Abstract
Purpose
To investigate the impact of the Ala1330Val (rs3736228, exon 18) and Val667Met (rs4988321, exon 9) polymorphisms of the low-density lipoprotein receptor-related protein 5 (LRP5) gene on peak bone mass in young men.
Methods
The Odense Androgen Study (OAS) is a population-based study comprising 783 Caucasian men aged 20-30 years. Genotyping was performed using real-time polymerase chain reaction (PCR) or fluorescence polarization. Bone mineral density (BMD) measurements were performed using dual-energy X-ray absorptiometry.
Results
The CC, CT, and TT genotypes in Ala1330Val were found in 75.6%, 21.8%, and 2.6% of the participants, respectively. Similarly, the GG, GA, and AA genotypes of Val667Met were found in 89.7%, 9.8%, and 0.5%, respectively. For the Ala1330Val polymorphism, no significant differences between the genotypes were found regarding BMD in the overall study population. However, when analysis was restricted to non-sedentary men (n = 589), a significant association between the number of T-alleles and BMD in the spine and whole body were found. Each copy of the T-allele changed the Z-score of the spine by (median and 95% confidence interval) −0.21 [95% CI: −0.40; −0.03] (p < 0.02). Analysis suggested an association between the AA genotype in the Val667Met polymorphism and increased body height and decreased BMD of the femoral neck; however, no significant gene-dose effect of the A-allele could be demonstrated in the whole population. When the analysis was restricted to non-sedentary subjects, however, each number of A-alleles was associated with a change in Z-score of −0.26 [95% CI: −0.51; −0.01] (p = 0.04). No further significant results emerged with haplotype analysis.
Conclusion
The Ala1330Val and Val667Met polymorphisms in the LRP5 gene are significantly associated with peak bone mass in physically active men.
Keywords: Association, Bone mineral density, Low-density lipoprotein receptor-related protein 5, Men, Polymorphisms
Family and twin studies have demonstrated that genetic factors account for 50–80% of the inter-individual variation in bone mineral density (BMD) in both women and men [1–9]. Polymorphisms in a number of genes affect the bone phenotype regarding BMD, bone size, or fracture risk [10]. Only a small part of the overall variation in BMD, however, has been explained by the polymorphisms identified so far.
The transmembrane proteins low-density lipoprotein receptor-related protein 5 (LRP5) and LRP6 are essential in Wnt signaling. They are expressed in many tissues including osteoblasts and have been implicated in the adaptive response of bone to mechanical load [11]. Located on chromosome 11q12–14, activating mutations in the LRP5 gene are responsible for rare conditions with a “high bone mass phenotype” [12, 13] as well as autosomal dominant osteopetrosis type 1 [14], and transgenic mice carrying the LRP5 G171V mutation have increased bone mass and bone biomechanical properties [15]. Conversely, inactivating mutations are responsible for the osteoporosis pseudoglioma syndrome [16] and this syndrome is recapitulated by LRP5 inactivation in mice [17].
In addition to these mutations, a number of polymorphisms have been described in the LRP5 gene (http://www.ncbi.nlm.nih.gov/SNP). One of these, the Ala1330Val (rs3736228, exon 18) polymorphism, has been associated with attenuated bone gain in prepubertal boys [18], decreased BMD in Japanese [19], American [20], Australian [21], and Dutch [22] women as well as in elderly Dutch men [22]. It has also been associated with an increased risk of fragility fractures in postmenopausal Australian women [21] and in elderly Dutch men [22]. No association, however, was found between this polymorphism and bone loss in elderly men and women [22]. Similarly, the Val667Met (rs4988321, exon 9) polymorphism was associated with attenuated bone gain in prepubertal boys but not in girls participating in a 1-year longitudinal Swiss study [18]. In the same study, this polymorphism was also associated with decreased lumbar spine bone mineral content and projected bone area and there was a trend toward lower BMD in the spine [18]. These associations were mainly driven by men [18] and in haplotype analysis including Ala1330Val, most of the effect was conferred by the Val667Met polymorphism. Moreover, in a case-control study by Ferrari et al. [23] on idiopathic osteoporosis in males, both LRP5 polymorphisms significantly increased the odds ratio for osteoporosis. Additionally, Val667Met was associated with low stature in adults of both sexes [18]. Thus, current evidence suggests that polymorphisms in the LRP5 gene may be involved in the control of peak bone mass and may increase the risk of osteoporosis in males [16,18, 23].
In the present study, we investigated the association between the Ala1330Val and Val667Met polymorphisms and peak bone mass in men in a population-based study.
Subjects and Methods
The Odense Androgen Study (OAS) is a population-based, prospective, observational study on the interrelationship between endocrine status, body composition, muscle function, and bone metabolism in young men. Details on the design and inclusion of participants are reported elsewhere [24]. In brief, 3,000 men aged 20–30 years were randomly selected from the civil registration database in Fuenen County, Denmark, and invited by mail to participate in the study. These subjects also received a questionnaire regarding smoking, medication, chronic diseases, drug abuse, etc. A total of 2,042 questionnaires were returned, and from these 859 men expressed interest in participation in the clinical part of the study. Nineteen withdrew before an appointment for inclusion was made. Ten men did not meet with the inclusion criteria (Caucasian and age between 20–29 years), and another 3 men gave their notice after the inclusion had stopped. Aside from these latecomers none of the responders were excluded. Of the remaining 827 eligible subjects, 43 did not attend for their appointment and did not wish to reschedule, but informed consent was obtained from 784 men, with dropout from 1 subject. Thus 783 Caucasian men gave written informed consent and were included in the OAS. These participants had similar age, body mass index (BMI), lifestyle, socio-economic status, and educational level as the background population, as evaluated by questionnaires and demographic data (manuscript in preparation). The protocol stipulated that subjects with massive substance or alcohol abuse, malignant disease or severe chronic disease be excluded; however, none of the responders fulfilled any of these criteria. The study was approved by the local Ethics Committee of Fuenen and Vejle Counties (file number 20010198), conducted according to the Helsinki Declaration, and declared in ClinicalTrials.gov: NCT00150163, registered September 6, 2005.
