Abstract
Multiwall carbon nanotube templates formed on the surfaces of planar interdigitated microelectrode arrays by means of AC electric field-guided assembly are being explored as potential substrates for tissue engineering. The objective of the present study is to examine whether surface patterns of aligned multiwall carbon nanotubes can have an effect on cell growth, morphology, and alignment. Bovine fibroblasts grown on aligned carbon nanotubes for a period of 2 weeks were found to have raised bodies and pronounced cell extensions for anchoring themselves to the substrate similar to that of the cells found in native tissues. On the other hand, cells grown on various control surfaces had a flat, circular morphology. The cell cultures were visualized by means of SEM imaging and the resulting morphologies were statistically analyzed and compared.
Keywords: AC electrokinetics, Carbon nanotube alignment, Dielectrophoresis, Microelectrodes, Patterned substrate, Tissue engineering
Introduction
Tissue engineering is a rapidly growing multidisciplinary field that seeks to develop biological substitutes to repair the function of a tissue that has been lost due to injury or disease. Since in vitro tissue formation typically requires a template that the cells can interact with, research has been invested in the creation of structures that mimic natural scaffolds in the body (El Ghalbzouri et al. 2004; Cooper et al. 2005; Meinel et al. 2004; Lu et al. 2004). One approach that attracts attention over the last few years is the so-called “bottom-up” template synthesis, whereby the desired substrate for cell attachment and tissue growth is created through the directed assembly of individual building nanoblocks (Alp et al. 2003).
Carbon nanotubes (CNTs) are among the nanomaterials that are currently being explored as candidates for “bottom up” synthesis of substrates for tissue engineering. Patterns on a substrate to create macromolecular structures with CNTs are present in the forms of patterned spots for CNT deposition (Gabay et al. 2005) and organized polystyrene beads coated with layers of CNT (Firkowska et al. 2006); however, limited information is available in the literature on organized CNTs themselves as a template for tissue engineering. Vertically grown multiwall carbon nanotubes (MWNTs) on the surface of a silicon substrate can be functionalized in a way to create a 3D network of cavities that favor cell attachment and growth (Correa-Duarte et al. 2004). Fibroblasts seeded on these templates were found to adhere and produce disorganized tissue. Moreover, stand-alone CNTs have been proposed as a growth substratum for cells and tissue. Zanello et al. (2006) have recently discovered that MWNTs were able to sustain osteoblast proliferation and bone formation in vitro. This proves that cells are able to interact with a non-degradable matrix such as CNTs without any observable detrimental effects.
CNTs are known to orient under the influence of an alternating current (AC) electric field and form aligned patterns on a flat surface (Yamamoto et al. 1998). Transportation of CNTs from the bulk of a suspension and subsequent concentration and alignment onto a flat surface can be achieved with dielectrophoresis, i.e., the translational motion that polarizable particles experience inside a spatially non-uniform electric field due to induced polarization effects (Pohl 1978). Chung et al. (2004) demonstrated a scalable and highly parallel process of electric field-induced CNT positioning and alignment that can lead to mass assembly of nanostructures and nanoscale devices. Furthermore, Chen and Zhang (2006) were recently able to dispersively align single walled carbon nanotubes (SWNTs) avoiding CNT entanglement between 2D co-planar parallel electrodes for potential applications in high performance nano-devices. Chen et al. (2001) have aligned CNTs between electrode gaps with a high degree of alignment and the appearance of good coverage.
The concept of utilizing dielectrophoretically aligned CNTs as an ordered substrate for tissue engineering still remains unexplored. The working hypothesis that CNTs can be tailored into a proper patterned substrate that may support cell attachment and growth and also promote formation of organized tissue has not been validated. The present article describes a first attempt to explore the response of cells grown on CNT templates. Specifically, we use planar interdigitated gold microelectrode arrays to guide the assembly of MWNTs into a directional surface pattern. The substrates thus formed were subsequently used for the seeding and growth of fibroblasts over a period of 2 weeks. Our objective was to determine whether oriented MWNT templates can have an effect on cell growth, morphology, and alignment. Cells cultured on bare glass and randomly deposited MWNTs provided a set of controls for determining cell morphology and substrate biocompatibility. The cell cultures were visualized by means of SEM imaging and the resulting morphologies were statistically compared.
