Summary
Social stress has long been known to affect physical and psychological health in humans and a variety of animal species. In mice, disruption of the social hierarchy (social disruption, SDR) resulted in significant changes in the phenotype and function of immune cells taken from the spleen. Interestingly, there were considerable individual differences in the development of this splenic response to SDR. Studies have suggested that these individual differences were mediated by behavioral factors such as social hierarchy. To test this hypothesis, social status within cages of male mice was identified before and after SDR. Results showed that in the majority of the cages social order was stable over time. In addition, examination of the association between social status and splenic function showed that the splenic response to SDR in subordinate mice was significantly augmented compared to dominants. This relationship between subordinate social status and the splenic response to social stress was more notable in cages with stable social hierarchies. To sum up, the current study showed a role for socio-behavioral factors in determining the response to stress. This study further demonstrated the complexity of factors playing a role in mediating the physiological response to social stress resulting in considerable individual differences in the response to stress.
Keywords: social hierarchy, social status, social disruption, glucocorticoid resistance, spleen, mice
Introduction
The response to stress varies considerably between individuals. In humans, personality and other dispositional traits play a role in shaping the psychological responses to stressful events. Animal studies further showed that stress responses can be modulated by a variety of host- and environmental-related factors (for review see Biondi 2001; Kemeny and Laudenslager, 1999). Several studies demonstrated individual differences in the immunological responses to stress. These studies showed a role for multiple factors such as the nature, duration and severity of the stressor in mediating these differences (for review see Avitsur et al., 2006).
Considerable individual differences were observed in response to a murine model of social stress - the social disruption (SDR). This model of social stress was developed based on the observations indicating that group-housed male mice tend to form social hierarchies. These hierarchies usually consist of a dominant, co-dominant and subordinate cage mates (Ginsburg & Allee, 1975). In SDR, these naturally occurring social hierarchies are repeatedly disrupted by an aggressive intruder mouse (Avitsur et al., 2001; Stark et al., 2001). During SDR, residents are attacked by the intruding aggressor and are repeatedly defeated. The residents attempt to escape and display the characteristic behavioral signs of fear and submissiveness. In a recent set of studies, it was demonstrated that SDR induced splenomegaly and altered the phenotype and function of immune cells taken from the spleen. SDR increased the number of CD11b+ cells in the spleen and induced a state of functional glucocorticoid (GC) resistance in mononuclear cells (Avitsur et al., 2001; 2002a; 2002b; Stark et al., 2001; 2002). LPS-stimulated splenocytes of SDR mice were less sensitive to the anti-proliferative /pro-apoptotic effects of corticosterone (Stark et al., 2001). Splenocytes also secreted high levels of the proinflammatory cytokines interleukin (IL)-6 and tumor necrosis factor (TNF)-α in the presence of LPS (Avitsur et al., 2003a; 2005; Stark et al., 2002). The mechanisms involved in the development of the splenic response to SDR have not been fully elucidated. Studies have shown that depletion of the CD11b+ cell population from splenocyte culture abolished the response (Stark et al., 2001). Additional studies illustrated that the splenic response to SDR was due, in part, to the failure of the glucocorticoid receptor to undergo nuclear translocation in the CD11b+ cells following exposure of the cells to corticosterone (Quan et al., 2003). Further studies demonstrated that SDR was associated with redistribution of leukocytes within the body. Specifically, SDR enhanced recruitment of immune cells from the bone marrow and the marginal pool to the spleen (Engler et al., 2004).
The splenic response to SDR varied significantly between individuals undergoing defeat in similar conditions (Avitsur et al., 2001, 2003b; Bailey et al., 2004, Kinsey et al., 2007). Following SDR, some of the mice exhibited significant changes in splenocytes phenotype and function, while their cage mates were hardly affected (Avitsur et al., 2001). Since these studies used inbred mice (C57BL/6), genetic differences were not likely to be the cause of the variability in the response to SDR. Therefore, environmental and behavioral factors were suggested to be involved (Avitsur et al., 2001). Several of these factors have been identified. First, physical defeat was found to be a key aspect in the response to social stress (Avitsur et al., 2002b; Bailey et al., 2004). Second, mice exhibiting higher levels of submissive behavior when defeated were more likely to exhibit a robust splenic response to SDR (Avitsur et al., 2001). Finally, previous experience in defeat increased the likelihood of mice to develop a strong splenic response following social stress (Avitsur et al., 2003b). Since early experience in defeat and increased tendency to exhibit submissive responses when attacked are characteristics of subordinate social status (Ginsburg and Allee, 1975), it was suggested that social status affected the severity of the splenic response to SDR. The objective of the present study was to characterize the relationship between social status and the splenic response to SDR. In this study, social status in cages of male mice was determined before and after SDR. Subsequently, the association between social status and the splenic response of mice to the stress was assessed.
Methods
Subjects
Subjects were male C57BL/6 mice (Charles River Inc.), aged 6-8 weeks and housed two per cage in an American Association for the Accreditation of Laboratory Animal Care (AAALAC) accredited facility. Mice were given free access to food and water and were maintained in polycarbonate cages, with shaved wood bedding, on a 12-hour light/dark cycle (lights on at 6 AM). Animal care procedures were approved by the Ohio State University Institutional Laboratory Animal Care and Use Committee. Twenty nine pairs of mice were included in this study; eleven pairs were assigned to control conditions and eighteen pairs underwent SDR procedure.
Stimulus Animals
For SDR, aggressive mice were used. Aggressors were singly housed C57BL/6 male mice selected on the basis of pre-experimental screening for aggressive behavior (Avitsur et al., 2001). For the resident-intruder test, young (4-5 weeks), C57BL/6 mice were used as intruders. These intruders had no previous record of aggressive behavior. All stimulus animals were purchased from Charles River Inc., housed in an AAALAC accredited facility with free access to food and water.
Social Disruption Stress (SDR)
Social disruption was previously described (Avitsur et al., 2001). Briefly, an aggressive mouse was introduced into the home cage of SDR mice for one hour beginning at 4:30 PM. Observations were made to ensure that the aggressors defeated the experimental subjects. Animals underwent six daily cycles of SDR. Control mice remained undisturbed in their home cages.