Genotyping
DNA was isolated from whole blood samples using QIAamp DNA Blood Midi kits (Qiagen, Valencia, CA, USA).
The Ala1330Val polymorphism was analyzed using Taqman primers and probes that were designed using Primer Express software (Applied Biosystems):
Sense: 5′-GACGGCGAGGCAGACTGT-3′
Antisense: 5′-GCAGGGCCAGGGTCTTG-3′
C probe: 5′-FAM-TCAAAGTCCGCCTCGT-NFQ-3′
T probe: 5′-VIC-CACAGTCCACCTCGTC-NFQ-3′
The real-time analysis was performed using the ABI PRISM 7700 Sequence Detection System. To validate the method, approximately 10% of the samples were repeated and in total 17 samples—at least 5 of each genotype—were sequenced.
The Val667Met polymorphism was analyzed using fluorescence polarization as described previously [25], using AcycloPrime-FP SNP Detection kits (Perkin Elmer Life Sciences, Boston, MA, USA). PCR fragments containing the polymorphisms were generated using standard conditions with the following PCR primers:
Sense: 5-gccTTcTTggTcTTcAccAg-3′
Antisense: 5′-cTTTgAggcAggAAcAgAgg-3′
Enzymatic clean-up and subsequent AcycloPrime-FP reaction with SNP primer (sense 5′-ccTcgAgAccAATAAcAAcgAc-3′) were performed according to the instructions of the kit manufacturer. Fluorescence polarization was read in a Wallac Victor multilabel plate reader (Perkin Elmer Life Sciences, Boston, MA, USA). Blank samples and samples with known genotype were included as negative and positive controls, respectively. A total of 10% of all samples were sequenced and genotyping confirmed in all cases.
Anthropometrics and Lifestyle
Body weight, body height, sitting height, and arm span were measured. Data regarding exercise habits were collected using a self-administered questionnaire. Non-sedentary lifestyle was defined as participation in any form of regular exercise.
Dual-Energy X-ray Absorptiometry (DXA)
BMD of the lumbar spine, hip, and whole-body was measured using a Hologic 4500 DXA-scanner (Waltham, MA, USA). The coefficient of variation (CV) for BMD is 1.5%, 1.5%, and 0.7% in the lumbar spine, total hip, and whole body, respectively. Lean body mass (LBM) and fat mass was obtained from the whole-body scans.
Biochemistry
Serum samples were drawn after an overnight fast between 08.00 and 10.00 hours and stored for later analyses at −80°C. Serum concentrations of osteocalcin were measured by a luminoimmunoassay (BRAHMS Diagnostica, Berlin, Germany) with an intra-assay and inter-assay CV of 5% and 8%, respectively.
Serum levels of type 1 collagen C-terminal telopeptide (1CTP) were measured using radioimmunoassay (RIA) (Orion Diagnostica, Espoo, Finland) with an intra-assay and inter-assay CV of 4% and 6%, respectively. Bone-specific alkaline phosphatase (bone-AP) concentration in serum was determined enzymatically after monoclonal antibody capture (Metra Biosystems, Mountain View, CA, USA). The intra-assay CV was 2% and the inter-assay CV 12% for this analysis. Serum levels of 25-OH-vitamin D were measured using a competitive radioimmunoassay (DiaSorin, Stillwater, MN, USA) with an intra-assay CV of 6% and an inter-assay CV of 15%. Serum IGF-I was determined in acid–ethanol extracts as described previously [26]. Intra- and inter-assay variation averaged less than 5% and less than 10%, respectively.
Statistics
Data are reported as the mean ± SD, median (range), or median [95% CI], as appropriate. BMD values are shown in absolute values. Regarding the allele dose-analyses, BMD values were transformed to Z-scores using the SD observed in our population. Hardy-Weinberg equilibrium was checked using chi-square analysis. Linkage disequilibrium (D’) between polymorphisms was evaluated by using GOLD software [27]. Haplotype analysis was undertaken using FAMHAP software for constructing haplotypes [28]. Comparisons between groups were performed using one-way ANOVA, unpaired t-test or similar nonparametric tests. Associations between parameters were analyzed using Pearson’s regression analysis and multiple linear regression analysis. Dichotomies were tested using the chi-square test. A p value of less than 0.05 was considered significant. Statistical analyses were performed using SPSS 11.5 and SPSS 12.0.
Results
Genotype Frequencies
For the Ala1330Val polymorphism, the CC genotype was found in 589 (75.6%), the CT genotype in 170 (21.8%), and the TT genotype in 20 (2.6%), respectively (Table 1) allele frequencies of 0.87 and 0.13 for the C- and T-alleles, respectively.
Table 1.
