Abstract
During HIV-1 infection, the CD8+ T lymphocyte response is critical to controlling the virus; indeed, the development of AIDS results, in large part, from the eventual failure of this response. The ability to measure the composite CD8+ T lymphocyte anti-viral activity is, therefore, an essential requirement in the evaluation of immune based therapies and potential vaccines. We report here the details of a reproducible assay that measures the ability of CD8+ T lymphocytes to suppress viral production by infected autologous CD4+ T lymphocytes. The assay is not limited to persons with any specific HLA type, and the use of bi-specific antibodies for cell expansion makes the assay feasible in situations where cell numbers may be limiting. The measurement of viral production over time provides a global readout of the CD8+ T lymphocyte overall function against HIV-1, which can be used for longitudinal assessment of individual HIV-infected persons in order to evaluate therapy, immune reconstitution, and new vaccines.
Keywords: HIV, CD8 T cell, viral inhibition, co-culture
1. Introduction
The CD8+ T lymphocyte is central to the control of HIV-1 infection. Data from humans, as well as macaque CD8+ T lymphocyte depletion studies, have extensively documented the essential role of HIV-specific CD8+ T lymphocyte cytotoxicity and non-lytic anti-viral mechanisms (Rasmussen et al., 2002; Robinson, 2003; DeVico & Gallo, 2004; Klein et al., 1995). Conversely, in other individuals, the progression to AIDS usually coincides with a decline in cytotoxic T lymphocyte (CTL) activity and poorly proliferatvie HIV-specific CD8+ T lymphocyte (Goulder et al., 1997). Indeed, based on these observations, there has been a paradigm shift in the approach to HIV-1 vaccine development, with increased emphasis on potent CD8+ T lymphocyte immunity. Various technical advances have enabled more quantitative and highly detailed analysis of virus-specific cellular immunity in humans, but there is still a need for a simple, reproducible all-encompassing single assay that can evaluate the composite of CD8+ T lymphocyte antiviral activity, which may help in the development of a universally accepted single measure of what successful immune reconstitution entails (Lieberman, 2004).
Many of the current methods of assessing CD8+ T lymphocyte function during chronic HIV-1 infection involve separate and distinct assays that are each restricted to a particular facet of anti-viral activity. Tetramer staining, which enumerates cells that can bind to a particular HLA/peptide complex, provides no functional information, and, furthermore, is limited to the few available HLA allele/peptide combinations (Shankar et al., 2000). The use of heterologous HIV-1-infected cells allows the detection of CD8+ T cell non-cytotoxic antiviral responses, which is only one component of the CD8+ T lymphocyte response in HIV-1 infected persons (Mackewicz, Garovoy, & Levy, 1998; Killian, Ng, Mackewicz, & Levy, 2005). Antigen-specific gamma interferon production, measured either by ELISPOT or intracellular staining, does not necessarily correlate with cytotoxicity (Appay et al., 2000). Chromium release or flow cytometry-based cytotoxic T lymphocyte (CTL) assays usually involve target cells that are HLA-matched at a single locus, and therefore do not reflect activity against all the relevant peptide/Class I complexes which the CD8+ T lymphocytes are capable of recognizing. The alternative approach, using autologous B cell lymphoblastoid cell lines (LCL) generated by EBV transformation (Ferrari et al., 1997; Sheehy, McDermott, Furlan, Klenerman, & Nixon, 2001), eliminates this problem, but LCL may not precisely mirror primary CD4+ T lymphocytes, the physiological targets of HIV-1 infection. Antigen-specific proliferation assays reflect just that, and may not necessarily provide information regarding the actual effector functions of the dividing HIV-specific CD8+ T lymphocytes. Moreover, many of the currently used assays for CD8+ T lymphocyte function involve response to selected HIV-1 peptides, and are therefore not accurate models of the overall HIV-1 response (Betts, Casazza, & Koup, 2001).
In earlier studies, we established a co-culture system to evaluate the ability of CTL clones to inhibit viral production by acutely infected HLA-matched cell lines (Yang et al., 1997). The endpoint involved ELISA evaluation of p24 production, which is an unbiased measure that encompasses the summation of CD8+ T lymphoctye anti-HIV-1 activity during the co-culture period, reflecting proliferation, cytolysis, and release of soluble anti-viral factors. This assay provided valuable new insights into antigen specificity and viral escape mechanisms, but nonetheless was limited to a specific HLA allele. In addition, the infection of CD4+ T cell lines which have been passaged in culture for long periods may not necessarily reflect the infection of primary CD4+ T lymphocytes. We have now developed a modified version of this co-culture system that can be used on a small amount of blood, from which populations of autologous CD8+ and CD4+ T lymphocytes are each expanded separately with bi-specific antibodies, in order to evaluate the composite of several CD8+ T lymphocyte anti-viral functions in HIV-1-infected persons of any HLA type. A similar assay, described in the pre-HAART era, also evaluated the anti-viral effect of CD8+ T lymphocytes on the outgrowth of HIV-1 from autologous CD4+ T lymphocytes, but differed from our protocol in that no exogenous virus was added (Hausner, Giorgi, & Plaeger-Marshall, 1993). The infection of the CD4+ T lymphocytes with a high titer of exogenous virus ensures that the majority of anti-viral activity being measured is constant between experiments, and reduces potential variability due to different HAART treatments.
