Abstract
A methanol extract of Alsomitra macrocarpa leaves and branches induced a marked alteration of cell morphology in a human stellate cell line (LX-2). Similar morphologic alterations were observed in several other cell lines. Active compound was purified from the extract and determined to be cucurbitacin E (Cuc E). It has been known that Cuc E causes marked disruption of the actin cytoskeleton, supporting our observation, but how Cuc E altered the actin cytoskeleton has not been elucidated. By using the standard fluorescence assay using copolymerization and depolymerization of native and pyrene labelled actin, this study revealed that Cuc E interacted directly with actin consequently stabilizing the polymerized actin. When NIH-3T3 cells exogenously expressing YFP-labeled actin were treated with Cuc E, firstly the aggregation of globular actin and secondly the aggregation of actin including disrupted fibrous actin in the cells was observed.
Keywords: Alsomitra macrocarpa, Cucurbitacin E, Cell morphology, Actin, Cytoskeleton
Introduction
Plants produce many kinds of secondary metabolites to protect themselves from other organisms. They have prospects of medicinal effects on human cells. We found that methanol extract of Alsomitra macrocarpa leaves and branches caused drastic cell morphology alterations in human cultured cells. A. macrocarpa is an Asian tropical climbing gourd with remarkable winged seeds, but little is known about biologically active compounds contained in this plant.
Cucurbitacins are obtained originally from Cucurbitaceae and are cytotoxic triterpenoid substances. Series of cucurbitacin cognates were identified and their pharmacological effects, such as anti-tumor, purgative, anti-inflammatory, and anti-fertility activities have also been reported (Chen et al. 2005). It has been reported that cucurbitacin E (Cuc E) possesses anti-tumor activity and caused alterations in cell morphology by disrupting actin cytoskeleton (Duncan et al. 1996), but how Cuc E alters the actin cytoskeleton has not been elucidated.
Many actin-targeting molecules were obtained from marine organisms, mushrooms, and molds, and these molecules are classified into two prominent types (Allingham et al. 2006); the first ones are actin filament destabilizers such as cytochalasins and latrunculins, and the other ones are actin filament stabilizers such as jasplakinolide and phalloidin. In the cultured mammalian cells, both cause actin filament aggregation (Hoehn et al. 1973; Usui et al. 2004). In this study, we report the purification of Cuc E from A. macrocarpa and evaluated direct interaction between Cuc E and purified actin.
Materials and methods
Cell lines, chemicals and biochemicals
LX-2 cells (human hepatic stellate cell line) were a generous gift from Prof. Friedman of Mount Sinai (Xu et al. 2005). NIH-3T3 cells (mouse embryo fibroblast cell line) were obtained from ATCC cell collection. Jasplakinolide was purchased from Calbiochem. Alexa Flour 568-phalloidin and Alexa Flour 488-goat anti-mouse IgG were purchased from Molecular Probes. Rabbit skeletal muscle actin and pyrene-labeled actin were purchased from Cytoskeleton.
Extraction and purification of Cuc E
Air-dried branches and leaves of A. macrocarpa were ground in a cross beater mill equipped with a 5 mm sieve. An aliquot (1 kg) was extracted at room temperature with methanol (2 × 1 L, 7 days). The methanol extract (36.7 g) was partitioned between ethyl acetate and water (1:1, v/v). The ethyl acetate soluble part (7.3 g) was submitted to liquid chromatography on silica gel (Wakogel C-200, 75–150 μm, 5 × 30 cm, Wako) using 10% step gradients from 0% to 100% ethyl acetate in hexane. The 50–60% ethyl acetate eluate (1.64 g) was chromatographed on Wakogel C-200 again (2.5 × 20 cm). 0.51 g of ethyl acetate eluate was obtained. Ten μg of the ethyl acetate eluate were purified with HPLC on YMC-AQ302 reverse phase column (5 μm, 150 × 4.6 mm) eluted with acetonitrile and H2O (45:55) at 1 mL min−1, and the activity for altering the cell morphology was examined. A part of ethyl acetate eluate of Wakogel C-200 (0.2 g) was applied on reverse phase TLC (25 mg/plate, RP-18 F254s, Merk) developed with methanol and H2O (7:3). The active spot with respect to the sample purified by HPLC was cut out and eluted by methanol to give 7.7 mg. The purity was confirmed by HPLC.