Covariates, BMD, and lifestyle (sedentary/non-sedentary) by LRP5 genotypes (Ala1330Val and Val667Met polymorphisms)
| Polymorphisms | ||||||||
|---|---|---|---|---|---|---|---|---|
| Ala1330Val | Val667Met | |||||||
| CC | CT | TT | p values (ANOVA) | GG | GA | AA | p values (ANOVA) | |
| n | 589 | 170 | 20 | – | 699 | 76 | 4 | – |
| Age (years) | 25.5 ± 2.8 | 25.3 ± 2.8 | 25.9 ± 2.7 | NS | 25.5 ± 2.8 | 25.3 ± 2.6 | 27.0 ± 1.8 | NS |
| Body height (cm) | 181.7 ± 6.8 | 181.5 ± 6.1 | 182.8 ± 7.7 | NS | 181.7 ± 6.8 | 181.3 ± 5.6 | 190.7 ± 6.3 | 0.02 |
| Arm span (cm) | 183.4 ± 8.2 | 183.1 ± 7.0 | 185.4 ± 8.9 | NS | 183.3 ± 8.0 | 182.6 ± 6.9 | 193.5 ± 6.8 | 0.04 |
| Body weight (kg) | 81.9 ± 11.8 | 81.1 ± 12.4 | 85.9 ± 16.7 | NS | 81.7 ± 12.1 | 82.2 ± 12.9 | 90.4 ± 12.2 | NS |
| Body mass index (kg/m2) | 24.8 ± 3.3 | 24.6 ± 3.7 | 25.6 ± 4.1 | NS | 24.8 ± 3.3 | 25.1 ± 4.3 | 24.8 ± 1.8 | NS |
| Total fat mass (kg) | 14.9 ± 6.2 | 14.9 ± 7.3 | 17.7 ± 8.5 | NS | 15.3 ± 6.2 | 15.1 ± 6.7 | 17.9 ± 7.9 | NS |
| Lean body mass (kg) | 64.0 ± 7.3 | 63.3 ± 6.8 | 65.3 ± 9.0 | NS | 64.7 ± 6.9 | 64.4 ± 6.0 | 70.4 ± 7.0 | NS |
| Units alcohol/week (n) | 11.4 ± 10.2 | 10.7 ± 8.6 | 12.3 ± 8.7 | NS | 11.0 ± 9.4 | 10.8 ± 8.1 | 6.5 ± 7.3 | NS |
| Cigarettes/day (n) | 0 [0–45] | 0 [0–35] | 0 [0–25] | NS | 0 [0–35] | 0 [0–30] | 0 [0–1] | NS |
| Serum 25-OH-D3 (nmol/l) | 65.1 ± 27.9 | 64.2 ± 26.9 | 67.9 ± 28.6 | NS | 65.2 ± 27.5 | 63.0 ± 29.1 | 60.0 ± 25.4 | NS |
| Serum IGF-I (μg/l) | 199.9 ± 52.8 | 202.4 ± 50.3 | 191.0 ± 36.1 | NS | 27.6 ± 7.5 | 25.5 ± 6.6 | 27.3 ± 7.8 | NS |
| Serum osteocalcin (mmol/l) | 3.0 ± 1.1 | 3.1 ± 1.3 | 3.0 ± 1.0 | NS | 3.1 ± 1.2 | 2.8 ± 1.0 | 2.3 ± 0.5 | NS |
| Serum 1CTP (μg/l) | 5.0 ± 1.4 | 4.9 ± 1,5 | 5.1 ± 1.3 | NS | 5.0 ± 1.4 | 4.9 ± 1.4 | 4.9 ± 0.9 | NS |
| Bone-AP (U/l) | 26. 6 ± 8.1 | 28.4 ± 10.0 | 26.7 ± 8.3 | NS | 26.7 ± 8.2 | 25.8 ± 7.0 | 33.4 ± 16.4 | NS |
| BMDspine (g/cm2) | 1.08 ± 0.12 | 1.07 ± 0.17 | 1.05 ± 0.13 | NS | 1.08 ± 0.12 | 1.07 ± 0.22 | 1.07 ± 0.15 | NS |
| BMDhip (g/cm2) | 0.95 ± 0.14 | 0.94 ± 0.14 | 0.94 ± 0.15 | NS | 0.95 ± 0.14 | 0.96 ± 0.15 | 0.84 ± 0.07 | 0.03 |
| BMDWB (g/cm2) | 1.22 ± 0.10* | 1.21 ± 0.09 | 1.21 ± 0.11 | NS | 1.22 ± 0.10 | 1.21 ± 0.11 | 1.20 ± 0.08 | NS |
| Sedentary/non-sedentary (n) | 150/440 | 37/133 | 4/16 | NS | 171/528 | 18/58 | 1/3 | NS |
No significant differences were found between the groups (ANOVA). This was true also if the CT and TT genotypes were pooled (t-test). Genotypes were in Hardy-Weinberg equilibrium (Ala1330Val: χ2 = 3.2, p = 0.07; Val667Met: χ2 = 1.48, p = 0.22)
*p = 0.05 comparing the CC genotype with CT + TT pooled; NS, not significant
Similarly, the GG, GA, AA genotypes of the Val667Met polymorphism were found in 699 (89.7%), 76 (9.8%), and 4 (0.5%), respectively. This corresponds to allele frequencies of 0.95 and 0.05 for the G- and A-alleles, respectively.
Both allele distributions were compatible with Hardy-Weinberg equilibrium (χ2 = 3.2, p = 0.07 and χ2 = 1.48, p = 0.22). The two polymorphisms were not in complete linkage disequilibrium (D’ = 0.955, R² = 0.33).
Covariates
Data on covariates in relation to genotypes are shown in Table 1. No significant differences regarding covariates (age, body height, body weight, BMI, sitting height (not shown), total fat mass, lean body mass, alcohol consumption, smoking, serum 25-OH-D3, or serum IGF-I) were seen between the genotypes of the two polymorphisms. Regarding the Val667Met genotypes, however, subjects with the AA genotype had significantly increased body height (190.7 ± 6.6 vs. 181.7 ± 6.8 and 181.3 ± 5.6 cm, p = 0.02) and arm span (193.5 ± 6.8 vs. 183.3 ± 8.0 and 182.6 ± 6.9 cm, p = 0.04).
