looping.
The roles of histone modification, DNA methylation and germ-line transcription in antigen receptor gene assembly
More then two decades ago, it was proposed that lineage- and developmental specific regulation of antigen receptor gene assembly, is regulated by chromatin accessibility of Ig and TCR loci to the V(D)J recombinase [27]. Consistent with this proposal are in vitro studies showing that the nucleosome substantially interferes with cleavage of recombination signal sequences by the RAG proteins [28,29]. Histone modification likely plays an important role in this process. A region containing the DHJH cluster initially becomes hyperacetylated in pre-pro-B cells, followed by acetylation of VH segments once DHJH joints have been generated [30]. Distinct chromatin modifiers regulate Igh accessibility. Ezh2, a H3K27 methyltransferase, normally associated with transcriptional inhibition, promotes IgH V(D)J joining of the distal VH regions [31]. The transcription factor Pax5 modulates histone-demethylation, whereas STAT5 promotes histone acetylation of distal VH regions [19,32]. Whereas these studies implicate epigenetic marking in the regulation of antigen receptor assembly, the precise mechanism by which they allow chromatin accessibility to the recombination machinery remains to be determined.
The most common modification of DNA involves the methylation of cytosine residues, mediated by a family of DNA methyltransferases that have the ability to promote the de novo methylation or alternatively modulate hemi-methylated DNA sequences. The role of methylation in antigen receptor assembly has been particularly well studied in the IgK loci [33]. During B-lineage development, demethylation occurs in a monoallelic fashion prior to the onset of IgK VJ gene rearrangement [34]. Interestingly, recent studies have indicated that the demethylated IgK allele is also selectively targeted by AID [35].
Both sense and anti-sense germ-line transcription have been shown to correlate well with Igh V(D)J joining [27,36]. Germ-line transcription is controlled by enhancer and promoter elements [3]. Pre-BCR signaling activates IgK germ-line transcription in large-pre-B cells, whereas TCRα germ-line transcription is induced during β-selection [3]. The presence of germ-line transcripts encoded by antigen receptor genes has raised the question whether they play a critical role in modulating antigen receptor gene assembly. Most compelling evidence for a role in germ-line transcription in antigen receptor gene assembly has been obtained from studies focused on the regulation of TCRα VJ rearrangement. Inhibition of transcriptional elongation severely perturbed TCRα VJ rearrangement as well as remodeling of Jα promoters [37].
Nuclear location, compaction, looping and antigen receptor gene assembly
During the past decade, structure preserving three-dimensional fluorescence in situ hybridization and high precision epifluorescence microscopy, have provided insight into the organization and nuclear localization of antigen receptor genes in developing lymphocytes (Figure 3). In non-lymphoid cells as well as T cells, the Igh locus is positioned in close proximity to the nuclear membrane, whereas in committed pro-B cells the Igh locus is located in central nuclear domains [38,39]. In peripheral B cells, the untranscribed Igh locus is associated with heterochromatic DNA domains, while the transcriptionally active Igh allele is located away from the heterochromatin [40].
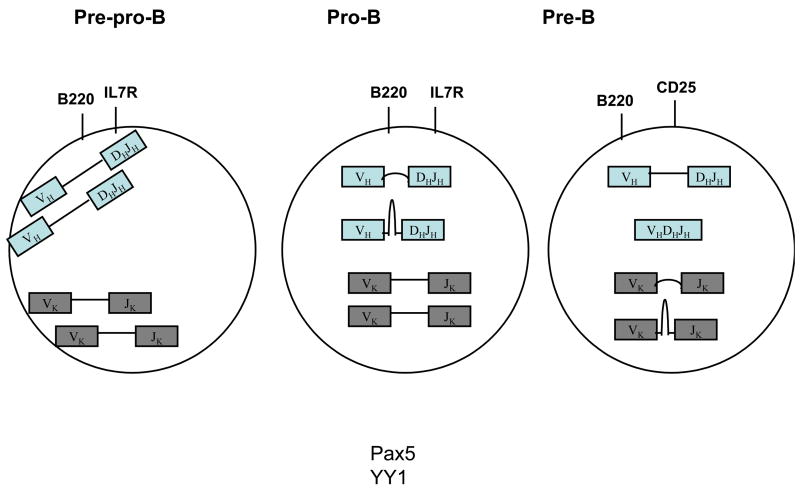
Figure 3.