Cell culture and immunofluorescence procedure
LX-2 cells were grown in Dulbecco’s Modified Eagle Medium (SIGMA) supplemented with 10% fetal bovine serum, 100 μg mL−1 of penicillin, and 100 unit mL−1 streptomycin (Gibco) at 37 °C in a humidified 5% CO2 atmosphere. After overnight incubation of cells seeded at 10% confluence, Cuc E dissolved in DMSO was added and cells were incubated at growth condition for appropriate time.
For immunofluorescence studies, drug treated cells grown on a cover glass were washed with PBS, fixed with 10% formalin neutral buffer solution (Wako) for 30 min at 37 °C. After wash with PBS, anti-tubulin antibody diluted 1:100 with PBS containing 10% goat serum was added and left for over night at 4 °C. The cover glass was washed with PBS and incubated with 1:150 diluted Alexa Flour 568-phalloidin, 1:200 diluted Alexa Flour 488-goat anti-mouse IgG, and 1 μg mL−1 of DAPI with PBS containing 10% goat serum for 1 h at room temperature in the dark. The cover glass was washed with PBS, mounted on a slide with anti fade solution, and examined with a Fluoview microscope (Olympus).
For flow cytometry, drug treated cells were fixed with cold 70% (v/v) ethanol and stained with 50 μg mL−1 propidium iodide. Total fluorescence intensity was determined with FACS Vantage (Becton Dickinson).
NIH-3T3 cells were grown in DMEM supplemented with 10% calf serum, 100 μg mL−1 of penicillin, and 100 unit mL−1 streptomycin at 37°C in a humidified 5% CO2 atmosphere. Plasmid encoding YFP-tagged actin (p401YFP–actin) was constructed by subcloning human β-actin cDNA amplified from human fetal liver cDNA into p401FLAG–YFP (Kioka 1999). NIH-3T3 cells were transfected with p401YFP–actin, followed by selection with 1000 µg mL−1 of G418. Expression of YFP–actin was confirmed by fluorescent microscopy and Western blotting. After overnight incubation of cells seeded at 20% confluence, Cuc E or Jasplakinolide dissolved in DMSO was added and YFP-signals were observed with 1X81 fluorescent microscope (Olympus) equipped with ORCA ER CCD camera (Hamamatsu).
Actin depolymerization and polymerization assay
Actin depolymerization was evaluated with a fluorescence spectroscopy. Actin and pyrene-labeled actin monomers were mixed in G buffer (5 mM Tris–HCl, pH 8.0, 0.2 mM ATP, 0.2 mM CaCl2, and 0.5 mM DTT) to obtain a stock solution (2 mg mL−1) of 10% pyrene-labeled actin. The stock solution was, beforehand, polymerized by the addition of 10% volume of polymerization buffer (20 mM MgCl2, 500 mM KCl and 10 mM ATP). Depolymerization was started by 20 times dilution of polymerized actin solution with G buffer containing an appropriate concentration of drug (final DMSO concentration, 1%) and monitored with a Power Scan HT fluorometer (Dainippon Sumitomo Pharma) with excitation at 360 nm and emission at 420 nm. The Actin polymerization assay was started by mixing 0.2 mg mL−1 actin (10% pyrene-labeled actin) in G buffer with the drug (final DMSO concentration, 1%) and 10% volume of polymerization buffer, and monitored with the fluorescence spectroscope.
Results
Purification and determination of the structure of Cuc E
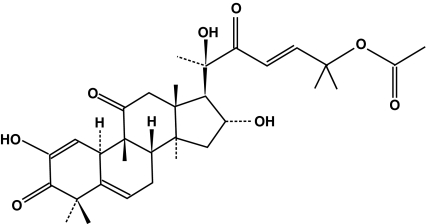
About 7.7 mg of pure compound was purified from 36.6 g of methanol extract of A. macrocarpa. The NMR data of this compound were identical to those of Cuc E (Fig. 1, MW 556) as previously reported; 1H NMR (Lavie et al. 1962) and 13C NMR (Velde and Lavie 1983).
Fig. 1.
