Summary
Previous studies in Drosophila have shown that heparan sulfate proteoglycans (HSPGs) are involved in both breathless (btl)- and heartless (htl)-mediated FGF signaling during embryogenesis. However, the mechanism(s) by which HSPGs control Btl and Htl signaling is unknown. Here we show that dally-like (dlp, a Drosophila glypican) mutant embryos exhibit severe defects in tracheal morphogenesis and show a reduction in btl-mediated FGF signaling activity. However, htl-dependent mesodermal cell migration is not affected in dlp mutant embryos. Furthermore, expression of Dlp, but not other Drosophila HSPGs, can restore effectively the tracheal morphogenesis in dlp embryos. Rescue experiments in dlp embryos demonstrate that Dlp functions only in Bnl/FGF receiving cells in a cell-autonomous manner, but is not essential for Bnl/FGF expression cells. To further dissect the mechanism(s) of Dlp in Btl signaling, we analyzed the role of Dlp in Btl-mediated air sac tracheoblast formation in wing discs. Mosaic analysis experiments show that removal of HSPG activity in FGF-producing or other surrounding cells does not affect tracheoblasts migration, while HSPG mutant tracheoblast cells fail to receive FGF signaling. Together, our results argue strongly that HSPGs regulate Btl signaling exclusively in FGF-receiving cells as co-receptors, but are not essential for the secretion and distribution of the FGF ligand. This mechanism is distinct from HSPG functions in morphogen distribution, and is likely a general paradigm for HSPG functions in FGF signaling in Drosophila.
Keywords: FGF, Heparan sulfate proteoglycans, Dally, Dally-like (Dlp), Trachea, Air sac tracheoblast, Signaling, Morphogen
Introduction
During metazoan development, the formation of complex body pattern is controlled by a handful of secreted signaling molecules, including members of the Wnt, Hedgehog (Hh), transforming growth factor-β (TGFβ) and fibroblast growth factor (FGF) families. FGFs comprise a large family of proteins that participate in numerous developmental and physiological processes including patterning, cell migration and proliferation (Coumoul and Deng, 2003; Itoh and Ornitz, 2004; Ornitz and Itoh, 2001; Thisse and Thisse, 2005). In Drosophila, two FGF receptors, Heartless (Htl) and Breathless (Btl), are expressed in distinct patterns and mediate different developmental events during embryogenesis. Htl is expressed in the early embryonic mesoderm where it is required for the dorsolateral migration of mesoderm cells following gastrulation (Beiman et al., 1996; Gisselbrecht et al., 1996; Michelson et al., 1998; Shishido et al., 1993; Shishido et al., 1997). Both pyramus and thisbe are identified as genes encoding the FGF ligands for Htl (Gryzik and Muller, 2004; Stathopoulos et al., 2004). btl is specifically expressed in tracheal cells (Klambt et al., 1992; Shishido et al., 1993), which migrate toward clusters of cells expressing the FGF ligand Branchless (Bnl). Bnl functions as a motogen and guidance molecule for tracheal cell migration during embryogenesis (Sutherland et al., 1996). It is currently unknown whether Bnl forms a gradient and whether such a presumptive gradient is essential for guiding the migration of tracheal cells (Affolter and Weijer, 2005).
Recently, Sato and Kornberg has characterized another Bnl/Btl-mediated event, the development of the air sac of the dorsal thorax (Sato and Kornberg, 2002). The air sac precursor cells, “the tracheoblasts”, develop just before metamorphosis and will ultimately serve the adult organism. In this system, Bnl is expressed in the columnar epithelia of wing imaginal discs, where it acts as a chemoattractant to guide the migration of the air sac tracheoblasts on the top of columnar epithelia (Sato and Kornberg, 2002). Further study showed that FGF signaling is essential for the leading tracheal cells, as cells defective in FGF signaling are excluded from the tip of the air sac (Cabernard and Affolter, 2005). Consistent with this, recent genetic mosaic analysis showed that Btl activity is also required for guiding the migration of the leading tracheal cells during larva tracheal development (Ghabrial and Krasnow, 2006). Drosophila embryonic tracheal branching occurs only after cell division ceases. However, air sac tracheoblasts develop and proliferate at the same time. Since mosaic clonal analysis can be used effectively in the imaginal discs, the air sac in the wing disc provides an excellent model system to dissect further the mechanism(s) of Bnl/Btl mediated tracheoblasts formation (Cabernard et al., 2004).
It has long been appreciated that FGF signaling is facilitated by heparan sulfate proteoglycans (HSPGs) (Ornitz, 2000). HSPGs are cell-surface and extracellular matrix (ECM) molecules composed of a protein core to which heparan sulfate (HS) glycosaminoglycan (GAG) chains are attached (Bernfield et al., 1999; Esko and Selleck, 2002; Hacker et al., 2005; Lin, 2004). HSPGs are implicated in many developmental signaling pathways both in Drosophila and in vertebrates (Lin, 2004). However, the mechanistic functions of HSPGs in these signaling pathways are not well-understood. In Drosophila, there are two glypicans (Division abnormally delayed (Dally) and Dally-like (Dlp)) (Baeg et al., 2001; Khare and Baumgartner, 2000; Nakato et al., 1995), one syndecan (Sdc) (Spring et al., 1994) and one perlecan (Terribly reduced optic lobes (Trol)) (Datta and Kankel, 1992; Voigt et al., 2002). Glypicans and syndecans are linked to the plasma membrane by a glycosylphosphatidylinositol (GPI) anchor or a transmembrane domain, respectively. Perlecans are secreted HSPGs that are mainly distributed in the ECM. Previously, we have shown that both Htl- and Btl-mediated signaling events are defective in embryos mutant for sugarless (sgl) and sulfateless (sfl), which encode two enzymes required for the biosynthesis of HSPG GAG chains (Hacker et al., 1997; Lin et al., 1999). This study provides the first in vivo evidence for a role of HSPGs in FGF signaling during development. However, two important questions remained to be addressed. First, which HSPG core protein is involved in Htl- and Btl- mediated FGF signaling? Among four Drosophila HSPGs, the glypicans Dally and Dlp are best characterized and are essential for signaling activities of morphogens including Wingless (Wg), Hh and Decapentaplegic (Dpp) (Hacker et al., 2005; Lin, 2004). It is currently unknown whether they are also required for FGF signaling. Second, as HSPGs control Wg, Hh and Dpp signaling mainly by regulating the distribution of these secreted molecules, it is important to determine whether HSPGs control FGF-dependent processes by similar mechanisms. This is a particularly interesting question as FGF functions as an extracellularly diffusible and/or matrix-bound guidance cues whose gradient might be essential for the directionality of tracheal morphogenesis.
