Abstract
Cell lines represent valuable tools for basic research and diagnostic applications as well as for the production of biological products such as antibodies or vaccines. For all cell culturists, a well-identified origin of their cell lines as well as the periodic re-examination of their identity should be a basic requirement. We established a simple polymerase chain reaction (PCR) to verify or identify rodent and human cell lines. Since mouse-, rat-, Chinese hamster- and Syrian hamster-derived cell lines represent the most frequently used rodent cell lines, our investigations were focused on these species. Our assay used oligonucleotide primers annealing to sequences within the β-actin and the β-globin gene and to repetitive DNA. Primers were designed mostly from intron sequences of the genes aiming to amplify only one specific DNA segment and thus enabling to exclude easily false DNA. More than 130 cells lines originating from the five species were analyzed in that study. Our PCR revealed specific profiles for all species investigated. No further methods like DNA sequencing or fragment length polymorphism analysis were needed to differentiate these species. The results introduce our PCR-assay as a rapid, specific and routinely feasible tool in order to identify or distinguish rodent cell lines from each other and from human cell lines.
Keywords: Cell culture techniques, Cell line authenticity, Cross-contamination, Species identification, Species PCR
Introduction
Continuous cell lines are widely used model systems in biology and experimental medicine and many laboratories cultivate cell lines from different species at the same time. One fundamental and irrevocable requirement for the utilization of cell lines is their well-identified origin as well as the exclusion of cross-contamination by prokaryotic or eukaryotic cells. Cross-contaminated or misidentified cultures might end up in invalid data leading to doubtful results and economically to a waste of time and money (Stacey et al. 2000).
Nevertheless the logical requirement for identification or authentication seems to be less recognized and underestimated. It had been estimated that about one-third of cell cultures were of a different tissue origin or species to that being claimed (Markovic and Markovic 1998). Therefore, special emphasis must be taken to (i) prevent such an ‘accident’ and (ii) to monitor regularly the identity of the cell line(s) in use. The importance of quality controls as an essential part of good laboratory practice has been continuously addressed (Nelson-Rees et al. 1981; Stevenson 1987; Zoon 1993; MacLeod et al. 2002; Stacey et al. 2000; Masters 2002).
Several methods had been established to monitor cell culture identity using immunological markers (Stulberg et al. 1976; O’Toole et al. 1983), isoenzyme analysis (Halton et al. 1983; Steube et al. 1995) and karyologic examination (Miller et al. 1971; Nelson-Rees et al. 1974). The development of DNA-fingerprinting techniques offered detection of individual human samples (Jeffreys et al. 1985; Stacey et al. 1992) and is now used as an international standard for authentication of human cell lines (Dirks et al. 1999; Masters et al. 2001).
Besides human cell lines, those from rodents comprise to the majority of cell lines used in research and development. However, only a few PCR-based methods for the identification of animals and animal cell lines have been described (Stacey et al. 1997; Parodi et al. 2002; Liu et al. 2003; Lopez-Andreo et al. 2005). Therefore, we have presented recently a preliminary report of a β-globin-based PCR focusing mainly on mouse and rat cell lines (Steube et al. 2003).
Now we extended the former study and analyzed more than 130 DNAs derived from rodent and human cell lines. We chose PCR amplification of both the globin and the actin gene and of repetitive sequences in order to identify and differentiate Syrian and Chinese Hamster cell lines, as well as those derived from other rodents and from human. Our data document a simple and robust PCR technique but also describe a few drawbacks possibly arising when relying on a PCR with only one target gene or primer pair.
Materials and methods
In order to minimize the risk of false PCR amplification, cell culture, DNA isolation, preparation of the reaction mix and final PCR were performed in different laboratories.
Cell lines and culture conditions
All cell lines are deposited at the German Collection of Microorganisms and Cell Cultures (DSMZ, Braunschweig, Germany). The cultures are mycoplasma-free and were cultivated without antibiotics at 37°C in a humidified atmosphere containing 5–10% CO2 using growth media (Invitrogen, Karlsruhe, Germany) supplemented with 10–20% fetal bovine serum (Sigma, Taufkirchen, Germany) and other nutrients, when applicable. Growth conditions and cell line characteristics are described in the electronic DSMZ Catalogue of Human and Animal Cell Lines (http://www.dsmz.de).
