Abstract
Protein delivery represents a powerful tool for experiments in live cells including studies of protein-protein interactions, protein interference with blocking antibodies, intracellular trafficking and protein or peptide biological functions. Most available reagents dedicated to the protein delivery allow efficient crossing of the plasma membrane. Nevertheless, the major disadvantage for these reagents is a weak release of the delivered protein into the cytoplasm. In this publication we demonstrate efficient protein delivery with a non-peptide based reagent, in human epithelial carcinoma HeLa cells and primary human skin fibroblasts. Using a fluorescent protein in combination with fluorescence microscopy and fluorescence-assisted cell sorting analysis, we show that the delivered protein is indeed released effectively in the cytoplasm, as expected for a dedicated carrier. Furthermore, we present a step-by-step method to optimize conditions for successful intracellular protein delivery.
Keywords: Intracellular release, Live cells, Non-viral technology, Protein delivery, Synthetic delivery reagent
Introduction
The increasing number of recombinant proteins available offers the possibility to study protein function and develop therapeutic candidates with intracellular targets. Furthermore, intracellular delivery of recombinant proteins to live cells offers a powerful alternative to gene or siRNA transfections. For such technology to be successful, the delivered protein needs to cross the plasma membrane and to be efficiently released in the cytoplasm. Methods such as electroporation, microinjection or macromolecular systems have been adapted to introduce proteins into cells by crossing cellular membranes (Bar-Sagi and Feramisco 1985; Campbell et al. 1995; Morris et al. 2001; Prochiantz 2000; Raptis et al. 1995; Schwarze and Dowdy 2000; Tinsley et al. 1998; Zelphati et al. 2001). However, the ability to introduce proteins (including antibodies and peptides) to the cytoplasm of live cells was greatly facilitated by the development of delivery reagents mainly based on peptides (Cell-Penetrating Peptide, CPP or Protein Transduction Domain, PTD). Usually, these reagents form non-covalent complexes with the protein to be delivered and carry it through the plasma membrane. As proteins differ widely in size, overall charge and structure, they influence complex formation between the protein of interest and the delivery reagent. Successful delivery therefore largely depends on the physicochemical properties of the protein to be delivered. Indeed, robust reagents enable protein/reagent complexes disassembly inside the cytoplasm and release of the native protein.
In this report, we present a practical approach to successful protein delivery with a non-peptide based reagent. We raise the importance of the releasing step in protein delivery, which represent a bottleneck for such experiment and is often neglected in the literature (Drin et al. 2003; Fischer et al. 2004; Ho et al. 2001; Richard et al. 2003; Saalik et al. 2004). We also analyse key parameters namely the volume of reagent, the amount of protein, the protein/reagent incubation time with the cells and finally, the time of analysis required to obtain an efficient release of the protein into the cytoplasm.
Materials
R-phycoerythrin (R-PE; ref P-801) and Histone-H1-AlexaFluor®488 (ref H-13188) proteins were obtained from Molecular Probes (Eugene, Oregon, USA). Anti-α-tubulin-FITC (F2168) antibody and Bovine Serum Albumin (BSA; ref A4919) were purchased from Sigma Chemical Co. (St. Louis, Missouri, USA). Recombinant Turbo Green Fluorescent Protein (rTurboGFP; ref FP552) was obtained from Evrogen (Moscow, Russia). The protein delivery reagent PULSin™ (ref 501–04) is a cationic amphiphile-based formulation prepared in water, registered trademark of Polyplus-transfection (Illkirch, France).
Methods
Cell culture
Human epithelial carcinoma cell line HeLa (ATCC CCL2) was cultured in Minimal Essential Medium (MEM, Eurobio, Courtaboeuf, France) at 37 °C in 5% CO2. Culture medium contained 10% heat-inactivated fetal bovine serum (FBS, Perbio, Brebières, France), penicillin (100 units/mL, Eurobio), streptomycin (100 μg/mL, Eurobio) and glutamine (2 mM, Eurobio).