In this study, we have further defined the molecular mechanisms of HSPG-mediated FGF signaling in Drosophila. Our analyses demonstrate that Dlp is essential for Btl-mediated tracheal development, but is not critical for Htl signaling. We further explore the mechanism of Dlp function in FGF signaling by rescue experiments in dlp mutant embryos and by mosaic analysis in air sac system. To our surprise, we found that Dlp controls Btl signaling mainly in FGF-receiving cells, but not in FGF-producing cells or its surrounding tissues. This mechanism of HSPG activity in FGF signaling differs from its role in morphogen signaling. Our new findings favour a model in which HSPGs act as co-receptors to facilitate FGF/FGFR interaction and/or stabilization in FGF-receiving cells.
MATERIALS AND METHODS
Drosophila strains
The following Drosophila mutant strains were used: dally80, dallyP2, dlpA187, htlAB42, sfll(3)03844, wgIG22, btlLG19, bnlP1, bnlP2, 1-eve-1. dally80, dlpA187 (Han et al., 2004b), htlAB42 (Gisselbrecht et al., 1996), sfll(3)03844 (Lin and Perrimon, 1999), wgIG22 (van den Heuvel et al., 1993), btlLG19 (Klambt et al., 1992), bnlP1 (Sutherland et al., 1996) are null alleles. dallyP2 and bnlP2 are enhancer-trap lacZ lines used to visualize dally and bnl expression (Lin and Perrimon, 1999; Sutherland et al., 1996). 1-eve-1 is a trachealess enhancer-trap line used to visualize tracheal cells. The following UAS and Gal4 lines were used for ectopic expression: UAS-dlp (Baeg et al., 2001), UAS-dally (Franch-Marro et al., 2005), UAS-syndecan (Johnson et al., 2004), UAS-perlecan (Voigt et al., 2002), UAS-dlp-GFP (Han et al., 2004b), UAS-btl-GFP (Sato and Kornberg, 2002), UAS-GFP (flybase), UAS-CD8-GFP (Lee and Luo, 1999); btl-Gal4 (Shiga et al., 1996), bnl-Gal4 (Kamimura et al., 2006), 69B-Gal4 (Brand and Perrimon, 1993), sim-Gal4 (Golembo et al., 1996), paired-Gal4 (Yoffe et al., 1995), tubP-Gal80 (Lee and Luo, 1999).
Generation of germline clones and marked disc clones
Females with germline clones were generated by the autosomal FLP-DFS technique (Chou and Perrimon, 1996). Negatively marked clones of mutant cells in the wing disc were generated by the FLP-FRT method (Golic, 1991; Xu and Rubin, 1993) and induced by subjecting first- or second-instar larvae to a heat shock at 37°C for 1 hour. Mutation in Minute on chromosome 3L was used to generate large clones of cells mutant for dlp, dally-dlp and sfl in disc columnar epithelial cells (Han et al., 2005). Positively marked clones of mutant cells in trachea were generated by the MARCM system (Lee and Luo, 1999). Mutant alleles were crossed to the MARCM strain (y w hsp70-flp; btl-gal4, UAS-CD8-GFP/CyO; tubP-gal80 FRT2A/TM6B); 2- to 10-hr-old embryos were heat shocked at 37 °C for 1.5 hour. Below, we list the genotypes used in our analysis.
Germline clones (Fig 1)
Figure 1. Dlp is required for bnl/btl-dependent tracheal cell migration during embryogenesis.

All embryos are oriented anterior to the left.
(A-D, H-I) Lateral views of stage15 embryos immunostained with 2A12 antibody. Insets show higher magnification of a single typical tracheal metamere. (A) Wild-type tracheal pattern. In maternal/zygotic null dlp embryo (B), virtually no tracheal branching occurs. This defect is completely paternally rescued (C). (D) bnl shows strong genetic interaction with dlp. One copy of bnl mutation can lead to strong tracheal defects combined with dlp maternal mutation. In wild-type embryos, overexpression of UAS-bnl by btl-Gal4 generates masses of fine branches (H). This phenotype is markedly suppressed in maternal/zygotic dlp mutant background (I).
(E-F) Lateral views of stage 11 embryos stained with the diphospho-MAPK-specific antibody. The strong expression observed in wild-type (E) tracheal pits is markedly reduced in the corresponding positions in maternal/ zygotic null dlp embryos (F).
(G) β-Gal antibody staining of stage 11 maternal/ zygotic dlp mutant embryos, which contain 1-eve-1. Tracheal pits form normally with respect to size and position in these mutant embryos.
(J-M) Ventral views of stage 9 embryos immunostained for Twi expression. Unlike in htl mutant embryos (K), these Twi-positive mesodermal cells migrate normally in maternal/ zygotic null dlp (L) or dally-dlp (M) mutant embryos.
dlpA187 germline clones: y w hsp70-flp; dlpA187 FRT2A/ P[OvoD1]FRT2A x dlpA187 FRT2A / TM6B dally80 dlpA187 germline clones: y w hsp70-flp; dally80 dlpA187 FRT2A/ P[OvoD1]FRT2A x dally80dlpA187 FRT2A / TM6B
trachealess expression in dlpA187mutant embryo: y w hsp70-flp; dlpA187 FRT2A/ P[OvoD1]FRT2A x dlpA187 FRT2A 1-eve-1/ TM6B
dlpA187genetic interaction with bnlP1: y w hsp70-flp; dlpA187 FRT2A/ P[OvoD1]FRT2A x bnlP1 / TM3 bnl expression in dlpA187mutant embryo: y w hsp70-flp; dlpA187 FRT2A/ P[OvoD1]FRT2A x dlpA187 FRT2A bnlP2/ TM6B
Overexpression of bnl in dlpA187mutant embryo: y w hsp70-flp; dlpA187 btl-Gal4 FRT2A/P[OvoD1]FRT2A x dlpA187 FRT2A UAS-bnl/ TM6B
For rescue experiments (Fig 2):
Figure 2. Rescue of dlp mutant embryos by ectopic expression of UAS-dlp in different domains or by other HSPG core proteins.

(A-L) Lateral view of stage 15 embryos stained with the tracheal lumenal antibody 2A12.