Chemical structures of cucurbitacin E isolated from A. macrocarpa
Effects of Cuc E on LX-2 cells
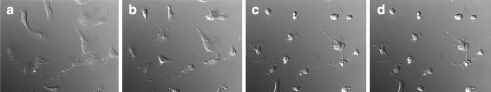
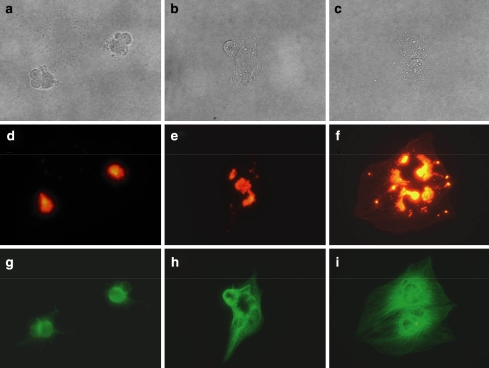
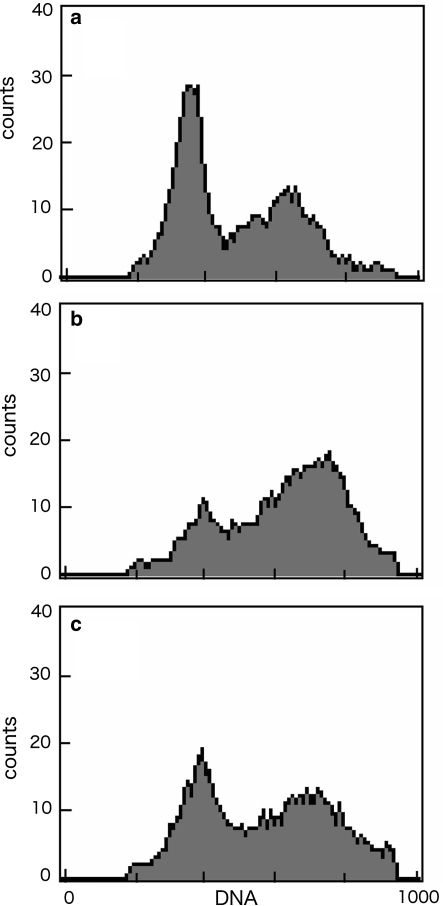
When LX-2 cells were cultured with the methanol extract of A. macrocarpa, the cell morphology was drastically altered (Fig. 2). LX-2 cells adhered to the dish but most of the cytoplasmic matrix gathered around the nucleus. The active compound in the methanol extract was Cuc E. As reported earlier (Duncan et al. 1996), we also observed that actin cytoskeleton was disrupted, while the microtubule network was not affected (data not shown). The morphology of the cells changed clearly at 10 nM of Cuc E, and actin aggregation was observed at 5 nM but the morphology of the cells was not altered. The LX-2 cells treated with Cuc E (20 nM) were alive for more than 24 h and when the drug was removed, cells restored its morphology again although actin spots were remained (Fig. 3). Recovery of cytokinesis after the removal of the drug was also investigated using flow cytometry. The DNA profile revealed that many cells contained two nuclei after 24 h treatment of Cuc E, (Fig. 4b). Then after 24 h incubation with drug-free medium, the peak profile shifted to that of Cuc E untreated cells (Fig. 4a and c).
Fig. 2.
Effect of Cuc E on the morphology of LX-2 cells. (a) 0, (b) 6, (c) 24, and (d) 60 min after addition of 30 μg mL−1 methanol extract of A. macrocarpa
Fig. 3.
Removal of Cuc E on morphology, filament actin, and tubulin of LX-2 cells. Cells were incubated with Cuc E (20 nM) for 24 h and medium was replaced with drug-free medium. Bright field images for (a) 0, (b) 6, and (c) 24 h after medium change. Alexa-phalloidin staining for (d) 0, (e) 6, and (f) 24 h. Tubulin staining for (g) 0, (h) 6, and (i) 24 h
Fig. 4.
Effects of Cuc E on the distribution of DNA content analyzed by flow cytometry. LX-2 cells were treated with Cuc E (a) 0 nM and (b) 20 nM for 24 h, and (c) 20 nM for 24 h treatment following 24 h incubation with drug-free medium
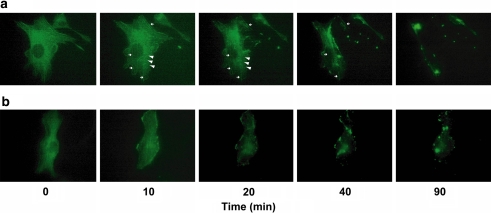
The results of time lapse analysis of YFP-actin expressed in NIH-3T3 cells exposed to the drug are shown in Fig. 5. By the treatment of Cuc E (500 nM), firstly many actin spots supposed to be made by globular actin (G-actin) aggregation, and secondly actin aggregates including disrupted fibrous actin (F-actin) were observed (Fig. 5a). On the other hand, F-actin was depleted from the central region of the cells by the treatment with 500 nM of Jasplakinolide (Fig. 5b).