Materials and methods
Microelectrodes
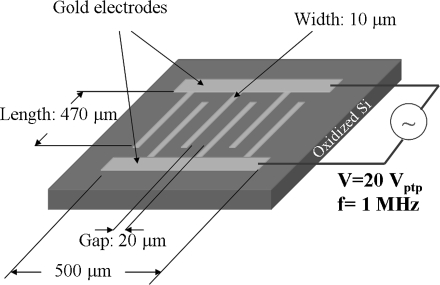
Planar, interdigitated microelectrode arrays (Fig. 1) were fabricated on the surfaces of oxidized silicon wafers (SiO2 thickness: 500 nm) using conventional silicon microfabrication techniques, namely photolithography and metal evaporation (gold deposition). The adhesion of the gold electrodes (thickness ∼100 nm) onto the substrate was enhanced with the deposition of a thin layer (20 nm) of titanium between the gold and silicon oxide. The spacing between the microelectrodes was 20 μm. The width and length of each electrode was 10 and 470 μm, respectively. The overall length and width of the array were both equal to 500 μm.
Fig. 1.
Schematic showing the main features of the microelectrode arrays used for carbon nanotube alignment
Carbon nanotubes
Multiwall carbon nanotubes (MWNTs) produced by chemical vapor deposition, with external diameter 20–40 nm, mean length of 10 μm, and purity >96% were purchased from MicroTechNano (Indianapolis, IN) and used without further purification. The nanotubes were suspended in distilled water with the aid of the non-ionic surfactant Pluronic® F127 Prill, a triblock copolymer [(Ethylene Oxide)97(Propylene Oxide)69(Ethylene Oxide)97] terminating in primary hydroxyl groups and having an average molecular weight of 12,600 (BASF, Florham Park, NJ). The initial dispersion was facilitated by ultrasound sonication of the samples for 4 h inside an ultrasound bath (VWR; Model: 50D). Remaining aggregates in the suspension were removed by centrifugation at 1,800 g for 4 min.
Dielectrophoretic alignment of carbon nanotubes
The chip was first mounted on a custom-designed stage that provided contacts with an external AC source. A silicone spacer was placed on the chip with the central gap enclosing the array pattern. The MWNT suspension was loaded into the silicone spacer and a sinusoidal voltage of 20 V (peak-to-peak) and 1 MHz was applied across the electrodes by using a function generator (BK Precision; Model 4040A). The applied voltage was monitored with a Tektronix 465 Oscilloscope (Beaverton, OR). At the end of the experiment, the aligned MWNT were washed six times with water and six times with ethanol (EtOH) before being air dried. During each washing step, 7 μl of the suspending medium were removed and replaced with an equal volume of fresh, clean medium. Once the washing of the carbon nanotubes was complete, the electric field was deactivated.
Cell isolation and culture
Bovine ligament fibroblasts were isolated from the central ligament of the metacarpal-carpal joint of 12–18 month old calves. Harvested ligaments were cleaned of extraneous fat and digested in 0.25% collagenase A (Roche Diagnostics Corporation, Laval, QC) for 40 h at 37 °C. Viable cells, determined from the Trypan Blue dye exclusion assay (Invitrogen Corporation, NY), were seeded at a density of 7,000 cells cm−2 on each surface investigated (glass or microelectrode arrays) and maintained in DMEM culture media (Sigma-Aldrich, Canada) supplemented with 5% fetal bovine serum. Cell cultures were grown in an incubator maintained at 37 °C and 95% relative humidity supplemented with 5% CO2: 95% atmospheric air. After 2 weeks in culture, cells attached to the surfaces were rinsed with phosphate buffer saline (PBS, pH 7.4) and fixed with 4% paraformaldehyde (Sigma-Aldrich, Canada) overnight at 4 °C. Cells were then dehydrated in graded ethanol solutions followed by chemical fixation through graded hexamethyldisilazane (HMDS) solutions (Sigma-Aldrich, Canada). Remaining HMDS was aspirated and the samples allowed to dry overnight in a fume hood. The samples were then mounted on aluminum stubs, pulse sputter coated with approximately a 20 Å layer of gold using an Anatech Hummer VI-A Sputter Coater (Denver, NC) and imaged with a Jeol JSM840 SEM (Peabody, MA) with HKL Flamenco EBSD data acquisition software (version 5.0.6.0).