Social Dominance
Social status of the two residents of each cage was assessed using the resident-intruder test. The behavior of mice during the test was videotaped and later coded and analyzed by a researcher blinded to treatment groups using the Observer Video-Pro software package (Noldus Information Technology, Sterling, VA). Cages that did not show a clear social order participated in all parts of the experiment but were omitted from the statistical analysis where indicated.
For the resident-intruder test, a young (4-5 weeks) non-aggressive mouse was introduced into the home cage of the experimental subjects for 10 minutes. The following behavioral measures were scored: social exploration of the intruder; attacks of the intruder; attacks of the other cage resident and submissive behavior. The dominant mouse was defined as the resident that exhibited the highest duration of aggression towards the intruder or the other cage resident and/or the lowest level of submissive behavior. If no aggression was displayed, then the dominant mouse was defined as the resident that exhibited the highest duration of social exploration of the intruder. If the difference in the duration of social exploration between cage residents was less than 5% of the duration of observation, then social status was not defined for this cage and the cage was omitted from the analysis.
Splenocyte Isolation And Culture Conditions
Spleens were harvested into ice-cold Hanks' balanced salt solution (HBSS). Splenocytes single cell suspensions were obtained and counted as previously described (Avitsur et al., 2005). Samples were resuspended (2.5×106 cells/ml) in supplemented RPMI medium (10% heat-inactivated FBS, 0.075% sodium bicarbonate, 10 mM Hepes buffer, 100 U/ml penicillin G, 100 μg/ml streptomycin sulfate, 1.5 mM L-glutamine, 0.00035% 2-mercaptoethanol). To stimulate the cells, 1 μg/ml LPS (Sigma) was added. To test the sensitivity of cells to inhibition by glucocorticoids, aliquots from each cell suspension were treated with corticosterone (Sigma, C2505, 0 - 5 μM) diluted in a buffer of 0.2% ethanol in supplemented RPMI. Cell suspensions were added in triplicate to flat-bottom 96-well plates at a volume of 100 μl/well, and plates were incubated at 37°C and 5% CO2. After 48 hours the cell viability assay was performed. Duplicate plates, containing 200 μl/well were cultured for 18 hours. Supernatants from these plates were harvested and kept frozen in -70°C until assayed for IL-6 and TNF-α.
Cell Viability Assay
The CellTiter 96 Aqueous non-radioactive proliferation assay kit was purchased from Promega (Madison, WI). The tetrazolium substrate solution was prepared according to the manufacturer's instructions, and 20 μl were added to each well of the 96-well plates. Living cells convert this substrate to formazan, producing a brown precipitate. The plates were incubated at 37°C and 5% CO2 for three hours and the resulting color changes were quantified by obtaining optical density readings at 490 nm on an ELISA plate reader. To account for differences in background activity of cells, the mean optical density (O.D.) of the three RPMI/FBS wells for a given treatment was subtracted from each of the corresponding stimulated values.
Cytokine Elisa
Interleukin-6 concentrations were measured by sandwich ELISA following a standard protocol from Pharmingen (San Diego, CA). Tumor Necrosis Factor-α concentrations were measured by an OptEIASet Mouse TNF-α (Mono/Poly) ELISA from Pharmingen (San Diego, CA) following the manufacturer's instructions with minor modifications (Avitsur et al., 2003a). IL-6 and TNF-α concentrations were quantified using standard curves generated using serial dilutions of recombinant IL-6 and TNF-α (Pharmingen), respectively. For statistical purposes, a sample falling below the detection limit of the ELISA was assigned the value corresponding to the sensitivity of that assay. The sensitivity range for IL-6 was 69-16,666 pg/ml, and for TNF-α 63-1000 pg/ml. For IL-6 ELISA, intra-assay variability was 6% and inter-assay variability was 9%. For TNF-α ELISA, intra-assay variability was 7.6% and inter-assay variability was 9.5%
Procedure
Subjects arrived at the OSU vivarium at the age of 6-8 weeks. Mice were housed two/cage for at least two weeks prior to behavioral testing to allow the establishment of social rank between cage residents. Social status of the two residents of each cage was assessed using the resident-intruder test. Social status in the cages was assessed twice prior to SDR. The compiled results from the two trials of the test were used to determine social order in each cage. Following behavioral measurements, cages were randomly assigned to control and SDR groups. SDR mice underwent six daily cycles of SDR, while controls remained undisturbed in their home cages. Following SDR, the resident-intruder test was performed again on all the animals. Two days later, all mice were sacrificed to assess splenic response to SDR. The timeline of the study is illustrated in Table 1.
Table 1. Timeline of experimental procedure.
| Day: | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AM | RI | RI | RI | SPL | |||||||||||
| PM | SDR | SDR | SDR | SDR | SDR | SDR |
RI = Resident Intruder test
SDR = Social Disruption
SPL = Assessment of the splenic response
Data Analysis
The effects of social status and SDR on splenic function were determined using the following tests: Spleen weights and splenocyte numbers were analyzed using a two-way ANOVA with stress (control/SDR) and social status (Dominant/Submissive) as between subject factors. Cell viability, IL-6 and TNF-α data were analyzed using a repeated measure ANOVA, with stress (control/SDR) and social status (Dominant/Submissive) as between subject factors and corticosterone concentration (0-5 μM) as a repeated measure factor. Fisher PLSD post hoc test was performed to assess differences between experimental groups where appropriate.
The effects of behavioral tests on splenic function were determined using the following tests: Spleen weights and splenocyte numbers were analyzed using a one-way ANOVA with behavioral testing (control/control without behavioral testing) as between subject factor. Cell viability, IL-6 and TNF-α data were analyzed using a repeated measure ANOVA, with behavioral testing (control/control without behavioral testing) as between subject factor and corticosterone concentration (0-5 μM) as a repeated measure factor. For all tests, the level of significance was set at p<0.05
Results
Splenic response to SDR
Similar to previous studies, SDR resulted in significant splenomegaly, increased number of mononuclear cells in the spleen, elevated secretion of TNF-α and IL-6 from LPS-stimulated splenocytes and increased viability of splenocytes in the presence of corticosterone (p<0.05 for all variables).
Role of social status in mediating the splenic response to SDR
Overall, the results of the resident-intruder test were found to be associated with the splenic response to SDR. Animals identified as subordinates using this test showed a more robust splenic response to SDR compared to dominants.