2. Materials and Methods
2.1. Blood samples and initial cell subset expansion
This study was approved by the UCLA Institutional Review Board. All donors were recruited according to UCLA Human Subjects guidelines, and provided informed consent. Blood samples were diluted 1:1 with RPMI containing 10% fetal bovine serum (FBS), and centrifuged for 10 minutes at 300g. The buffy coat was collected, diluted 1:1 in complete RPMI/10% FBS (10 mM Hepes, 2mM glutamine, 50 IU/ml pen/strep), and was layered over Ficoll (1:3 volume to volume), followed by a 30 minute centrifugation at 850g. The peripheral blood mononuclear cell (PBMC) layer was carefully removed and washed twice in RPMI/10%, after which the cell concentration was adjusted to 2×106/ml. To expand the separate populations of CD8+ and CD4+ T cells, the PBMC were plated in 24 well plates (2 ml/well) to which was added 1 μg/ml of either CD3/CD4 or CD3/CD8 bi-specific antibody. These bi-specific antibodies (the generous gift of Dr. Johnson T. Wong to Dr. Otto Yang) expand the CD4+ T lymphocytes or CD8+ T lymphocytes, respectively; the purity of the expanded populations is > 90 %, as determined by flow cytometry (Ibarrondo et al., 2005; Yang et al., 1997). During the 11–14 day expansion period, cells are maintained in 50 units IL-2/ml and sub-cultivated as needed, first into additional 2 ml wells, then into T 25 flasks, to maintain a concentration of approximately 1×106/ml. Alternatively, if sufficient amount of blood is obtained, the subsets can be separated by other methods, such as FACS sorting or magnetic bead techniques, and activated with anti-CD3/CD28 or anti-CD2/CD3/CD28 antibodies.
2.2. Preparation of cells for co-culture
CD4+ and CD8+ T lymphocytes are counted; at least 6×105 CD4 and 5.25×105 CD8 T lymphcytes are needed per condition (e.g., pre-post treatment comparison). The CD4+ T cells are centrifuged (300g for 10 min) in a 15 ml centrifuge tube and resuspended in 200 μl of complete RPMI media, to which wild type HIV-1 NL4-3 virus at an MOI of 0.01–0.1 is added. Tubes are incubated at 37° C/5% CO2 for 4 hours, with intermittent shaking. After the 4-hour incubation, the infected CD4+ T lymphocytes are washed twice with complete RPMI media (300g for 10 min) and resuspended at a concentration of 5×105/mL in IL-2 supplemented (20–50 units/ml) complete RPMI media.
The CD8+ T lymphoctyes are used at three different concentrations, to achieve effector:target ratios of 2:1, 1:1, and 0.5:1. Accordingly, these cells are divided into three 15 mL tubes, each containing 3×105 cells, 1.5×105 cells, and 0.75×105 cells, respectively. The cells are then centrifuged at 300g for 10 min, and each pellet resuspended in 300 μl of IL-2 supplemented (20–50 units/mL) complete RPMI media.
2.3. Autologous co-cultures
The co-cultures are set up in round-bottomed 96-well plates (Corning Costar, Corning, NY, catalog # 3799). The CD4+ T lymphocytes are plated in 12 wells (i.e., one well for each of the 3 effector:target ratios plus control well, each in triplicate) for each condition of CD8+ lymphocyte to be assayed. To each of the 3 control wells, 100 μl of IL-2 supplemented (20–50 units/mL) complete RPMI media is added. These wells will contain no effector cells and will be used to determine the maximum amount of viral production for each condition. To the nine remaining wells containing CD4+ cells, 100μl of CD8+ T lymphocytes is added, at the appropriate concentration, each concentration in triplicate. The plate is incubated for 4 days at 37° C/5% CO2. After 4 days, 100 μl of media is carefully removed from each well (without disturbing the cells) and frozen at −20° C for the p24 ELISA. The media is replaced with 100μl of fresh IL-2 (20–50 units/mL) supplemented complete RPMI media. After an additional 3 days (i.e., day 7 of co-culture), this process is repeated, and the samples are again stored at −20 C. These steps are repeated on day 10 of co-culture; this is last time point to be tested, so it is not necessary to replace media once removed. At this point, the cells can be either discarded, or, if desired, saved for any additional analysis.
2.4 Assay for p24
The amount of HIV-1 released by the CD4+ T lymphocytes can be evaluated by ELISA, EIA or real-time PCR. In this study, we used a commercial p24 ELISA kit (Perkin Elmer, Boston, MA, catalog # NEK050B), which can detect levels of p24 ranging from 12.5 to 400 pg/mL. At least three dilutions of each culture supernatant are tested. Suggested dilutions are 1:5, 1:10, 1:50 for samples from days 4 and 7, and 1:10, 1:100, and 1:1000 for the day 10 samples. Samples from the control wells were similarly diluted. At least one of these dilutions must fall within the linear range of the standard curve for the ELISA. The assay is performed according to the manufacturer’s instructions, and the plates are read at 490 or 492 nm, with a reference filter > 600 nm.