Isolation of genomic high molecular weight DNA
Cells were grown to sub-confluence or sub-maximal density, harvested and washed two times with phosphate-buffered saline (PBS). Approximately 4 × 106 cells were resuspended in 200 μl PBS and kept frozen until the isolation procedure. The cells were lysed and genomic DNA was isolated by use of the High Pure PCR Template Preparation Kit (Roche, Mannheim). The isolated DNA was eluted in 300 μl of 10 mM Tris pH 8.5 and kept at 4°C after determination of the concentration.
PCR amplification and primers
Oligonucleotide primers listed in Table 1 were obtained commercially from Invitrogen. The reaction mix contained 1.5 mM MgCl2, 50 mM KCL, 10 mM Tris–HCl pH 8.3, each 100 μmol of the four deoxynucleotides (TaKaRa-Lonza, Verviers, Belgium) and usually 40 pmol of the various primers. PCR tubes (50 μl) containing 50–100 ng DNA and 1.5 units of TaKaRA hot start polymerase were covered with light mineral oil and amplification was started with 2 min heating to 94°C, followed by 3 min at 72°C and subsequently by 35 cycles of denaturation (94°C for 30 s), annealing (temperature see Table 1) and extension (72°C for 60 s) using a Perkin Elmer DNA Thermal Cycler (Applied Biosystems, Weiterstadt, Germany). All buffers and primers were aliquoted and a designated set of pipettes were used. Usually, a negative control with destilled water was included in the PCR and the amplifications were carried out repeatedly during a period of 2 years. Each 10 μl of the products were electrophoresed on a 1.2% agarose gel (Ultra pure grade, Invitrogen), stained with ethidium bromide (Sigma), visualized under UV-light and photographed. The DNA molecular weight standards from MBI Fermentas (St. Leon-Rot, Germany) and Invitrogen were applied as size marker.
Table 1.
Primers used in the PCR amplifications
| Primer designation | 5′ to 3′ sequence | Species | Position | Location | EMBL-gene bank accession number |
|---|---|---|---|---|---|
| c-globin-F | cct gtg ggg aaa ggt gaa c | M, R | 2759–2777 | Exon 1 | J00413 and X06701 |
| glo-mus-R | ata cca gat acc tgc agg ctt at | M | 3735–3757 | Intron 2 | J00413 |
| glo-rat-R1 | agg gca tat cca cac atc atc | M, R | 841–861 | Intron 2 | X06701 |
| glo-rat-F | gat gat gtg tgg ata tgc cct g | R | 841–861 | Intron 2 | X06701 |
| glo-rat-R2 | aat tcc ttg ccc agg tgg | R | 1412–1430 | Exon 3 | X06701 |
| glo-sHam-F | agg tga tcc act cct tcg ct | SH | 251–270 | cDNA | X57030 |
| glo-sHam-R | tgt tct cta ggg aac aag tga ctt c | SH | 529–552 | cDNA | X57030 |
| act-rat-F | ggc ttt agg agc ttg aca ata ctg | R | 2118–2140 | Intron 3 | J0091 |
| act-rat-R | gca ttg gtc acc ttt aga tgg a | R | 2620–2641 | Intron 4 | J0091 |
| act-bHam-1F | ata ttg aga aca tcg ttc ccc | CH, SH | 1417–1427 | Intron 1 | U20114 |
| act-bHam-1R | cca caa gta gtc aag gca ggt | CH, SH | 2082–2102 | Intron 2 | U20114 |
| act-bHam-2F | acc tgc ctt gac tac ttg tgg | CH, SH | 2082–2102 | Intron 2 | U20114 |
| act-bHam-2R | agg cta agg atg ctt agc tca | CH, SH | 2860–2880 | Intron 4 | U20114 |
| act-cHam-F | acc tgc ctt gac tac ttg tgg | CH | 2082–2103 | Intron 2 | U20114 |
| act-cHam-R | ttg gtc acc att aga tga ag | CH | 2601–2620 | Intron 3 | U20114 |
| Act-hum-F | cta caa tga gct gcg tgt gg | H | 1494–1514 | Exon 2 | M10277 |
| Act-hum-R | taa ccc tca tgt cag gca ga | H | 2491–2510 | Intron 4 | M10277 |
| HamRepS-F | gtg tat tcc agg agc agc agc c | Rod | 250–272 | repet S | AB185084 |
| HamRepS-R | act ggt cct ctg cag gtg ca | Rod | 671–690 | repet S | AB185084 |
CH, Chinese hamster; H, Human; M, Mouse; R, Rat; Rod, Rodents; SH, Syrian hamster
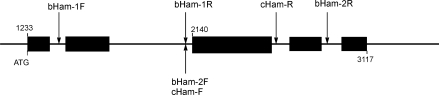
A schematic diagram of the hamster actin gene and the relative position of the primers are depicted in Fig. 1.