Data and statistical analyses
SEM images were analyzed using image analysis software (ImageJ U.S. National Institutes of Health, Bethesda, Maryland, USA, http://www.rsb.info.nih.gov/ij/) in order to quantify the percent coverage of aligned MWNTs between the electrodes and also to assess the morphology of cells grown on the various substrates. Images were converted to 8-bit grayscale and then thresholded to distinguish the aligned MWNTs or cells from the background. Thresholded images were then analyzed to determine the percent coverage of MWNTs or cell morphological parameters (circularity, axis length and area of the cell nuclei). Statistical analyses of the data were performed by either a two-way or three-way analysis of variance (ANOVA) depending on the particular experiment using SPSS 14.0 for Windows (SPSS Inc., Chicago, IL). In all statistical analyses, significance was associated with p-values less than 0.05.
Results and discussion
Preparation of carbon nanotube templates
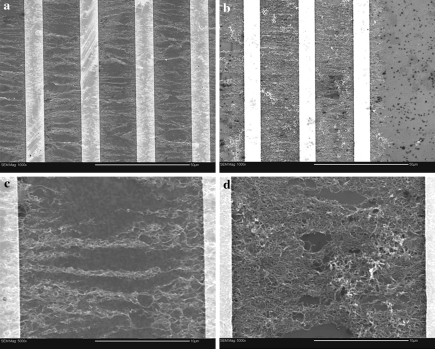
Formation of MWNT templates took place through dielectrophoretic alignment of carbon nanotubes from their suspensions in aqueous solutions of Pluronic® F127. The process was monitored with a reflected visible light microscope. Immediately after the activation of the electric field, carbon nanotubes started to migrate from the bulk of the dispersion, forming visible bundles on the surface that were aligned in a direction perpendicular to the edges of the microelectrodes. The phenomenon resulted in significant accumulation of carbon nanotubes on the surface of the microelectrode chip. After approximately 20 min of dielectrophoresis, and when no further visible change in surface coverage could be observed, the suspension was removed and the assembled templates were rinsed with copious amounts of water and ethanol. Microscopic observations of the aligned MWNT structures before and after rinsing indicated that this particular method of medium removal did not result in noticeable distortion of the aligned MWNT formations. The characteristics of the resulting templates (surface coverage, alignment quality) were found to be statistically dependent on the surfactant (Pluronic® F127) concentration used in the preparation of the carbon nanotube suspensions (p < 0.02). SEM images of the templates formed from suspensions containing 0.01 and 0.4 wt% surfactant can be seen in Fig. 2. In the low magnification images (Fig. 2a, b), distinct differences are observed in surface coverage of MWNTs. Image analysis indicated that dielectrophoresis in suspensions containing 0.01 wt.% surfactant resulted in surface coverage of 32% (±2), whereas suspensions containing 0.4 wt.% surfactant produced surface coverage of 80% (±6). Higher magnification images show that dielectrophoresis of MWNT at low surfactant concentrations (Fig. 2c) results in a thin layer of perpendicularly aligned MWNT bundles bridging the electrode gaps, while higher concentration (Fig. 2d) resulted in a dense network of individual MWNTs, characterized by a lower degree of alignment. It is important to mention here that the original concentration of stably suspended carbon nanotubes was approximately the same in all samples (0.014 ± 0.002 mg ml−1, as determined from spectrophotometry measurements). Therefore, the differences observed in surface coverage should not be attributed to the amount of carbon nanotubes available for alignment, but rather to the quality of the carbon nanotube dispersions (individual tubes vs. bundles). Since the role of the surfactant molecules is to adsorb onto the MWNT surfaces and, by doing so, break up aggregates during sonication, it is expected that a higher surfactant concentration would result in a better (finer) dispersion. The higher degree of alignment observed in the case of carbon nanotube bundles is explained by considering that dielectrophoresis scales with the third power of the object’s characteristic dimension. Kim et al. (2006) also observed that, when aligning MWNTs suspended in aqueous solutions of sodium dodecyl sulfate or cetyltrimethylammonium bromide, a matted network of MWNTs formed after the removal of the suspension droplet with a gentle stream of nitrogen.