2.5. Calculations and Data Analysis
Triplicate wells showed values that varied by no more than 10%. For each condition, the mean value of the triplicate wells is calculated. Data is presented as percent inhibition, calculated according to the following formula:
3. Results and Discussion
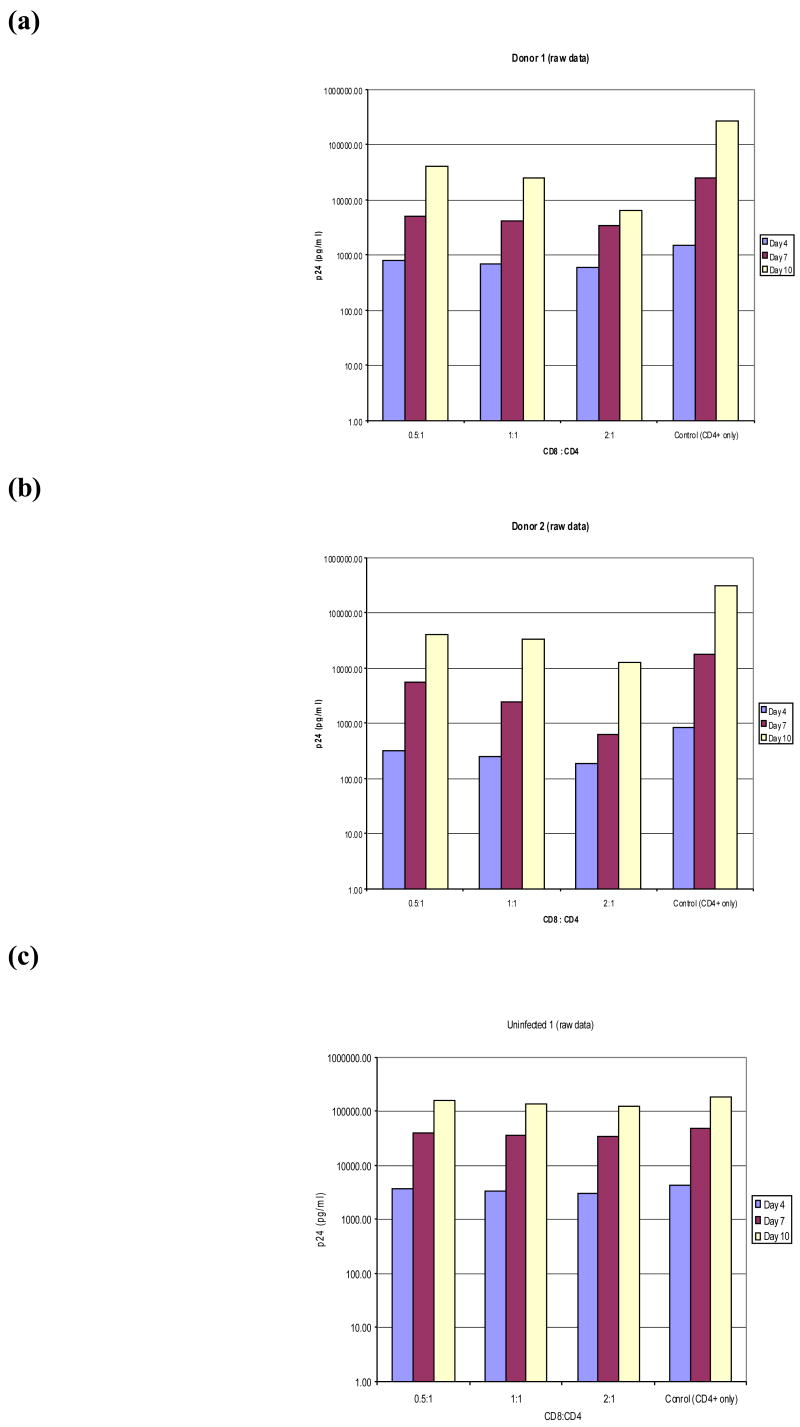
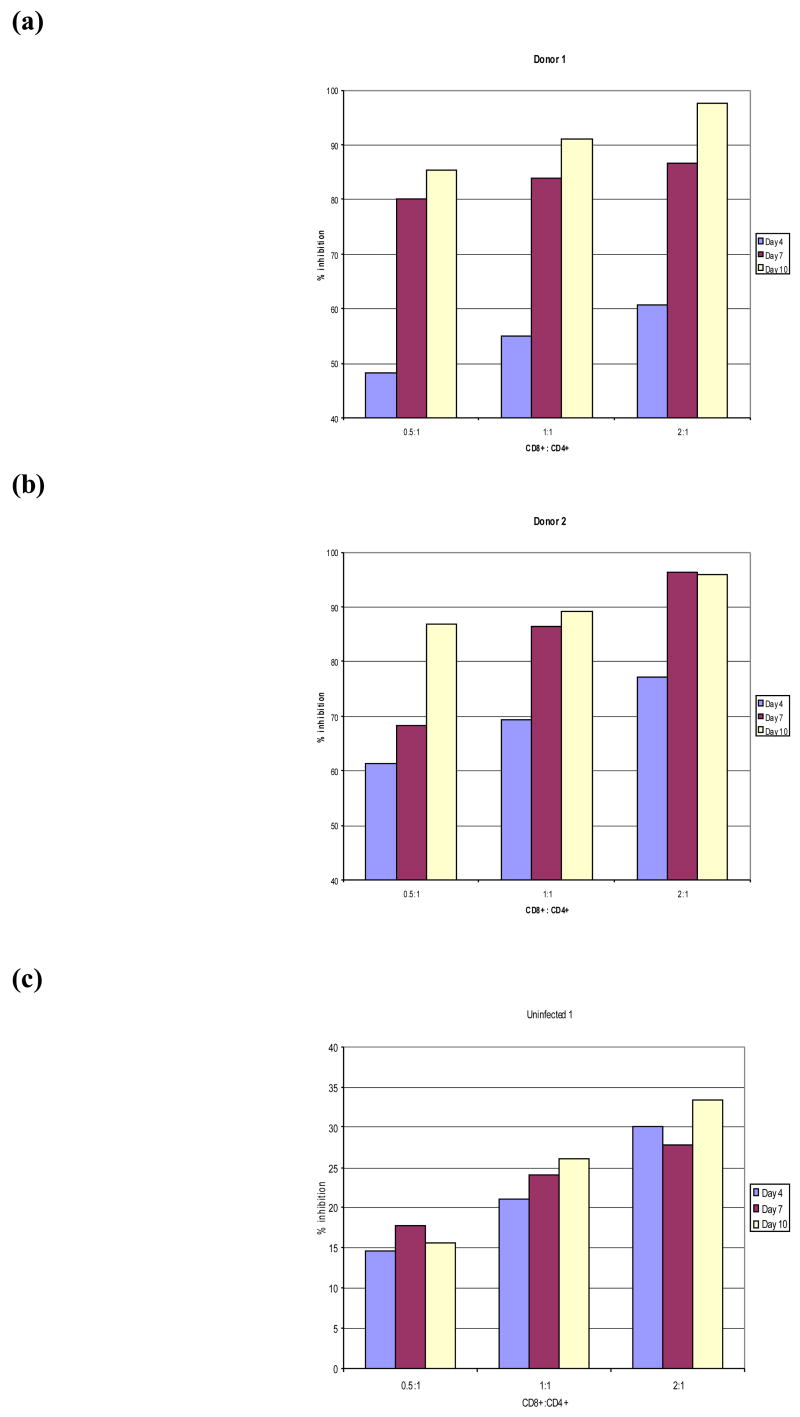
We have evaluated viral inhibition effects of CD8+ T lymphocytes from nine HIV-1-infected individuals who had a wide spectrum of viral loads and CD4+ T lymphocyte counts, and from 3 uninfected individuals (Table 1). Figures 1 and 2 show data from two representative HIV-infected and one uninfected donor. The actual p24 ELISA levels are shown in Figure 1, and the corresponding calculated viral inhibition values are displayed in Figure 2. The results for the HIV-infected individuals show that the presence of CD8+ T lymphocytes in wells with autologous HIV-1-infected CD4+ T lymphocytes results in a substantial reduction in the amount of virus. Indeed, the mean inhibition observed in co-cultures from the 9 HIV-infected donors was 73% (S.D.=11.6), as compared to 23.7% (S.D.