Association between social status measured prior to SDR and splenic response to SDR
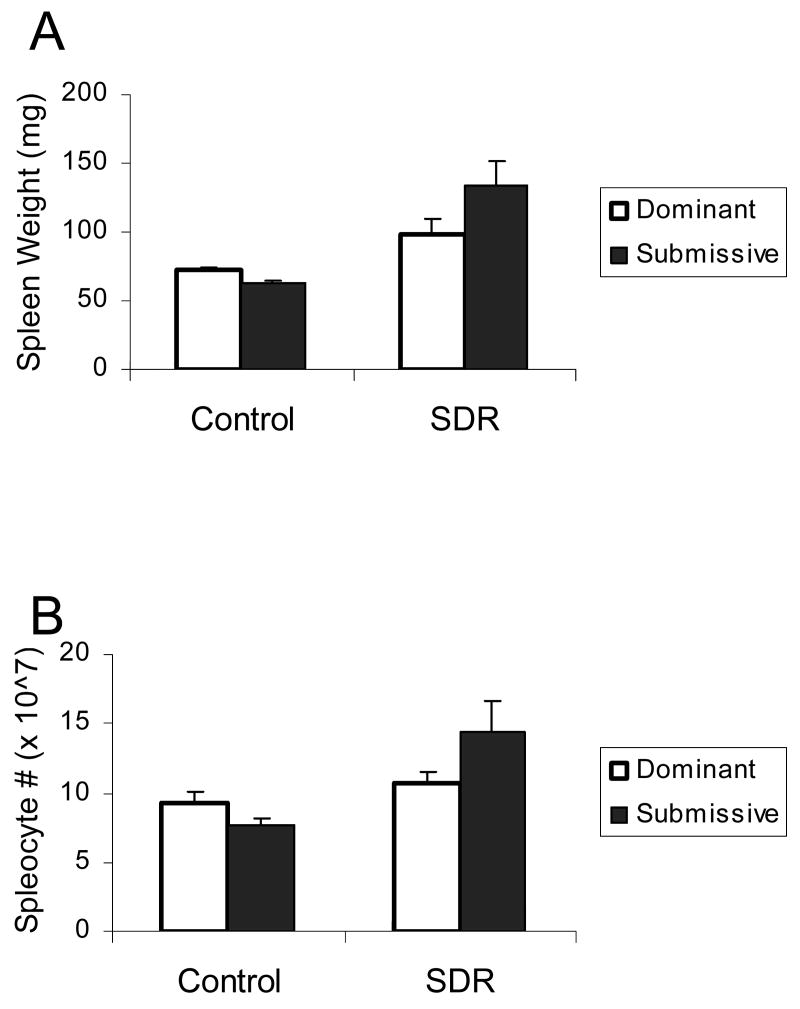
The following analyses include 10 control and 14 SDR cages that showed a clear social status prior to SDR in the resident-intruder test. Two-factor ANOVA did not indicate a role for social status in modulating the effect of SDR on spleen weight and the number of mononuclear cells in the spleen (p>0.05). Repeated measures ANOVA did not reveal an interaction between the effects of SDR, social status and corticosterone on cell viability and levels of TNF-α and IL-6. There was, however, an interaction between the effect of social status and corticosterone on IL-6 levels (F(5,170)=2.668, p<0.05) indicating that IL-6 levels were higher in cells taken from SDR-submissive mice compared to all other groups when untreated or treated with 0.005 μM corticosterone (Fig. 1).
Figure 1.
Splenic response in pair housed male mice. Social status (Dominant / Submissive) was identified prior to SDR using the resident-intruder test. Pairs of mice underwent six nightly SDR cycles (SDR, n=14 pairs) or remained undisturbed in their home cages (Control, n=10 pairs). Data are presented as mean ± S.E.M. (A.) Spleen weights (gr); (B.) Splenocyte numbers; (C.) Cell viability (optical density, OD) of LPS-stimulated splenocytes treated with corticosterone (0-5 μM); (D.) TNF-α and (E.) IL-6 levels (pg/ml) secreted from LPS-stimulated splenocytes treated with corticosterone (0-5 μM); * Significantly different from all other groups (p<0.05).
Association between social status measured after SDR and splenic response to SDR
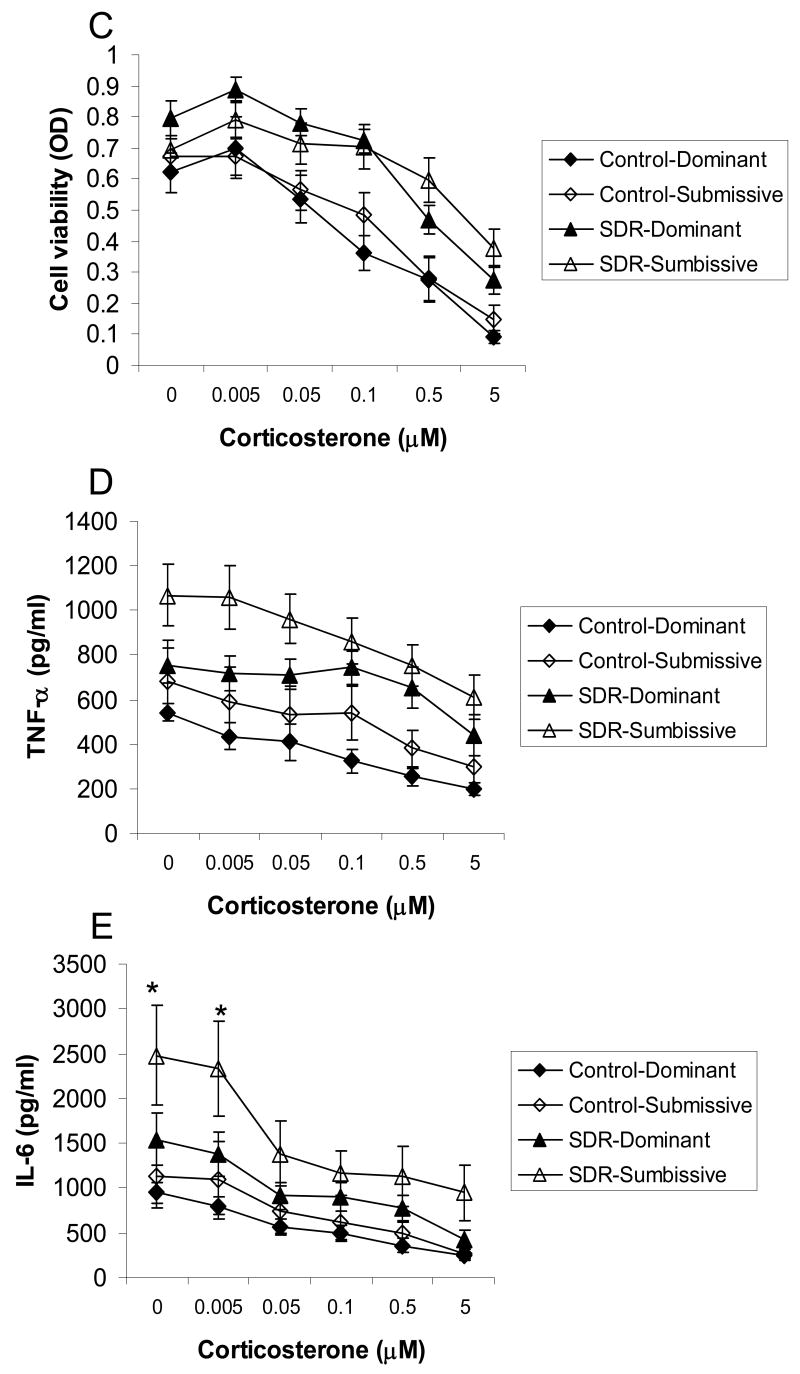
The following analyses include 10 control and 14 SDR cages that showed a clear social status following SDR in the resident-intruder test. Two-factor ANOVA revealed a significant interaction between the effects of SDR and social status on spleen weight (F(1,44)=7.36, p<0.01) indicating that spleens were heavier in SDR-submissive mice compared to all other groups. Repeated measures ANOVA revealed an interaction between the effects of SDR, social status and corticosterone on concentrations of IL-6 (F(5,170)=2.337, p<0.05) indicating that IL-6 production was higher in cells taken from SDR-submissive mice compared to all other groups, whether untreated or treated with 0.005 μM corticosterone. Additionally, cells from SDR submissive mice secreted more IL-6 than control-submissive and SDR-dominant mice when treated with 0.05 or 0.5 μM corticosterone (Fig. 2).