Fig. 2.
SEM images showing dielectrophoretically aligned carbon nanotubes in the gaps between microelectrodes. (a) and (c): Low (×1000) and high (×5000) magnification images, respectively, obtained from MWNT suspensions in 0.01 wt% F127; (b) and (d): low (×1000) and high (×5000) magnification images, respectively, obtained from MWNT suspensions in 0.4 wt% F127. Scale bars: 50 μm (a) and (b), 10 μm for (c) and (d)
A number of MWNT templates formed on microelectrode arrays were subsequently tested in terms of their ability to support growth of fibroblasts, as discussed below. The results (cell morphology, substrate biocompatibility) were compared against those obtained from cells cultured on bare glass or randomly deposited MWNTs (control substrates).
Cells grown on control substrates
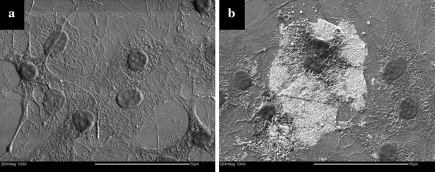
SEM images of cells cultured on bare glass for 2 weeks are shown in Fig. 3a. The cell attachment on the surface appeared disorganized. The cells had a spread out, flat “pancake”-like appearance with dendritic (arm-like) extensions from the cell membrane anchoring the cells to the glass surface. The majority of the cell nuclei had a near-circular shape. Typical fibroblast morphology on glass is composed of regions of extensively spread cytoplasm (Grinnell and Bennett 1981), which was seen here. The disorganized cytoplasm network shown in Fig. 3a, most likely composed of actin (Meng et al. 2006), indicates that the cells have grown over and under each other. The membrane boundary between cells is difficult to discern within the network. The difference between any extracellular matrix produced by the cells and the cytoplasm was indistinguishable.
Fig. 3.
Fibroblasts cultured on glass substrate (a) and carbon nanotube mats (white area in b)
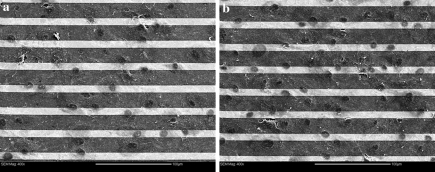
Cells grown on a glass substrate in the presence of randomly deposited MWNTs were imaged by SEM and are shown in Fig. 3b. The deposition occurred from a MWNTs suspension in EtOH that was left to dry onto the glass surface. The light grey areas are the aggregates of MWNTs. The cell nuclei are similar in size and the cells exhibited the same flat, spread-out appearance as those cultured on glass. These cells also exhibited typical fibroblast morphology. Some cells from the same culture were also found to interact with the MWNT aggregates with dendritic extensions grown over the MWNTs. It was unclear as to the extent of cell attachment to the MWNTs, but many cells interacted freely with the MWNT aggregates and were capable of attachment and growth on MWNTs. Zanello et al. (2006) found that osteoblasts had initiated the process of bone formation when attached to MWNTs and appeared spherical, with developed cytoplasmic prolongations. Figure 4 shows fibroblasts grown on microelectrode arrays lined with MWNTs that were randomly deposited from solutions containing F127 at concentrations of 0.01 wt% (Fig. 4a) and 0.4 wt% (Fig. 4b). As can be seen, these cells had the same flattened morphology as those grown on glass and randomly deposited MWNTs. The nuclei were circular and similar in size (p < 0.05). An additional observation that can be made from Fig. 4 is that the cell nuclei appear to be encased into a continuous solid matrix, where holes in the matrix allow the visualization of the original surface. The chemical composition of this matrix remains unknown for the moment. Immuno-fluorescent staining of the samples using antibodies against collagen type I did not yield conclusive results, due to a significant amount of non-specific binding of the primary antibody onto the gold electrodes and potentially the silica substratum.