BMD in Relation to Age
In the spine, no relationship between BMD and age could be detected. Maximum BMD in the total hip was observed around the age of 22 years. In subjects above this age BMD was significantly lower (ANOVA, p < 0.05). In subjects aged 30 years, BMD was 5.5% lower that the observed maximum.
Genotypes in Relation to BMD
Regarding the Ala1330Val polymorphism, no significant differences between BMD in the spine, hip, or whole body were found between subjects with the CC, CT, and TT genotypes. When the CT and TT genotypes were pooled, whole-body BMD was significantly lower in the CT + TT compared with the CC genotype (1.21 ± 0.10 vs. 1.22 ± 0.10, p = 0.05).
Regarding the Val 667Met polymorphism, a significant difference in hip BMD was observed, since subjects with the AA genotype had lower BMD (0.84 ± 0.07 vs. 0.95 ± 0.14 and 0.96 ± 0.15, p = 0.03).
Table 1 also shows the distribution of genotypes in relation to sedentary/non-sedentary lifestyle, but no significant difference in lifestyle was found between any of the genotypes.
Table 2 shows the gene-dose effect on BMD expressed as the change in Z-score of each copy of the T- or A-allele, respectively. The analysis was performed both with and without adjustment for BMI, smoking (0/1), any continuous medication (0/1), and serum 25-OH-D3 in the overall study population (multiple regression analysis). Regarding the Ala1330Val polymorphism, whole-body BMD tended to decrease with each copy of the T-allele (p = 0.07) in the overall study population (n = 779). No significant association between number of T-alleles and BMD at the other skeletal sites was found. Regarding the Val667Met polymorphism, no significant gene-dose effect could be demonstrated in the overall population.
Table 2.
Multiple regression analysis determining the change in BMD Z-score for each copy of the T- or A-allele
| Polymorphism | ||||
|---|---|---|---|---|
| Ala1330Val | Val667Met | |||
| Z-score | Unadjusted | Adjusteda | Unadjusted | Adjusteda |
| Total population (n = 779) | ||||
| BMDspine | −0.11 (−0.29; 0.06)NS | −0.10 (−0.27; 0.06)NS | −0.044 (−0.28; 0.19)NS | −0.054 (−0.27; 0.16)NS |
| BMDhip | −0.03 (−0.20; 0.11)NS | −0.02 (−0.17; 0.11)NS | 0.018 (−0.20; 0.23)NS | 0.002 (−0.22; 0.21)NS |
| BMDWB | −0.13 (−0.27; 0.11)p = 0.07 | −0.12 (−0.25; 0.01)p = 0.07 | −0.085 (−0.30; 0.13)NS | −0.11 (−0.33; 0.10)NS |
| Non-sedentary (n = 589) | ||||
| BMDspine | −0.21 (−0.40; −0.03)p = 0.02 | −0.20 (−0.37; −0.02)p = 0.01 | −0.26 (−0.51; −0.01)p = 0.04 | −0.23 (−0.46; −0.002)p = 0.048 |
| BMDhip | −0.08 (−0.26; 0.09)NS | −0.06 (−0.22; 0.10)NS | −0.08 (−0.33; 017)NS | −0.07 (−0.32; 0.19)NS |
| BMDWB | −0.17 (−0.33; −0.01)p = 0.04 | −0.15 (−0.30; 0.01)p = 0.07 | −0.17 (−0.41; 0.08)NS | −0.18 (−0.44; 0.07)NS |
Data are shown as mean effect and 95% confidence interval. Z-scores were derived from the study population itself
aAdjusted for BMI (ln-transformed), lean body mass (ln-transformed), smoking (0/1), any continuous medication (0/1), and serum 25-OH-D3
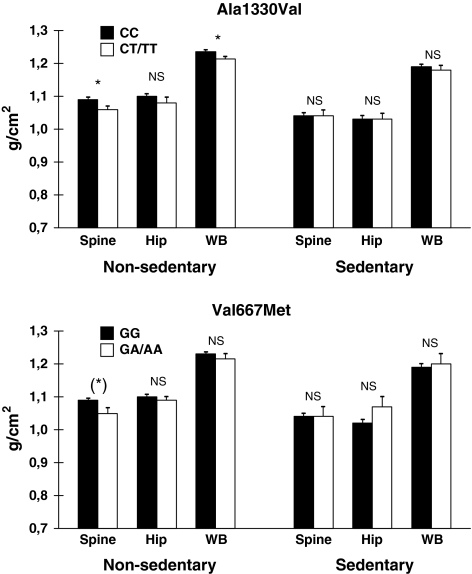
When analysis was restricted to non-sedentary men (n = 589), however, a significant correlation between the number of T-alleles at the Ala1330Val polymorphism and BMD in the spine was found in both the unadjusted (−0.21 [95% CI: −0.40; −0.03], p = 0.02) (Fig. 1) and adjusted analysis (−0.20 [95% CI: −0.37; −0.02], p = 0.01) (Table 2). Similarly, each copy of the A-allele in the Val667Met polymorphism decreased the lumbar spine BMD in unadjusted (−0.26 [95% CI: −0.51; −0.01], p = 0.04) and adjusted (−0.23 [95% CI: −0.46; −0.002], p = 0.048) analysis.
Fig. 1.
BMD of the lumbar spine, total hip, and whole body in relation to the genotype regarding the Ala1330Val (upper panel) and the Val667Met (lower panel) polymorphisms in the LRP5 gene. Participants with a non-sedentary (left) and sedentary lifestyle (right) are displayed. Data are shown as the mean ± SE. (*)p < 0.10, *p < 0.05
Genotypes in Relation to Bone Markers
No significant differences were observed between genotypes of the Ala1330Val or Val667Met polymorphisms regarding bone markers (Table 1).