Figure 2.
Splenic response in pair housed male mice. Social status (Dominant / Submissive) was identified after SDR using the resident-intruder test. Pairs of mice underwent six nightly SDR cycles (SDR, n=14 pairs) or remained undisturbed in their home cages (Control, n=10 pairs). Data are presented as mean ± S.E.M. (A.) Spleen weights (gr); (B.) Splenocyte numbers; (C.) Cell viability (optical density, OD) of LPS-stimulated splenocytes treated with corticosterone (0-5 μM); (D.) TNF-α and (E.) IL-6 levels (pg/ml) secreted from LPS-stimulated splenocytes treated with corticosterone (0-5 μM); * Significantly different from all other groups (p<0.05).
Stability of the social status identified using the resident-intruder test
Social status was assessed twice: before and after SDR. Correlations between social status determined before and after SDR was 0.66 for controls (p<0.01) and 0.39 for SDR cages. Comparing the results of the resident-intruder tests performed at these two time points revealed several differences. Social order measured before SDR changed in two control and three SDR cages compared to the later time point. Additionally, two of the SDR cages that showed a clear social status before SDR did not show a clear status after SDR. Thus, of the 10 control cages showing a clear social order prior to SDR, only eight cages showed the identical social order when measured several days later. Out of the 14 SDR cages showing a clear social order prior to SDR, nine cages showed an identical social order after the stress (80% of control cages compared to 64% of the SDR). Chi Square comparison between control and SDR cages did not reveal a significant difference in the frequency of stable/unstable hierarchies (p>0.1). The finding that social order was stable in some of the cages and unstable in others suggested that the stable and unstable cages may have been inherently different from a socio-behavioral perspective. Thus, we sought to examine whether the stability of the social order played a role in mediating the splenic response to SDR. The number of cages that exhibited a change in social status was too small to allow proper statistical analysis. However, the number of cages with stable hierarchies was sufficient to allow a separate analysis of this sub-population and therefore a separate analysis of the splenic response of this group was preformed.