Fig. 5.
Time-lapse analysis of the actin cytoskeleton in situ. The image of YFP-actin-expressing NIH-3T3 cells. Drugs were added at time 0. (a) Cuc E (500 nM). White arrow heads indicate G-actin aggregation and small allows indicate F-actin. (b) Jasplakinolide (500 nM)
Effect of Cuc E on F-actin stabilization in vitro
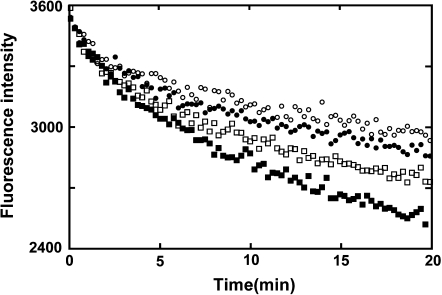
The interaction between Cuc E and purified actin was investigated with a fluorescent assay in which copolymerization of actin and pyrene-labeled actin showed enhancement of fluorescence. Initially, the effect of Cuc E on polymerization of actin was examined, but enhancement of polymerization by Cuc E was not observed (data not shown). As for the effect on actin depolymerization, the decrease of fluorescence intensity, according to the depolymerization of actin fiber, was suppressed by Cuc E in a dose-dependent manner (Fig. 6).
Fig. 6.
Effect of Cuc E on the actin depolymerization. Polymerized actin (10% pyrene-labeled actin, 2 mg mL−1) was diluted 20 times by G-buffer at room temperature (final concentration 4.7 μM). The samples were mixed to give final concentrations of 90 (open circle), 18 (closed circle), 9 (open square), and 0 μM (closed square) of Cuc E
Discussion
Cucurbitacins are cytotoxic triterpenoid substances derived from plants with medicinal properties known since antiquity (Lavie and Glotter 1971). In Turkey, the fruit juice of Ecballium elaterium containing Cuc B is used as a folk medicine for the treatment of sinusitis (Yesilada et al. 1988). Immunomodulatory activity of Cuc E isolated from Ecballium elaterium was reported (Attard et al. 2005). In this study, we isolated Cuc E from the branches and leaves of A. macrocarpa.
A marked alteration of cell morphology was observed not only in LX-2 cells but also in other cells, PC12, Hela, and HSC-T6 cells exposed to Cuc E (data not shown). The growth inhibition and morphology changes for a variety of tumor cell lines because of the disruption of the actin cytoskeleton by Cuc E were reported (Duncan et al. 1996). However, it was not clear if Cuc E interacts with actin directly or indirectly through some actin binding proteins. The results of the actin depolymerization and polymerization study in vitro using purified and fluorescence labeled actin showed that Cuc E suppressed the actin depolymerization, although it did not affect polymerization (data not shown). The molecules that interact with actin are largely classified into two groups, actin filament stabilizers and destabilizers. Actin stabilizers paradoxically stabilize actin filaments in vitro, but in vivo it can disrupt actin filaments. It is known that jasplakinolide, an actin filament stabilizer isolated from the marine sponge Jaspisjohnstoni, causes the accumulation of F-actin aggregates in cells (Lee et al. 1998). Bubb et al. (2000) proposed that this phenomenon is caused by formation of multiple actin filament nuclei with fiber propagation limited by the resulting shortage of monomeric actin in the cells. By the use of NIH-3T3 cells expressing YFP-actin, the effect of Cuc E on actin cytoskeleton was elucidated. As described by Bubb et al. (2000) exposure of cells to jasplakinolide results in almost complete depletion of F-actin from the central region of the cells and displayed thick bundles and aggregates at the cell margins (Fig. 5b), while exposure of cells to Cuc E resulted in firstly aggregation of G-actin and secondly aggregation of actins including disrupted F-actin (Fig. 5a). The different appearance of actin aggregates may be caused by the different mechanisms of interaction of these drugs with actin; F-actin polymerizing and stabilizing capacities are found for jasplakinolide (Bubb et al. 1994; Holzinger and Meindl 1997; Sheikh et al. 1997; Lee et al. 1998), while only F-actin stabilizing capacity for Cuc E by the standard fluorescent assay (Fig. 6). Further studies are needed to clarify the binding structure of actin to Cuc E.
Acknowledgment
We gratefully acknowledge the kind provision of LX-2 and HSC-T6 cells by Dr. Scott L. Friedman, Division of Liver Diseases, Mount Sinai School of Medicine, New York, NY and USA.