Fig. 1.
β-Actin gene structure and relative position of the primers. Schematic diagram of the β-actin gene structure of Chinese hamster according to the published sequences of the EMBL data base (accession number: U20114). Relative sizes of exons (filled bars) and introns are given; arrows show the approximate primer localization and numbers indicate the position of the nucleotide in the U20114 sequence
Results and discussion
Verification of cell lines used as controls
Authenticity testing is an indispensable requirement before and during any use of cell cultures. As international cell repository, our aim was to provide a reproducible, fast and simple PCR method to identify the animal species of cell lines most commonly used in cell culture laboratories, i.e. human, mouse, rat, and hamster. Human cells are most abundantly in cell culture labs and have been the subject of many reports on contaminations (Masters 2000; Drexler et al. 2003). Even the panel of the so called NCI-60 cell lines presumably contains about 10% of false classified tissue origins (Wang et al. 2006).
Our human cell lines were originally authentified by DNA fingerprinting and chromosomal analysis (Dirks et al. 1999, MacLeod et al. 1999). To utilize them as controls for the species-PCR we developed several primer pairs based on the human β-actin sequence (gene bank accession number M10277); only human DNA, but not from other species, led to amplified PCR products with these actin primers (data reported elsewhere).
Next, we re-evaluated our recently described β-globin primer pairs for the analysis of rodent cell lines (Steube et al. 2003). One primer pair specifically detected mouse DNA (Fig. 2a), another mouse and rat simultaneously (Fig. 2b) and the third one dominantly rat (Fig. 2c). DNAs from hamster, human, and African green monkey cell lines were not amplified significantly by use of these β-globin primers. The DNA probes are located in the introns as well as in the exons of the β-globin gene. Although the β-globin of mouse and rat share a very high sequence homology in the protein coding regions, there seems to exist sufficient variability within the intron regions of the genes to enable a differentiation between the two rodents. Lockley and Bardsley (2002) used similarly a combination of intron and exon primer for their discrimination of poultry.
Fig. 2.
PCR analysis of genomic DNA from different cell lines with primers designed from mouse and rat β-globin sequences. PCRs were performed with the following oligonucleotid primer pairs: (a) c-globin-F and glo-mus-R; (b) c-globin-F and glo-rat-R1; (c) glo-rat-F and glo-rat-R2. Amplified DNA fragments were detected after ethidium bromide staining of 1.2% agarose gels. Lane 1, K-562 (ACC 10); lane 2, mouse test sample sent to DSMZ; lane 3, L-929 (ACC 2); lane 4, PC-12 (ACC 159); lane 5, NIH-3T3 (ACC 59); lane 6, COS-7 (ACC 60); lane 7, RGE (ACC 262); lane 8, CHO-K1 (ACC 110); lane 9, negative control (water). H, Hamster; M, Mouse; P, Primate; R, Rat; W, Water control (no DNA). The positions of the DNA molecular weight markers of 500 and 1,000 bp are indicated
However, we then designed several primer pairs complementary only to intron sequences and chose the rat β-actin. The same rat cell lines which have been detected correctly by the globin-PCR were identified also by these novel actin primers (one example in Fig. 3). The PCR yielded amplified fragments only from rat DNA (lanes 1, 4–6) but not from mouse or hamster DNA (lanes 2, 3 and 7), respectively. The size of the PCR product estimated after gel electrophoresis was in good agreement with the lengths calculated on the basis of the published gene bank sequence data (552 bp for rat).