Haplotype Analysis
The effect of haplotypes of the Ala1330Val and Val667Met polymorphisms on BMD adjusted for BMI, lean body mass, smoking, drugs, and vitamin D are shown in Table 3. No significant association between BMD and haplotypes was found. This was also the case when sedentary and non-sedentary men were analyzed separately (not shown).
Table 3.
The effect of haplotypes of Ala1330Val and Val667Met polymorphisms on BMD adjusted for BMI, lean body mass, smoking, drugs, and vitamin D
| Haplotypes | |||||
|---|---|---|---|---|---|
| Ala1330Val | C | T | |||
| Val667Met | G | A | G | A | p |
| n | 1331 | 3 | 129 | 81 | |
| BMDspine (g/cm2) | 0.008 ± 0.96 | −0.42 ± 0.65 | −0.09 ± 0.90 | −0.04 ± 1.60 | NS |
| BMDhip (g/cm2) | −0.002 ± 1.01 | 0.97 ± 1.48 | −0.0005 ± 0.95 | −0.04 ± 1.0 | NS |
| BMDWB (g/cm2) | 0.02 ± 1.01 | 0.70 ± 0.59 | −0.10 ± 0.87 | −0.14 ± 1.04 | NS |
Data are shown as the mean ± SD. No significant associations between BMD and haplotypes were found when sedentary and non-sedentary men were analyzed separately (not shown)
Discussion
We found a significant association between the Ala1330Val and the Val667Met polymorphisms in the LRP5 gene and BMD in young men, especially when physical activity was taken into account as confounder. This finding supports a number of recent papers reporting significant associations between polymorphisms in this gene and BMD [18, 19, 21, 22]. Some of these studies involved or were restricted to men [18, 22, 29, 30]. Moreover, Ferrari et al. [18] suggested that the polymorphisms in the LRP5 gene are associated with bone gain in prepubertal boys. Only one study [25] failed to demonstrate an association between variations in the LRP5 gene and BMD, but most likely this is due to their restricted sample size (n = 219).
Major disruption of the LRP5 gene (homozygous nonsense or frame-shift mutations) resulting in loss-of-function has been demonstrated to be responsible for the osteoporosis pseudoglioma syndrome [16]. Conversely, a number of dominantly inherited gain-of-function mutations result in high bone mass phenotypes [12–14] and autosomal dominant osteopetrosis type 1 [14]. Minor changes in this gene, i.e., frequently occurring polymorphisms, may also be of importance in the regulation of bone mass as suggested by the association studies mentioned above. Further support comes from mapping a quantitative trait locus for BMD to chromosome 11q12–13 in some [31, 32] but not all studies [7, 33, 34]. In a case-control study comprising 78 men with idiopathic osteoporosis and 86 controls, Ferrari et al. [23] found that Ala1330Val and Val667Met conferred an odds ratio for osteoporosis of 2.98 (95% CI: 1.03–8.81) and 3.27 (95% CI: 1.43–7.44), respectively. Also, a single prospective study [21] comprising 1,301 elderly Australian women demonstrated that the c.3357A > G (rs556442) polymorphism was associated with decreased BMD and increased incidence of fractures. This polymorphism located in exon 15 is synonymous but was found to be in linkage disequilibrium with Ala1330Val [21]. Similarly, Mizuguchi et al. [19] found a linkage equilibrium block comprising exons 7 to 18. In our study, no significant effect of Ala1330Val or Val667Met on BMD was found apart from the ANOVA analysis which suggested that the AA genotype at Val667Met was associated with low BMD of the hip and increased body height. The number of participants with this genotype, however, was low (n = 4) and we found no significant gene-dose effect to back this finding. In non-sedentary subjects, however, we found a significant association between Ala1330Val genotype and the Val667Met and Z-score BMD in the spine both in unadjusted analysis and when adjusted for BMI, smoking, continuous medication, and serum levels of 25-OH-D3. Further, we observed an association between Ala1330Val and whole-body BMD in the unadjusted analysis. This interaction between lifestyle (sedentary/non-sedentary), LRP5 genotype, and BMD has not previously been reported. Such an association is fully in line with the proposed role of LRP5 as a player in adapting bone to weight-bearing. LRP5 acts as a co-receptor for Wnt proteins and is expressed in the osteoblast and a number of other cell types. The Ala1330Val (exon 18) polymorphism results in a substitution of alanine with the chemically similar valine. No functional studies have been performed on this polymorphism; however, the polymorphism is located within the second LDL-repeat of LRP5 [22] and this region is involved in the ligand binding of the LDL-receptor [35]. Also, preliminary results have shown that drugs that inhibit Dkk binding to LRP5 increase bone formation in vitro [36] and LRP5 seems to be involved in the adaptive response of bone to mechanical load and may be partly responsible for the “mechanostat” [11]. It is presently unclear why association between the Ala1330Val and Val667Met genotypes and BMD was found only in the spine and not in the hip in the non-sedentary group. Differences between the skeletal sites regarding loading pattern (compression vs. bending) and bone architecture (trabecular vs. cortical) may be important.
In our study, the numbers of T-alleles and A-alleles of the Ala1330Val and Val667Met polymorphisms, respectively, were associated with a decrease in BMD of the lumbar spine of 0.2 SD in the non-sedentary participants (i.e., approximately 2.5%). Previous studies have assessed this impact to be from 1% [20] to 15% [18] in women and men and women, respectively. While a number of genes have been associated with bone mass in women (e.g., PTH [37], alpha-1 chain of type 1 collagen [10], vitamin D receptor [38], transforming growth factor beta-1 [39], interleukin-6 [40], and osteoprotegerin [41]), only a few genes have been shown to be associated with peak bone mass in men. We have previously reported that the MTHFR gene is associated with peak bone mass in the same series of men as reported here [42]. Each of these polymorphisms including the LRP5 gene only accounts for a fraction of the variability in BMD; however, the 2.5% impact suggested by our study is not trivial.