Spectrum of Igh and Igl topologies and nuclear location in pre-pro-B, pro-B and pre-B cells. Indicated are the nuclear membrane localization of the Igh and Igl loci in pro-B cells. Igh locus contraction is observed on both alleles in pro-B cells, presumably mediated by looping of the intervening sequences. Mono-allelic looping bringing the Igh V, D and J elements into close proximity has been observed at the pro--B cells but not at the pre-pro-B cell stage. The IgL loci are also proposed to undergo mono-allelic looping (note that this still has to be demonstrated).
The IgK locus is also repositioned during B-lineage maturation. In pre-pro-B cells, the IgK locus is associated with the nuclear membrane, whereas in committed pro-B cells, the IgK loci move to centrally located nuclear domains [41,42]. At the small pre-B cell stage, one IgK allele is localized at the centromeric regions, whereas the other allele is repositioned away from the nuclear membrane and centromeric regions. The association of the IgK locus with centromeric heterochromatin is mediated by a silencer element, termed Sis, located within the IgK locus [43].
The Igh locus undergoes substantial large-scale genomic reorganization during B cell development. The Igh locus in T-lineage cells shows an extended configuration, whereas the Igh topology is contracted in central nuclear areas of pro-B cells [38,44]. Recent observations have demonstrated that two transcriptional regulators, Pax5 and YY1, modulate Igh locus contraction (Figure 3) [19,21].
Pax5 is required to promote distal, but not proximal, V(D)J gene rearrangement [20]. Whereas the distal Igh VH regions fail to recombine in Pax5-ablated cells, they are accessible to the recombination machinery [20]. Interestingly, the spatial distances separating the distal VH regions from the CH regions were substantially increased in Pax5-deficient pro-B cells [19]. Thus, Pax5 may act in pro-B cells, at least in part, by increasing the frequency by which distal VH regions encounter DHJH elements.
Recent observations have suggested another player, YY1, involved in the control of Igh V(D)J gene rearrangement. YY1 is a zinc-finger containing protein, that has remained conserved throughout invertebrate and vertebrate evolution. It is ubiquitously expressed but since it interacts with elements present in the Ig enhancers, it has been suggested to play critical roles in immunoglobulin gene regulation [45,46,47]. Recent data have shown that B cell development in YY1-deficient mice is blocked prior to the onset of Igh V(D)J gene rearrangement [21]. As described for Pax5-ablated mice, the defect in V(D)J gene rearrangement is most severe in the distal VH regions. Furthermore, the spatial distances separating the distal VH regions from the CH elements are substantially increased in YY1 pro-B cells when compared to wild type pro-B cells [21]. YY1 does not modulate the expression of Pax5, indicating that YY1 acts independently of Pax5 to control locus contraction and V(D)J gene rearrangement [21].
How do Pax5 and YY1 modulate IgH topology? Although still to be proven, it is conceivable that the Igh locus is organized in clouds of loops. Pax5 and YY1 may act to modulate either loop size or the spacing between putative loops. Alternatively, the Igh locus might be organized into clusters of loops and Pax5 and/or YY1 may modulate the spacing between clusters of loops, perhaps through the induction of de novo loops (Figure 3). Obviously, it will be important to determine how the Igh locus is organized in 3D-space.
Allelic exclusion and nuclear geography
The analysis of the nuclear organization of Igh genes has also provided insight into the mechanisms that underlie the allelic exclusion process [33]. Mono-allelic looping involving Igh VH and DHJH elements occurs with substantially higher frequencies in committed pro-B cells as compared to non-B lineage cells (Figure 3) [44]. Mono-allelic looping might be caused by the low probability of VH regions to encounter DHJH elements in pro-B cells and/or allelic differences in chromatin structure [44]. Similarly, allelic differences and rare induction of enhancer activity may also contribute to the allelic exclusion mechanism in the IgK locus [48].
