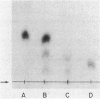
Abstract
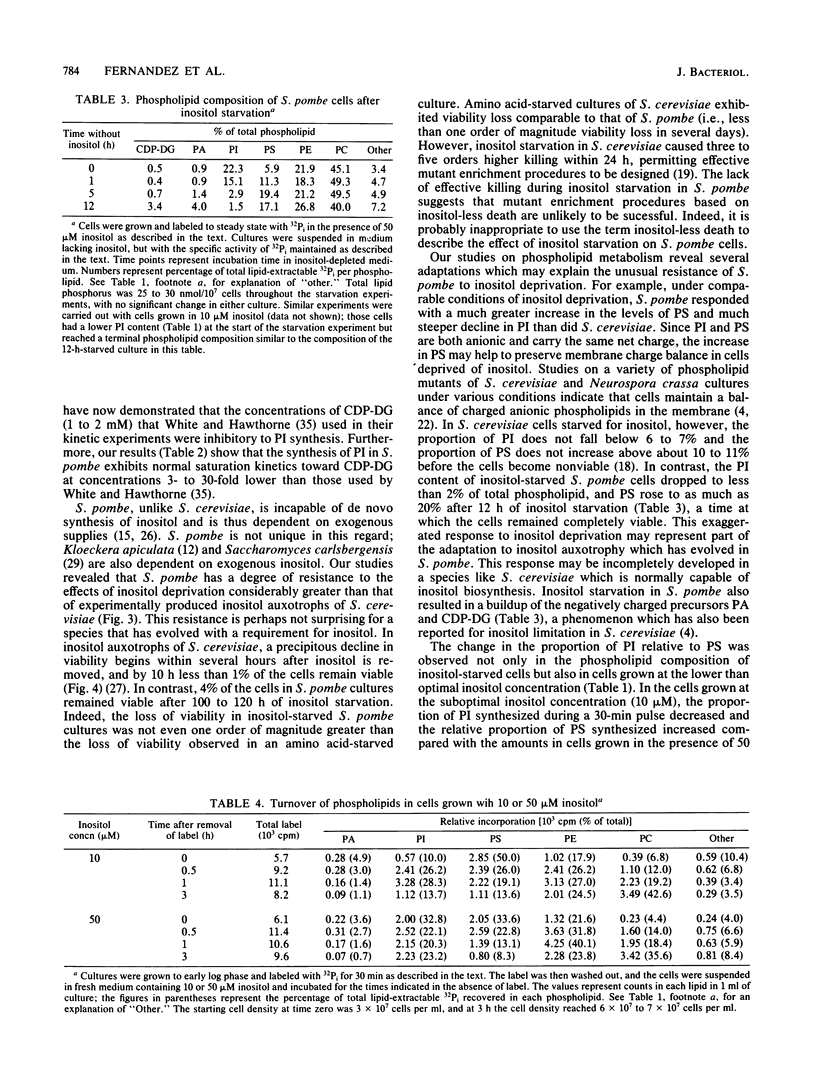
Phospholipid metabolism in the fission yeast Schizosaccharomyces pombe was examined. Three enzymes of phospholipid biosynthesis, cytidine diphosphate diacylglycerol synthase (CDP-DG), phosphatidylinositol (PI) synthase, and phosphatidylserine (PS) synthase, were characterized in extracts of S. pombe cells. Contrary to an earlier report, we were able to demonstrate that CDP-DG served as a precursor for PI and PS biosynthesis in S. pombe. S. pombe is naturally auxotrophic for the phospholipid precursor inositol. We found that S. pombe was much more resistant to loss of viability during inositol starvation than artificially generated inositol auxotrophs of Saccharomyces cerevisiae. The phospholipid composition of S. pombe cells grown in inositol-rich medium (50 microM) was similar to that of S. cerevisiae cells grown under similar conditions. However, growth of S. pombe at low inositol concentrations (below 30 microM) affected the ratio of the anionic phospholipids PI and PS, while the relative proportions of other glycerophospholipids remained unchanged. During inositol starvation, the rate of PI synthesis decreased rapidly, and there was a concomitant increase in the rate of PS synthesis. Phosphatidic acid and CDP-DG, which are precursors to these phospholipids, also increased when PI synthesis was blocked by lack of exogenous inositol. The major product of turnover of inositol-containing phospholipids in S. pombe was found to be free inositol, which accumulated in the medium and could be reused by the cell.