We found no association between either projected bone area at the spine or hip, or body weight and any of the polymorphisms; however, our data demonstrated a significant impact of the AA genotype of the Val667Met polymorphism and body height as previously reported by Ferrari et al. [18] and Koay et al. [29]. In our study, the number of subjects with the AA genotype was, however, very low (n = 4). Thus, this finding clearly needs confirmation in other study designs.
The allele frequencies found in our study corresponded closely to those reported in Dutch, Australian, Japanese, and Korean populations [19, 21, 22, 30].
Our study has some limitations. We only tested two polymorphisms in the LRP5 gene. We cannot, therefore, rule out the possibility that the associations found in our study could be due to other polymorphisms in the LRP5 gene or even in neighboring genes in linkage with the polymorphisms tested. Indeed, Bollerslev et al. [21] found that several of the polymorphisms in the LRP5 gene were in linkage disequilibrium with each other in Australian women. In our study, haplotype analyses were negative. This might be due to the limited number of participants. Testing large arrays of polymorphisms or haplotypes in relation to many outcome variables (i.e., bone mass at several skeletal sites), however, carry an increased risk of detecting spurious associations. Also, our study had a limited size. Given the observed genotype frequency and BMD values, our study had the power to detect differences in BMDof the spine between CC and CT + TT of 0.037 g/cm2 (alpha = 0.05 and beta = 0.80). Similarly, our study had the power to detect differences of 0.070 g/cm2 between GG and GA + AA in the overall analysis.
Our study also has several strengths. First, it was population-based. In contrast to most similar studies [18, 20] that recruited participants by advertisements, we recruited our participants by direct mailing on the basis of the National Personal Registry that includes all Danish citizens. This enabled us to account meticulously for the recruitment of our participants. Thus, our sample was not skewed with respect to key socio-economic parameters [24]. Second, our population had a relatively homogeneous genetic background both due to a small influx to our population and since participants with a first- or second-generation immigrant background were excluded on the basis on data from our national registry. Third, we selected young men aged 20–30 years since these were expected to have reached their peak bone mass. This was confirmed by our data demonstrating an infinitesimal decrease in BMD by age. Moreover, very few were afflicted with chronic diseases or had long-term exposure of environmental factors that may affect bone mass.
We conclude that the Ala1330Val (exon 18) and Val667Met (exon 9) polymorphisms of the LRP5 gene are significantly associated with lumbar spine peak bone mass in physically active men, potentially accounting for 2.5% of BMD in the spine. This gene-environment interaction provides support for LRP5 as a mediator of load-induced bone formation and suggests that this gene is involved in the pathogenesis of osteoporosis in men.
Acknowledgments
Mrs. Irmelin Krabbe, Donna Artbuckle, Bente Tøt, Kirsten Westerman, Rikke Kiilshøj, Annette Madsen, and Fenna de Freitas are thanked for skillful technical assistance. The project was supported by WADA (World Anti Doping Agency). Moreover, Novo Nordisk A/S, Pfeizer A/S, Kulturministeriets Udvalg for Idrætsforskning (The Danish Ministry of Culture), Overlægerådets Legatudvalg, Odense University Hospital, The Research Foundation and Clinical Institute, University of Southern Denmark contributed financial support. This project was also funded by a research grant from the European Union (GENOMOS) and by a research grant (G.0117.06) from the Fonds voor wetenschappelijk onderzoek (FWO), both to W.V.H. S.B. holds a specialization scholarship from the Institute for the Promotion of Innovation through Science and Technology in Flanders (IWT-Vlaanderen). W.B. holds a post-doctoral fellowship from the FWO.
Data from this study were presented at the 33rd European Symposium on Calcified Tissues, Prague, May 13, 2006.
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Pocock NA, Eisman JA, Hopper JL, Yeates MG, Sambrook PN, Eberl S (1987) Genetic determinants of bone mass in adults: A twin study. J Clin Invest 80:706–710 [DOI] [PMC free article] [PubMed]
- 2.Nguyen TV, Blangero J, Eisman JA (2000) Genetic epidemiological approaches to the search for osteoporosis genes. J Bone Miner Res 15:392–401 [DOI] [PubMed]
- 3.Flicker L, Hopper JL, Rodgers L, Kaymakci B, Green RM, Wark JD (1995) Bone density determinants in elderly women: A twin study. J Bone Miner Res 10:1607–1613 [DOI] [PubMed]
- 4.Krall EA, Dawson-Hughes B (1993) Heritable and life-style determinants of bone mineral density. J Bone Miner Res 8:1–9 [DOI] [PubMed]