Other levels of allelic exclusion of antigen receptor genes involve feedback mechanisms, mediated by pre-BCR and pre-TCR signaling. Pre-BCR mediated signaling results in the decontraction of the Igh locus [41]. Spatial distances separating the VH regions from the DHJH elements in B cells expressing a pre-BCR have not been directly measured, but it seems likely that these distances would be increased as well upon pre-BCR signaling. As described above, larger spatial separation would result in decreasing probabilities for VH regions to encounter DHJH elements, contributing to the allelic exclusion mechanism. It has been proposed that additional mechanisms act to regulate the Igh allelic exclusion process. Once a functional V(D)J joint has been generated, the second Igh allele relocates to the heterochromatic region, possibly preventing continued Igh V(D)J gene rearrangement [41]. Mono-allelic activation and mono-allelic silencing have also been suggested to play critical roles in the mechanisms that control TCR allelic exclusion [49]. The TCRβ and TCRα loci show substantial locus contraction in the DN and DP cell stage, respectively. Decontraction of the TCRβ locus was observed in DP thymocytes. As described for the Ig loci, mono-allelic association of the TCRβ locus with heterochromatic regions was observed [44]. The critical issue is now to determine whether mono-allelic looping involving variable and DJ elements is controlled by epigenetic mechanisms or alternatively by mono-allelic activation of enhancer activity. It is conceivable that the probability of V regions to encounter DJ elements in developing lymphocytes is low preventing bi-allelic rearrangements. Statistical analysis describing cumulative frequency distributions of spatial distances separating the V and DJ elements should provide insight into the probabilistic nature of antigen receptor gene rearrangement.
Consistent with a probabilistic nature of antigen receptor gene rearrangement are the observations that the dosage of E2A proteins is rate limiting with regard to TCRγ VJ and TCRδ V(D)J gene rearrangement [50]. Similarly, in pre-B lineage cells, receptor editing is highly sensitive to the dosage of the E2A proteins [25]. Thus the low dosage of the E2A protein may prevent bi-allelic Igh enhancer/promoter activation [48]. Epigenetic mechanisms likely contribute to the allelic exclusion process by allowing or suppressing the ability of transcriptional regulators to interact with regulatory elements in antigen receptor loci [33].
Collectively, these studies suggest that allelic exclusion is regulated, at least in part, by four distinct epigenetic mechanisms: (1) Mono-allelic looping. (2) Mono-allelic activation of enhancer activity. (3) De-contraction of antigen receptor genes mediated by pre-BCR or pre-TCR signaling. (4) Association of antigen receptor genes with hetero-chromatic regions.
Organization of antigen receptor loci
Much has been learned during the past decade about the nuclear localization and contraction mechanisms of antigen receptor genes. However, how the Ig and TCR genes are organized in 3D-space is unknown. Understanding the topology and mechanics of antigen receptor assembly will require a statistical description focused on the average size and shape rather than a static structure. Ultimately, it should be possible to describe the spectrum of antigen receptor conformations in terms of statistical mechanics, providing a physical explanation for the mechanisms that underlie the assembly of antigen receptor genes.
Acknowledgments
I thank Suchit Jhujhunwala, Menno van Zelm, Roy Riblet, Mandy Peak, Tobias Knoch and Doug Smith for stimulating discussions regarding chromatin topology. Work on this subject in the author’s laboratory was supported by the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