Association between social status in cages with stable order and splenic response to SDR
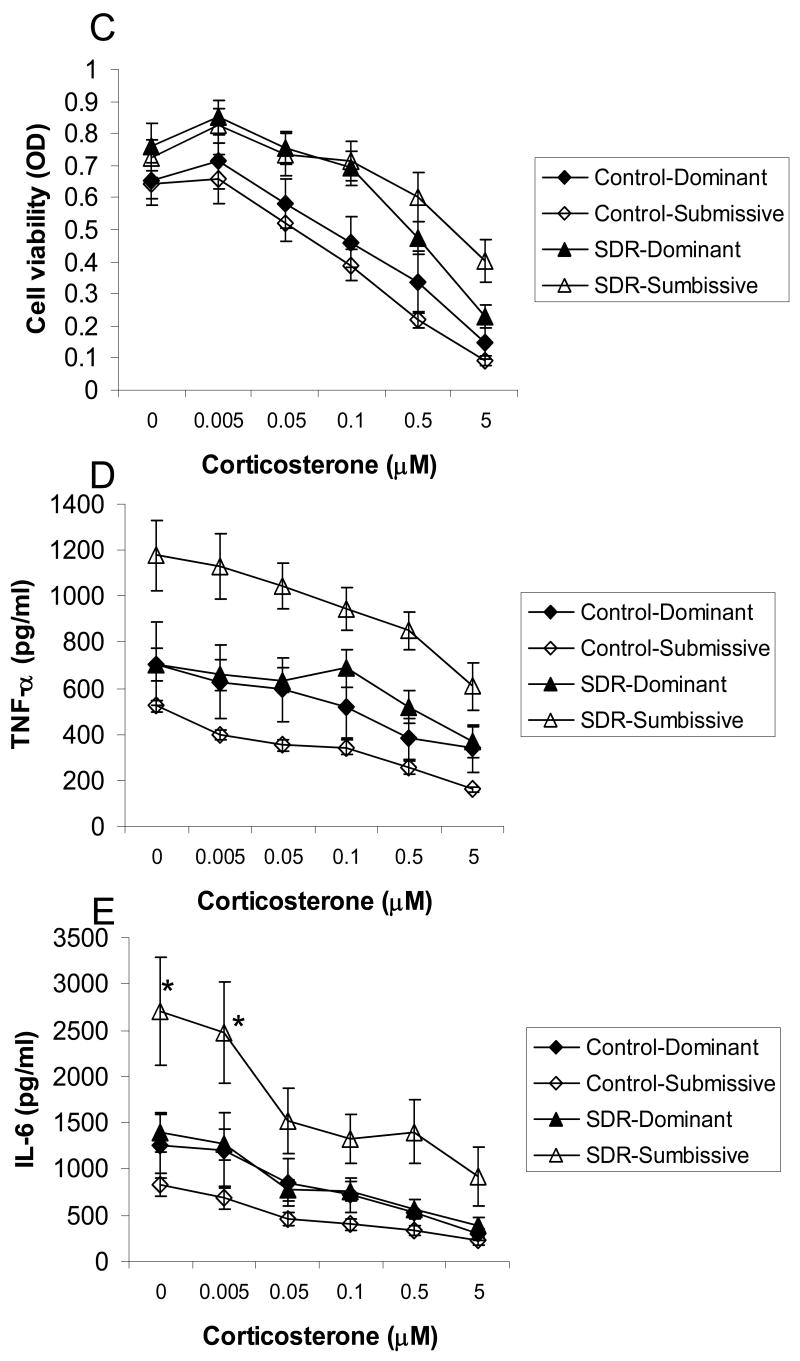
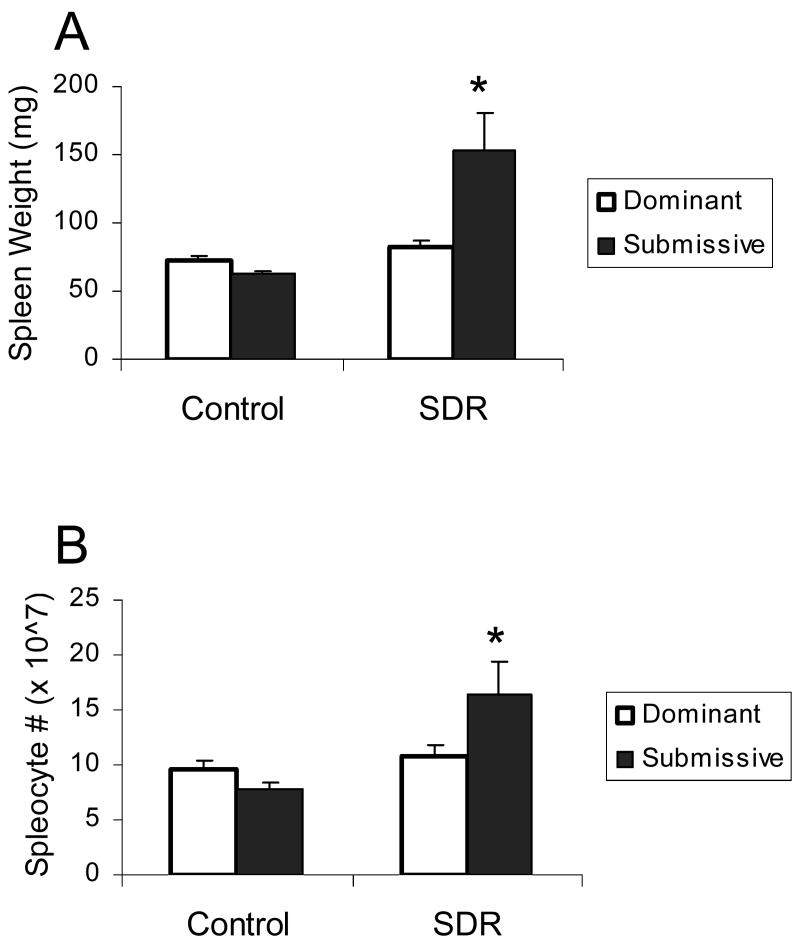
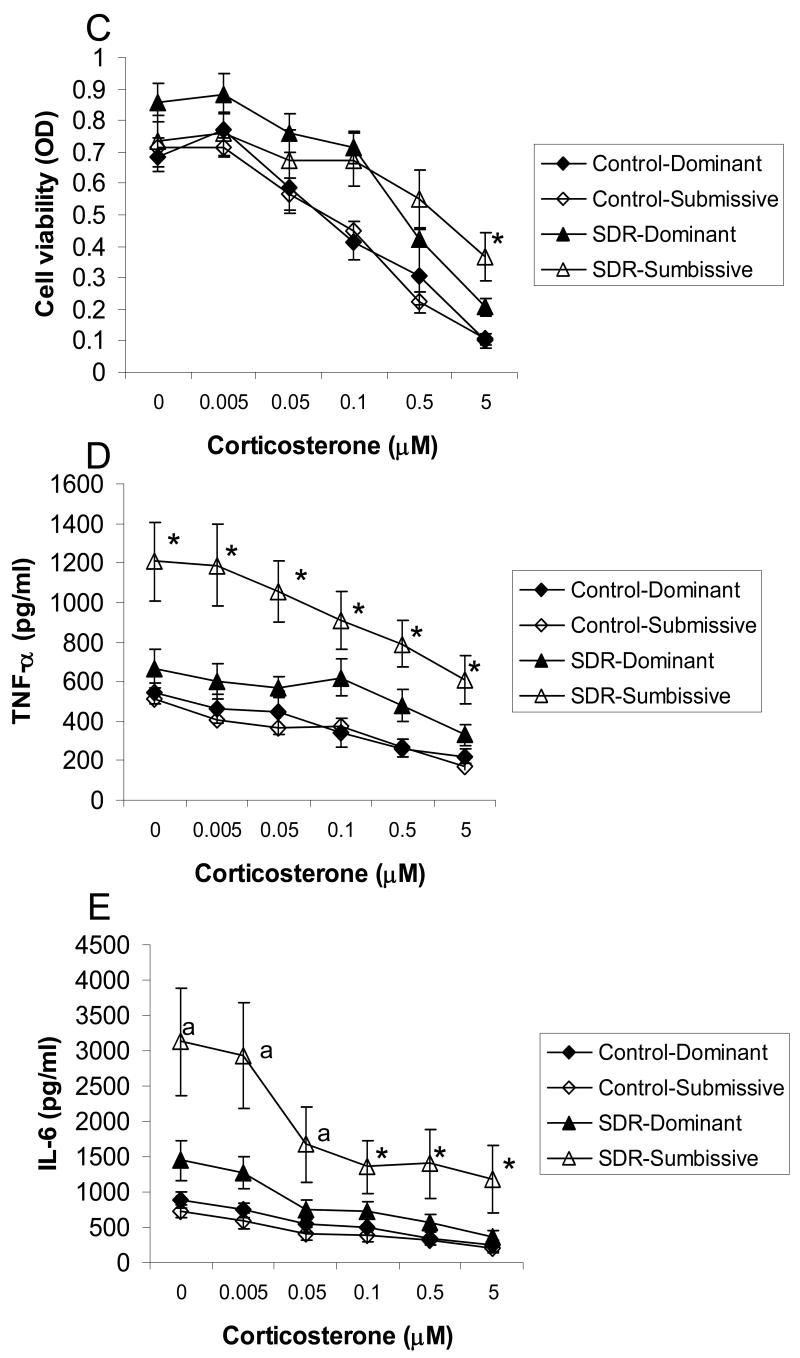
Examining the splenic response in cages with stable social hierarchies showed significant interactions between the effects of SDR and social status on spleen weight and the number of splenic mononuclear cells (F(1,30)= 7.305 and 4.23, respectively, p<0.05, Fig. 3 A and B). Post hoc analyses revealed that spleens were larger and the number of splenocytes was higher in SDR-submissive mice compared to all other groups. Additionally, repeated measures ANOVA revealed an interaction between the effects of SDR, social status, and corticosterone on cell viability, levels of TNF-α and IL-6 (F(5,110)=3.095, 2.492 and 2.396, respectively, p<0.05, Fig. 3 C-E). Post hoc analysis of cell viability revealed a differential response to corticosterone in SDR and control mice depending on social status. In control mice, the three highest doses of corticosterone significantly reduced viability of splenocytes, whereas in SDR mice the two highest doses were effective in suppressing viability. Additionally, viability of cells taken from SDR-submissive mice treated with the highest concentration of corticosterone was significantly higher than viability of cells taken from controls. In contrast, viability of cells taken from SDR-dominant mice treated with this concentration of corticosterone was not different than that of controls. Post hoc analysis revealed that TNF-α concentrations were higher in cells taken from SDR-submissive mice compared to all other groups, regardless of corticosterone treatment. Levels of TNF-α secreted from SDR-dominant mice were not different from that of controls. Similar post hoc analysis showed that IL-6 concentrations were higher in cells taken from SDR-submissive mice compared to controls in the presence of all corticosterone concentrations. In addition, IL-6 levels were higher in cells taken from SDR-submissive mice compared to SDR-dominant in the presence of the three highest concentrations of corticosterone. Levels of IL-6 were not different in cells taken from SDR-dominant mice compared to controls.
Figure 3.