Primary human skin fibroblasts (Biopredic International, Rennes, France) were cultured in complete medium (Biopredic International) at 37 °C in 5% CO2.
Protein delivery procedure
Cells were plated in order to reach approximately 70–80% confluency the day of protein delivery experiment. For one well of a 24-well plate, 0.5–8 μg of purified protein was diluted in 100 μL of Hepes buffer (20 mM, pH 7.4) in a 1.5 mL microcentrifuge tube, under sterile conditions. In each tube, 1–8 μL of delivery reagent were added to the protein solution. After a brief homogenization with a vortex, the protein/reagent mix was incubated for 15 min at room temperature to allow complexes formation. The cells were washed with 1 mL of PBS and 900 μL of culture medium without serum were added to each well. After addition of complexes onto each well, the plate was gently mixed and further incubated at 37 °C. After 4 h, the incubation medium was removed and replaced with 1 mL of fresh complete medium (containing serum). Protein delivery was analysed immediately or at later time points.
Fluorescence microscopy
In order to observe intracellular protein delivery in 24-well plates, the culture medium was removed from the cells and replaced with PBS (1 mL). Cells were observed by fluorescence microscopy using a Nikon Eclipse TE2000-S microscope and appropriate filter sets. Pictures were taken using a Nikon Coolpix 5400 camera.
Flow cytometry
After protein delivery procedure, HeLa cells were resuspended in PBS and submitted to microcapillary flow cytometry analysis. Data were gathered using a capillary flow cytometer (Guava Personal Cell Analysis-96 system) equipped with a 488 nm diode laser (Guava Technologies, Hayward, California, USA). R-PE fluorescence was collected at 583/42 nm.
Data were analysed using CytoSoft (Guava Technologies) and WinMDI 2.8 (freeware). Forward scatter gating was set to exclude dead cells and debris. A minimum of 2,000 events, representative of at least 80% of the whole cell suspension, was collected for each histogram. Analytical gates were chosen such that <1% of control cells fell within the positive region.
Results and discussion
Cationic amphiphilic-based delivery reagent allows complex formation with proteins via both electrostatic and hydrophobic interactions. The protein delivery mechanism using such reagents is similar to that of gene delivery (Dalkara et al. 2004; Kopatz et al. 2004; Zelphati et al. 2001). Protein/reagent complexes interact with the cell surface by binding to heparan sulfate proteoglycans (Labat-Moleur et al. 1996; Mislick and Baldeschwieler 1996). Complexes are internalized by endocytosis. Then, cationic amphiphilic-based reagent induces endosomes escape followed by the complexes disassembly.
When delivering proteins to cells, the objective is to release in the cytoplasm a native protein able to diffuse and reach its intracellular target. This implies that, once in the cytoplasm, the protein must dissociate efficiently from the delivery reagent. One method to determine protein delivery and diffusion inside the cytoplasm is to follow protein localisation by fluorescence microscopy.
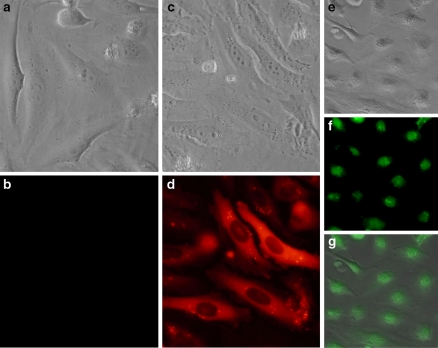
We have used R-PE (240 kDa), an autofluorescent protein as an example. R-PE alone is not able to cross the plasma membrane of HeLa cells (Figs. 1a, b). A cationic amphiphilic-based delivery reagent was added to R-PE solution to allow complexes formation as described in the Methods section. The addition of protein/reagent complexes onto HeLa cells leads to protein uptake and delivery observed as a diffuse intracytoplasmic fluorescent signal (Figs. 1c, d). As expected for a 240 kDa protein that is unable to cross the nuclear pores, R-PE remains within the cytoplasm but is excluded from the nucleus. On the contrary, when a fluorescent-derivative of the Histone-H1 is delivered into HeLa cells using the same reagent, the fluorescent signal is located into the nucleus compartment (Figs. 1e–g), as expected for such a protein. As a result, our fluorescence microscopy analysis demonstrates unambiguously that delivered proteins are released within the cytoplasm and can diffuse towards its intracellular target or being actively transported into the nucleus when containing appropriates NLS signals. Such experiments are therefore a prerequisite prior to fluorescence-assisted cell sorting (FACS) analysis where cell containing proteins sequestrated in the endosomes (which are not released in the cytoplasm and thus unable to resume cellular function) are still counted as positive. Unfortunately, most current data on protein delivery obtained using CPP were carried out only by flow cytometry (Drin et al. 2003; Fischer et al. 2004; Ho et al. 2001; Richard et al. 2003; Saalik et al. 2004). As a result, the efficacy of those peptides remains ambiguous.