- 1.Hesslein DG, Schatz DG. Factors and forces controlling V(D)J recombination. Adv Immunol. 2001;78:169–232. doi: 10.1016/s0065-2776(01)78004-2. [DOI] [PubMed] [Google Scholar]
- 2.Mostoslavsky R, Alt FW, Bassing CH. Chromatin dynamics and locus accessibility in the immune system. Nat Immunol. 2003;4:603–606. doi: 10.1038/ni0703-603. [DOI] [PubMed] [Google Scholar]
- 3.Jung D, Gillourakis C, Mostoslavsky R, Alt FW. Mechanism and control of V(D)J recombination at the immunoglobulin heavy chain gene. Annual Rev Immunol. 2006;24:541–570. doi: 10.1146/annurev.immunol.23.021704.115830. [DOI] [PubMed] [Google Scholar]
- 4.Alt FW, Yancopoulos GD, Blackwell TK, Wood C, Thomas E, Boss M, Coffman N, Rosenberg N, Tonegawa S, Baltimore D. Ordered rearrangement of immunoglobulin heavy chain variable region segments. EMBO J. 1984;3:1209–1219. doi: 10.1002/j.1460-2075.1984.tb01955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nussenzweig MC, Shaw AC, Sinn E, Danner DB, Hohnes HC, Morse HC, III, Leder P. Allelic exclusion in transgenic mice that express the membrane form of Ig M. Science. 1988;236:816–819. doi: 10.1126/science.3107126. [DOI] [PubMed] [Google Scholar]
- 6.Manz J, Denis K, Witte O, Brinster R, Storb U. Feedback inhibition of immunoglobulin gene rearrangement by membrane but not secreted μ heavy chains. J Exp Med. 1988;168:1363–1381. doi: 10.1084/jem.168.4.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.von Boehmer H. Selection of the T-cell repertoire: receptor-controlled checkpoints in T-cell development. Adv Immunol. 2004;84:201–238. doi: 10.1016/S0065-2776(04)84006-9. [DOI] [PubMed] [Google Scholar]
- 8.Busslinger M. Transcriptional control of early B cell development. Annu Rev Immunol. 2004;22:55–79. doi: 10.1146/annurev.immunol.22.012703.104807. [DOI] [PubMed] [Google Scholar]
- 9.Ikawa T, Kawamoto H, Goldrath AW, Murre C. E proteins and Notch signaling cooperate to promote T cell lineage specification and commitment. J Exp Med. 2006;15:1329–1342. doi: 10.1084/jem.20060268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bertolino E, Reddy K, Medina KL, Parganas E, Ihle J, Singh H. Regulation of interleukin 7-dependent immunoglobulin heavy-chain variable gene rearrangements by transcription factor STAT5. Nat Immunol. 2005;6:836–43. doi: 10.1038/ni1226. [DOI] [PubMed] [Google Scholar]
- 11.Roessler S, Györy I, Imhof S, Spivakov M, Williams RR, Busslinger M, Fisher AG, Grosschedl R. Distinct promoters mediate the regulation of Ebf1 gene expression by IL-7 and Pax5. Mol Cell Biol. 2007;2:2253–2258. doi: 10.1128/MCB.01192-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maier H, Ostraat R, Gao H, Fields S, Shinton SA, Medina K, Ikawa T, Murre C, Singh H, Hardy RR, Hagman J. Early B cell factor cooperates with Runx1 and mediates epigenetic changes associated with mb-1 transcription. Nat Immunol. 2004;5:1069–1077. doi: 10.1038/ni1119. •In hematopoietic stem cells, the mb-1 promoter is hypermethylated. However, the promoter is progressively demethylated upon maturing into the pro-B cell compartment. Beyond the pro-B cell stage the mb-1 promoter is completely demethylated. Demethylation and chromatin remodeling at the mb-1 promoter is modulated by EBF. These observations suggest a regulatory network in which EBF acts upstream of Pax5 to promote B-lineage specification. [DOI] [PubMed] [Google Scholar]
- 13.Rothenberg EV. Regulatory factors for initial T lymphocyte lineage specification. Curr Opin Hematol. 2007;14:322–329. doi: 10.1097/MOH.0b013e3281de72a8. [DOI] [PubMed] [Google Scholar]
- 14.Hoflinger S, Kesavan K, Fuxa M, Hutter C, Heavey B, Radtke F, Busslinger M. Analysis of Notch1 function by in vitro T cell differentiation of Pax5 mutant lymphoid progenitors. J Immunol. 2004;15:3935–3944. doi: 10.4049/jimmunol.173.6.3935. •Evidence is presented demonstrating that Notch1 mediated signaling acts to induce the expression of GATA3 and TCF1. These data link Notch signaling, GATA3 and TCF1 in a common pathway to regulate T-lineage maturation. [DOI] [PubMed] [Google Scholar]