Splenic response in pair housed male mice in cages with stable social status. Social status (Dominant / Submissive) was identified before and after SDR using the resident-intruder test. Only cages with identical social order before and after stress were included in this analysis. Pairs of mice underwent six nightly SDR cycles (SDR, n=8 pairs) or remained undisturbed in their home cages (Control, n=9 pairs). Data are presented as mean ± S.E.M. (A.) Spleen weights (gr); (B.) Splenocyte numbers; (C.) Cell viability (optical density, OD) of LPS-stimulated splenocytes treated with corticosterone (0-5 μM); (D.) TNF-α and (E.) IL-6 levels (pg/ml) secreted from LPS-stimulated splenocytes treated with corticosterone (0-5 μM);* Significantly different from all other groups (p<0.05). a Significantly different from SDR-dominant group (p<0.05).
Assessment of bite wounds
SDR-induced bite-wounds played a role in the development of the splenic response (Avitsur et al., 2001). Thus, wounds were documented following stress in cages with stable social status. According to ethical guidelines, animals did not suffer severe or debilitating injuries. Some of the SDR mice exhibited minor-to-mild bite wounds on the back or tail. Table 2 illustrates the frequency of wounds in SDR cages with stable social order. Briefly, all subordinates were wounded to some extent, whereas 22% of the dominants were not wounded. In addition, minor wounds were observed in 56 % of the dominants and 33% of the subordinates.
Table 2. Frequency of bite wounds in SDR cages with stable social order.
| Submissive (n=9) | Dominant (n=9) | |
|---|---|---|
| No wounds | 0% | 22% |
| Minor wounds | 33% | 56% |
| Mild wounds | 67% | 22% |
Effect of behavioral examinations on splenic function
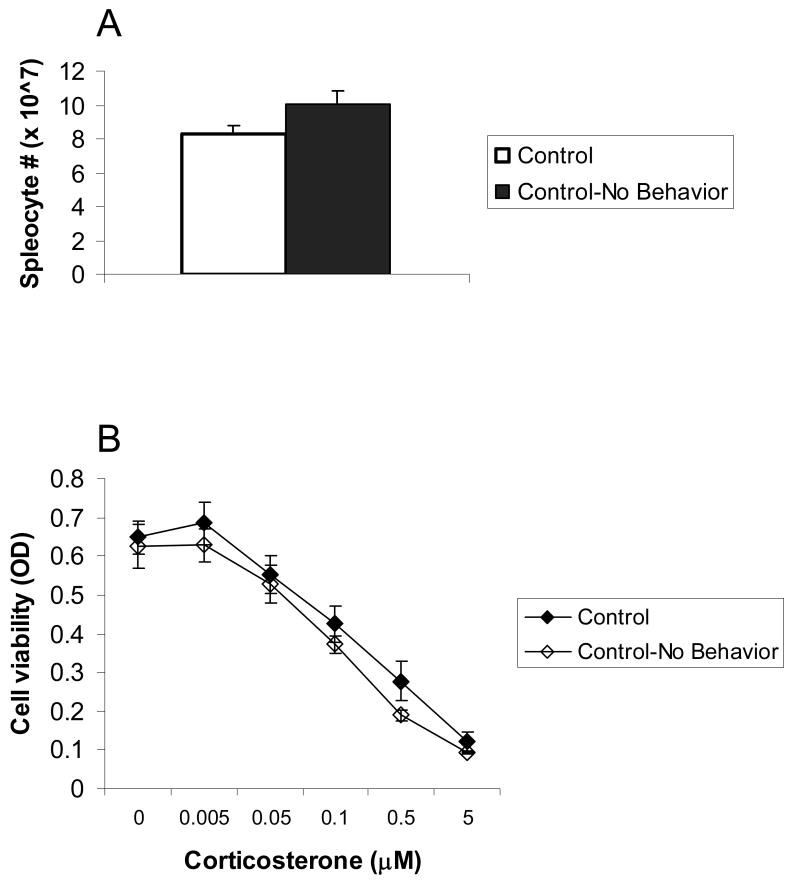
To confirm that the behavioral tests had no effect on splenic function an additional group of naïve mice was examined. These mice (n=8) were left undisturbed in their home cages until sacrificed alongside all other subjects. Splenic function of these mice was compared to that of control mice that underwent behavioral testing to assess social status. Results showed that splenic function was not significantly altered by behavioral testing. Sample data demonstrating splenocytes numbers and cell viability in these two control groups (with and without behavioral testing) are presented in Fig. 4.