Fig. 4.
SEM images of cells grown on microelectrodes covered with randomly deposited MWNTs originating from aqueous dispersions in (a) 0.01 wt% and (b) 0.4 wt% Pluronic F127. Scale bars: 100 μm
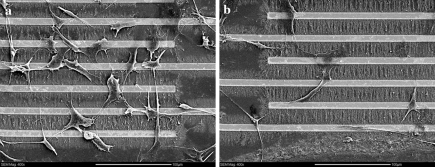
Cells grown on aligned MWNTs
Cells grown on microelectrode arrays covered by aligned MWNTs were also imaged by SEM. The morphology of cells grown on templates with 32% (Fig. 5a) and 80% surface coverage (Fig. 5b) was in striking contrast to those grown on control substrates. These cells had long dendritic extensions (pseudopodia) to anchor themselves onto the gold electrodes as well as the aligned MWNTs. It appeared that the cell extensions were the primary attachment mechanisms as the main body of the cell was raised. They did not exhibit the typical fibroblast morphology (flat and spread) as observed on glass surfaces (Grinnell and Bennett 1981). Many cells in Fig. 5 have extensions that terminated and attached to the gold electrodes instead of the MWNTs. Also, some cells have developed “hairy”, appearance which could indicate the cell attaching itself to the surface with cytoplasmic projections extending from the cytoskeleton similar to that observed when the cells were grown on the glass surfaces. Most importantly, the morphology of these cells appeared to be typical of fibroblasts found in native tissues (Petersen et al. 1999). Zanello et al. (2006) found that osteoblasts cultured on MWNTs exhibited neurite-like growths that would “anchor” the cell to the MWNT matrix. Correa-Duarte et al. (2004) also found that L929 mouse fibroblasts cultured on MWNT network cavities exhibited both a stretched morphology and cytoplasmic extensions to attach to the MWNTs, much like that presented here. Furthermore, the nuclei were difficult to visualize, but are more ellipsoidal than round. More cells (334 vs. 107, on average) were present on MWNTs aligned from a suspension of low than high surfactant concentration. These observations were consistent between the two series (duplicates) of experiments performed.
Fig. 5.
SEM images of cells grown on microelectrodes covered with aligned MWNTs originating from aqueous dispersions in (a) 0.01 wt% and (b) 0.4 wt% Pluronic F127. Scale bars: 100 μm
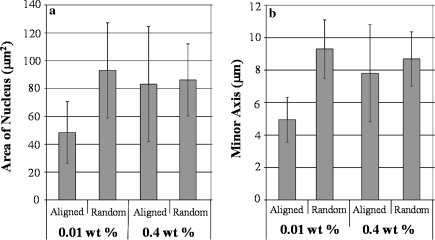
The shape parameters of cells grown on microelectrode arrays were quantified by image analysis and compared to determine the effects of MWNT alignment and surface coverage. Both these effects were found to be statistically significant (p < 0.03). By measuring the shape and area of the cell nuclei (which, generally, follows the shape of the cell as a whole) a comparison between elongated cells and cells with typical morphology was possible. As Fig. 6 shows, significant differences were found with respect to the minor axis length and surface area of cell nuclei. Cells grown on aligned MWNT substrates displayed smaller cell nuclei area (p < 0.01) and minor axis length (p < 0.01).
Fig. 6.
Statistical analysis comparing area of nucleus (a) and minor axis length (b) of cells grown on aligned and randomly dispersed carbon nanotube substrates
Although no firm explanation can be offered for these intriguing results, we believe that the observed cell morphology is caused by the alternating hydrophilic and hydrophobic domains created from MWNT patterning onto the silicon dioxide surface, as well as by the dimensions of these domains. It appears that the variable surface topography allowed proteins from the culture medium to adhere to specific areas, thus forcing a selective cell attachment behavior. Random deposition of MWNT on microelectrodes resulted in surface coverage that was too small to create the same effect and the cells were presented with a relatively uniform hydrophilic substrate.