=1.5) in cultures from uninfected donors, suggesting that the major viral reduction effects measured in this assay are mediated by HIV-specific CD8+ T lymphocytes. Repeated ELISA measurements on the same culture supernatants were highly reproducible.
Table 1.
Summary of clinical status and viral inhibition data for HIV-1-infected and uninfected donors. (Inhibition data are for CD8:CD4 ratio of 1:1)
| Donor | Viral Load (copies/ml of plasma) | CD4+ count (×106/l) | Inhibition (Day 4) | Inhibition (Day 7) | Inhibition (Day 10) | Mean Inhibition |
|---|---|---|---|---|---|---|
| HIV 1 | 200 | 304 | 56 | 83 | 91 | 77 |
| HIV 2 | 608,000 | 98 | 69 | 87 | 89 | 82 |
| HIV 3 | 35,000 | 122 | 79 | 85 | 90 | 85 |
| HIV 4 | 400 | 281 | 69 | 85 | 91 | 82 |
| HIV 5 | 400 | 333 | 61 | 82 | 91 | 78 |
| HIV 6 | 400 | 572 | 53 | 77 | 93 | 75 |
| HIV 7 | 31,300 | 115 | 41 | 50 | 78 | 56 |
| HIV 8 | 14,000 | 178 | 63 | 69 | 81 | 71 |
| HIV 9 | 50 | 658 | 43 | 47 | 67 | 52 |
| Uninfected 1 | N/A | N/A | 21 | 25 | 26 | 24 |
| Uninfected 2 | N/A | N/A | 19 | 22 | 24 | 22 |
| Uninfected 3 | N/A | N/A | 24 | 24 | 25 | 25 |
Figure 1. Level of viral production measured by the autologous T cell co-culture method.