Fig. 1.
The intracellular delivery of fluorescent proteins (R-PE and Histone-H1) mediated by a non-peptide based reagent. Two μg of R-PE was diluted in 20 mM Hepes buffer and added with HeLa cells in a 24-well. The plate was incubated for 16 h at 37 °C and cells were analysed by phase-contrast (a) and fluorescence microscopy (b) An amount of 2 μg of R-PE was delivered into HeLa cells after formation of complexes with 4 μL of delivery reagent. Intracellular protein delivery was analyzed after 16 h incubation at 37 °C by phase-contrast (c) and fluorescence microscopy (d) An amount of 4 μg of Histone-H1-AF®488 was delivered into HeLa cells after formation of complexes with 3 μl of delivery reagent. Intracellular protein delivery was analyzed 20 h later. Superimposition (g) of images obtained by phase-contrast (e) and fluorescence microscopy (f) was obtained using Adobe Photoshop software
In order to optimise the delivery, the following parameters need to be adjusted: the amount of the protein, the volume of reagent and the incubation time of protein/reagent complexes onto the cells. In addition, the kinetics of intracellular delivery and release in the cytoplasm should be determined. Each parameter is in part determined by the physicochemical properties of the protein and the cell type used for the delivery experiment. An optimisation procedure will be presented here for R-PE.
Effective protein delivery relies on the formation of electrostatic and hydrophobic interactions between protein and delivery reagent. Thus, the first parameter to consider for efficient complex formation is the volume of delivery reagent per μg of protein needed to form non-covalent complexes. R-PE alone was unable to cross plasma membrane of HeLa cells (Fig. 2a) when observed by fluorescence microscopy. For a given amount of 2 μg of R-PE, we tested several volumes of reagent to complex the protein. Complexes prepared with 1 μL of reagent only allowed delivery of R-PE into a small fraction of HeLa cells (Fig. 2b), whilst those prepared with 2 μL drastically increased the number of fluorescent cells containing R-PE throughout the cytoplasm (Fig. 2c). When using 4 μL of delivery reagent (Fig. 2d), an intense signal was observed in the cytoplasm. R-PE delivery using higher volume (6 μL) of reagent was comparable to that obtained with 4 μL of reagent indicating that a plateau was reached (data not shown). It is noteworthy that higher volumes (such as 8 μL) of reagent induced cytotoxicity in HeLa cells (data not shown). As a result, we show that the volume of delivery reagent is a key parameter to optimise for efficient protein delivery and to prevent cell toxicity. In the case of R-PE, the optimal amount of delivery reagent to be used is 4 μL per μg of protein.
Fig. 2.