(A-D, F-G) dlp mutant embryos are rescued by ectopic expression of UAS-dlp in whole ectoderm cells (69B-Gal4) (A), FGF expression cells (bnl-Gal4) (B), tracheal cells (btl-Gal4) (C), both FGF expression and tracheal cells (D), ventral midline cells (sim-Gal4) (F), or ectoderm in every other segment (prd-Gal4) (G). Ectopic expression in whole ectoderm can almost completely rescue dlp embryos (A). Expressions in FGF expression cells or ventral midline cells fail to rescue (B, F). btl-Gal4 rescued embryos develop an extensive tracheal network which has an abnormal pattern (C). This is possibly due to segmentation defect associated with other signaling pathways. In fact, this phenotype is similar to that in Wg mutant embryos (E). Embryos rescued by both btl-Gal4 and bnl-Gal4 are similar to those rescued by btl-Gal4 alone (D). Ectopic expression in ectoderm of every other segment can rescue most of the tracheal defect with alternative truncations in dlp embryos (G). This phenotype is very similar to that in btl mutant embryos rescued by prd-Gal4/UAS-btl-GFP (I). (J-L) dlp mutant embryos ectopically expressing UAS-dally (J), UAS-syndecan (K) and UAS-perlecan (L) by prd-Gal4. None of these HSPG core proteins is able to rescue dlp embryos compared to Dlp expression (G).
(M-N”’) Stage 11 (M-M”’) and 13 (N-N”’) dlp embryos rescued by prd-Gal4/UAS-dlp stained for trachealess-lacZ (M, N), Dlp (M’, N’) and diphospho-MAPK (M”’, N”’). The first two channels are merged in M” and N” to indicate overlapping region (arrows).
btlLG19 prd-Gal4 /TM6B x btlLG19 UAS-btl-GFP / TM6B
y w hsp70-flp; dlpA187 btl-Gal4 FRT2A/ P[OvoD1]FRT2A x dlpA187 UAS-dlpFRT2A / TM6BAZ
y w hsp70-flp; dlpA187 bnl-Gal4 FRT2A/ P[OvoD1]FRT2A x dlpA187 UAS-dlpFRT2A / TM6BAZ
y w hsp70-flp; dlpA187 bnl-Gal4 FRT2A/ P[OvoD1]FRT2A x btl-Gal4-UAS-CD8-GFP/+; dlpA187 UAS-dlpFRT2A /+
y w hsp70-flp; dlpA187 UAS-dlp FRT2A/ P[OvoD1]FRT2A x dlpA187 69B-Gal4 FRT2A / prd-Gal4 UAS-GFP
y w hsp70-flp; dlpA187FRT2A/ P[OvoD1]FRT2A x sim-Gal4-UAS-dlp/+; dlpA187 UAS-eGFP /+
y w hsp70-flp; dlpA187 UAS-dlp FRT2A/ P[OvoD1]FRT2A x dlpA187 prd-Gal4 FRT2A / TM6B
MAPK staining: y w hsp70-flp; dlpA187 prd-Gal4 FRT2A/ P[OvoD1]FRT2A x UAS-dlp/+; dlpA187 FRT2A 1-eve-1/+
y w hsp70-flp; dlpA187 prd-Gal4 FRT2A/ P[OvoD1]FRT2A x UAS-dallyflag /+; dlpA187 UAS-eGFP FRT2A / +
y w hsp70-flp; dlpA187 prd-Gal4 FRT2A/ P[OvoD1]FRT2A x UAS-syndecan /+; dlpA187 UAS-eGFP FRT2A / + y w hsp70-flp; dlpA187 prd-Gal4 FRT2A/ P[OvoD1]FRT2A x UAS-perlecan /Y; dlpA187 UAS-eGFP FRT2A / +
Wing disc clones mutant for dally-dlp or sfl marked by absence of GFP (Fig 4)
Figure 4. HSPGs are dispensable in FGF/Bnl producing cells and surrounding cells.
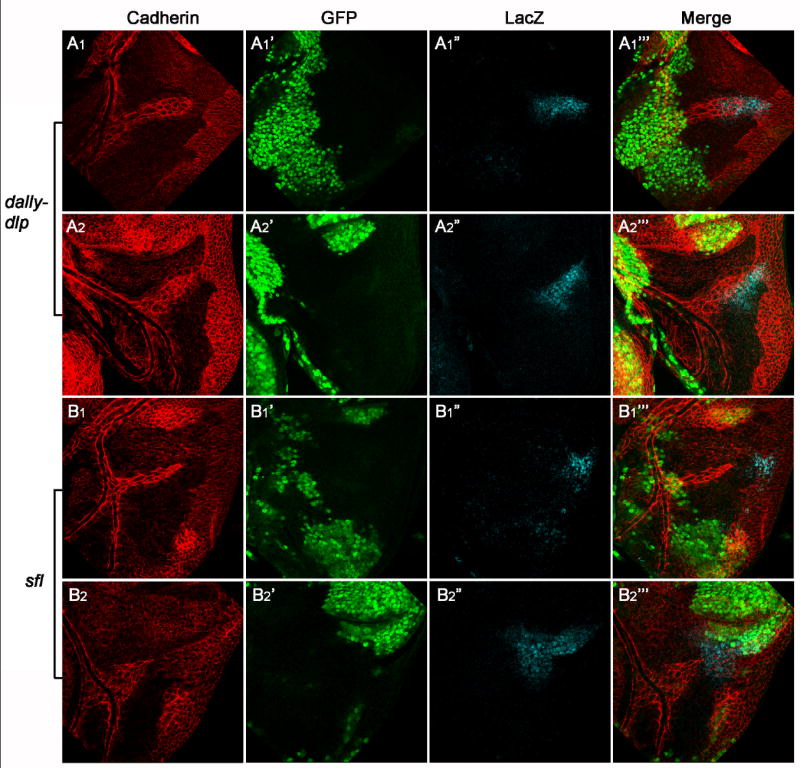
All wing discs are oriented ventral up, anterior to the left. All mutant clones are generated in the columnar epithelial layer, which are marked by the absence of GFP (the second column). E-Cadherin staining (the first column) is used to outline migrating air sac tracheoblasts. bnl expression is demonstrated by β-Gal staining (the third column) utilizing a bnl-lacZ line. The E-Cadherin staining images are taken at the tracheoblast layer, while the GFP and β-Gal staining pictures are taken at the columnar epithelial layer. Different focal planes are merged in the fourth column. For dally-dlp or sfl, two representative mutant clones are shown. Big dally-dlp (A1-A2”’) or sfl (B1-B2”’) mutant clones in columnar epithelial cell layer do not interfere tracheoblast cell migration. bnl expression is also not affected in these mutant clones.
y w hsp70-flp/ y w hsp70-flp; hsp70-Myc-GFP M(3)i55 FRT2A/ dally80 dlpA187 FRT2AbnlP2
y w hsp70-flp/ y w hsp70-flp; hsp70-Myc-GFP M(3)i55 FRT2A/ sfll(3)03844FRT2AbnlP2
Tracheal clones mutant for dlp, dally-dlp, sfl or wild type clones marked by the presence of GFP (Fig.5)
Figure 5. Both dally and dlp are essential in FGF/Bnl receiving cells.