PBMC were stimulated with bi-specific antibodies to produce pure CD4+ and CD8+ T lymphocyte cultures. After 14 days, the CD4+ cells were infected for 4 hours with wild type HIV-1 virus and placed in a 96 well plate. The CD8+ T lymphocytes were added to the wells containing the infected CD4+ cells at three different ratios (0.5:1, 1:1, and 2:1), each in triplicate. Media only was added to three wells as a positive control for maximum viral production in the absence of CD8+ T lymphocytes. Viral production was measured by p24 ELISA at three time points, as described in Materials and Methods. Panels a and b show the actual p24 production at three different timepoints from HIV+ Donors 1 and 2, respectively. Panel c shows values for Uninfected Donor 1.
Figure 2. Viral inhibition.
The degree of viral inhibition was calculated according to the formula:
1−(mean p24 levels for experimental wells/mean p24 for control wells) × 100
Panels a and b show the calculated percent inhibition at three different time-points for HIV+ Donors 1 and 2, respectively. Panel c shows the calculated percent inhibition at three different time-points for Uninfected donor 1.
The autologous co-culture assay thus provides a practical method of evaluating the composite HIV-specific effector function of CD8+ T lymphocytes from HIV-1-infected individuals. The single measurement of p24 levels at several time-points during the co-culture period provides an unbiased readout that reflects the multiple types of anti-viral activity of the CD8+ T lymphocytes. These functions most likely comprise proliferation, lysis of infected cells, and the production of both known and as-yet unidentified anti-viral cytokines and chemokines (Levy, 2003; DeVico et al., 2004). In addition, the technique of expanding the CD8+ T lymphocytes with bi-specific antibodies permits the assay to be used in situations where the initial cell population is scarce. Importantly, CD8+ T lymphocytes expanded in this manner and tested in an ELISpot assay have been shown to reflect the T cell receptor repertoire and anti-HIV function of the original ex vivo cell population (Jones et al., 2003).
In the limited sample of HIV-infected donors tested thus far, the degree of viral inhibition correlated neither with the viral load (p= 0.283) nor with CD4+ T lymphocyte count (p=0.12). This is not unexpected, since plasma viral load does not necessarily reflect total viral burden (Anton et al., 2003) or the length of time that the CD8+ T lymphocytes have been fighting the virus. The co-culture assay, therefore, would be most appropriate for evaluating changes over time within a single individual, rather than for inter-individual comparisons.
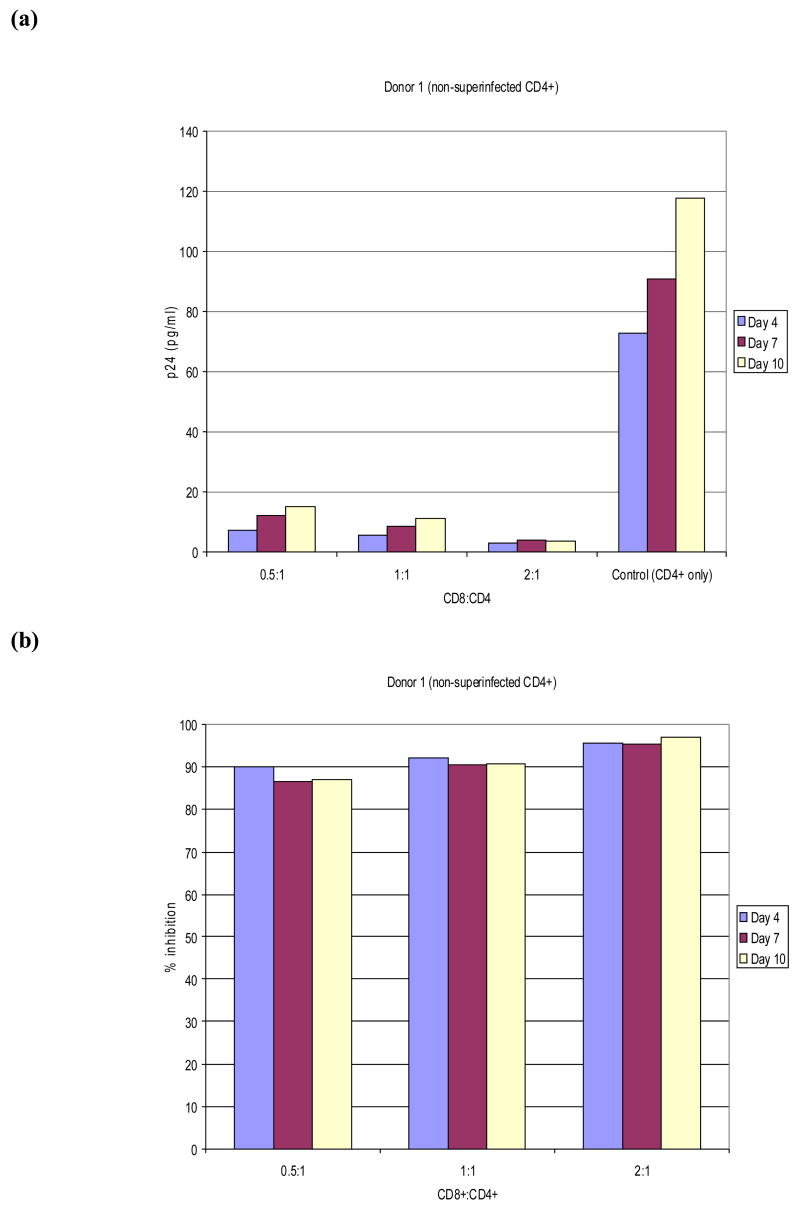
The viral inhibition readout in our assay is most likely reflecting the large amount of exogenous virus added, rather than endogenous HIV-1, particularly for those persons in whom HAART is successful. This was confirmed in experiments on three of the HIV-1-infected donors, in which we compared p24 levels and viral inhibition in co-cultures not infected with exogenous virus to those which did receive exogenous virus. A representative experiment is shown in Figure 3, which demonstrates that the p24 levels are minimal when no exogenous virus is added, and that the small amount of virus produced in culture by the endogenously infected CD4+ cells is completely inhibited by autologous CD8+ T lymphocytes. These data are consistent with earlier kinetic studies on viral production by CD4+ T lymphocytes from HIV-infected individuals, which documented that viral levels are just beginning to rise by 10–14 days (Walker, Moody, Stites, & Levy, 1986). The addition of exogenous virus has also been used in assays aimed at evaluating non-cytotoxic anti-HIV responses of CD8+ T lymphocytes, which have been reported to correlate with disease status(Killian et al., 2005).
Figure 3. Level of viral production and calculated levels of viral inhibition measured by the autologous T cell co-culture method in the absence of NL4-3 superinfection.