Fig. 3.
Analysis of genomic DNA from rodent cell lines with a specific rat primer pair targeting rat β-actin. PCRs were performed with oligonucleotid primers act-rat-F and act-rat-R which are specific for rat. Amplified DNA fragments were detected after ethidium bromide staining of a 1.2% agarose gel. Lane 1, PC-12 (ACC 159); lane 2, MC3T3-E1 (ACC 210); lane 3, C2C12 (ACC 565); lane 4, RGE (ACC 262); lane 5, RBL-2H3 (ACC 312); lane 6, A-10 (ACC 132); lane 7, BHK-21 (ACC 61). H, Hamster; M, Mouse; R, Rat. The arrow indicates the DNA molecular weight marker of 500 bp
Additionally, a second set of rat actin primers revealed the same species-specific results (data not shown). In subsequent experiments analyzing a large number of different cell lines and species we confirmed the efficiency of these primers. All rat-specific primers show no sequence identity with β-actin genes of the other species tested, which explains the specificity of the reaction.
Based on these new data, we suggest analyzing rat cell lines with the β-actin primers, while the first β-globin primer is sufficient for mouse DNA.
Hamster PCR
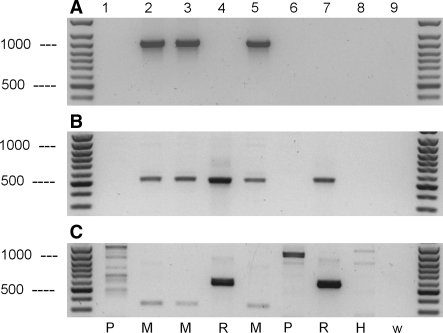
Due to incomplete genomic sequences, it has been a problem to distinguish cell lines of Chinese hamster (CH, Cricetulus griseus) from Syrian hamster (SH, Mesocricetus auratus). From these species, respectively, the widely used cell lines CHO and BHK-21 were established. In a recent report, Parodi et al. (2002) introduced a specific PCR for the detection of CH, but not for SH. In case of CH, the complete β-actin gene sequences (Gene bank no. U20114) opened the opportunity to design intron primers specifically for CH as schematically depicted in Fig. 1. The primer pairs act-bHam 1 and act-bHam 2 detected successfully all CH cell lines analyzed, while DNA from mice, primates and rats were not recognized as targets (Fig. 4). Interestingly, besides the CH, also the SH were identified by means of these primers although the nucleotide sequences were derived from introns of the CH gene. This suggests a very high sequence homology for β-actin between the two hamster species. Based on the published CH sequence, one should expect a DNA fragment of 685 bp when primer pair bHam 1 is used (Fig. 4a) and one of 797 bp with primer pair bHam 2 (Fig. 4b), respectively. The sizes of the PCR products estimated from the agarose gels fitted quite well with the expected sizes.
Fig. 4.
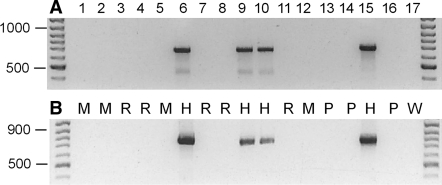
Analysis of genomic DNA from different cell lines using actin primer pairs detecting both, Chinese and Syrian (Golden) hamster cell lines. Primer: (a) act-bHam-1F and act-bHam-1R; (b) primer: act-bHam-2F and act-bHam-2R. Amplified DNA fragments were detected after ethidium bromide staining of 1.2% agarose gels. Lane 1, 1G1 (ACC 424); lane 2, 2E10H2 (ACC 178); lane 3, A-10 (ACC 132); lane 4, A-S-30D (ACC 208); lane 5, B-16V (ACC 370); lane 6, CHO-DHFR (ACC 126); lane 7, C6-BU-1 (ACC 108); lane 8, GH3 (ACC 464); lane 9, HPD-1NR (ACC 314); lane 10, HPD-2NR (ACC 293); lane 11, MMQ (ACC 484); lane 12, MS-5 (ACC 441); lane 13, HELA (ACC 57); lane 14, COS-1 (ACC 63); lane 15, V-79 (ACC 335); lane 16, HL-60 (ACC 3); lane 17, water control (no DNA). H, Hamster; M, Mouse; P, Primate; R, Rat; W, Water control (no DNA). The arrows indicate the DNA molecular weight markers of 500, 900 and 1,000 bp
To provide evidence that the PCR product resulting from the cell line BHK-21 (SH) was indeed related to β-actin, we determined its nucleotide sequence. Conducting a BLAST search (Altschul et al. 1997 and http://www.ncbi.nlm.nih.gov) we tracked a 1,128 bp long mRNA sequence (Gene Bank accession number AJ312092) from β-actin of Mesocricetus auratus. The nucleotides from position 361 to 982 of this sequence were completely identical to our PCR product. However, at position 803 of this mRNA sequence, our corresponding PCR product contained an insertion of 85 nucleotides which had no homology to any other known sequences. Since in the CH (sequence U20114) gene intron-3 with 98 bp is located at the corresponding position and since the flanking exons of this intron were highly homologous to our actin PCR product of cell line BHK-21, we strongly suggest that the detected 85 bp insertion is indeed an intron of the β-actin from Mesocricetus auratus. The overall identity of both SH and CH sequences within this part of the gene was found to be 96% and the new SH sequence was sent to the NCBI.