Full text
PDF

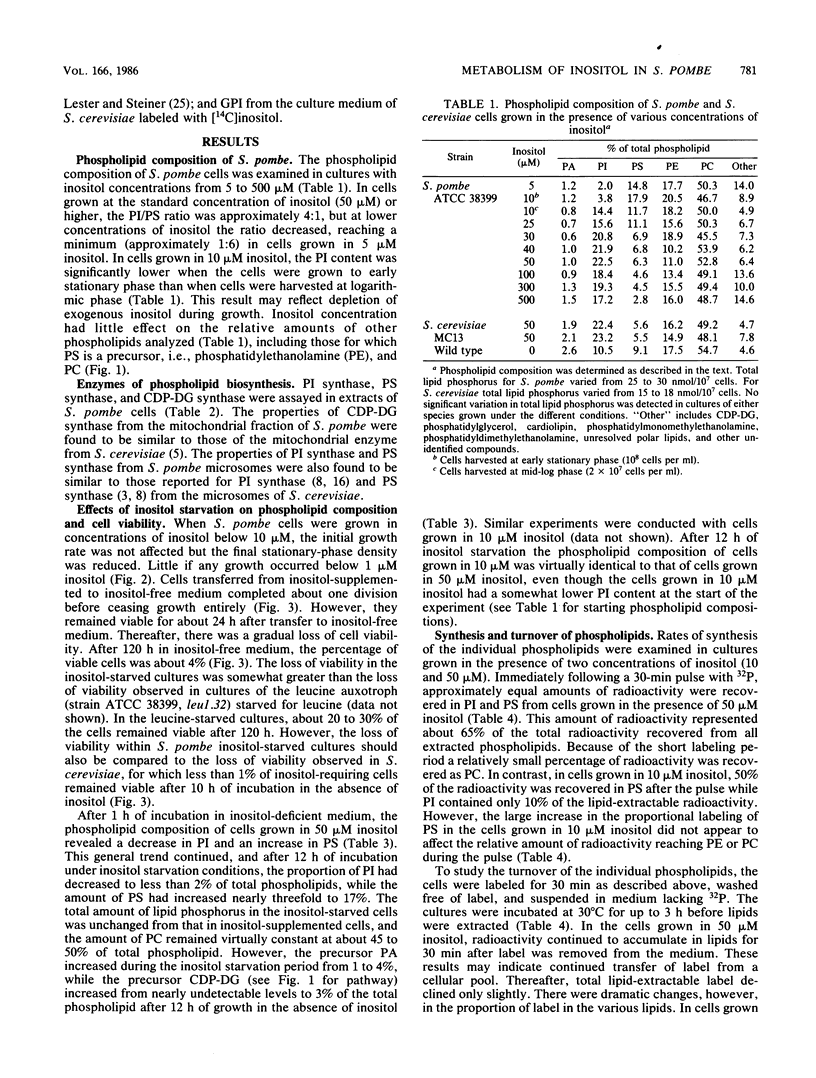
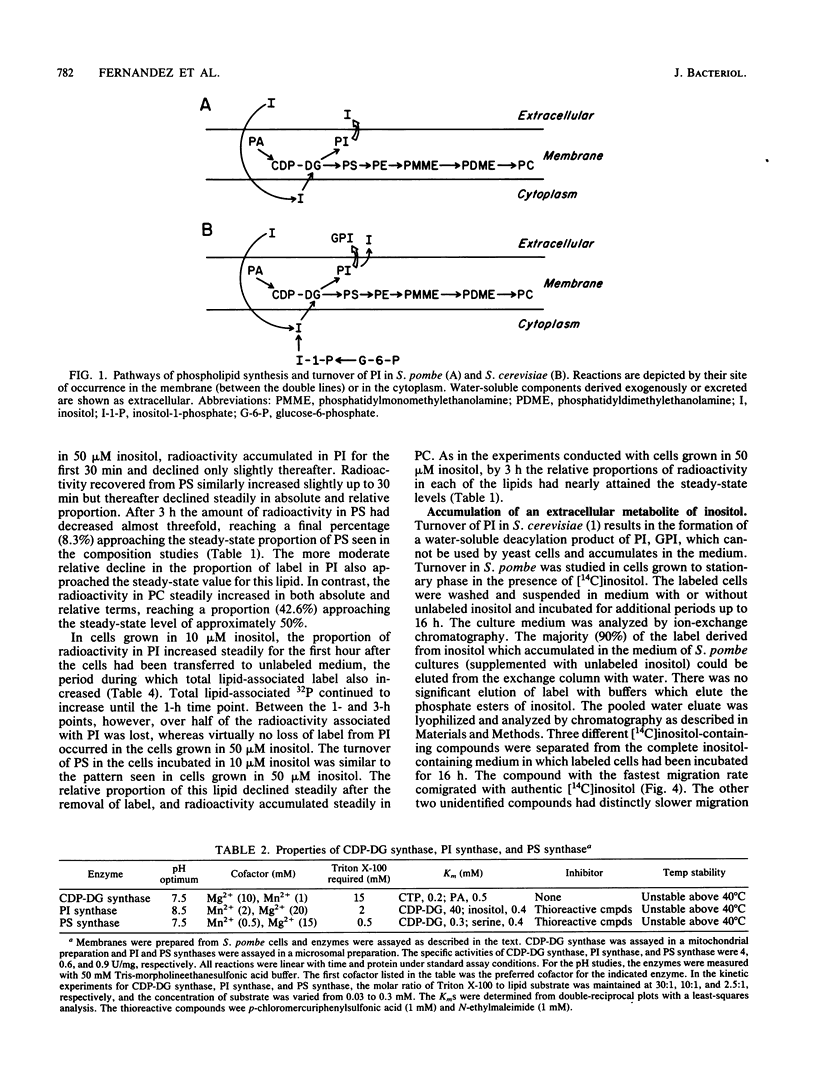
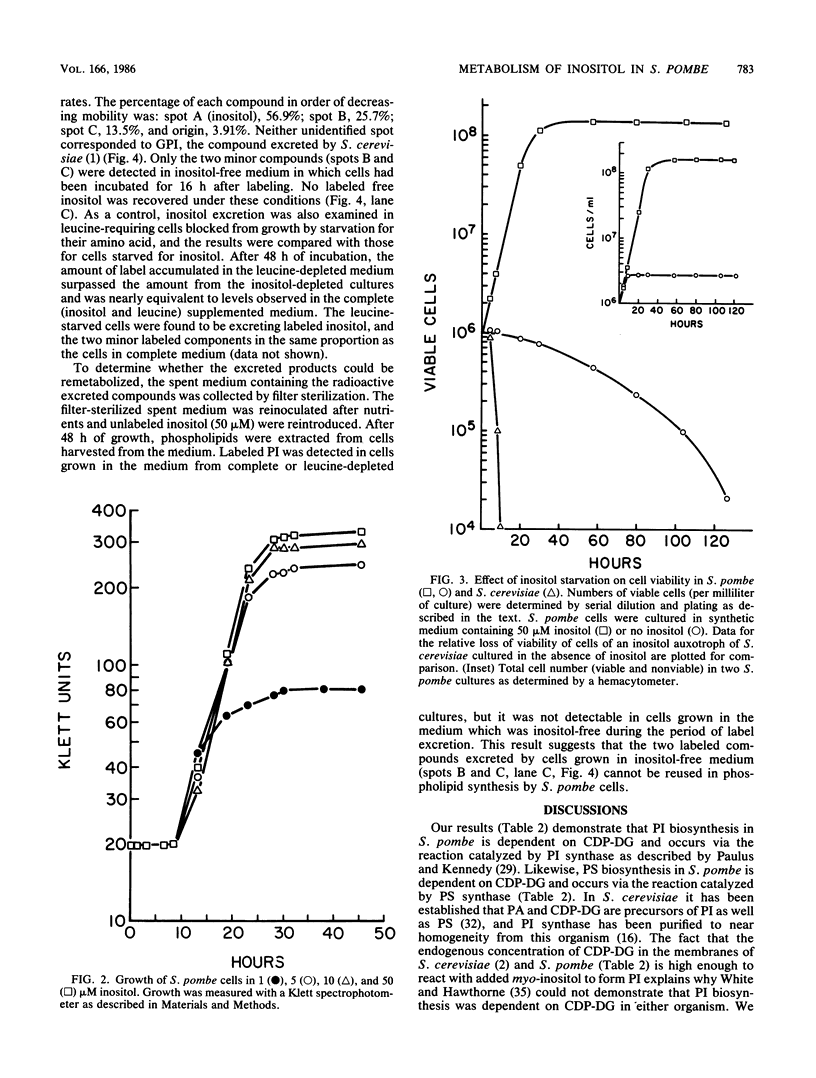


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angus W. W., Lester R. L. The regulated catabolism of endogenous and exogenous phosphatidylinositol by Saccharomyces cerevisiae leading to extracellular glycerophosphorylinositol and inositol. J Biol Chem. 1975 Jan 10;250(1):22–30. [PubMed] [Google Scholar]
- Atkinson K., Fogel S., Henry S. A. Yeast mutant defective in phosphatidylserine synthesis. J Biol Chem. 1980 Jul 25;255(14):6653–6661. [PubMed] [Google Scholar]
- Bae-Lee M. S., Carman G. M. Phosphatidylserine synthesis in Saccharomyces cerevisiae. Purification and characterization of membrane-associated phosphatidylserine synthase. J Biol Chem. 1984 Sep 10;259(17):10857–10862. [PubMed] [Google Scholar]
- Becker G. W., Lester R. L. Changes in phospholipids of Saccharomyces cerevisiae associated with inositol-less death. J Biol Chem. 1977 Dec 10;252(23):8684–8691. [PubMed] [Google Scholar]
- Belendiuk G., Mangnall D., Tung B., Westley J., Getz G. S. CTP-phosphatidic acid cytidyltransferase from Saccharomyces cerevisiae. Partial purification, characterization, and kinetic behavior. J Biol Chem. 1978 Jul 10;253(13):4555–4565. [PubMed] [Google Scholar]
