Abstract
Despite numerous studies showing neurotrophic and neuroprotective effects of estrogen in animal models, the long-term effects of estrogen use on brain morphology in older women are not known. Thus, we compared ventricular, cerebrospinal fluid, white matter, and gray matter volumes estimated from magnetic resonance images of postmenopausal women with more than 20 years exposure to unopposed estrogen, women who were not on estrogen, and young healthy women. Estrogen users had significantly smaller ventricles and greater white matter volumes than non-users, but hormone exposure did not affect gray matter volumes. Young healthy women had significantly smaller ventricles, less cerebrospinal fluid and more gray matter than both groups of older women. However, they had comparable white matter volumes to older women on estrogen. These findings suggest that long-term estrogen protects against white matter loss in aging. This adds to findings from other studies suggesting estrogen is neuroprotective of the hippocampus and other regions in older women.
Keywords: estrogen, aging, white matter, gray matter, atrophy, magnetic resonance imaging
1. Introduction
The hallmarks of human brain aging include a loss of white matter [21;22] and gray matter [20]. Cortical changes are not due to significant neuronal loss, because neuron numbers are relatively stable [19]. Instead, loss of cortical gray and white matter tissue volumes are likely the result of loss of dendritic arbors, synapse loss, neuronal shrinkage and the degradation of the myelin as well as other glial changes [17;25]. Whether estrogen loss or replacement at menopause alters the course of brain aging in healthy older women is unknown. Thus, we examined brain morphological differences among women with very long-term estrogen use (EW), women who did not use estrogen (NONE) and healthy younger women (YW).
Estradiol modifies the response to lesions and ischemic stroke [34] and lesions and can change the pattern and physiology of the interactions among neurons, thus essentially remodeling brain circuits [32]. Ovariectomy results in a profound loss of synaptic density in the CA1 region of the hippocampus [13] and prefrontal cortex in nonhuman primates [27]. These actions suggest that estradiol can play both neuroprotective and neurotrophic roles that may be of particular importance in aging. These effects differ depending on dose, timing, length of treatment and whether estradiol is co-administered with progesterone (for review see [28]).
Information on the functional effects of estrogen in aging come from studies of women on hormone treatment, and there are contradictory results. Observational studies from postmenopausal women on long-term estrogen show a reduced risk of developing Alzheimer’s disease [26] and preservation or enhancement of cognitive abilities, particularly memory [9], suggesting neuroprotective effects of estrogen. Higher bioavailable estradiol, but not absolute estradiol or change in levels are predictive better cognitive function in aging [33]. In contrast, approximately five years of estrogen treatment, when initiated many years after menopause, is not protective against dementia [24]. The varied results between the observational and treatment studies of postmenopausal hormone use may be due to a number of differences in the subject samples as well as the study designs.
There are few quantitative structural neuroimaging studies of the effects of long-term hormone use, and again, there are conflicting results. There is a trend for greater atrophy, as assessed by larger ventricles, in current hormone users, as opposed to past users or those who never used hormones. No differences between groups were found for other measures of atrophy such as sulcal widening or white matter lesions [16]. Hormone users do not differ in ventricular volume, but have smaller white matter hyperintensities [23] and ventricular volume was less likely to increase with time in hormone users as compared to non-users [3]. Hippocampal volumes are larger with long-term estrogen use as opposed to tamoxifen, but hormone users do not differ from those on no hormone therapy [5]. More recently, voxel-based morphometry shows a sparing of gray matter in prefrontal, parietal, and temporal regions, with white matter sparing adjacent to the lateral ventricles in the temporal lobes in current and past users of hormones in contrast to women who have never used hormones [6]. Sparing is related to length of hormone use. In contrast, a quantitative MRI study in a restricted age sample (60 to 64 years) finds no differences in regional volumes, white or grey matter among current, past or never users [15].
It is unclear whether continuous estrogen use from menopause to old age has different protective effects than intermittent use or when hormone use is initiated later in life. Thus, additional information is needed to complete the transition from estrogen effects at the cellular level in animal models, to effects on cognition in older women. This study provides preliminary data on the effects of long-term estrogen-alone on brain morphometry in estimated tissue volumes in older women.
2. Methods
2.1 Participants
Quantitative analysis of brain images of young healthy women (YW), were compared to healthy older postmenopausal women (estrogen users EW; nonusers NONE; See Table 1 for participant characteristics). Participants were non-smokers with no history of neurodegenerative disease or stroke, or significant alcohol use. Written informed consent was obtained from all participants.