All mutant clones are generated in the air sac tracheoblast cells. The clones are positively marked by CD8-GFP; the entire air sac is outlined with E-Cadherin staining.
(A1-A3) Three representative wild-type clones, which are located in the tip region of migrating tracheoblasts (the most distal part of air sac).
(B1-B3) Three representative dlp mutant clones located in the tip region of migrating tracheoblasts.
(C1-C3) Three representative dally mutant clones located in the tip region of migrating tracheoblasts.
(D1-D3) Three representative dally-dlp mutant clones. These clones never reach the tip of air sac.
(E1-E3) Three representative dally-dlp mutant clones rescued by UAS-dlp. They can localize in the tip region of migrating tracheoblasts.
(F1-F3) Three representative sfl mutant clones. These clones never reach the tip of air sac.
(G) Statistic data demonstrate ratio of clones that contribute to the tip of air sac. Among 45 Wild-type clones, 33% contribute to the tip region. Among 58 dlp mutant clones, 38% contribute to the tip region. Among 39 dally mutant clones, 25% contribute to the tip region. Among 45 dally-dlp or 30 sfl mutant clones, none of them reach the tip region. Among 38 dally-dlp mutant clones rescued by UAS-dlp, 24% contribute to the tip region.
y w hsp70-flp/ y w hsp70-flp; btl-gal4 UAS-CD8-GFP/+; tubP-gal80 FRT2A/ FRT2A
y w hsp70-flp/ y w hsp70-flp; btl-gal4 UAS-CD8-GFP/+; tubP-gal80 FRT2A/ dlpA187 FRT2A
y w hsp70-flp/ y w hsp70-flp; btl-gal4 UAS-CD8-GFP/+; tubgal80 FRT2A/ dally80 FRT2A
y w hsp70-flp/ y w hsp70-flp; btl-gal4 UAS-CD8-GFP/+; tubP-gal80 FRT2A/ dally80 dlpA187 FRT2A
y w hsp70-flp/ y w hsp70-flp; btl-gal4 UAS-CD8-GFP/UAS-dlp; tubP-gal80 FRT2A/ dally80 dlpA187 FRT2A
y w hsp70-flp/ y w hsp70-flp; btl-gal4 UAS-CD8-GFP/+; tubP-gal80 FRT2A/ sfll(3)03844 FRT2A
Immunostainings
Fixation of embryos and imaginal discs as well as antibody staining procedure were performed as described (Belenkaya et al., 2002; Hacker et al., 1997; Han et al., 2004a). Primary antibodies were used at the following dilutions: mouse anti-tracheal lumen antibody mAb2A12 (1:5) (Iowa Developmental Studies Hybridoma Bank; IDSHB), mouse anti-diphospho-MAPK (1:200) (Sigma), rabbit anti-Twist (1:1000) (Michelson et al., 1998), rabbit anti-Dlp (1:200) (Baeg et al., 2001), rat anti-E-Cadherin (1:5) (IDSHB), rabbit anti-β-Gal (1:500) (Cappel), mouse anti-β-Gal (1:3000) (Roche), chicken anti-β-Gal (1:1000) (Abcam), rabbit anti-GFP Alexa Fluor 488 (1:1000) (Molecular Probes), mouse anti-GFP (1:200) (Chemicon), mouse anti-Dlp (1:50)(Lum et al., 2003), rabbit anti-Sal (1:150)(Belenkaya et al., 2004). The primary antibodies were detected by fluorescent- conjugated secondary antibodies from Jackson ImmunoResearch Laboratories or ABC kit from Vectastain.
Results
Dlp is required for Btl-dependent tracheal development during embryogenesis
To dissect the molecular mechanisms by which HSPGs control FGF signaling in Drosophila, we first asked which HSPG is involved in Htl- and Btl-mediated signaling. As dlp mutant embryos are embryonic lethal (Han et al., 2004b), we suspected that Dlp may be involved in FGF signaling during embyogenesis. Therefore, we analyzed tracheal branching and mesodermal migration phenotypes associated with dlp null mutant embryos. dlp homozygous mutant embryos derived from females lacking maternal dlp activity (referred to as dlp embryos hereafter) were generated by “FLP-DFS” technique (Chou and Perrimon, 1996). To visualize Drosophila embryonic tracheal system, we used a monoclonal antibody 2A12 which recognizes tracheal lumen. We observed severe tracheal branching defects in dlp embryos (Fig. 1B). This defect is very similar to those observed in embryos mutant for bnl, btl (Fig. 2H), sgl or sfl (Klambt et al., 1992; Lin et al., 1999; Sutherland et al., 1996), suggesting that Dlp is the main HSPG required for Btl signaling during tracheal development. The following three lines of evidence further support our hypothesis. First, tracheal defect associated with dlp embryos is completely paternally rescuable as one copy of paternal wild-type dlp can rescue the tracheal defect associated with dlp embryos (Fig. 1C). However, when the paternal chromosome contains a bnl mutation, multiple truncations and branching defects were observed in the trachea of these embryos, suggesting that dlp shows strong genetic interaction with bnl (Fig. 1D). Second, Btl signaling is required for activation of MAPK in the tracheal pits at stage 11 (Gabay et al., 1997a; Gabay et al., 1997b). Btl-dependent MAPK activity visualized by diphospho-MAPK-specific antibody staining is shown in ten tracheal pits (T1-A7) in a wild-type embryo at stage 11 (Fig. 1E). This Btl-dependent MAPK activity is strikingly reduced in dlp embryos (Fig. 1F). The reduced Btl-dependent MAPK activity is not due to lack of tracheal anlagen, as tracheal anlagen determined by the enhancer trap fly line 1-eve-1 is normal in dlp embryos (Fig. 1G) (Perrimon et al., 1991). Finally, in wild-type embryos, ectopic expression of bnl in tracheal cells hyperactivates Btl signaling, leading to a marked increase in fine tracheal branching (Fig. 1H). This effect is suppressed in dlp embryos (Fig. 1I). This data also suggests that Dlp is required in FGF-receiving cells. It is worthwhile to note that Bnl expression is not defective in dlp embryos (see Figure S1 in the supplemental materials). In addition, dlp embryos exhibited more severe morphological defects than btl or bnl mutant embryos. This is due to patterning defects associated with Wg and Hh signalling as Dlp is required for Hh and Wg signalling during embryogenesis (Baeg et al., 2001; Desbordes and Sanson, 2003; Franch-Marro et al., 2005; Han et al., 2004b; Kirkpatrick et al., 2004). Taken together, our results argue that Dlp is essential for Bnl/Btl-dependent FGF signaling during embryonic tracheal development.