- 5.Smith DM, Nance WE, Kang KW, Christian JC, Johnston CC Jr (1973) Genetic factors in determining bone mass. J Clin Invest 52:2800–2808 [DOI] [PMC free article] [PubMed]
- 6.Young D, Hopper JL, Nowson CA, Green RM, Sherwin AJ, Kaymakci B, Smid M, Guest CS, Larkins RG, Wark JD (1995) Determinants of bone mass in 10- to 26-year-old females: A twin study. J Bone Miner Res. 10:558–567 [DOI] [PubMed]
- 7.Peacock M, Koller DL, Fishburn T, Krishnan S, Lai D, Hui S, Johnston CC, Foroud T, Econs MJ (2005) Sex-specific and non-sex-specific quantitative trait loci contribute to normal variation in bone mineral density in men. J Clin Endocrinol Metab 90:3060–3066 [DOI] [PubMed]
- 8.Videman T, Batti MC, Gibbons LE, Vanninen E, Kaprio J, Koskenvuo M (2002) The roles of adulthood behavioural factors and familial influences in bone density among men. Ann Med 34:434–443 [DOI] [PubMed]
- 9.Naganathan V, Macgregor A, Snieder H, Nguyen T, Spector T, Sambrook P (2002) Gender differences in the genetic factors responsible for variation in bone density and ultrasound. J Bone Miner Res 17:725–733 [DOI] [PubMed]
- 10.Jin H, Ralston SH (2005) Genetics of osteoporosis. Curr Rheumatol Rep 7:66–70 [DOI] [PubMed]
- 11.Akhter MP, Wells DJ, Short SJ, Cullen DM, Johnson ML, Haynatzki GR, Babij P, Allen KM, Yaworsky PJ, Bex F, Recker RR (2004) Bone biomechanical properties in LRP5 mutant mice. Bone 35:162–169 [DOI] [PubMed]
- 12.Little RD, Carulli JP, Del Mastro RG, Dupuis J, Osborne M, Folz C, Manning SP, Swain PM, Zhao SC, Eustace B, Lappe MM, Spitzer L, Zweier S, Braunschweiger K, Benchekroun Y, Hu X, Adair R, Chee L, FitzGerald MG, Tulig C, Caruso A, Tzellas N, Bawa A, Franklin B, McGuire S, Nogues X, Gong G, Allen KM, Anisowicz A, Morales AJ, Lomedico PT, Recker SM, Van Eerdewegh P, Recker RR, Johnson ML (2002) A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am J Hum Genet 70:11–19 [DOI] [PMC free article] [PubMed]
- 13.Boyden LM, Mao J, Belsky J, Mitzner L, Farhi A, Mitnick MA, Wu D, Insogna K, Lifton RP (2002) High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med 346:1513–1521 [DOI] [PubMed]
- 14.Van Wesenbeeck L, Cleiren E, Gram J, Beals RK, Benichou O, Scopelliti D, Key L, Renton T, Bartels C, Gong Y, Warman ML, De Vernejoul MC, Bollerslev J, Van Hul W (2003) Six novel missense mutations in the LDL receptor-related protein 5 (LRP5) gene in different conditions with an increased bone density. Am J Hum Genet 72:763–771 [DOI] [PMC free article] [PubMed]
- 15.Babij P, Zhao W, Small C, Kharode Y, Yaworsky PJ, Bouxsein ML, Reddy PS, Bodine PV, Robinson JA, Bhat B, Marzolf J, Moran RA, Bex F (2003) High bone mass in mice expressing a mutant LRP5 gene. J Bone Miner Res 18:960–974 [DOI] [PubMed]
- 16.Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, Wang H, Cundy T, Glorieux FH, Lev D, Zacharin M, Oexle K, Marcelino J, Suwairi W, Heeger S, Sabatakos G, Apte S, Adkins WN, Allgrove J, Arslan-Kirchner M, Batch JA, Beighton P, Black GC, Boles RG, Boon LM, Borrone C, Brunner HG, Carle GF, Dallapiccola B, De Paepe A, Floege B, Halfhide ML, Hall B, Hennekam RC, Hirose T, Jans A, Juppner H, Kim CA, Keppler-Noreuil K, Kohlschuetter A, Lacombe D, Lambert M, Lemyre E, Letteboer T, Peltonen L, Ramesar RS, Romanengo M, Somer H, Steichen-Gersdorf E, Steinmann B, Sullivan B, Superti-Furga A, Swoboda W, van den Boogaard MJ, Van Hul W, Vikkula M, Votruba M, Zabel B, Garcia T, Baron R, Olsen BR, Warman ML (2001) LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell 107:513–523 [DOI] [PubMed]
- 17.Kato M, Patel MS, Levasseur R, Lobov I, Chang BH, Glass DA, Hartmann C, Li L, Hwang TH, Brayton CF, Lang RA, Karsenty G, Chan L (2002) Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J Cell Biol 157:303–314 [DOI] [PMC free article] [PubMed]
- 18.Ferrari SL, Deutsch S, Choudhury U, Chevalley T, Bonjour JP, Dermitzakis ET, Rizzoli R, Antonarakis SE (2004) Polymorphisms in the low-density lipoprotein receptor-related protein 5 (LRP5) gene are associated with variation in vertebral bone mass, vertebral bone size, and stature in whites. Am J Hum Genet 74:866–875 [DOI] [PMC free article] [PubMed]
- 19.Mizuguchi T, Furuta I, Watanabe Y, Tsukamoto K, Tomita H, Tsujihata M, Ohta T, Kishino T, Matsumoto N, Minakami H, Niikawa N, Yoshiura K (2004) LRP5, low-density-lipoprotein-receptor-related protein 5, is a determinant for bone mineral density. J Hum Genet 49:80–86 [DOI] [PubMed]
- 20.Koller DL, Ichikawa S, Johnson ML, Lai D, Xuei X, Edenberg HJ, Conneally PM, Hui SL, Johnston CC, Peacock M, Foroud T, Econs MJ (2005) Contribution of the LRP5 gene to normal variation in peak BMD in women. J Bone Miner Res. 20:75–80 [DOI] [PubMed]
- 21.Bollerslev J, Wilson SG, Dick IM, Islam FM, Ueland T, Palmer L, Devine A, Prince RL (2005) LRP5 gene polymorphisms predict bone mass and incident fractures in elderly Australian women. Bone 36:599–606 [DOI] [PubMed]