Efficient intracellular protein delivery is dependent on the volume of delivery reagent used. An amount of 2 μg of R-PE was delivered into HeLa cells after formation of complexes with increasing volume of delivery reagent (a: 0 μL; b: 1 μL; c: 2 μL and d: 4 μL). The cells were analysed 7 h after addition of the complexes onto the cells
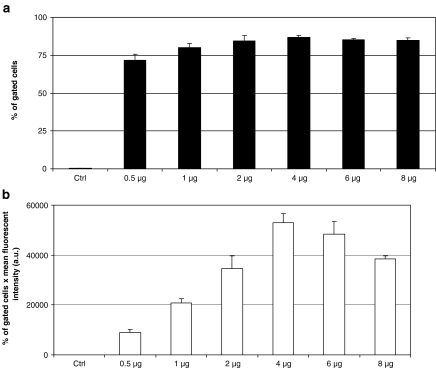
In addition to the volume of reagent, the optimal amount of protein to be used per well needs to be determined. The efficiency of protein delivery in HeLa cells was analysed by fluorescence microscopy (data not shown) and subsequently quantified by FACS analysis (Figs. 3a, b). A non-fluorescent protein (1 μg), Bovine Serum Albumin (BSA), was used as a negative control (Ctrl). Variable amounts of R-PE were incubated with 4 μL of delivery reagent to form complexes and then added to HeLa cells. For each amount of protein tested, the percentage of fluorescent cells (Fig. 3a) was approximately 80%, except for 0.5 μg of R-PE. There was no significant difference from 1 to 8 μg of R-PE per well of a 24-well plate. However, the intracellular amount of delivered fluorescent protein per cell increased proportionally to the amount of R-PE used to form complexes up to 4 μg (Fig. 3b). Delivering higher amount of R-PE above 4 μg (6 or 8 μg) leads to a lower intracellular amount of protein delivered per cell, mainly due to cytotoxicity. Therefore, this experiment shows that a minimal amount (1 μg) of protein is needed to obtain high percentage (>75%) of cells containing the delivered protein (Fig. 3a). Nevertheless, 1 μg of R-PE is not sufficient to reach the highest amount of protein delivered per cell (Fig. 3b). As a result, measuring both the delivery efficiency and the amount of protein delivered per cell is required to determine the optimal conditions. We tested various cell lines with optimal conditions of R-PE delivery and determined that the efficiency varies from 60% in human primary fibroblasts, to 80% in CHO cells and more than 95% in NIH-3T3 cells.
Fig. 3.
The amount of protein greatly influences the delivery efficiency. Cell sorting experiments were carried out onto HeLa cells after intracellular protein delivery. A volume of 4 μL of delivery reagent was added to various amounts of R-PE (1–8 μg) or 1 μg of BSA (negative control, Ctrl). Complexes were added to cells and further incubated for 20 h before FACS analysis. Gates were set to distinguish between autofluorescence of control cells and highly fluorescent cells. Means of percentage of fluorescent cells ± SEM are shown (a), n = 3 per point. Results are also presented as the percentage of gated fluorescent cells per mean intensity of fluorescence (b), n = 3 per point
This optimisation was carried out for several proteins delivered into HeLa cells. The results are shown in Table 1 indicating that the optimal conditions for the protein amount and the volume of reagent differ from one protein to another one.
Table 1.
Amount of protein and volume of reagent giving the highest protein delivery in the cytoplasm for R-PE (R-phycoerythrin), antibody (anti-α-tubulin-FITC) or GFP (Recombinant TurboGFP) and in the nucleus of HeLa cells for Histone (Histone-H1-AlexaFluor®488)
| R-PE | Antibody | GFP | Histone | |
|---|---|---|---|---|
| Amount of protein (μg) | 1 | 1 | 3 | 4 |
| Volume of delivery reagent (μL) | 4 | 2.5 | 4 | 3 |
An important step for protein delivery is the binding of protein/reagent complexes to the cellular membrane. For adherent cells, this step is simply achieved by covering the monolayer upon addition of the complexes to the cells. The cell uptake efficiency depends to the size of the complexes and their ability to sediment onto the cells and to stick to the surface. The size of complexes formed by the biomolecule and the reagent has to be at least 100 nm in diameter to be able to sediment efficiently on the cellular layer. Indeed, it has been demonstrated (Chuck et al. 1996; Erbacher et al. 1998) that particles of 100 nm or below have a fast Brownian motion which prevents sedimentation onto cells. The size of complexes varies depending on the protein tested and thus determines, in part, the incubation time required for sedimentation to occur efficiently. We determined the size of the complexes formed between the delivery reagent and the R-PE by dynamic light scattering measurements (DLS) and found a mean diameter of 500 nm (data not shown).