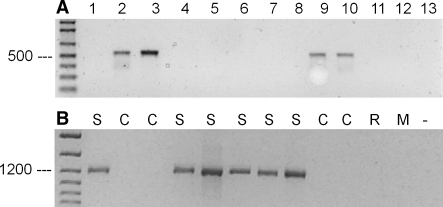
The specific identification of CH was finally achieved by use of an alternative reverse actin primer, cHam-R, using sequences of the above mentioned intron-3 (Fig. 5a). As corresponding forward primer, cHam-F of intron-2 (identical to bHam2F), was sufficient for that analysis. Both PCR products from CHO-K1 DNA (primer pairs, bHam-1 and cHam) were sequenced and 100% identity with Gene bank no U-20114 (β-actin for Cricetulus griseus) was found, indicating the correctness of the PCR analysis. Since the genomic β-actin sequence for the SH is not available, a specific identification of SH based on actin primer remains to be difficult. However, we showed recently the specific detection of SH by β-globin primer pairs (Steube et al 2003). The additional PCR-analysis applying these globin primers should unequivocally differentiate between SH and CH. This is documented in Fig. 5b: DNA from SH cell lines could be identified by β-globin primers (while the CH cells have been detected with the β-actin primers; Fig. 5a). DNAs from other species were not amplified by these primers.
Fig. 5.
PCR analysis of genomic DNA from different cell lines with primer pairs each specific for Chinese or Syrian (Golden) hamster. Primer: (a) act-cHam-F and act-cHam-R; (b) glo-sHam-F and glo-sHam-R. Amplified DNA fragments were detected after ethidium bromide staining of 1.2% agarose gels. Lane 1, BHK-21 (ACC 61); lane 2, CHO-K1 (ACC 110); lane 3, CHO (ACC -); lane 4, HAP-T1 (ACC 222); lane 5, HKT-1097 (ACC 445); lane 6, HPD-1NR (ACC 314); lane 7, HPD-2NR (ACC 293); lane 8, M3E3/C3 (ACC 340); lane 9, V-79 (ACC 335); lane 10, CHO-DHFR (ACC 126); lane 11, PC-12 (ACC 159); lane 12, NIH-3T3 (ACC 59); lane 13, water control (no DNA). C, Chinese hamster; M, Mouse; R, Rat; S, Syrian hamster. The arrows indicate the DNA molecular weight markers of 500 and 1,200 bp
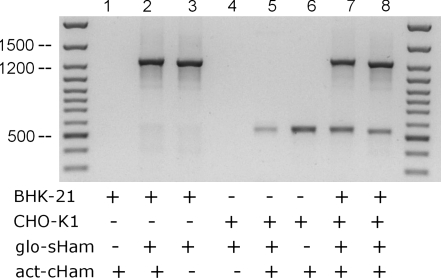
Finally, we sequenced the product of the BHK-21 globin PCR. A 1,243 bp long fragment was obtained in which the flanking parts were highly homologous to the mRNA of beta-like γ-globin (no X64179 of Mesocricetus auratus), while the inner part seemed to be an approximately 940 bp long unpublished intron. The corresponding long introns (no.2) of human, mouse and rat β-globin are 849, 654 and 636 bp respectively. In case of the hamsters, no genomic sequence data are available for β-globin. The efficiency of these two primer pairs to clearly differentiate between both hamster species in a DNA mixture was proven by a PCR with combinations of both DNAs and both primer pairs. Figure 6 documents that BHK-21 DNA was detected only by β-globin primer, CHO-K1 only by β-actin and not vice versa whereas a mixture of both DNAs produced two amplification products due to both primer pairs.