- 15.Barndt RJ, Dai M, Zhuang Y. Functions of E2A-HEB heterodimers in T-cell development revealed by a dominant negative mutation of HEB. Mol Cell Biol. 2000;20:6677–6685. doi: 10.1128/mcb.20.18.6677-6685.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murre C. Helix-loop-helix proteins and lymphocyte development. Nat Immunol. 2005;6:1079–1086. doi: 10.1038/ni1260. [DOI] [PubMed] [Google Scholar]
- 17.Engel I, Murre C. E2A proteins enforce a proliferation checkpoint in developing thymocytes. EMBO J. 2004;23:202–211. doi: 10.1038/sj.emboj.7600017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stanhope-Baker P, Hudson KM, Shaffer AL, Constantinescu A, Schlissel MS. Cell type-specific chromatin structure determines the targeting of V(D)J recombinase activity in vitro. Cell. 1996;14:887–899. doi: 10.1016/s0092-8674(00)81272-6. •The V(D)J recombinase is expressed in both developing B- and T-lineage cells. However, the rearrangement of antigen receptor genes is tightly regulated. Using an in vitro approach, the investigators demonstrate that the lineage- and stage-specificity of recombination is reflected in the chromatin accessibility. [DOI] [PubMed] [Google Scholar]
- 19.Fuxa M, Skok J, Souabni A, Salvagiotto G, Roldan E. Busslinger M: Pax5 induces V-DJ rearrangements and locus contraction of the immunoglobulin heavy-chain gene. Genes Dev. 2004;18:411–422. doi: 10.1101/gad.291504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hesslein DG, Schatz DG. Factors and forces controlling V(D)J recombination. Adv Immunol. 2001;78:169–232. doi: 10.1016/s0065-2776(01)78004-2. [DOI] [PubMed] [Google Scholar]
- 21.Liu H, Schmidt-Supprian M, Shi Y, Hobeika E, Barteneva N, Jumaa H, Pelanda R, Reth M, Skok J, Rajewsky K, Shi Y. Yin Yang 1 is a critical regulator of B cell development. Genes Dev. 2007;21:1179–1189. doi: 10.1101/gad.1529307. ••YY1 is a zinc-finger containing protein, which has the ability to bind to immunoglobulin enhancer elements. In this study, the role of YY1 in B cell development, YY1-deficient mice were generated. B cell development in YY1-deficient mice was blocked at the pro-B cell stage. Ig V(D)J rearrangements involving distal VH regions were severely perturbed. However, the expression patterns of Pax5, E2A and EBF were not affected in the absence of YY1. These data raise the possibility that YY1 is involved in modulating Igh topology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Romanow WJ, Langerak AW, Goebel P, Wolvers-Tettero IL, van Dongen JJ, Feeney SJ, Murre C. E2A and EBF act in synergy with the V(D)J recombinase to generate a diverse immunoglobulin repertoire in nonlymphoid cells. Mol Cell. 2000;5:343–353. doi: 10.1016/s1097-2765(00)80429-3. [DOI] [PubMed] [Google Scholar]
- 23.Goebel P, Janney N, Valenzuela JR, Romanow WJ, Murre C, Feeney AJ. Localized gene-specific induction of accessibility to V(D)J recombination induced by E2A and early B cell factor in nonlymphoid cells. J Exp Med. 2001;194:645–656. doi: 10.1084/jem.194.5.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inlay MA, Tian H, Lin T, Xu Y. Important roles for E protein binding sites within the immunoglobulin K chain intronic enhancer in activating VκJκ rearrangement. J Exp Med. 2006;203:1721–1732. doi: 10.1084/jem.20041135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quong M, Martensson A, Langerak AW, Rivera RR, Nemazee D, Murre C. Receptor editing and marginal zone B cell development are regulated by the helix-loop-helix protein, E2A. J Exp Med. 2004;199:1113–1120. doi: 10.1084/jem.20031180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Z, Espinoza CR, Yu Z, Stephan R, He T, Williams GS, Burrows PD, Hagman J, Feeney AJ, Cooper MD. Transcription factor Pax5 (BSAP) transactivates the RAG-mediated V(H)-to-D(H)J(H) rearrangement of immunoglobulin genes. Nat Immunol. 2006;7:616–24. doi: 10.1038/ni1339. [DOI] [PubMed] [Google Scholar]