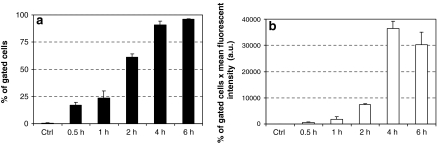
Since serum induced dissociation of protein/delivery reagent complexes (data not shown) and is known to inhibit delivery mediated by cationic lipids (Farhood et al. 1992), all previously described protein delivery experiments were performed in the absence of serum. For R-PE, we have shown that the optimal incubation time of cells in presence of protein/reagent complexes in the absence of serum was 4 h (Fig. 4) after which the culture medium is replaced with complete culture medium. Longer incubation times such as 6 h in the absence of serum lead to reduced cell viability. A 4 h incubation time of cells without serum is therefore the optimal condition for a high percentage of fluorescent cells with reduced cytotoxicity.
Fig. 4.
The incubation time of protein/delivery reagent complexes with cells influences the delivery efficiency. Delivery reagent (4 μL) was added to R-PE (1 μg) or BSA (1 μg, Ctrl) to form complexes. Complexes were thereafter added onto HeLa cells and further incubated for the indicated time (30 min to 6 h). FACS analysis was carried out 16 h post-delivery. The experiment was carried out as described in the figure 3
When using fragile cells, the incubation time of the complexes with cells can be reduced advantageously by a centrifugation step (for 5 min at 190 g) immediately after adding the complexes in the wells. The centrifugation enhances sedimentation of the complexes onto the cells thus reducing incubation time in the absence of serum to 30 min at 37 °C after which complete medium containing serum is added. This alternative is particularly well-suited for sensitive cell types which do not withstand the absence of serum for several hours.
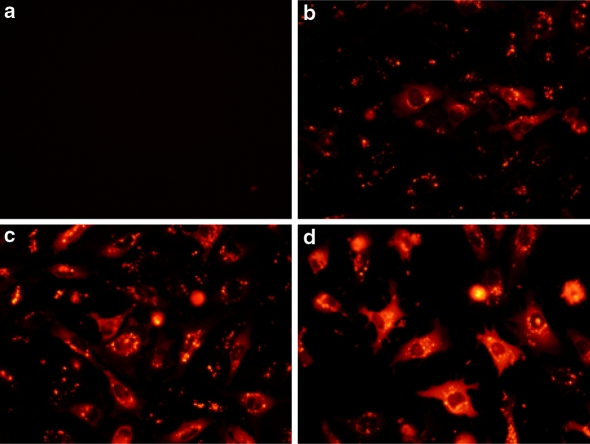
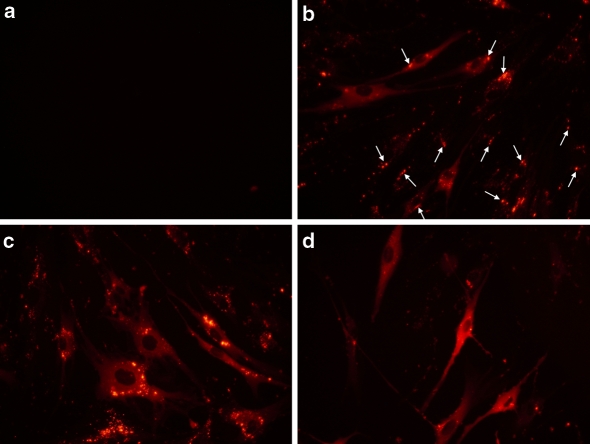
Upon internalisation of the complexes by the cells, the protein needs to be released in the cytoplasm, an active process which in part depends on the cell type. In human primary fibroblasts, the addition of R-PE alone did not induce any protein delivery (Fig. 5a). In contrast, 4 h after addition of R-PE/delivery reagent complexes on primary fibroblasts (Fig. 5b), a fluorescent signal was mainly located as aggregates sequestered in endosomes (white arrows in Fig. 5b). However, 8 h after protein delivery (Fig. 5c), a diffuse fluorescent signal was observed in the cytoplasm, due to the efficient release of the R-PE from the endosomes and diffusion in the cytoplasm. Finally, on the next day (20 h, Fig. 5d) the protein was observed in the whole cytoplasm showing that the release was completed. As a result, the post-delivery kinetic determines the optimal window for protein release in the cytoplasm enabling the free protein to reach its intracellular target by diffusion.