Fig. 6.
Efficiency of the primers to detect Syrian and Chinese Hamster DNA within a mixture of both. Equal amounts of DNA from Syrian hamster (BHK-21), Chinese hamster (CHO-K1) and a combination of both were analyzed by PCR with act-cHam primer alone (lanes 1, 6), glo-sHam primer alone (lanes 3, 4), or a combination of both primer pairs (lanes 2, 5, 7, 8) as marked with a plus (+) or minus (−) symbol in the figure. The arrows indicate the DNA molecular weight markers of 500, 1,200 and 1,500 bp
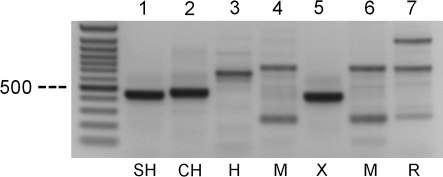
As a recognized cell bank it is not only important to confirm the presumed species of an animal cell line but also to offer an identification service for unknown DNA samples within a frame of commonly used species. Therefore primer pairs targeting DNA from various species simultaneously and revealing PCR products with different sizes could be a “golden standard”. Thacker et al. (1988) first discriminated between subclones of CH cell lines by hypervariable probes and later extended this idea to detect more than one species by use of a single alu-primer and was able to differentiate between mouse and CH cell lines (Thacker 1994). However, multiple PCR products may cause problems in reproducibility and interpretation of the results. By our search for such a ‘one-for-all’ system we developed primer pairs from repetitive sequences present in the subfamily of hamster (Cricetinae) described by Yamada et al. (2006). One primer pair was found to be sufficient for our purposes. An unknown sample sent to the DSMZ was compared by our species-PCR with known DNAs of CH, SH, human, mouse and rat (Fig. 7). The PCR product of the unknown DNA migrated in the agarose gel very close to the position of the SH amplicon, suggesting it very likely to be derived from SH. Further PCRs with the above evaluated primers for β-actin and β-globin proved that the unknown sample was indeed from SH.
Fig. 7.
PCR analysis of genomic DNA from different cell lines with primer pairs designed from repetitive sequences of Syrian hamster. Amplified DNA fragments were detected after ethidium bromide staining of 1.2% agarose gels. Lane 1, BHK-21 (ACC 61); lane 2, CHO-K1 (ACC 110); lane 3, T-24 (ACC 376); lane 4, L-929 (ACC 2); lane 5, unknown sample sent to DSMZ; lane 6, NIH-3T3 (ACC 59); lane 7, RBL-2H3 (ACC 312). CH, Chinese hamster; H, Human; M, Mouse; R, Rat; SH, Syrian hamster; X, Unknown sample. The arrows indicate the DNA molecular weight markers of 500 bp
From our results analyzing these closely related species we suggest that a PCR based on intron-derived primers is more advantageous than a PCR relying on exon primers.
Lessa and Appelbaum (1993) summarized DNA screening techniques involving a PCR amplification of genomic DNA. They used primers derived from aldolase annealing to two different exons. They reported simultaneous amplification of 2–4 products of various sizes, depending on the animal species analyzed. Recently Stacey et al. (1997) and Liu et al. (2003) followed that procedure and adapted it for certain animal cell lines of different phylogenetic relationship. The amplified products of DNA from diverse species analyzed on agarose gels resulted either in single or multiple bands with various intensities.