We further asked whether Dlp is involved in mesodermal cell migration controlled by Htl which requires HSPGs for its signaling (Lin et al., 1999). Mesodermal cell migration can be visualized by Twist (Twi) staining. After invagination through the ventral furrow at gastrulation, Twi-positive mesodermal cells migrate along the ectoderm in a dorsolateral direction (Fig. 1J). In htl mutant embryos, mesoderm migration does not occur properly, resulting in an irregular pattern of Twi-positive cells in the dorsal margin (Fig. 1K) (Beiman et al., 1996; Gisselbrecht et al., 1996; Michelson et al., 1998). Interestingly, Twi-positive cells have an even distribution of migrating margins, suggesting that mesodermal cell migration is normal in dlp embryos (Fig. 1L). Similarly, mesodermal cell migration is normal in dally-dlp double mutant embryos, which are derived from females with germline clones (Fig. 1M). These data argue that neither Dally nor Dlp is required in Htl-dependent mesodermal cell migration. Alternatively, Dally and Dlp may be redundant with Syndecan or Perlecan in Htl signaling.
Mechanism of Dlp function in FGF signaling during embryonic tracheal development
To further determine the mechanism and specificity of Dlp in FGF signaling, we performed three series of rescue experiments. First, we asked which cells require Dlp in FGF signaling. To address this question, we ectopically expressed a transgene dlp in different domains to rescue dlp embryos by the Gal4-UAS system (Brand and Perrimon, 1993). When UAS-dlp is expressed in the whole embryonic ectoderm by 69B-Gal4 (for all Gal4 expression pattern, see Figure S2 in the supplemental materials) in dlp embryos, the tracheal defect is almost completely rescued (Fig. 2A). However, when UAS-dlp is induced in FGF expression cells by bnl-Gal4, almost no rescue was observed compared to dlp embryos (Fig. 2B). Interestingly, when UAS-dlp is only expressed by btl-Gal4 in FGF receiving cells, the tracheal cells, we observed significant rescue of the tracheal defect (Fig. 2C). Furthermore, when we provide UAS-dlp in both FGF expression and receiving cells by a combination of bnl-Gal4 and btl-Gal4, the tracheal phenotype appears very similar to that rescued by btl-Gal4 alone (Fig. 2D). These data together argue that Dlp is only required in FGF receiving cells, but not in FGF producing cells. The rescue is not complete probably due to patterning defect in these embryos, as Dlp is also required for signaling activities of Wg and Hh (Baeg et al., 2001; Desbordes and Sanson, 2003; Franch-Marro et al., 2005; Han et al., 2004b; Kirkpatrick et al., 2004). In fact, the btl-Gal4 rescued tracheal phenotype is similar to that in a Wg mutant embryo (Fig. 2E). In particular, the dorsal trunk tracheal cells are less rescued in these embryos (Fig. 2C, D). This is likely due to a reduced Spalt (Sal) expression in these embryos (See Figure S3 in the supplemental materials) as Sal expression is regulated by Wg signaling and is required for dorsal trunk tracheal development (Kuhnlein and Schuh, 1996; Llimargas, 2000).
Above experiments suggest a role of Dlp in tracheal cells for FGF signaling. Next, we asked whether Dlp functions cell-autonomously in FGF signaling as Dlp acts non-autonomously over a long range in Hh signaling in embryo (unpublished data). The following three experiments argue that Dlp is required autonomously in tracheal cells. First, the tracheal defect can not be rescued by ectopic expression of UAS-dlp in ventral midline cells by singleminded-Gal4 (sim-Gal4), which have certain distance from tracheal cells (Fig. 2F). Second, when UAS-dlp is expressed by paired-Gal4 (prd-Gal4), which is expressed in every other segment, the MAPK activity can only be restored autonomously in tracheal cells which overlap with Dlp expression domain (Fig. 2M-M”’). Third, the tracheal phenotype rescued by prd-Gal4 /UAS-dlp (Fig. 2G) is almost identical to those in btl mutant embryos background rescued by prd-Gal4 /UAS-btl-GFP (Fig. 2H-I), suggesting Dlp functions similarly to Btl as a co-receptor. Interestingly, the rescue of dlp or btl by expression of prd-Gal4 /UAS-dlp or UAS-btl-GFP is quite complete, even in tracheal pits which do not overlap significantly with prd-Gal4 expression domains. We took a closer look at those embryos and found that in stage 13 the even-numbered tracheal trees overlap significantly with prd-Gal4 expression domain (T2 and A1 in Fig. 2N-N”’). Although the main body of odd-numbered tracheal trees do not lie under the prd-Gal4 expression domain, the tips of these tracheal trees can still overlap with the prd domain and restore MAPK activity (T3 in Fig. 2N-N”’). From these findings, we conclude that Dlp functions autonomously in tracheal cells and it appears to be required in just a few tip cells.
Finally, we asked whether ectopic expression of other HSPGs can restore tracheal defect in dlp embryos. UAS-dally, UAS-syndecan or UAS-perlecan are ectopically expressed by prd-Gal4. In striking contrast with dlp expression, none of these HSPGs can rescue the tracheal defects associated with dlp embryos (Fig. 2J-L). Similarly, ectopic expression of these HSPGs by btl-Gal4 in tracheal cells also fails to restore tracheal defects (data not shown). These data demonstrate that Dlp can provide specific activity for Btl signaling during embryogenesis.
Both Dlp and Dally are up-regulated in the air sac tracheoblast cells of the wing disc
Our embryonic data suggest that HSPGs are only required in FGF receiving cells as co-receptors, we further ask if this is a general paradigm in other FGF mediated events in Drosophila. Recent studies demonstrated that Bnl/Btl signaling is essential for the formation of the air sac tracheoblasts in the wing disc (Cabernard and Affolter, 2005; Sato and Kornberg, 2002). In this system, Btl is expressed in tracheoblast cells while Bnl is expressed in the underlying columnar epithelial cells acting as a chemoattractant to induce the migration of tracheoblasts (Fig. 3A) (Sato and Kornberg, 2002). As mosaic mutant clones can be induced independently in tracheoblast cells and their underlying columnar epithelial cells, the wing air sac system provides an excellent model system to further define the functions of HSPGs in tracheal cells or in underlying columnar epithelial cells.
Figure 3. Both dlp and dally are expressed in the air sac tracheoblasts.

(A) Schematic drawing of wing imaginal disc and associated air sac tracheoblast cells in the late third instar larvae.
(B-B”) Air sac tracheoblasts are outlined by E-Cadherin staining (B). They migrate towards the gradient of Bnl expression, which is shown by β-Gal staining using a bnl-lacZ line (B’). Images from different focal planes are merged in (B”).