- 22.van Meurs JB, Rivadeneira F, Jhamai M, Hugens W, Hofman A, van Leeuwen JP, Pols HA, Uitterlinden AG (2006) Common genetic variation of the low-density lipoprotein receptor-related protein 5 and 6 genes determines fracture risk in elderly white men. J Bone Miner Res 21:141–150 [DOI] [PubMed]
- 23.Ferrari SL, Deutsch S, Baudoin C, Cohen-Solal M, Ostertag A, Antonarakis SE, Rizzoli R, De Vernejoul MC (2005) LRP5 gene polymorphisms and idiopathic osteoporosis in men. Bone 37:770–775 [DOI] [PubMed]
- 24.Nielsen TL, Wraae K, Brixen K, Hermann AP, Andersen M, Agen C (2006) Prevalence of overweight, obesity, and physical inactivity in 20–29 year-old, Danish men. relation to sociodemography, physical dysfunction, and low socioeconomic status: The Odense Androgen Study. Int J Obesity 30:805–815 [DOI] [PubMed]
- 25.Hsu TM, Chen X, Duan S, Miller RD, Kwok PY (2001) Universal SNP genotyping assay with fluorescence polarization detection. Biotechniques 31:560, 562, 564–560,8, passim [DOI] [PubMed]
- 26.Frystyk J, Dinesen B, Orskov H (1995) Non-competitive time-resolved immunofluorometric assays for determination of human insulin-like growth factor I and II. Growth Regul 5:169–176 [PubMed]
- 27.Abecasis GR, Cookson WO (2000) GOLD–graphical overview of linkage disequilibrium. Bioinformatics 16:182–183 [DOI] [PubMed]
- 28.Becker T, Knapp M (2004) Maximum-likelihood estimation of haplotype frequencies in nuclear families. Genet Epidemiol 27:21–32 [DOI] [PubMed]
- 29.Koay MA, Woon PY, Zhang Y, Miles LJ, Duncan EL, Ralston SH, Compston JE, Cooper C, Keen R, Langdahl BL, MacLelland A, O’Riordan J, Pols HA, Reid DM, Uitterlinden AG, Wass JA, Brown MA (2004) Influence of LRP5 polymorphisms on normal variation in BMD. J Bone Miner Res 19:1619–1627 [DOI] [PubMed]
- 30.Koh JM, Jung MH, Hong JS, Park HJ, Chang JS, Shin HD, Kim SY, Kim GS (2004) Association between bone mineral density and LDL receptor-related protein 5 gene polymorphisms in young Korean men. J Korean Med Sci 19:407–412 [DOI] [PMC free article] [PubMed]
- 31.Koller DL, Rodriguez LA, Christian JC, Slemenda CW, Econs MJ, Hui SL, Morin P, Conneally PM, Joslyn G, Curran ME, Peacock M, Johnston CC, Foroud T (1998) Linkage of a QTL contributing to normal variation in bone mineral density to chromosome 11q12–13. J Bone Miner Res 13:1903–1908 [DOI] [PubMed]
- 32.Koller DL, Econs MJ, Morin PA, Christian JC, Hui SL, Parry P, Curran ME, Rodriguez LA, Conneally PM, Joslyn G, Peacock M, Johnston CC, Foroud T (2000) Genome screen for QTLs contributing to normal variation in bone mineral density and osteoporosis. J Clin Endocrinol Metab 85:3116–3120 [DOI] [PubMed]
- 33.Ralston SH, Galwey N, MacKay I, Albagha OM, Cardon L, Compston JE, Cooper C, Duncan E, Keen R, Langdahl B, McLellan A, O’Riordan J, Pols HA, Reid DM, Uitterlinden AG, Wass J, Bennett ST (2005) Loci for regulation of bone mineral density in men and women identified by genome wide linkage scan: the FAMOS study. Hum Mol Genet 14:943–951 [DOI] [PubMed]
- 34.Deng HW, Xu FH, Huang QY, Shen H, Deng H, Conway T, Liu YJ, Liu YZ, Li JL, Zhang HT, Davies KM, Recker RR (2002) A whole-genome linkage scan suggests several genomic regions potentially containing quantitative trait Loci for osteoporosis. J Clin Endocrinol Metab 87:5151–5159 [DOI] [PubMed]
- 35.Russell DW, Brown MS, Goldstein JL (1989) Different combinations of cysteine-rich repeats mediate binding of low density lipoprotein receptor to two different proteins. J Biol Chem 264:21682–21688 [PubMed]
- 36.Liu P, Zhang Y, Li X, Zheng J, Wu D (2005) Enhancement of bone formation by small molecule compounds that disrupt Dkk-LRP5/6 interaction. ASBMR (abstract) 1062
- 37.Hosoi T, Miyao M, Inoue S, Hoshino S, Shiraki M, Orimo H, Ouchi Y (1999) Association study of parathyroid hormone gene polymorphism and bone mineral density in Japanese postmenopausal women. Calcif Tissue Int 64:205–208 [DOI] [PubMed]
- 38.Uitterlinden AG, Fang Y, van Meurs JB, Pols HA, van Leeuwen JP (2004) Genetics and biology of vitamin D receptor polymorphisms. Gene 338:143–156 [DOI] [PubMed]
- 39.Yamada Y (2001) Association of polymorphisms of the transforming growth factor-beta1 gene with genetic susceptibility to osteoporosis. Pharmacogenetics 11:765–771 [DOI] [PubMed]
- 40.Audi L, Garcia-Ramirez M, Carrascosa A (1999) Genetic determinants of bone mass. Horm Res 51:105–123 [DOI] [PubMed]
- 41.Arko B, Prezelj J, Kocijancic A, Komel R, Marc J (2005) Association of the osteoprotegerin gene polymorphisms with bone mineral density in postmenopausal women. Maturitas 51:270–279 [DOI] [PubMed]
- 42.Abrahamsen B, Jorgensen HL, Nielsen TL, Andersen M, Haug E, Schwarz P, Hagen C, Brixen K (2005) MTHFR c.677C > T polymorphism as an independent predictor of peak bone mass in Danish men-results from the Odense Androgen Study. Bone 38:215–219 [DOI] [PubMed]