Abbreviations
- A. macrocarpa
Alsomitra macrocarpa
- Cuc E
Cucurbitacin E
- F-actin
Fibrous actin
- G-actin
Globular actin
References
- Allingham JS, Klenchin VA, Rayment I (2006) Actin-targeting natural products: structures, properties and mechanisms of action. Cell Mol Life Sci 63:2119–2134 [DOI] [PMC free article] [PubMed]
- Attard E, Brincat MP, Cuschieri A (2005) Immunomodulatory activity of cucurbitacin E isolated from Ecballium elaterium. Fitoterapia 76:439–441 [DOI] [PubMed]
- Bubb MR, Senderowicz AM, Sausville EA, Duncan KL, Korn ED (1994) Jasplakinolide, a cytotoxic natural product, induces actin polymerization and competitively inhibits the binding of phalloidin to F-actin. J Biol Chem 269:14869–14871 [PubMed]
- Bubb MR, Spector I, Beyer BB, Fosen KM (2000) Effects of jasplakinolide on the kinetics of actin polymerization. An explanation for certain in vivo observations. J Biol Chem 275:5163–5170 [DOI] [PubMed]
- Chen JC, Chiu MH, Nie RL, Cordell GA, Qiu SX (2005) Cucurbitacins and cucurbitane glycosides: structures and biological activities. Nat Prod Rep 22:386–399 [DOI] [PubMed]
- Duncan KL, Duncan MD, Alley MC, Sausville EA (1996) Cucurbitacin E-induced disruption of the actin and vimentin cytoskeleton in prostate carcinoma cells. Biochem Pharmacol 52:1553–1560 [DOI] [PubMed]
- Hoehn H, Sprague CA, Martin GM (1973) Effects of cytochalasin B on cultivated human diploid fibroblasts and its use for the isolation of tetraploid clones. Exp Cell Res 76:170–174 [DOI] [PubMed]
- Holzinger A, Meindl U (1997) Jasplakinolide, a novel actin targeting peptide, inhibits cell growth and induces actin filament polymerization in the green alga Micrasterias. Cell Motil Cytoskeleton 38:365–372 [DOI] [PubMed]
- Kioka N, Sakata S, Kawauchi T et al. (1999) Vinexin: a novel vinculin-binding protein with multiple SH3 domains enhances actin cytoskeletal organization. J Cell Biol 144:59–69 [DOI] [PMC free article] [PubMed]
- Lavie D, Shvo Y, Gottlieb OR, Glotter E (1962) The Constituents of Ecbullium elaterium L. XV. The Structures of Elatericin A and Related Cucurbitacins. J Org Chem 27:4546–4557 [DOI]
- Lavie D, Glotter E (1971) The cucurbitanes, a group of tetracyclic triterpenes. Fortschr Chem Org Naturst 29:307–362 [DOI] [PubMed]
- Lee E, Shelden EA, Knecht DA (1998) Formation of F-actin aggregates in cells treated with actin stabilizing drugs. Cell Motil Cytoskeleton 39:122–133 [DOI] [PubMed]
- Sheikh S, Gratzer WB, Pinder JC, Nash GB (1997) Actin polymerisation regulates integrin-mediated adhesion as well as rigidity of neutrophils. Biochem Biophys Res Commun 238:910–915 [DOI] [PubMed]
- Usui T, Kazami S, Dohmae N, Mashimo Y, Kondo H, Tsuda M, Terasaki AG, Ohashi K, Kobayashi J, Osada H (2004) Amphidinolide h, a potent cytotoxic macrolide, covalently binds on actin subdomain 4 and stabilizes actin filament. Chem Biol 11:1269–1277 [DOI] [PubMed]
- Vande V, Lavie D (1983) 13C NMR Spectroscopy of Cucurbitacins. Tetrahedron 39:317–321 [DOI]
- Xu L, Hui AY, Albanis E, Arthur MJ, O'Byrne SM, Blaner WS, Mukherjee P, Friedman SL, Eng FJ (2005) Human hepatic stellate cell lines, LX-1 and LX-2: new tools for analysis of hepatic fibrosis. Gut 54:142–151 [DOI] [PMC free article] [PubMed]
- Yesilada E, Tanaka S, Sezik E, Tabata M (1988) Isolation of an anti-inflammatory principle from the juice of Ecballium elaterium. J Nat Prod 51:504–508 [DOI] [PubMed]