- 27.Yancopoulos G, Alt F. Developmentally controlled and tissue-specific expression of unrearranged VH gene segments. Cell. 1985;40:271–281. [PubMed] [Google Scholar]
- 28.Kwon J, Imbalzano AN, Mathews A, Oettinger MA. Accessibility of nucleosomal DNA to V(D)J cleavage is modulated by RSS positioning and HMG1. Mol Cell. 1998;2:829–839. doi: 10.1016/s1097-2765(00)80297-x. [DOI] [PubMed] [Google Scholar]
- 29.Golding A, Chandler S, Ballestar E, Wolffe AP, Schlissel MS. Nucleosome structure completely inhibits in vitro cleavage by the V(D)J recombinase. EMBO J. 1999;18:3712–23. doi: 10.1093/emboj/18.13.3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chowdhury D, Sen R. Stepwise activation of the immunoglobulin heavy chain gene locus. EMBO J. 2001;20:6394–6403. doi: 10.1093/emboj/20.22.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Su IH, Basavaraj A, Krutchinsky AN, Hobert O, Ullrich A, Chait BT, Tarakhovsky A. Ezh2 controls B cell development through histone H3 methylation and Igh rearrangement. Nat Immunol. 2003;4:124–31. doi: 10.1038/ni876. • Ezh2 is a H3K27 methyltransferase that is associated with transcriptional repression. The investigators provide evidence that Ezh2 is required to promote, rather than inhibit, IgH V(D)J rearrangement that involve distal VH regions. [DOI] [PubMed] [Google Scholar]
- 32.Johnson K, Pflugh DL, Yu D, Hesslein DG, Lin KI, Bothwell AL, Thomas-Tikhonenko A, Schatz DG, Calame K. B cell-specific loss of histone 3 lysine 9 methylation in the V(H) locus depends on Pax5. Nat Immunol. 2004;5:853–61. doi: 10.1038/ni1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bergman Y, Cedar H. 2004: A stepwise epigenetic process controls immunoglobulin allelic exclusion. Nat Rev Immunol. 2004;10:753–761. doi: 10.1038/nri1458. [DOI] [PubMed] [Google Scholar]
- 34.Mostoslavsky R, Singh N, Kirillov A, Pelanda R, Cedar H, Chess A, Bergman Y. Kappa chain monoallelic demethylation and the establishment of allelic exclusion. Genes Dev. 1998;15:1801–11. doi: 10.1101/gad.12.12.1801. •Evidence is provided for mono-allelic marking of the IgK loci. The IgK locus is CpG methylated in pro-B cells. Upon developmental progression the IgK alleles undergo progressive mono-allelic demethylation. It is proposed that methylation of CpG residues is important for the allelic exclusion mechanism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fraenkel S, Mostoslavsky R, Novobrantseva TI, Pelanda R, Chaudhuri J, Esposito G, Jung S, Alt FW, Rajewsky K, Cedar H, Bergman Y. Allelic ‘choice’ governs somatic hypermutation in vivo at the immunoglobulin kappa-chain locus. Nat Immunol. 2007 doi: 10.1038/ni1476. in press. [DOI] [PubMed] [Google Scholar]
- 36.Bolland DJ, Wood AL, Johnston CM, Bunting SF, Morgan Chakalova GL, Fraser PJ, Corcoran AE. Antisense intergenic transcription in V(D)J recombination. Nat Immunol. 2004;5:630–637. doi: 10.1038/ni1068. [DOI] [PubMed] [Google Scholar]
- 37.Abarrategui I, Krangel MS. Regulation of T cell receptor-alpha gene recombination by transcription. Nat Immunol. 2006;7:1109–115. doi: 10.1038/ni1379. •Previous observations have indicated that germ-line transcription is associated with antigen receptor gene rearrangements. Here unambiguous evidence is provided that transcriptional elongation is required to promote TCRα VJ gene rearrangement. [DOI] [PubMed] [Google Scholar]
- 38.Kosak ST, Skok JA, Medina KL, Riblet R, Beau MML, Fisher AG, Singh H. Subnuclear compartmentalization of immunoglobulin loci during lymphocyte development. Science. 2002;296:158–162. doi: 10.1126/science.1068768. •This is the first paper demonstrating contraction of the IgH locus upon commitment to the B cells lineage. Additionally, it is shown that the IgH alleles in non-B cells are located at the nuclear membrane, whereas in B cells the IgH alleles are positioned in centrally nuclear domains. [DOI] [PubMed] [Google Scholar]