Table 1.
Participant Characteristics
| Older Women | YW | ||
|---|---|---|---|
| EW Group^ | NONE Group^ | ||
| N | 10 | 10 | 10 |
| Age | 70.1 (4.1) | 73.4 (3.5) | 30.6 (6.9) |
| Years Education | 14.3 (2.1) | 15.1 (3.4) | - |
| Years Postmenopausal | 25.4 (7.7) | 24.6 (5.7) | - |
| Mini-Mental State Exam1 | 28.1 (1.4) | 28.4 (.84) | - |
| Geriatric Depression Scale2 | 2.8 (3.4) | 3.5 (3.7) | - |
| WAIS-R Vocabulary# | 11.7(1.8) | 11.9 (1.4) | - |
| Years on hormone therapy | 23.1 (9.3) | - | - |
| Hormone Regimen | |||
| CEE3 | 9/10 | None | |
| Estradiol4 | 1/10 | ||
Mean Values (Standard Deviation); EW= Estrogen users; NONE= No hormone use; YW=Young Women EW and NONE groups were matched on all characteristics except hormone use (p’s> .05)
Wechsler Adult Intelligence Scale Revised scaled score [31]
[7]
[36]
Conjugated Equine Estrogen (Premarin); 6 women were on .625mg/day; 3 were on .625mg/day but with individualized dosing schemes: on estrogen for 25 days and off for 5 days; alternating daily .625 and .90 mg/day; .625mg. for five days/week
(Estrace) .2mg four days/wk
2.2 Magnetic Resonance Imaging and Region of Interest (ROI) Analysis
MR brain images were acquired using a 1.5-T Horizon MR system (GE Medical Systems, Waukesha, Wis). The imaging protocol consisted of eight 5-mm-thick oblique axial slices superior and parallel to a line between the anterior and posterior commissures, including diencephalic and cortical regions superior to the temporal lobes. A single rater, blinded to group status (D.M.H.) used a “seed growing” method that uses image contrast (ANALYZE AVW 3.0; BIR Mayo Clinic) to outline ROI’s (lateral and third ventricles, white matter, cerebrospinal fluid; CSF, intracranial volume, ICV; See Figure). The lateral and third ventricles were present in four of the eight brain slices. ROIs were normalized by ICV on each of the eight slices to correct for potential confounding factors that head size may have across age or between groups [22]. A basic volume estimate was calculated as the total area of the ROI multiplied by the slice thickness [29]
Figure 1.
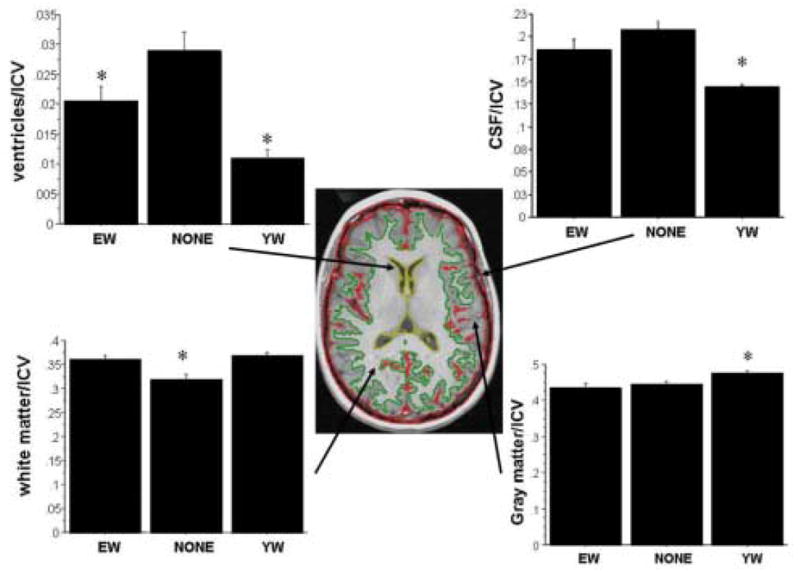
Central figure is a representative MRI slice to illustrate ROIs. Ventricles (yellow border), CSF (red border; defined as the strip along the perimeter of the brain from cortical gray matter to the skull as well as sulcal islands of CSF within the parenchyma), white matter (green border, delineated outward from the ventricles until it outlined the contrast border between gray and white matter); and gray matter (calculated as: ICV − CSF + WM + ventricles) volumes were normalized by dividing by intracranial volume (ICV). ICV from each slice included everything inside the innermost edge of the skull (white band). Intra-rater reliability was high (repeated measures p’s> .15 for all regions) as were correlations among repeated measurements for each of the ROI’s (all r’s>.93). Graphs are comparisons of regions of interest among women using estrogen (EW), those not using estrogen (NONE), and young women (YW) (ANOVA). On the graphs, an asterisk denotes significant differences among groups (p<.05). Error bars are standard error of mean. NONE have larger ventricles than both EW and YW. EW have smaller ventricles than NONE but larger ventricles than YW. YW have less CSF than EW and NONE women who do not differ from each other. EW and YW have more white matter than NONE, but did not differ from each other. YW have more gray matter than EW and NONE, who did not differ from each other.