PBMC were isolated from blood from HIV+ donors and stimulated with bi-specific antibodies to produce pure CD4+ and CD8+ T lymphocyte cultures. After 14 days the CD8+ T lymphocytes were added to the wells containing autologous CD4+ cells at three different ratios (0.5:1, 1:1, and 2:1), each in triplicate. Media only was added to three wells as a positive control for maximum viral production in the absence of CD8+ T lymphocytes. Viral production was measured by p24 ELISA at three time points, as described in Materials and Methods. Panel a shows the actual p24 production at three different time-points from HIV+ Donor 1 and Panel b shows the calculated percent inhibition using the formula described in the Figure 2 legend.
One caveat to our own assay system is the variability of endogenous HIV-1 between individuals, and the potential effect of HAART. However, our intent is to produce a measurement of CD8+ T lymphocyte antiviral function against HIV-1; using a single clonal strain of the virus minimizes the bias of sequence variation. For example, the virus could develop escape mutations in vivo that would result in reduced in vitro antiviral activity against HIV-1, without reducing that actual antiviral function of CTL. Using a fixed strain of the virus can allow better comparisons for the same individual over time. For tested persons in whom a large amount of endogenous HIV-1 is present, the expanded CD4+ T lymphocytes can be produced in vitro in the presence of antiretrovirals, to prevent overgrowth by the endogenous virus (Wilson et al., 1995). Clearly, in vivo suppression of virus is far more complex a process than is captured by our assay, but the protocol we describe does provide a reliable method for longitudinal assessment of individual HIV-infected persons in the context of testing therapies, immune reconstitution and new vaccines.
A central feature of the autologous CD8/CD4 co-culture system is that it encompasses CD8+ T lymphocyte recognition of viral epitopes displayed in the context of all the MHC Class I molecules presented by the autologous CD4+ T lymphocytes. The specific combination of different HLA alleles, which may influence antigen-presentation and/or T cell recognition, is unique to that individual, and therefore probably closely mirrors the interactions that are occurring in vivo within that person. Importantly, the CD4/CD8 co-culture assay can be used to evaluate CD8+ T lymphocyte anti-viral function in donors of any HLA type, since it relies on the autologous CD4+ T lymphocytes, rather than a limited number of available CD4+ T cell lines. The autologous CD8/CD4 viral inhibition assay, therefore, is amenable to use in clinical settings, and may serve as a novel practical strategy to monitor the immune functional status of HIV-1-infected individuals.
The CD8+ T lymphocytes used in the co-culture system represent the full spectrum of antigen specificities present in each donor; there is no pre-selection of cells that recognize HIV. This situation mimics the in vivo scenario, where HIV-specific CD8+ T lymphocytes must function in the context of T lymphocytes with multiple specificities and activities. One key cell population of CD8+ T lymphocytes that may be relevant is the CD8+CD28− T cell subset, which has been shown to have suppressive activity. A dramatic example of the suppressor function of this subset is in allogeneic organ transplant recipients, where the presence of these cells allowed substantial reduction in the dose of immunosuppressive drugs required to prevent rejection (Cortesini et al., 2002). In HIV-1-infected persons, there are often high proportions of CD8+CD28− T lymphocytes as well (Effros et al., 1996), many of which are specific for HIV, but also for cytomegalovirus (Weekes, Carmichael, Wills, Mynard, & Sissons, 1999). Thus, the presence of CD8+CD28− T lymphocytes, irrespective of specificity, in the co-culture assay is physiologically relevant, and if these cells do, in fact, function in some way to suppress the antiviral activity of HIV-specific CD8+ T lymphocytes, this will be reflected in the final p24 readout.