- Berridge M. J. Rapid accumulation of inositol trisphosphate reveals that agonists hydrolyse polyphosphoinositides instead of phosphatidylinositol. Biochem J. 1983 Jun 15;212(3):849–858. doi: 10.1042/bj2120849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carman G. M., Matas J. Solubilization of microsomal-associated phosphatidylserine synthase and phosphatidylinositol synthase from Saccharomyces cerevisiae. Can J Microbiol. 1981 Nov;27(11):1140–1149. doi: 10.1139/m81-179. [DOI] [PubMed] [Google Scholar]
- Carman G. M., Zaniewski R. L., Cousminer J. J. CDP-diacylglycerol synthase activity in Clostridium perfringens. Appl Environ Microbiol. 1982 Jan;43(1):81–85. doi: 10.1128/aem.43.1.81-85.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbertson M. R., Donahue T. F., Henry S. A. Control of inositol biosynthesis in Saccharomyces cerevisiae: properties of a repressible enzyme system in extracts of wild-type (Ino+) cells. J Bacteriol. 1976 Apr;126(1):232–242. doi: 10.1128/jb.126.1.232-242.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbertson M. R., Henry S. A. Inositol-requiring mutants of Saccharomyces cerevisiae. Genetics. 1975 May;80(1):23–40. doi: 10.1093/genetics/80.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWSON R. M., WHITE R. W., FREINKEL N. The fate of mesoinositol during the growth of an inositol-dependent yeast Kloeckera brevis. J Gen Microbiol. 1962 Feb;27:331–339. doi: 10.1099/00221287-27-2-331. [DOI] [PubMed] [Google Scholar]
- Downes C. P., Michell R. H. The polyphosphoinositide phosphodiesterase of erythrocyte membranes. Biochem J. 1981 Jul 15;198(1):133–140. doi: 10.1042/bj1980133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H., OYAMA