3. Results
3.1 Effects of estrogen
EW, NONE and YW groups differed significantly on ventricular size, CSF, white matter and gray matter volumes (F’s2,27> 5.0; p’s <.02; Analysis of Variance. StatView 1998; SAS Cary North Carolina; see Figure). Post hoc comparisons showed that NONE had larger ventricles than both EW and YW (p’s<.03), and EW had larger ventricles than YW (p=.009). YW had significantly less CSF and more grey matter than either EW and NONE (p’s< .04) who did not differ from each other (p’s >.10). EW and YW had significantly more white matter than NONE (p’s<.002), but EW and YW did not differ from each other (p> .10). White matter differences were not solely an artifact of ventricular volumes as the same group differences occurred for slices superior to the lateral ventricles (data not shown). The marginal age differences (Table 1) do not explain volumetric differences between EW and NONE groups, as group differences remained the same when age was used as a covariate.
3.2. Effects of age
Age was positively correlated with ventricular size across all participants (r=.72; p<.001), as well as the YW (r=.75; p=.01) alone, but was marginally correlated in NONE (r=.58; p=.08), and not correlated in the EW (p> .10). Age was negatively correlated with white and gray matter across all participants (r’s>−.52; p’s<.01). Age was negatively correlated with white matter in EW women (r=−.68; p=.03), but not significantly correlated in the other groups (p> .10) nor was age correlated with gray matter in any of the individual groups (p> .10). There was a significant positive correlation between age and CSF across all groups (r=.66; p<.001) but correlations were not significant for any individual group. As expected, age and ICV were not correlated.
4. Discussion
The major finding of this study is that long-term unopposed estrogen use preserves white matter in older women. Ventricular enlargement, which commonly accompanies age-related brain atrophy, is also less pronounced in estrogen users. The underlying mechanism is unknown but white matter preservation and reduced ventricular size may have the same underlying mechanism, that is, preservation of fibers coming from cortex to distant or subcortical regions and/or preservation of myelin. Much more attention has focused on white matter lesions in aging, which was not the focus here. White matter lesions as well as atrophy are associated with cognitive decline in old age [11]. The functional implications of the white matter volume loss reported here, requires further study.
There is neurobiological support for estrogen effects on white matter preservation. Estrogen receptors are present on neuroglia including astrocytes, oligodendrocytes, and microglia [for review see, [18]]. Estrogen alters oligodendrocyte and astrocyte morphology in culture, and causes synthesis of myelin basic protein and glial fibrillary protein [10] and increases expression of the estrogen beta receptor in cortical microglia [14]. Estrogen also has an anti-inflammatory role and promotes remyelination [2].
Effects of estrogen on gray matter volume were not found, although older women had less gray matter than young women. This suggests that the measure was sufficiently sensitive to detect loss of grey matter but the variability in the cortical ribbon combined with the small sample size may not permit detection of subtle preservation or acceleration of grey matter loss. Alternatively it could be that estrogen use does not preserve gray matter in the regions examined but does in regions not examined such as the temporal lobes [4]. Our results may seem counter intuitive given the extensive loss of synaptic density and activity changes in the hippocampus after estrogen depletion in animal models [35]. However, those studies did not examine hippocampal volume and this study did not examine the hippocampus. Recent studies of synaptic loss after androgen deprivation in male nonhuman primates shows that there is a simultaneous increase in glial processes and dendritic diameter which results in no net change in the volume of the hippocampus [12]. Thus, the remaining question is whether grey matter in temporal lobe regions as well as the hippocampus is selectively affected by estrogen use as compared to other cortical grey matter. Further studies are needed to confirm this idea.