(C-C”) Air sac tracheoblasts are outlined by btl-Gal4, UAS-CD8-GFP (C’), and are counterstained with E-Cadherin (C).
(D-D”) Dlp immunostaining shows Dlp is expressed in columnar epithelial cells (D’). (D) shows β-Gal staining using a bnl-lacZ line. The first two channels are merged in (D”) to indicate relative position of Dlp and Bnl expression.
(E-E””) Dlp immunostaining shows that Dlp protein is expressed in the air sac tracheoblasts (E’), which is marked by btl-Gal4, UAS-cytoplasmic GFP (E). In contrast, dlp is not expressed in mesodermal cells (E’), which are outlined by Twist staining (E”). (E) and (E’) are merged in (E”’); all three channels are merged in (E””).
(F) Image of living tracheoblasts which express UAS-dlp-GFP by btl-Gal4. Dlp-GFP is localized in multiple filopodia extending from these cells (arrows).
(G-G”) β-Gal staining using a dally-lacZ line demonstrates that dally is also expressed in the air sac tracheoblasts (F’). Dally expression is absent from mesodermal muscle precursor cells.
We marked the air sac tracheoblasts using UAS-CD8-GFP driven by the btl-Gal4 (Fig. 3C’). We also stained the tracheoblasts by E-Cadherin, the adherent junction marker (Fig. 3B, C). It is worthwhile to note that although these cells are changing their morphology dynamically, they maintain intact polarity as demonstrated by E-Cadherin staining. The expression of Bnl was visualized by LacZ staining utilizing a bnl-LacZ line (Sutherland et al., 1996) (Fig. 3B’, D). From the composite image of E-Cadherin and LacZ staining in two cell layers (Fig. 3B”), we observed Bnl expression in underlying epithelium and tracheoblast formation toward the direction of Bnl expression cells.
As a first step to determine the role of Dlp in this process, we examined dlp expression using an anti-Dlp antibody (Lum et al., 2003). We found that Dlp is expressed in the plasma membrane of the tracheoblast cells (Fig. 3E-E”’). Importantly, a Dlp-GFP fusion protein is distributed in the multiple filopodia extending from the leading air sac cells (Fig. 3F, arrows). The expression pattern of Dlp suggests its possible involvement in reception of FGF. Dlp is also expressed abundantly in the columnar epithelial layer including the Bnl expression cells (Fig. 3D’, D”). In contrast, Dlp is not expressed in Htl-positive muscle precursor cells (Fig. 3E’-E””) which surround tracheal cells in the notum region of the wing discs.
We also examined Dally expression using dallyP2, a dally enhancer trap lacZ line. Dally is also highly expressed in the tracheoblast cells (Fig. 3G’, G”). Interestingly, Dally expression is also absent in muscle cells (Fig. 3G’, G”). The absence of both Dlp and Dally in Htl-expressing cells suggests that they are not involved in Htl signaling in the wing disc. This result is consistent with the embryonic data that Dlp and Dally are not essential for Htl signaling during mesodermal cell migration (Fig. 1J-M).
Removal of HSPGs in FGF producing cells and other surrounding columnar epithelia cells does not affect tracheoblast formation
The wing disc air sac can be used to determine whether HSPGs are required for FGF signaling in tracheal cells (btl expressing cells) or/and their underlying columnar epithelial cells which produce Bnl (Fig.3). We and others have previously shown that HSPGs are essential for the distribution of HSPG-binding morphogen molecules including Wg, Hh and Dpp (Baeg et al., 2004; Belenkaya et al., 2004; Bornemann et al., 2004; Franch-Marro et al., 2005; Han et al., 2004a; Han et al., 2004b; Han et al., 2005; Kirkpatrick et al., 2004; Kreuger et al., 2004; Takei et al., 2004). HSPGs might be essential for the distribution of Bnl and possibly the gradient formation of Bnl in the columnar epithelial cells. Alternatively, HSPGs might be only required for Bnl/Btl signaling in FGF receiving cells, the tracheoblast cells. To distinguish these possibilities, we generated big HSPG mutant clones which cover completely the Bnl expressing cells and their surrounding columnar epithelial cells. First, large clones mutant for dlp (data not shown) or dally-dlp double mutant in the columnar epithelium cells were generated (Fig. 4 A1-A1”’, A2-A2”’). In these clones Bnl expression is not affected as shown by bnl-LacZ (Fig. 4 A1”, A2”). Interestingly, we do not detect any defects in air sac tracheoblast formation even when the mutant clones cover almost the whole notum region (Fig. 4 A1, A2). We also generated large clones mutant for sfl which is required for the GAG biosynthesis of all HSPGs (Lin and Perrimon, 1999). Consistent with the results from dally-dlp clones, tracheoblasts can develop normally on the top of sfl defective columnar epithelium cells including Bnl producing cells (Fig. 4 B1-B2”’). More than 20 big clones of dally-dlp and sfl, which cover the whole Bnl producing cells and their surrounding cells, were examined and virtually identical results were observed. Collectively, these data argue that the presence of HSPGs in underlying columnar epithelial cells including Bnl producing cells is not critical for FGF-mediated air sac tracheoblast formation.
Both Dally and Dlp are required for tracheoblast cells to receive FGF signaling
Next, we asked whether HSPGs are required for FGF signaling in FGF receiving cells, the air sac cells. Our embryonic data suggest that activation of FGF signaling in only a few tip cells can rescue tracheal development quite completely (Fig. 2G, H, I, N-N”’). Consistent with our result, recent studies by others (Cabernard and Affolter, 2005; Ghabrial and Krasnow, 2006) suggested that in larval dorsal branches and in air sac tracheoblasts, FGF signaling is dispensable for all the trailing tracheal cells, but is only required in the leading or tip cells. Tracheal cells defective in FGF signaling are unable to locate at the tip region of the air sac. Thus, we used this ‘no tip cell’ phenotype as a read-out for FGF signaling defect in tracheoblast cells. We generated homozygous mutant clones in the air sac using the MARCM technique, in which only the mutant cells expressing CD8-GFP can be recognized (Lee and Luo, 1999).