A possible explanation for the limited cell adhesion observed might also be sought on surfactant molecules that were not removed after repeated washings with de-ionized water and ethanol, but remained adsorbed onto the surface of the assembled MWNT templates and surrounding area. Poly(ethyleneoxide) (PEO), a main constituent of Pluronic surfactants that is also known for its surface antifouling properties (Palsson and Bhatia 2003), might have played a role in preventing protein adsorption from the culture medium on the substrate and subsequent cell adhesion. This hypothesis, however, requires further investigation because it is not supported by the results obtained from cells growing on randomly deposited carbon nanotubes on microelectrodes (also deposited from the same surfactant-containing suspensions; cf. Fig. 4).
Another observation that can be made from the SEM images is that cells cultured onto aligned MWNTs show limited, if any, signs of preferential orientation. One possible explanation may be that the feature size of the aligned template is too small to induce a cellular response that can lead to cell alignment. The length scales common to contact guidance are within the submicro- to micro-meter range. Manwaring et al. (2004) found similar results to ours in terms of orientation when culturing meningeal cells on nano-grooved surfaces with various groove sizes. At a groove size of 10 nm, the researchers reported that the cells had equal probability to orient to any direction. However, only at larger groove sizes of 248 and 945 nm they observed that 45% and 70% of the cells, respectively, had a long axis within 10° of the groove direction. Since the diameter of the MWNTs used in this work was approximately 20–40 nm, it was likely that the fibroblast cells were not influenced by the substrate orientation to exhibit morphological directionality. Another possible reason for the absence of definitive cell orientation might be the small length scale of the individual carbon nanotube patterns, which were interrupted by the closely spaced microelectrodes. As seen in Fig. 5, many cells were found to span across multiple gap spacings and electrodes. Carbon nanotube alignment in microelectrodes with separation of 100 μm (as opposed to 20 μm, shown here) did not result in substantial surface coverage and was not pursued further.
Conclusions
In this work, a first attempt was made to explore the growth, morphology, and alignment of cells seeded on substrates that were formed through the electric field-directed patterning of multiwall carbon nanotubes on flat surfaces. It was shown that, by utilizing the principle of dielectrophoresis, templates of aligned multiwall carbon nanotubes can be readily and reproducibly formed in microelectrode arrays. The amount of surfactant used for the dispersion of carbon nanotubes in water was found to play a significant role in both the quality of nanotube alignment and surface coverage achieved. Cells grown on microelectrodes covered with dielectrophoretically aligned MWNT exhibited morphology that resembles that of fibroblasts found in native tissues, however, they were lacking directionality. It is believed that the observed cell morphology is caused by the alternating hydrophilic/hydrophobic domains created during MWNT patterning on silicon dioxide, however this hypothesis warrants further investigation. On the other hand, the morphologies of cells grown on various control surfaces (bare glass, random nanotubes on glass, or random nanotubes deposited on microelectrodes from surfactant-containing suspensions) are in stark contract with the above.
The ideas of using larger inter-electrodes gaps (that produce continuous templates, much larger than the cell size) and MWNT with various diameters (as a means to control the pitch or periodicity of the resulting template) are currently being tested by our group. Formation of MWNT templates for cell culture through dielectrophoresis inside organic solvents is also being investigated as an alternative to the use of surfactants.
Acknowledgements
Financial support for this work was provided by Ontario Centres of Excellence (OCE)/Collaborative Program of Sectorial Interest (#NM60131). Fabrication of the microelectrodes was performed at the Cornell Nanofabrication Facility (a member of the National Nanofabrication Users Network), Cornell University, Ithaca, NY. Infrastructure funding for this project was provided by Canada Foundation for Innovation (CFI) and Ontario Innovation Trust (OIT).