Other methods like fragment length polymorphism analysis and their variations require a restriction of the genomic DNA followed by PCR amplification of random or defined DNA sequences and eventually of a final sequencing step. All PCR with these methods might lead to multiple amplification products and bear the risk of amplified DNA patterns with mixed intensities or missing or artificial bands on the agarose gels. PCRs creating several bands may cause problems in reproducibility and interpretation as shown by Milanesi et al. (2003) and Lopez-Andreo et al. (2005). The use of these methods for the analyses of non-human cell lines and for the detection of interspecies contamination is further limited by the necessity to design extensive protocols and electronic data processing for each species to be analyzed.
Conclusion
We introduced an efficient and robust PCR assay for the detection of the most commonly used species in cell culture which can be easily and quickly performed in most laboratories. Our applied PCR assays need no subsequent sequencing step or DNA restriction and no computer-based analysis system. While one species-specific primer pair may be sufficient for the confirmation of an expected species, a combination of two or more primer pairs should be performed for identification of an unknown cell line and to minimize artificial side effects.
References
- Altschul SF, Madden TL, Schaeffer AA, Zhang J, Zhang Z, Miller W, Lipma DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nuc Acid Res 25:3389-3402 [DOI] [PMC free article] [PubMed]
- Dirks WG, MacLeod RAF, Jaeger K, Milch H, Drexler HG (1999) First searchable database for DNA profiles of human cell lines: sequential use of fingerprint techniques for authentication. Cell Mol Biol 45:841–853 [PubMed]
- Drexler HG, Dirks WG, MacLeod RAF, Quentmeier H, Steube KG, Uphoff CC (2001) Catalogue of human and animal cell lines. Braunschweig 2001, electronic form at http://www.dsmz.de
- Drexler HG, Dirks WG, Matsuo Y, MacLeod RAF (2003) False leukemia-lymphoma cell lines: an update on over 500 cell lines. Leukemia 17:416–426 [DOI] [PubMed]
- Halton DM, Peterson WD Jr., Hukku B (1983) Cell culture quality control by rapid isoenzymatic characterization. In Vitro 19:16–24 [DOI] [PubMed]
- Jeffreys AJ, Wilson V, Thein SL (1985) Individual-specific ‘fingerprints’ of human DNA. Nature 316:76–79 [DOI] [PubMed]
- Lessa EP, Applebaum G (1993) Screening techniques for detecting allelic variation in DNA sequences. Mol Ecol 2:119–129 [DOI] [PubMed]
- Liu MY, Lin SC, Liu H, Candal F, Vafai A (2003) Identification and authentication of animal cell culture by polymerase chain reaction amplification and DNA sequencing. In Vitro Cell Dev Biol Anim 39:424–427 [DOI] [PubMed]
- Lockley AK, Bardslay RG (2002) Intron variability and actin gene can be used to discriminate between chicken and turkey DNA. Meat Sci 61:163–169 [DOI] [PubMed]
- Lopez-Andreo M, Lugo L, Garrido-Pertierra A, Prieto MI, Puyet A (2005) Identification and quantitation of species in complex DNA mixtures by real-time polymerase chain reaction. Anal Biochem 339:73–82 [DOI] [PubMed]
- MacLeod RAF, Dirks WG, Matsuo Y, Kaufmann M, Milch H, Drexler HG (1999) Widespread intraspecies cross-contamination of human tumor cell lines arising at source. Int J Cancer 83:555–563 [DOI] [PubMed]
- MacLeod RAF, Dirks WG, Dexler HG (2002) Persistent use of misidentified cell lines and its prevention. Genes Chromosomes Cancer 33:103–105 [DOI] [PubMed]
- Markovic O, Markovic N (1998) Cell cross-contamination in cell cultures: the silent and neglected danger. In Vitro Cell Dev Biol Anim 34:1–8 [DOI] [PubMed]
- Masters JR (2000) Human cancer cell lines: facts and fantasy. Nat Rev Mol Cell Biol 1:233–236 [DOI] [PubMed]