First, we induced clones of wild-type cells to examine how wild-type cells behave during air sac formation. As expected, clones of wild-type cells were found in all the locations of the air sac. Among the 45 clones examined, more than 30% of clones contain cells located at the tip of the air sac (Fig. 5A1-A3, G). Next, we generated dlp homozygous mutant clones in air sac cells. Of the 58 clones examined, more than 30% of clones reach the tip region (Fig. 5B1-B3, G). This ratio is similar to wild-type clones suggesting that Dlp is dispensable for the tip air sac cells to respond to FGF signaling. This result is surprising as Dlp is required for embryonic tracheal development and is specifically expressed in air sac cells. We reasoned that Dally might play a redundant role in this circumstance. Therefore, we examined dally or dally-dlp double mutant clones. 25% of dally clones (n=39) are observed in the tip region while none of dally-dlp clones (n=45) is able to locate at the tip region (Fig. 5C1-D3, G). Furthermore, expression of Dlp in dally-dlp mutant clones can rescue this defect (Fig. 5E1-E3, G). These data suggest that both Dally and Dlp are required for the tips air sac cells to response to FGF signaling. We also examined sfl mutant clones and found none of them (n=30) is able to populate the tip region, suggesting that HS chains in Dally and Dlp are essential for FGF signaling (Fig. 5F1-F3, G). It is noteworthy to mention that the size of dally-dlp and sfl mutant clones are not significantly reduced compared to wild-type clones (Fig. 5D1-D3, F1-F3) (see supplemental data S4). These data argue that EGF signaling pathway is not affected in these mutant clones as EGF signaling is important for air sac cell proliferation and survival (Cabernard and Affolter, 2005). Collectively, our results argue strongly that HSPGs are required for air sac precursor cells to relay FGF signals, while they are dispensable in columnar epithelial cells including FGF producing cells. These results also are consistent with our embryonic data, demonstrating that HSPGs regulate FGF signaling in tracheal cells (the FGF-receiving cells) in both systems.
Discussion
There are three main important findings in this work. First, we have identified Dlp as an essential molecule required for tracheal development. Dlp is required for Btl-mediated tracheal branching during embryogenesis while both Dlp and Dally are involved in the formation of air sac tracheoblasts in the wing disc. Second, our data show that other HSPGs cannot replace Dlp for Btl signaling during embryogenesis and that both Dlp and Dally are not essential for Htl-mediated mesodermal cell migration. These data demonstrate that different FGFs may require different HSPGs to execute their effective signaling activities during development. Third and most importantly, we provide strong evidence that Dlp controls Btl signaling only in FGF-receiving cells in both embryonic and larval tracheal systems. This mechanism of HSPG activity in FGF signaling is very different from its roles in regulating the signalling activities of morphogens including Wnt, Hh and Dpp. Together, our new findings further define novel mechanisms and the specificities of HSPGs in FGF signaling during development.
Dlp is the major HSPG involved in Btl-mediated FGF signaling
Extensive biochemical and cell culture studies suggest that HSPGs are the part of the FGF/FGFR signaling complex (Ornitz, 2000; Ornitz and Itoh, 2001). However, the mechanisms of HSPGs in FGF signaling during development are less known. Previously, we have shown that embryos mutant for two HSPG biosynthesis enzymes, sgl and sfl, exhibit defects in both Btl- and Htl-mediated FGF signaling (Lin et al., 1999). An important issue remaining to be solved is which HSPG core proteins are involved in these signaling events. The data in this work provide strong evidence for the first time that Dlp is the key molecule required for Btl signaling during embryonic tracheal development, while both Dlp and Dally are involved in the Btl mediated air sac tracheoblasts formation in the wing disc. Our results provide several novel insights into the specificity of individual HSPG in FGF signaling. First, Dlp is involved in Btl signaling, but not in Htl signaling. These findings indicate that different FGF/FGFR complexes may require different HSPGs for their signaling activities. Second, Dlp is highly active and specific for Btl signaling; overexpression of the other three Drosophila HSPGs fail to rescue tracheal defects in dlp embryos. The specific activity of Dlp in Btl signaling could be due to the Dlp protein core or the HS GAG chains attached to the Dlp core protein. In this regard, it is especially surprising that Dally, which has 22% identity with Dlp and also bears a GPI anchor, cannot rescue tracheal phenotypes associated with dlp embryos. As Dlp is involved in several other signaling pathways such as Hh (Desbordes and Sanson, 2003; Han et al., 2004b), Wg (Baeg et al., 2001; Baeg et al., 2004; Franch-Marro et al., 2005; Han et al., 2005; Kirkpatrick et al., 2004; Kreuger et al., 2004), and Dpp (Belenkaya et al., 2004), it is unlikely that Dlp core protein interacts with the ligands directly. In this regard, it is worthwhile to note that ectopic expression of Dally also fails to rescue Hh signaling in dlp embryos (data not shown). We propose that Dlp may have unique HS GAG chains that might provide high and specific activity for ligands such as Bnl and Hh.
The biosynthesis of HS GAG chains is determined by the HSPG protein core in which the GAG attachment sites and other protein parts such as the N-terminal cystenine-rich domain control both quantity and quality of the attached GAG chains (Esko and Selleck, 2002; Lin, 2004). Detailed structure and functional studies of Dlp will further help to define specific requirements of the core protein or GAG attachment sites in FGF signaling. Furthermore, the unique GAG chains may be modified by specific enzymes. In this regard, it is particularly important to note that 6-O sulfation of HS is critical for Btl signaling, as Drosophila heparan sulfate 6-O-sulfotransferase is specifically expressed in embryonic tracheal system and is required for Btl signaling during embryogenesis (Kamimura et al., 2001). More recent study showed that the overall sulfation level is more important than strictly defined HS fine structures for FGF signaling in some developmental contexts (Kamimura et al., 2006). In this regard, we suggest that Dlp may be the optimal substrate for sulfation enzymes during embryogenesis. Therefore, the activity of Dlp in FGF signaling during embryogenesis cannot be replaced by other HSPGs including Dally, Syndecan and Perlecan.
Although Dlp is essential for Btl signaling during embryogenesis, both Dally and Dlp are involved in Btl signaling in air sac tracheoblast cells. Similarly, our previous studies showed that both Dally and Dlp are involved in regulating Wg, Hh and Dpp distribution in the wing disc (Belenkaya et al., 2004; Han et al., 2004b; Han et al., 2005). The different functions of the same HSPG in embryos and discs may reflect temporal and developmental stage dependent regulation of HSPG functions (Allen and Rapraeger, 2003).