In sum, we have described an assay that provides a composite measure of anti-HIV-1 function by CD8+ T lymphocytes against autologous infected CD4+ T lymphocytes, which can be used to evaluate anti-viral function of HIV-1-infected individuals, regardless of their HLA type. The assay relies on p24 levels, which we evaluated by a commercial ELISA, but can also be tested by EIA, as described by Castillo et al. (Castillo et al., 2000), or even by real time PCR, allowing standardization and application in a variety of clinical settings. The ability to provide a single metric of CD8+ T lymphocyte function that captures the effective protection potential provides a highly useful assay to evaluate immune status and the effect of certain drug regimens. We ourselves our using this system to screen and characterize novel immunomodulatory pharmacologic telomerase activating compounds (Fauce et al., 2006), and the assay may be similarly relevant to the evaluation of new therapeutic vaccines (John, Arango-Jaramillo, Self, & Schwartz, 2004).
Acknowledgments
We are grateful for the participation of donors from the UCLA CARE Clinic, and to Dr. Beth Jamieson for helpful comments on the manuscript. The research was supported by the following NIH grants: AI060362 (RBE), AI043203 (OOY), AI52031 (SRF), and by TA Therapeutics, Ltd (RBE).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anton PA, Mitsuyasu RT, Deeks SG, Scadden DT, Wagner B, Huang C, et al. Multiple measures of HIV burden in blood and tissue are correlated with each other but not with clinical parameters in aviremic subjects. AIDS. 2003;17:53–63. doi: 10.1097/00002030-200301030-00008. [DOI] [PubMed] [Google Scholar]
- Appay V, Nixon DF, Donahoe SM, Gillespie GM, Dong T, King A, et al. HIV-specific CD8+ T cells produce antiviral cytokines but are impaired in cytolytic function. Journal of Experimental Medicine. 2000;192:63–75. doi: 10.1084/jem.192.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts MR, Casazza JP, Koup RA. Monitoring HIV-specific CD8+ T cell responses by intracellular cytokine production. Immunol Lett. 2001;79:117–125. doi: 10.1016/s0165-2478(01)00273-5. [DOI] [PubMed] [Google Scholar]
- Castillo RC, Arango-Jaramillo S, John R, Weinhold K, Kanki P, Carruth L, et al. Resistance to human immunodeficiency virus type 1 in vitro as a surrogate of vaccine-induced protective immunity. J Infect Dis. 2000;181:897–903. doi: 10.1086/315300. [DOI] [PubMed] [Google Scholar]
- Cortesini R, Renna-Molajoni E, Cinti P, Pretagostini R, Ho E, Rossi P, et al. Tailoring of immunosuppression in renal and liver allograft recipients displaying donor specific T-suppressor cells. Hum Immunol. 2002;63:1010–1018. doi: 10.1016/s0198-8859(02)00442-1. [DOI] [PubMed] [Google Scholar]
- DeVico AL, Gallo RC. Control of HIV-1 infection by soluble factors of the immune response. Nat Rev Microbiol. 2004;2:401–413. doi: 10.1038/nrmicro878. [DOI] [PubMed] [Google Scholar]
- Effros RB, Allsopp R, Chiu CP, Wang L, Hirji K, Harley CB, et al. Shortened telomeres in the expanded CD28-CD8+ subset in HIV disease implicate replicative senescence in HIV pathogenesis. AIDS/Fast Track. 1996;10:F17–F22. doi: 10.1097/00002030-199607000-00001. [DOI] [PubMed] [Google Scholar]
- Fauce S, Jamieson BD, Chin AC, Ng H, Yang OO, Harley CB, et al. Telomerase-based pharmacologic enhancement of anti-viral function in HIV disease. American Association of Immunologists Annual Meeting Abstract Book, 26.May 12, 2006. [Google Scholar]
- Ferrari G, Humphrey W, McElrath MJ, Excler JL, Duliege AM, Clements ML, et al. Clade B-based HIV-1 vaccines elicit cross-clade cytotoxic T lymphocyte reactivities in uninfected volunteers. Proc Natl Acad Sci USA. 1997;94:1396–1401. doi: 10.1073/pnas.94.4.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulder PJ, Phillips RE, Colbert RA, McAdam S, Ogg G, Nowak MA, et al. Late escape from an immunodominant cytotoxic T-lymphocyte response associated with progression to AIDS. Nature Medicine. 1997;3:212–217. doi: 10.1038/nm0297-212. [DOI] [PubMed] [Google Scholar]