- Masters JR, Thomson JA, Daly-Burns B, Reid YA, Dirks WG, Packer P, Toji LH, Ohno T, Tanabe H, Arlett CF, Kelland LR, Harrison M, Virmani A, Ward TH, Ayres KL, Debenham PG (2001) Short tandem repeat profiling provides an international reference standard for human cell lines. Proc Natl Acad Sci USA 98:8012–8017 [DOI] [PMC free article] [PubMed]
- Masters JR (2002) False cell lines: the problem and the solution. Cytotechnology 39:17–22 [DOI] [PMC free article] [PubMed]
- Milanesi E, Ajmone-Marsan P, Bignotti E, Losio N, Bernardi J, Checdani E, Soncini M, Ferrari M (2003) Molecular detection of cell line cross contamination using amplified fragment polymorphism DNA fingerprinting technology. In Vitro Cell Dev Biol Anim 39:124–130 [DOI] [PubMed]
- Miller OJ, Miller DA, Allderdice PW, Dev VG, Grewal MS (1971) Quinacrine fluorescent karyotypes of human diploid and heteroploid cell lines. Cytogenetics 10:338–346 [DOI] [PubMed]
- Nelson-Rees WA, Flandermeyer RR, Hawthorne PK (1974) Banded marker chromosomes as indicators of intraspecies cellular contamination. Science 184:1093–1096 [DOI] [PubMed]
- Nelson-Rees WA, Daniels DW, Flandermeyer RR (1981) Cell cross-contamination in cell cultures. Science 212:446–452 [DOI] [PubMed]
- O’Toole CM, Povey S, Hepburn P, Franks LM (1983) Identity of some human bladder cancer cell lines. Nature 301:429–430 [DOI] [PubMed]
- Parodi B, Aresu O, Bini D, Lorenzini R, Schena F, Visconti P, Cesaro M, Ferrera D, Andreotti V, Ruzzon T (2002) Species identification and confirmation of human and animal cell lines: a PCR-based method. Biotechniques 32:432–440 [DOI] [PubMed]
- Stacey GN, Bolton BJ, Doyle A (1992) DNA fingerprinting transforms the art of cell authentication. Nature 357:261–262 [DOI] [PubMed]
- Stacey GN, Hoelzl H, Stephenson JR, Doyle A (1997) Authentication of animal cell cultures by direct visualization of repetitive DNA, aldolase gene PCR and isoenzyme analysis. Biologicals 25:75–85 [DOI] [PubMed]
- Stacey GN, Masters JR, Hay RJ, Drexler HG, MacLeod RAF, Freshney RI (2000) Cell contamination leads to inaccurate data: we must take action now. Nature 403:356 [DOI] [PubMed]
- Steube KG, Grunicke D, Drexler HG (1995) Isoenzyme analysis as a rapid method for the examination of the species identity of cell cultures. In Vitro Cell Dev Biol Anim 31:115–119 [DOI] [PubMed]
- Steube KG, Meyer C, Uphoff CC, Drexler HG (2003) A simple method using beta-globin polymerase chain reaction for the species identification of animal cell lines—a progress report. In Vitro Cell Dev Biol Anim 39:468–475 [DOI] [PubMed]
- Stevenson R (1987) Development of cell banking in the US 1960–1985: a strategic approach to quality control. Adv Cell Cult 51:267–288
- Stulberg CS, Peterson WD Jr., Simpson WF (1976) Identification of cells in culture. Am J Hematol 1:237–242 [DOI] [PubMed]
- Thacker J, Webb MB, Debenham PG (1988) Fingerprinting cell lines: use of human hypervariable DNA probes to characterize mammalian cell cultures. Somat Cell Mol Genet 14:519–525 [DOI] [PubMed]
- Thacker J (1994) Fingerprinting of mammalian cell lines with a single PCR primer. BioTechniques 16:252–253 [PubMed]
- Wang H, Huang S, Shou J, Su EW, Onyia JE, Liao B, Li S (2006) Comparative analysis and integrative classification of NCI60 cell lines and primary tumors using expression profiling data. BMC Genomics 7:166 [DOI] [PMC free article] [PubMed]
- Yamada K, Kamimura E, Kondo M, Tsuchiya K, Nishida-Umehara C, Matsuda Y (2006) New families of site-specific repetitive DNA sequences that comprise constitutive heterochromatin of the Syrian hamster (Mesocricetus auratus). Chromosoma 115:36–49 [DOI] [PubMed]
- Zoon KC (1993) Points to consider in the characterization of cell lines used to produce biologicals. Center for Biological Evaluation and Research, Food and Drug Administration, Rockville, MD, 7–8