- 39.Yang Q, Riblet R, Schildkraut CL. Sites that direct nuclear compartmentalization are near the 5′ end of the mouse immunoglobulin locus. Mol Cell Biol. 2005;25:6021–6030. doi: 10.1128/MCB.25.14.6021-6030.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Skok JA, Brown KE, Azuara V, Caparros ML, Baxter J, Takacs K, Dillon N, Gray D, Perry RP, Merkenschlager M, Fisher AG. Nonequivalent nuclear location of immunoglobulin alleles in B lymphocytes. Nat Immunol. 2001;2:848–854. doi: 10.1038/ni0901-848. [DOI] [PubMed] [Google Scholar]
- 41.Roldan E, Fuxa M, Chong W, Martinez D, Novatchkova M, Busslinger M, Skok J. Locus ‘decontraction’ and centromeric recruitment contribute to allelic exclusion of the immunoglobulin heavy-chain gene. Nat Immunol. 2005;6:31–41. doi: 10.1038/ni1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goldmit M, Ji Y, Skok J, Roldan E, Jung S, Cedar H, Bergman Y. Epigenetic ontogeny of the IgK locus during B cell development. Nat Immunol. 2005;6:198–203. doi: 10.1038/ni1154. • Here evidence is provided that in pre-B cells the IgK locus is associated with either active chromatin or centromeric heterochromatin. Interestingly, the interaction with heterochromatin is associated with Ikaros and HP1 binding. [DOI] [PubMed] [Google Scholar]
- 43.Liu Z, Widlak P, Zou P, Xiao F, Oh M, Li S, Chang MY, Shay JW, Garrard WT. A recombination silencer that specifies heterochromatin positioning and Ikaros association in the immunoglobulin K locus. Immunity. 2006;24:405–415. doi: 10.1016/j.immuni.2006.02.001. •The investigators identify a novel regulatory element, named sis, required for the targeting of the IgK locus to centromeric heterochromatin. It is proposed that Sis is involved in the monoallelic IgK silencing. [DOI] [PubMed] [Google Scholar]
- 44.Sayegh C, Jhunjhunwala S, Riblet R, Murre C. Visualization of looping involving the immunoglobulin heavy-chain locus in developing B cells. Genes Dev. 2005;19:322–327. doi: 10.1101/gad.1254305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park M, Atchinson MI. Isolation of a candidate repressor/activator, NF-E1 (YY1), that binds to the immunoglobulin K 3′ enhancer and the immunoglobulin heavy chain mE1 site. Proc Natl Acad Sci. 1991;88:9804–9808. doi: 10.1073/pnas.88.21.9804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gordon SJ, Saleque S, Birnstein BK. Yin Yang is a lipopolysaccharide-inducible activator of the murine 3′ Igh enhancer, hs3. J Immunol. 2003;170:5549–5557. doi: 10.4049/jimmunol.170.11.5549. [DOI] [PubMed] [Google Scholar]
- 47.Calame K, Atchison M. YY1 helps to bring loose ends together. Genes Dev. 2007;15:1145–1152. doi: 10.1101/gad.1559007. [DOI] [PubMed] [Google Scholar]
- 48.Liang HE, Hsu LY, Cado D, Schlissel MS. Variegated transcriptional activation of the immunoglobulin K locus in pre-B cells contributes to the allelic exclusion of light-chain expression. Cell. 2004;118:19–29. doi: 10.1016/j.cell.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 49.Skok JA, Gisler R, Novatchkova M, Farmer D, de Laat W, Busslinger M. Reversible contraction by looping of the Tcrα and Tcrβ loci in rearranging thymocytes. Nat Immunol. 2007;8:378–387. doi: 10.1038/ni1448. • Previous data obtained by the investigators have demonstrated that the IgH alleles undergo decontraction upon developing into the pre-B cell compartment. The studies described in this paper extend these observations to the TCR loci. The TCRβ and TCRα loci underwent long-range contraction in the DP and DN compartment, respectively. It is suggested that locus decontraction contributes to the allelic exclusion mechanism of TCR genes. [DOI] [PubMed] [Google Scholar]
- 50.Bain G, Romanow WJ, Albers K, Havran WL, Murre C. Positive and negative regulation of V(D)J recombination by the E2A proteins. J Exp Med. 1999;1999;189:289–300. doi: 10.1084/jem.189.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]