V. I., LEVY M., FREEMAN A. E. Myo-Inositol as an essential growth factor for normal and malignant human cells in tissue culture. J Biol Chem. 1957 May;226(1):191–205. [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Henry S. A., Donahue T. F., Culbertson M. R. Selection of spontaneous mutants by inositol starvation in yeast. Mol Gen Genet. 1975 Dec 30;143(1):5–11. doi: 10.1007/BF00269415. [DOI] [PubMed] [Google Scholar]
- Henry S. A., Klig L. S., Loewy B. S. The genetic regulation and coordination of biosynthetic pathways in yeast: amino acid and phospholipid synthesis. Annu Rev Genet. 1984;18:207–231. doi: 10.1146/annurev.ge.18.120184.001231. [DOI] [PubMed] [Google Scholar]
- Hubbard S. C., Brody S. Glycerophospholipid variation in choline and inositol auxotrophs of Neurospora crassa. Internal compensation among zwitterionic and anionic species. J Biol Chem. 1975 Sep 25;250(18):7173–7181. [PubMed] [Google Scholar]
- Johnson B. F. Growth of the fission yeast, Schizosaccharomyces pombe, with late, eccentric, lytic fission in an unbalanced medium. J Bacteriol. 1967 Jul;94(1):192–195. doi: 10.1128/jb.94.1.192-195.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LESTER H. E., GROSS S. R. Efficient method for selection of auxotrophic mutants of Neurospora. Science. 1959 Feb 27;129(3348):572–572. doi: 10.1126/science.129.3348.572. [DOI] [PubMed] [Google Scholar]
- Lester R. L., Steiner M. R. The occurrence of diphosphoinositide and triphosphoinositide in Saccharomyces cerevisiae. J Biol Chem. 1968 Sep 25;243(18):4889–4893. [PubMed] [Google Scholar]