Fig. 5.
Kinetic of intracellular R-PE delivery into human primary fibroblasts. One μg of R-PE diluted in 100 μL Hepes buffer (20 mM, pH 7.4) was incubated with 4 μL of delivery reagent for 15 min at room temperature. The cells were analyzed 4 (b), 8 (c) and 20 h (d) after addition of protein/reagent complexes onto cells. As a control (a), R-PE alone was added onto human primary fibroblasts and analysed 20 h after addition of the protein onto cells
In conclusion, we have presented a practical approach for protein delivery into live cells and we have demonstrated that the determination of optimal conditions is required for efficient delivery. Routinely, we recommend using 1–2 μg of R-PE and 2–4 μL of delivery reagent to obtain an efficient protein delivery. Furthermore, we provide evidence for an effective release of protein in the cytoplasm when introduced into many cell types by a non-peptide based delivery reagent. Recent data supports the potency of this delivery method to introduce functional proteins such as active blocking antibody into human cancer cell line (Cassinelli et al. 2006) or into mouse Sertoli cells (Ying et al. 2007) and active peptides into neurones (Leshchyns’ka et al. 2006).
Acknowledgments
We are grateful to Drs C. Muller and J. Peluso for their help in FACS analysis and to Drs J.-P. Behr and J.-F. Williamson for critical reading of the manuscript.
Abbreviations
- BSA
Bovine serum albumin
- CPP
Cell-penetrating peptide
- DLS
Dynamic light scattering
- FACS
Fluorescence-assisted cell sorting
- PTD
Protein transduction domain
- R-PE
R-phycoerythrin
References
- Bar-Sagi D, Feramisco JR (1985) Microinjection of the ras oncogene protein into PC12 cells induces morphological differentiation. Cell 42:841–848 [DOI] [PubMed]
- Campbell PL, McCluskey J, Yeo JP, Toh BH (1995) Electroporation of antibodies into mammalian cells. Methods Mol Biol 48:83–92 [DOI] [PubMed]
- Cassinelli G, Lanzi C, Petrangolini G, Tortoreto M, Pratesi G, Cuccuru G, Laccabue D, Supino R, Belluco S, Favini E, Poletti A, Zunino F (2006) Inhibition of c-Met and prevention of spontaneous metastatic spreading by the 2-indolinone RPI-1. Mol Cancer Ther 5:2388–2397 [DOI] [PubMed]
- Chuck AS, Clarke MF, Palsson BO (1996) Retroviral infection is limited by Brownian motion. Hum Gene Ther 7:1527–1534 [DOI] [PubMed]
- Dalkara D, Zuber G, Behr JP (2004) Intracytoplasmic delivery of anionic proteins. Mol Ther 9:964–969 [DOI] [PubMed]
- Drin G, Cottin S, Blanc E, Rees AR, Temsamani J (2003) Studies on the internalization mechanism of cationic cell-penetrating peptides. J Biol Chem 278:31192–31201 [DOI] [PubMed]
- Erbacher P, Zou S, Bettinger T, Steffan AM, Remy JS (1998) Chitosan-based vector/DNA complexes for gene delivery: biophysical characteristics and transfection ability. Pharm Res 15:1332–1339 [DOI] [PubMed]