- Hausner MA, Giorgi JV, Plaeger-Marshall S. A reproducible method to detect CD8 T cell mediated inhibition of HIV production from naturally infected CD4 cells. Journal of Immunological Methods. 1993;157:181–187. doi: 10.1016/0022-1759(93)90085-l. [DOI] [PubMed] [Google Scholar]
- Ibarrondo FJ, Anton PA, Fuerst M, Ng HL, Wong JT, Matud J, et al. Parallel human immunodeficiency virus type 1-specific CD8+ T-lymphocyte responses in blood and mucosa during chronic infection. J Virol. 2005;79:4289–4297. doi: 10.1128/JVI.79.7.4289-4297.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John R, Arango-Jaramillo S, Self S, Schwartz DH. Modeling partially effective HIV vaccines in vitro. J Infect Dis. 2004;189:616–623. doi: 10.1086/381393. [DOI] [PubMed] [Google Scholar]
- Jones N, Agrawal D, Elrefaei M, Hanson A, Novitsky V, Wong JT, et al. Evaluation of antigen-specific responses using in vitro enriched T cells. Journal of Immunological Methods. 2003;274:139–147. doi: 10.1016/s0022-1759(02)00510-0. [DOI] [PubMed] [Google Scholar]
- Killian MS, Ng S, Mackewicz CE, Levy JA. A screening assay for detecting CD8+ cell non-cytotoxic anti-HIV responses. J Immunol Methods. 2005;304:137–150. doi: 10.1016/j.jim.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Klein MR, van Baalen CA, Holwerda AM, Kerkhof G, Bende RJ, Keet IP, et al. Kinetics of Gag-specific cytotoxic T lymphocyte responses during the clinical course of HIV-1 infection: a longitudinal analysis of rapid progressors and long-term asymptomatics. Journal of Experimental Medicine. 1995;181:1365–1372. doi: 10.1084/jem.181.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy JA. The search for the CD8+ cell anti-HIV factor (CAF) Trends Immunol. 2003;24:628–632. doi: 10.1016/j.it.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Lieberman J. Tracking the killers: how should we measure CD8 T cells in HIV infection? AIDS. 2004;18:1489–1493. doi: 10.1097/01.aids.0000131320.75396.4d. [DOI] [PubMed] [Google Scholar]
- Mackewicz CE, Garovoy MR, Levy JA. HLA compatibility requirements for CD8(+)-T-cell-mediated suppression of human immunodeficiency virus replication. J Virol. 1998;72:10165–10170. doi: 10.1128/jvi.72.12.10165-10170.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen RA, Hofmann-Lehmann R, Li PL, Vlasak J, Schmitz JE, Reimann KA, et al. Neutralizing antibodies as a potential secondary protective mechanism during chronic SHIV infection in CD8+ T-cell-depleted macaques. AIDS. 2002;16:829–838. doi: 10.1097/00002030-200204120-00002. [DOI] [PubMed] [Google Scholar]
- Robinson HL. T cells versus HIV-1: fighting exhaustion as well as escape. Nat Immunol. 2003;4:12–13. doi: 10.1038/ni0103-12. [DOI] [PubMed] [Google Scholar]
- Shankar P, Russo M, Harnisch B, Patterson M, Skolnik P, Lieberman J. Impaired function of circulating HIV-specific CD8(+) T cells in chronic human immunodeficiency virus infection. Blood. 2000;96:3094–3101. [PubMed] [Google Scholar]
- Sheehy ME, McDermott AB, Furlan SN, Klenerman P, Nixon DF. A novel technique for the fluorometric assessment of T lymphocyte antigen specific lysis. J Immunol Methods. 2001;249:99–110. doi: 10.1016/s0022-1759(00)00329-x. [DOI] [PubMed] [Google Scholar]
- Walker CM, Moody DJ, Stites DP, Levy JA. CD8+ lymphocytes can control HIV infection in vitro by suppressing virus replication. Science. 1986;234:1563–1566. doi: 10.1126/science.2431484. [DOI] [PubMed] [Google Scholar]
- Weekes MP, Carmichael AJ, Wills MR, Mynard K, Sissons JG. Human CD28-CD8+ T cells contain greatly expanded functional virus-specific memory CTL clones. Journal of Immunology. 1999;162:7569–7577. [PubMed] [Google Scholar]
- Wilson CC, Wong JT, Girard DD, Merrill DP, Dynan M, An DD, et al. Ex vivo expansion of CD4 lymphocytes from human immunodeficiency virus type 1-infected persons in the presence of combination antiretroviral agents. J Infect Dis. 1995;172:88–96. doi: 10.1093/infdis/172.1.88. [DOI] [PubMed] [Google Scholar]
- Yang OO, Kalams SA, Trocha A, Cao H, Luster A, Johnson RP, et al. Suppression of human immunodeficiency virus type 1 replication by CD8+ cells: evidence for HLA class I-restricted triggering of cytolytic and noncytolytic mechanisms. J Virol. 1997;71:3120–3128. doi: 10.1128/jvi.71.4.3120-3128.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]