- Megnet R. A method for the selection of auxotrophic mutants of the yeast Schizosaccharomyces pombe. Experientia. 1964 Jun 15;20(6):320–321. doi: 10.1007/BF02171071. [DOI] [PubMed] [Google Scholar]
- PAULUS H., KENNEDY E. P. The enzymatic synthesis of inositol monophosphatide. J Biol Chem. 1960 May;235:1303–1311. [PubMed] [Google Scholar]
- Paltauf F., Johnston J. M. Lipid metabolism in inositol-deficient yeast, Saccharomyces carlsbergensis. I. Influence of different carbon sources on the lipid composition of deficient cells. Biochim Biophys Acta. 1970 Dec 15;218(3):424–430. [PubMed] [Google Scholar]
- STRAUSS B. S. Cell death and unbalanced growth in Neurospora. J Gen Microbiol. 1958 Jun;18(3):658–669. doi: 10.1099/00221287-18-3-658. [DOI] [PubMed] [Google Scholar]
- Steiner M. R., Lester R. L. In vitro studies of phospholipid biosynthesis in Saccharomyces cerevisiae. Biochim Biophys Acta. 1972 Feb 21;260(2):222–243. doi: 10.1016/0005-2760(72)90035-5. [DOI] [PubMed] [Google Scholar]
- White G. L., Hawthorne J. N. Phosphatidic acid and phosphatidylinositol metabolism in Schizosaccharomyces pombe. Biochem J. 1970 Apr;117(2):203–213. doi: 10.1042/bj1170203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YARBROUGH H. F., Jr, CLARK F. M. Utilization of inositol, an essential metabolite for Schizosaccharomyces pombe. J Bacteriol. 1957 Mar;73(3):318–323. doi: 10.1128/jb.73.3.318-323.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]