A recent voxel based morphometry study shows that postmenopausal women who have ever used hormones have sparing of gray matter throughout cortex, and like our study, significantly smaller ventricles and sparing of white matter (although the white matter sparing was specifically in the temporal lobes), in contrast to those who had never used hormone therapy [6]. Two recent studies using two different quantitative methods (voxel based morphometry and ROI segmentation with volumetric quantification) show that white matter atrophy has a curvilinear relationship with age with an acceleration of atrophy in the later decades of life (> 65 years;[1;30]). Indeed hormone use is not related to white matter volumes in women 60–64 years [15]. Cortical gray matter loss is linear without acceleration in the latter decades in contrast to the temporal lobe and hippocampus that show a curvilinear relationship with acceleration of atrophy in the later decades. Thus, one possibility is that very long-term estrogen use partially protects against this final phase of accelerated age-related brain atrophy.
Limitations of this study are the small sample size, its cross-sectional design and the lack of data for some brain regions. We cannot know if women who take estrogen differ in some other lifestyle variable that may also affect brain morphology or aging, the so-called “healthy user bias”. Only randomized clinical trials can resolve this potential bias which would be difficult if not impossible to mount from menopause to late aging. What would be of interest is whether limited hormone use at the time of the perimenopause plays a more critical role in neuroprotection than when started much later in life [37]. Our study addresses only women on estrogen-alone and not other formulations such as those with progesterone. This is important as neurobiological evidence suggests that estrogen in combination with progesterone has very different effects on a number of physiological processes, than estrogen alone [8]. This study was limited to cortical regions superior to the temporal lobe thus precluding assessment of temporal lobe structures and other important brain regions. In addition, tissue classification is by an image contrast method, and without microscopic confirmation of tissue boundaries. Improved MRI methods that quantify gray matter and white matter by the tissue’s biological characteristics in the future will likely elucidate the underlying mechanism of morphological differences in the elderly due to hormone use.
Acknowledgments
This study was supported in part by PHS Grants NIH R01AG12611, R01AG18843 and the OHSU General Clinical Resource Center NCRR 5M01 RR000334.
Footnotes
Disclosure Statement
We have no actual or potential conflicts of interest to disclose. The OHSU IRB approved subject participation in this study and subjects signed written informed consent documents.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Duy M. Ha, Email: duyha@fas.harvard.edu.
Jingang Xu, Email: xuj@ohsu.edu.
Jeri S. Janowsky, Email: janowskj@ohsu.edu.
Reference List
- 1.Allen JS, Bruss J, Brown CK, Damasio H. Normal neuroanatomical variation due to age: the major lobes and a parcellation of the temporal region. Neurobiol Aging. 2005;26:1245–1260. doi: 10.1016/j.neurobiolaging.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 2.Bebo BF, Jr, Fyfe-Johnson A, Adlard K, Beam AG, Vandenbark AA, Offner H. Low-dose estrogen therapy ameliorates experimental autoimmune encephalomyelitis in two different inbred mouse strains. J Immunol. 2001;166:2080–2089. doi: 10.4049/jimmunol.166.3.2080. [DOI] [PubMed] [Google Scholar]
- 3.Cook IA, Morgan ML, Dunkin JJ, David S, Witte E, Lufkin R, Abrams M, Rosenberg S, Leuchter AF. Estrogen replacement therapy is associated with less progression of subclinical structural brain disease in normal elderly women: a pilot study. Int J Geriatr Psychiatry. 2002;17:610–618. doi: 10.1002/gps.644. [DOI] [PubMed] [Google Scholar]
- 4.Eberling JL, Wu C, Haan MN, Mungas D, Buonocore M, Jagust WJ. Preliminary evidence that estrogen protects against age-related hippocampal atrophy. Neurobiol Aging. 2003;24:725–732. doi: 10.1016/s0197-4580(02)00056-8. [DOI] [PubMed] [Google Scholar]