Figure 4.
The effect of behavioral examination on splenic function. Two control groups were compared: Control (n=22) – mice that underwent all behavioral tests to assess social status; Control- No Behavior (n=8) – naïve mice that did not undergo behavioral examination. Results showed that there was no significant difference between the splenic function of these groups. Data are presented as mean ± S.E.M. (A.) Splenocyte numbers; (B.) Cell viability (optical density, OD) of LPS-stimulated splenocytes treated with corticosterone (0-5 μM).
Discussion
Defeat in the social disruption stress model is known to induce splenomegaly and alter phenotype and function of splenocytes in male mice (Avitsur et al., 2001; 2002a; 2002b; Stark et al., 2001; 2002). Studies have documented large individual differences between genetically identical mice in the splenic response to SDR. Increased splenic response was associated with submissive responses to aggressive attacks and with previous experience of defeat (Avitsur et al., 2001; 2003b). Based on these studies, it was hypothesized that social status played a key role in mediating the response to SDR. The results of present study suggested a role for subordinate social status in the modulation of the splenic immunological response to SDR. The results further suggested a role for the stable social order in mediating this complex interaction.
Dominancy in the wild entails greater access to limited resources such as territory, food and breeding females (Miczek et al. 2001). Dominant mice in the wild patrol, mark, and defend their territories and exclude or dominate other males. The resident-intruder test reflected this aspect of dominancy demonstrating the tendency of the dominant resident to attack and chase away an invading intruder (Koyama and Kamimura, 1999). The resident-intruder test was found to be associated with the splenic response to the social stress. Mice identified as subordinates by this test were more likely to develop a robust splenic response to SDR. Other test of dominancy, examining access to limited resources of food or breeding females were not associated with the splenic response to SDR (data not shown). SDR models the social interactions between male competitors fighting over territory and driving out potential invaders. The ability of a mouse to successfully attack and chase away an intruder (measured in the resident-intruder test) is related to the behavioral responses associated with this stress model and therefore may also be relevant for the immunological response in such situations. Indeed, previous studies indicated that loss of territory affected immune function. In the chronic psychosocial stress model, mice that were attacked and defeated by an intruder mouse showed blunted interleukin-2, splenocytes proliferation, and IgG antibody responses to immunization with keyhole limpet hemocyanine, as compared to controls (Bartolomucci et al., 2003). These effects were not seen in non-defeated mice, suggesting that the loss of territorial resources influenced the individual immune responses to the stressor. The ability of a resident mouse to struggle for food or breeding females may be less relevant for the behavioral strategies mice need to cope with SDR and thus is less likely to be associated with the splenic response to this stress model.
According to the resident-intruder test, social order remained stable in most cages and changed in several others. In cages with stable social order, the association between subordinate social status and the splenic response to SDR was more notable compared to the analysis of the entire population. This finding suggests that the development of a robust splenic response to SDR was modulated not only by subordinate social status but also by factors associated with the stability of the social order. Thus, the individual differences in the splenic response to SDR were influenced by complex interactions between the behavioral and environmental factors affecting these mice prior to the introduction of the stress, and by factors associated with the social interactions within the cages during the stress.
Social hierarchy in cages of male mice may be unstable and affected by aggressive interactions (Ginsburg and Allee, 1975; Miczek, et al., 2001; Wimer and Fuller, 1966). The present study allowed the assessment of the stability of the dominance order within a cage by comparing the results of the dominancy tests conducted at two time points: before and after SDR. In control cages, the correlation between the two trials was high (r=0.66, p<0.01) indicating that dominancy was relatively stable over time in control conditions. In addition, the stability over time of the dominance order in SDR cages was lower (r=0.39, p>0.1), although the frequency of status changes was not significantly different from that of controls. These findings may suggest that attacks and defeat during SDR had a minor effect on the stability of social order.
Behavioral observations performed during SDR confirmed that all stressed mice were attacked and defeated by the aggressor. A previous study has shown that there were notable individual differences in the behavioral response to defeat. Defeated mice may respond to aggressive attacks with the submissive posture, while their cage mates may show a more active response and run away from the aggressor (Avitsur et al., 2001). Furthermore, mice that tend to exhibit a submissive response were also more likely to show a robust splenic response to SDR (Avitsur et al., 2001). Studies have also demonstrated that the physical aspects of the defeat and the resulting bite-wounds are important for the development of the splenic response to SDR (Avitsur et al., 2001; Bailey et al., 2004). Together, these findings may suggest that the more active response may have an advantage in that it reduces the ability of the aggressor to catch and physically attack its opponents. Based on this conclusion, a possible interpretation of the present findings may be that dominants were more likely to show an active response to defeat and therefore received less bite wounds. Consequently, although being behaviorally defeated, dominants were less likely to develop a robust splenic response to SDR. To examine this hypothesis, the type and severity of bite wounds after SDR were assessed. According to ethical guidelines, the SDR procedure was designed and conducted so that animals did not suffer severe or debilitating injuries. Yet, SDR often resulted in minor-to-mild bite wounds on the animals' backs and tails. Examination of the wounds showed that all subordinates were wounded to some extent, whereas 22% of the dominants were not wounded. In addition, most of the dominants showed only a few very superficial wounds. In contrast, in subordinates 33% showed few superficial wounds and the rest suffered a number of mild bite wounds. Thus, the data suggested that subordinate mice showed more severe bite wounds compared to dominants. Together, these findings suggest that the behavioral response of dominant and submissive mice to defeat may have had an effect on the likelihood of being injured and thus may have played a role in determining the severity of the splenic response to SDR.