- Farhood H, Bottega R, Epand RM, Huang L (1992) Effect of cationic cholesterol derivatives on gene transfer and protein kinase C activity. Biochim Biophys Acta 1111:239–246 [DOI] [PubMed]
- Fischer R, Kohler K, Fotin-Mleczek M, Brock R (2004) A stepwise dissection of the intracellular fate of cationic cell-penetrating peptides. J Biol Chem 279:12625–12635 [DOI] [PubMed]
- Ho A, Schwarze SR, Mermelstein SJ, Waksman G, Dowdy SF (2001) Synthetic protein transduction domains: enhanced transduction potential in vitro and in vivo. Cancer Res 61:474–477 [PubMed]
- Kopatz I, Remy JS, Behr JP (2004) A model for non-viral gene delivery: through syndecan adhesion molecules and powered by actin. J Gene Med 6:769–776 [DOI] [PubMed]
- Labat-Moleur F, Steffan AM, Brisson C, Perron H, Feugeas O, Furstenberger P, Oberling F, Brambilla E, Behr JP (1996) An electron microscopy study into the mechanism of gene transfer with lipopolyamines. Gene Ther 3:1010–1017 [PubMed]
- Leshchyns’ka I, Sytnyk V, Richter M, Andreyeva A, Puchkov D, Schachner M (2006) The adhesion molecule CHL1 regulates uncoating of clathrin-coated synaptic vesicles. Neuron 52:1011–1025 [DOI] [PubMed]
- Mislick KA, Baldeschwieler JD (1996) Evidence for the role of proteoglycans in cation-mediated gene transfer. Proc Natl Acad Sci U S A 93:12349–12354 [DOI] [PMC free article] [PubMed]
- Morris MC, Depollier J, Mery J, Heitz F, Divita G (2001) A peptide carrier for the delivery of biologically active proteins into mammalian cells. Nat Biotechnol 19:1173–1176 [DOI] [PubMed]
- Prochiantz A (2000) Messenger proteins: homeoproteins, TAT and others. Curr Opin Cell Biol 12:400–406 [DOI] [PubMed]
- Raptis LH, Liu SK, Firth KL, Stiles CD, Alberta JA (1995) Electroporation of peptides into adherent cells in situ. Biotechniques 18:104, 106, 108, 110 passim [PubMed]
- Richard JP, Melikov K, Vives E, Ramos C, Verbeure B, Gait MJ, Chernomordik LV, Lebleu B (2003) Cell-penetrating peptides. A reevaluation of the mechanism of cellular uptake. J Biol Chem 278:585–590 [DOI] [PubMed]
- Saalik P, Elmquist A, Hansen M, Padari K, Saar K, Viht K, Langel U, Pooga M (2004) Protein cargo delivery properties of cell-penetrating peptides. A comparative study. Bioconjug Chem 15:1246–1253 [DOI] [PubMed]
- Schwarze SR, Dowdy SF (2000) In vivo protein transduction: intracellular delivery of biologically active proteins, compounds and DNA. Trends Pharmacol Sci 21:45–48 [DOI] [PubMed]
- Tinsley JH, Hawker J, Yuan Y (1998) Efficient protein transfection of cultured coronary venular endothelial cells. Am J Physiol 275:H1873–H1878 [DOI] [PubMed]
- Ying M, Chen B, Tian Y, Hou Y, Li Q, Shang X, Sun J, Cheng H, Zhou R (2007) Nuclear import of human sexual regulator DMRT1 is mediated by importin-beta. Biochim Biophys Acta 1773:804–813 [DOI] [PubMed]
- Zelphati O, Wang Y, Kitada S, Reed JC, Felgner PL, Corbeil J (2001) Intracellular delivery of proteins with a new lipid-mediated delivery system. J Biol Chem 276:35103–35110 [DOI] [PubMed]