- 5.Eberling JL, Wu C, Tong-Turnbeaugh R, Jagust WJ. Estrogen- and tamoxifen-associated effects on brain structure and function. Neuroimage. 2004;21:364–371. doi: 10.1016/j.neuroimage.2003.08.037. [DOI] [PubMed] [Google Scholar]
- 6.Erickson KI, Colcombe SJ, Raz N, Korol DL, Scalf P, Webb A, Cohen NJ, McAuley E, Kramer AF. Selective sparing of brain tissue in postmenopausal women receiving hormone replacement therapy. Neurobiol Aging. 2005;26:1205–1213. doi: 10.1016/j.neurobiolaging.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 7.Folstein M, Folstein S, McHugh P. Mini-mental state: A practical method of grading the cognitive status of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 8.Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobs D, Tang M, Stern Y, Sano M, Marder K, Bell K, Schofield P, Dooneief G, Gurland B, Mayeux R. Cognitive function in nondemented older women who took estrogen after menopause. Neurology. 1998;50:368–373. doi: 10.1212/wnl.50.2.368. [DOI] [PubMed] [Google Scholar]
- 10.Jung-Testas I, Renoir M, Bugnard H, Greene GL, Baulieu EE. Demonstration of steroid hormone receptors and steroid action in primary cultures of rat glial cells. J Steroid Biochem Mol Biol. 1992;41:621–631. doi: 10.1016/0960-0760(92)90394-x. [DOI] [PubMed] [Google Scholar]
- 11.Koga H, Yuzuriha T, Yao H, Endo K, Hiejima S, Takashima Y, Sadanaga F, Matsumoto T, Uchino A, Ogomori K, Ichimiya A, Uchimura H, Tashiro N. Quantitative MRI findings and cognitive impairment among community dwelling elderly subjects. J Neurol Neurosurg Psychiatry. 2002;72:737–741. doi: 10.1136/jnnp.72.6.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leranth C, Prange-Kiel J, Frick KM, Horvath TL. Low CA1 spine synapse density is further reduced by castration in male non-human primates. Cereb Cortex. 2004;14:503–510. doi: 10.1093/cercor/bhh012. [DOI] [PubMed] [Google Scholar]
- 13.Leranth C, Shanabrough M, Redmond DE., Jr Gonadal hormones are responsible for maintaining the integrity of spine synapses in the CA1 hippocampal subfield of female nonhuman primates. J Comp Neurol. 2002;447:34–42. doi: 10.1002/cne.10230. [DOI] [PubMed] [Google Scholar]
- 14.Li R, Shen Y, Yang LB, Lue LF, Finch C, Rogers J. Estrogen enhances uptake of amyloid beta-protein by microglia derived from the human cortex. J Neurochem. 2000;75:1447–1454. doi: 10.1046/j.1471-4159.2000.0751447.x. [DOI] [PubMed] [Google Scholar]
- 15.Low LF, Anstey KJ, Maller J, Kumar R, Wen W, Lux O, Salonikas C, Naidoo D, Sachdev P. Hormone replacement therapy, brain volumes and white matter in postmenopausal women aged 60–64 years. Neuroreport. 2006;17:101–104. doi: 10.1097/01.wnr.0000194385.10622.8e. [DOI] [PubMed] [Google Scholar]
- 16.Luoto R, Manolio T, Meilahn E, Bhadelia R, Furberg C, Cooper L, Kraut M. Estrogen replacement therapy and MRI-demonstrated cerebral infarcts, white matter changes, and brain atrophy in older women: the Cardiovascular Health Study. J Am Geriatr Soc. 2000;48:467–472. doi: 10.1111/j.1532-5415.2000.tb04990.x. [DOI] [PubMed] [Google Scholar]
- 17.Marner L, Nyengaard JR, Tang Y, Pakkenberg B. Marked loss of myelinated nerve fibers in the human brain with age. J Comp Neurol. 2003;462:144–152. doi: 10.1002/cne.10714. [DOI] [PubMed] [Google Scholar]
- 18.Mor G, Nilsen J, Horvath T, Bechmann I, Brown S, Garcia-Segura LM, Naftolin F. Estrogen and microglia: A regulatory system that affects the brain. J Neurobiol. 1999;40:484–496. doi: 10.1002/(sici)1097-4695(19990915)40:4<484::aid-neu6>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 19.Morrison J, Hof P. Life and death of neurons in the aging brain. Science. 1997;278:412–419. doi: 10.1126/science.278.5337.412. [DOI] [PubMed] [Google Scholar]