Acknowledgments
The authors thank Jacqui Verity, Amy Hufnagle and Elizabeth Sheridan for excellent help.
Author Disclosure: This work was supported by an NIH/NIMH grant RO1 MH46801 and the Mind, Brain, Body and Health Network (MBBH). The NIH and MBBH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Conflict Of Interest: The authors state that there are not conflicts of interest that could inappropriately influence or be perceived to influence this work.
Drs. Avitsur, Bailey, Padgett and Sheridan developed the conceptual framework in which this work was done and developed the theoretical background of the study. Dr. Avitsur designed the study, oversaw all aspects of the experimental manipulation, data analysis and wrote the majority of the manuscript. Dr. Kinsey assisted in designing the study and interpretation of the data. Ms. Bidor analyzed the behavioral data and carried out the statistical analysis. All authors contributed to and have approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Avitsur R, Kavelaars A, Heijnen C, Sheridan JF. Social stress and the regulation of tumor necrosis factor-alpha secretion. Brain Behav Immun. 2005;19(4):311–317. doi: 10.1016/j.bbi.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Avitsur R, Padgett DA, Dhabhar FS, Stark JL, Kramer KA, Engler H, Sheridan JF. Expression of glucocorticoid resistance following social stress requires a second signal. J Leuk Biol. 2003a;74:507–513. doi: 10.1189/jlb.0303090. [DOI] [PubMed] [Google Scholar]
- Avitsur R, Padgett DA, Sheridan JF. Social interactions, stress and immunity. Neurol Clin. 2006;24:483–491. doi: 10.1016/j.ncl.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Avitsur R, Stark JL, Dhabhar FS, Kramer KA, Sheridan JF. Social experience alters the response to social stress in mice. Brain Behav Immun. 2003b;17:426–437. doi: 10.1016/s0889-1591(03)00034-5. [DOI] [PubMed] [Google Scholar]
- Avitsur R, Stark JL, Dhabhar FS, Padgett DA, Sheridan JF. Social disruption induced glucocorticoid resistance: Kinetics and site specificity. J Neuroimmunol. 2002a;124:54–61. doi: 10.1016/s0165-5728(02)00010-3. [DOI] [PubMed] [Google Scholar]
- Avitsur R, Stark JL, Dhabhar FS, Sheridan JF. Social stress alters splenocyte phenotype and function. J Neuroimmunol. 2002b;132:66–71. doi: 10.1016/s0165-5728(02)00310-7. [DOI] [PubMed] [Google Scholar]
- Avitsur R, Stark JL, Sheridan JF. Social stress induces glucocorticoid resistance in subordinate animals. Horm Behav. 2001;39:247–257. doi: 10.1006/hbeh.2001.1653. [DOI] [PubMed] [Google Scholar]
- Bailey MT, Avitsur R, Engler H, Padgett DA, Sheridan JF. Physical defeat reduces the sensitivity of murine splenocytes to the suppressive effects of corticosterone. Brain Behav Immun. 2004;18(5):416–24. doi: 10.1016/j.bbi.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Bartolomucci A, Sacerdote P, Panerai AE, Pederzani T, Palanza P, Parmigiani S. Chronic psychosocial stress-induced down-regulation of immunity depends upon individual factors. J Neuroimmunol. 2003;141:58–64. doi: 10.1016/s0165-5728(03)00220-0. [DOI] [PubMed] [Google Scholar]
- Biondi M. Effects of stress on immune function: an overview. In: Ader R, Felten DL, Cohen N, editors. Psychoneuroimmunology. San Diego, Calif.: Academic Press; 2001. pp. 189–226. [Google Scholar]
- Engler H, Bailey MT, Engler A, Sheridan JF. Effects of repeated social stress on leukocyte distribution in bone marrow, peripheral blood and spleen. J Neuroimmunol. 2004;48:106–115. doi: 10.1016/j.jneuroim.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Ginsburg B, Allee WC. Some effects of conditioning on social dominance and subordination in inbred strains of mice. In: Schein MW, editor. Social hierarchy and dominance. New York: Halsted Press; 1975. pp. 282–303. [Google Scholar]
- Kemeny ME, Laudenslager ML. Introduction beyond stress: the role of individual difference factors in psychoneuroimmunology. Brain Behav Immun. 1999;13(2):73–75. doi: 10.1006/brbi.1999.0562. [DOI] [PubMed] [Google Scholar]
- Kinsey SG, Bailey MT, Sheridan JF, Padgett DA, Avitsur R. Repeated social defeat causes increased anxiety-like behavior and alters splenocyte function in C57BL/6 and CD-1 mice. Brain Behav Immun. 2007;21(4):458–466. doi: 10.1016/j.bbi.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama S, Kamimura S. Lowered sperm motility in subordinate social status of mice. Physiol Behav. 1999;65:665–669. doi: 10.1016/s0031-9384(98)00205-4. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Maxson SC, Fish EW, Faccidomo S. Aggressive behavioral phenotypes in mice. Behav Brain Res. 2001;125:167–181. doi: 10.1016/s0166-4328(01)00298-4. [DOI] [PubMed] [Google Scholar]
- Quan N, Avitsur R, Stark JL, He L, Lai W, Dhabhar F, Sheridan JF. Molecular mechanisms of glucocorticoid resistance in splenocytes of socially stressed male mice. J Neuroimmunol. 2003;137:51–58. doi: 10.1016/s0165-5728(03)00042-0. [DOI] [PubMed] [Google Scholar]
- Stark JL, Avitsur R, Hunzeker J, Padgett DA, Sheridan JF. Interleukin-6 and the development of social disruption-induced glucocorticoid resistance. J Neuroimmunol. 2002;124:9–15. doi: 10.1016/s0165-5728(02)00004-8. [DOI] [PubMed] [Google Scholar]
- Stark JL, Avitsur R, Padgett DA, Campbell KA, Beck FM, Sheridan JF. Social stress induces glucocorticoid resistance in macrophages. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1799–R1805. doi: 10.1152/ajpregu.2001.280.6.R1799. [DOI] [PubMed] [Google Scholar]
- Wimer RE, Fuller JL. Patterns of behavior. In: Green EL, editor. Biology of the laboratory mouse. McCraw Hill, NY: 1966. pp. 629–653. [Google Scholar]