References
- Alp B, Andrews JS, Mason VP, Thompson IP, Wolowacz R, Markx GH (2003) Building structured biomaterials using AC electrokinetics. IEEE Eng Med Biol Mag 22:91–97 [DOI] [PubMed]
- Chen C, Zhang Y (2006) Manipulation of single-wall carbon nanotubes into dispersively aligned arrays between metal electrodes. J Phys D Appl Phys 39:172–176 [DOI]
- Chen XQ, Saito T, Yamada H, Matsushige K (2001) Aligning single-wall carbon nanotubes with an alternating-current electric field. Appl Phys Lett 78:3714–3716 [DOI]
- Chung J, Lee K-H, Lee J, Ruoff RS (2004) Toward large-scale integration of carbon nanotubes. Langmuir 20:3011–3017 [DOI] [PubMed]
- Cooper JA, Lu HH, Ko FK, Freeman JW, Laurencin CT (2005) Fiber-based tissue-engineered scaffold for ligament replacement: design considerations and in vitro evaluation. Biomaterials 26:1523–1532 [DOI] [PubMed]
- Correa-Duarte MA, Wagner N, Rojas-Chapana J, Morsczeck C, Thie M, Giersig M (2004) Fabrication and biocompatibility of carbon nanotube-based 3D networks as scaffolds for cell seeding and growth. Nano Lett 4:2233–2236 [DOI]
- El Ghalbzouri A, Lamme EN, van Blitterswijk C, Koopman J, Ponec M (2004) The use of PEGT/PBT as a dermal scaffold for skin tissue engineering. Biomaterials 25:2987–2996 [DOI] [PubMed]
- Firkowska I, Olek M, Pazos-Peréz N, Rojas-Chapana J, Giersig M (2006) Highly ordered MWNT-based matrixes: topography at the nanoscale conceived for tissue engineering. Langmuir 22:5427–5434 [DOI] [PubMed]
- Gabay T, Jakobs E, Ben-Jacob E, Hanein Y (2005) Electro-chemical properties of carbon nanotube based multi-electrode arrays. Physica A 350:611–621 [DOI] [PubMed]
- Grinnell F, Bennett MH (1981) Fibroblast adhesion on collagen substratain the presence and absence of plasma fibronectin. J Cell Sci 48:19–34 [DOI] [PubMed]
- Kim Y, Hong S, Jung S, Strano M.S, Choi J, Baik S. (2006) Dielectrophoresis of surface conductance modulated single-walled carbon nanotubes using catanionic surfactants. Phys Chem B 110:1541–1545 [DOI] [PubMed]
- Lu Q, Ganesan K, Siminescu DT, Ryavahare NR (2004) Tissue engineering of blood vessels: characterization of smooth-muscle cells for culturing on collagen-and-elastin-based scaffolds. Biomaterials 25:5227–5237 [DOI] [PubMed]
- Manwaring ME, Walsh JF, Tresco PA (2004) Contact guidance induced organization of extracellular matrix. Biomaterials 25:3631–3638 [DOI] [PubMed]
- Meinel L, Karageorgiou V, Hofmann S, Fajardo R, Snyder B, Li C, Zichner L, Langer R, Vunjak-Novakovic G, Kaplan DL (2004) Engineering bone-like tissue in vitro using human bone marrow stem cells and silk scaffolds. J Biomed Mater Res 71A:25–34 [DOI] [PubMed]
- Meng J, Song L, Meng J, Kong H, Zhu G, Wang C, Xu L, Xie S, Xu H (2006) Using single-walled carbon nanotubes nonwoven films as scaffolds to enhance long-term cell proliferation in vitro. J Biomed Mater Res 79A:298–306 [DOI] [PubMed]
- Palsson BO, Bhatia SN (2003) Tissue engineering. CRC Press, Boca Raton
- Petersen W, Tillmann B (1999) Structure and vascularization of the cruciate ligaments of the human knee joint. Anat Embryol 200:325–334 [DOI] [PubMed]
- Pohl HA (1978) Dielectrophoresis. Cambridge University Press, Cambridge
- Yamamoto K, Akita S, Nakayama Y (1998) Orientation and purification of carbon nanotubes using ac electrophoresis. J Phys D Appl Phys 31:L34–L36 [DOI]
- Zanello LP, Zhao B, Hu H, Haddon RC (2006) Bone cell proliferation on carbon nanotubes. Nano Lett 6:562–567 [DOI] [PubMed]