- 20.Raz N, Gunning F, Head D, Dupuis J, McQuain J, Briggs S, Loken W, Thornton A, Acker J. Selective aging of the human cerebral cortex observed in vivo: Differential vulnerability of the prefrontal gray matter. Cerebral Cortex. 1997;7:268–282. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- 21.Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. J Neurosci. 2003;23:3295–3301. doi: 10.1523/JNEUROSCI.23-08-03295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salat D, Kaye J, Janowsky J. Prefrontal gray and white matter volumes in healthy aging and Alzheimer disease. Archives of Neurology. 1999;56:338–344. doi: 10.1001/archneur.56.3.338. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt R, Fazekas F, Reinhart B, Kapeller P, Fazekas G, Offenbacker H, Eber B, Schumacher M, Freidl W. Estrogen replacement therapy in older women: A neuropsychological and brain MRI study. J Am Geriatr Soc. 1996;44:1307–1313. doi: 10.1111/j.1532-5415.1996.tb01400.x. [DOI] [PubMed] [Google Scholar]
- 24.Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, Fillit H, Stefanick ML, Hendrix SL, Lewis CE, Masaki K, Coker LH. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2947–2958. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- 25.Sloane JA, Hollander W, Moss MB, Rosene DL, Abraham CR. Increased microglial activation and protein nitration in white matter of the aging monkey. Neurobiol Aging. 1999;20:395–405. doi: 10.1016/s0197-4580(99)00066-4. [DOI] [PubMed] [Google Scholar]
- 26.Tang M, Jacobs D, Stern Y, Marder K, Schofield P, Gurland B, Andrews H, Mayeux R. Effect of oestrogen during menopause on risk and age at onset of Alzheimer’s disease. The Lancet. 1996;348(9025):429–432. doi: 10.1016/S0140-6736(96)03356-9. Ref Type: Newspaper. [DOI] [PubMed] [Google Scholar]
- 27.Tang Y, Janssen WG, Hao J, Roberts JA, McKay H, Lasley B, Allen PB, Greengard P, Rapp PR, Kordower JH, Hof PR, Morrison JH. Estrogen replacement increases spinophilin-immunoreactive spine number in the prefrontal cortex of female rhesus monkeys. Cereb Cortex. 2004;14:215–223. doi: 10.1093/cercor/bhg121. [DOI] [PubMed] [Google Scholar]
- 28.Turgeon JL, McDonnell DP, Martin KA, Wise PM. Hormone therapy: physiological complexity belies therapeutic simplicity. Science. 2004;304:1269–1273. doi: 10.1126/science.1096725. [DOI] [PubMed] [Google Scholar]
- 29.Uylings HB, van Eden CG, Hofman MA. Morphometry of size/volume variables and comparison of their bivariate relations in the nervous system under different conditions. J Neurosci Methods. 1986;18:19–37. doi: 10.1016/0165-0270(86)90111-1. [DOI] [PubMed] [Google Scholar]
- 30.Walhovd KB, Fjell AM, Reinvang I, Lundervold A, Dale AM, Eilertsen DE, Quinn BT, Salat D, Makris N, Fischl B. Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiol Aging. 2005;26:1261–1270. doi: 10.1016/j.neurobiolaging.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 31.Wechsler D. Wechsler Adult Intelligence Scale - Revised. San Antonio, TX: Psychological Corp., Harcourt Brace Jovanovich; 1981. [Google Scholar]
- 32.Woolley C, Wenzel H, Schwartzkroin P. Estradiol increases the frequency of multiple synapse boutons in the hippocampal CA1 region of the adult female rat. J Comp Neurol. 1996;373:108–117. doi: 10.1002/(SICI)1096-9861(19960909)373:1<108::AID-CNE9>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 33.Yaffe K, Lui L, Grady D, Cauley J, Kramer J, Cummings S. Cognitive decline in women in relation to non-protein-bound oestradiol concentrations. The Lancet. 2000;356:708–712. doi: 10.1016/S0140-6736(00)02628-3. [DOI] [PubMed] [Google Scholar]
- 34.Yang SH, Liu R, Wu SS, Simpkins JW. The use of estrogens and related compounds in the treatment of damage from cerebral ischemia. Ann N Y Acad Sci. 2003;1007:101–107. doi: 10.1196/annals.1286.010. [DOI] [PubMed] [Google Scholar]
- 35.Yankova M, Hart SA, Woolley CS. Estrogen increases synaptic connectivity between single presynaptic inputs and multiple postsynaptic CA1 pyramidal cells: a serial electron-microscopic study. Proc Natl Acad Sci USA. 2001;98:3525–3530. doi: 10.1073/pnas.051624598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yesavage J, Brink T, Rose T. Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res. 1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 37.Zandi PP, Carlson MC, Plassman BL, Welsh-Bohmer KA, Mayer LS, Steffens DC, Breitner JC. Hormone replacement therapy and incidence of Alzheimer disease in older women: the Cache County Study. JAMA. 2002;288:2123–2129. doi: 10.1001/jama.288.17.2123. [DOI] [PubMed] [Google Scholar]