Mechanism(s) of HSPG function in FGF signaling
While it is well established that HSPGs can regulate FGF signaling by facilitating FGF/FGFR interaction (Ornitz, 2000), it is unknown whether HSPGs can also control FGF distribution, thereby modulating FGF signaling. This is a particularly important issue as in many developmental contexts FGF ligand is produced in one type of cell and acts on other cells to initiate its biological activity (Thisse and Thisse, 2005; Zhang et al., 2006). One important finding of this work is that HSPGs control tracheal morphogenesis by regulating FGF signaling only in FGF-receiving cells, but not by regulating the secretion or distribution of FGF ligand in its producing cells and surrounding cells. Several important results support our conclusion: 1. dlp mutant embryos can suppress the phenotype of overexpressing Bnl in the tracheal cells. 2. Ectopic expression of Dlp in tracheal cells, rather than FGF expression cells, can effectively restore tracheal defects associated with dlp embryos. 3. Embryos rescued by prd-Gal4/ UAS-dlp in dlp backbround is very similar to btl mutant embryos rescued by prd-Gal4/ UAS-btl-GFP. 4. HSPGs are required for FGF signaling in its receiving cells in the air sac, but are dispensable in the columnar epithelial layer which includes FGF producing cells and other surrounding cells. Our detailed analyses thus demonstrate for the first time the specific and distinct requirement of HSPGs in FGF signaling during tracheal development. Moreover, our embryonic and larval data together suggest this is likely a general mechanism for HSPG function in FGF signaling in Drosophila.
Two major models are proposed for the role of HSPGs in FGF signaling (Lin, 2004; Ornitz, 2000). In one model, low affinity HS/GAG chains on the cell surface limit the diffusion of FGF ligand, thereby increasing its local concentration and the probability that it will interact with high-affinity FGFRs. In the second model, HSPGs facilitate the dimerization or oligomerization of FGF ligands thereby inducing receptor clustering and signal transduction. Our experimental data cannot exclude either of these mechanisms. However, our results are in favour of the second case as we show that HSPGs are not required in FGF concentration gradient in FGF producing cells, but are essential in FGF-receiving cells. Finally, a recent study showed that dynamin-mediated vesicle internalization is a crucial step to regulate FGF signaling in Drosophila tracheal system (Dammai et al., 2003). Mutants in awd (abnormal wing disc) or shi (shibire), which encodes for a nucleoside diphosphate kinase and Drosophila dynamin, respectively, have increased levels of Btl in tracheal cell surface, increased FGF signaling activity and ectopic tracheal branching. In this regard, HSPGs may control FGF signaling by stabilizing the FGF/FGFR complex from degradation or internalization in FGF receiving cells. Further experiments using HSPG and awd/shi double mutants are needed to test this possibility.
Comparison of HSPG functions in FGF and morphogen signaling
Over the past several years, extensive studies in Drosophila and other model systems have established the essential roles of HSPGs in developmental signaling pathways including Wg, Hh and Dpp (Hacker et al., 2005; Lin, 2004). In Drosophila embryo and wing imaginal disc, HSPGs are involved in the transport of morphogens including Wg, Hh and Dpp by a restricted diffusion mechanism (Belenkaya et al., 2004; Han et al., 2004b; Han et al., 2005; The et al., 1999). Narrow stripes of clones mutant for HSPGs can impede the movement of morphogens to further cells. However, in all of these cases, the first mutant cells adjacent to the morphogen source can still transduce signals arguing that HSPGs are not essential for morphogen signaling activity, but rather control the distributions or local concentrations of morphogens (Belenkaya et al., 2004; Han et al., 2004b; Han et al., 2005). Our novel results from this work point out a major difference for a role of HSPGs in FGF signaling from their roles in morphogen signaling, as removal of HSPGs (dally-dlp or sfl) from FGF receiving cells can effectively block FGF signaling. Although the graded FGF activity may play an essential role in tracheal morphogenesis (Affolter and Weijer, 2005), our data from this work argue that the main function of HSPGs in FGF signaling is not to regulate the distribution of FGF ligand. Consistent with the different roles of HSPGs in FGF and morphogen signaling, we found that Dlp acts cell-autonomously in FGF signaling while it functions non-autonomously in Hh signaling in embryos (Unpublished data). Our results suggest that Bnl transportation may be different from morphogen movement in the epithelial cells of the wing pouch. Indeed, morphogen molecules diffuse through the same layer of cells, columnar epithelial cells, while FGF is transported between different layers of tissues, from columnar epithelia to tracheoblasts. Moreover, leading air sac cells are always in close proximity with underlying columnar epithelia. They also extend multiple filopodia toward ligand gradient and presumably actively pursue the FGF ligands (Sato and Kornberg, 2002) while wing disc morphogens including Wg, Hh and Dpp need to transport many cell diameters from their sources to reach their receiving cells. Studies in vertebrate also suggest that a graded distribution of FGF8 protein can be generated by the decay of fgf8 mRNA and this RNA gradient is translated into a protein gradient (Dubrulle and Pourquie, 2004). In this case, no active transport mechanism is required to form a FGF gradient. In mammalian limb and lung development different FGFs are often expressed in different layers of cells, such as epithelium and mesenchyme, and signal through each other (Thisse and Thisse, 2005; Zhang et al., 2006). It is interesting to determine whether HSPGs function similarly in these systems as in Drosophila.
Supplementary Material
Acknowledgments
We thank M. Krasnow, S. Cohen, J. Vincent, S. Hou, T. Kornberg, M. Sato, H. Nakato and the Bloomington Stock Center for Drosophila stocks; P. Beachy, A. Michelson and the Iowa Developmental Studies Hybridoma Bank (IDSHB) for antibodies; H. Liu for technical assistance. We thank Tanya Belenkaya and Caitlin Maynard for comments on the manuscript. This work was supported partially by NIH grant 2R01GM063891, Research Scholar Grant RSG-07-051 from American Cancer Society, March of Dimes foundation to X. L. D. Y is an Albert J. Ryan fellow and is supported by a predoctoral fellowship from American Heart Association.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Affolter M, Weijer CJ. Signaling to cytoskeletal dynamics during chemotaxis. Dev Cell. 2005;9:19–34. doi: 10.1016/j.devcel.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Allen BL, Rapraeger AC. Spatial and temporal expression of heparan sulfate in mouse development regulates FGF and FGF receptor assembly. J Cell Biol. 2003;163:637–48. doi: 10.1083/jcb.200307053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeg GH, et al. Heparan sulfate proteoglycans are critical for the organization of the extracellular distribution of Wingless. Development. 2001;128:87–94. doi: 10.1242/dev.128.1.87. [DOI] [PubMed] [Google Scholar]
- Baeg GH, et al. The Wingless morphogen gradient is established by the cooperative action of Frizzled and Heparan Sulfate Proteoglycan receptors. Dev Biol. 2004;276:89–100. doi: 10.1016/j.ydbio.2004.08.023. [DOI] [PubMed] [Google Scholar]
- Beiman M, et al. Heartless, a Drosophila FGF receptor homolog, is essential for cell migration and establishment of several mesodermal lineages. Genes Dev. 1996;10:2993–3002. doi: 10.1101/gad.10.23.2993. [DOI] [PubMed] [Google Scholar]
- Belenkaya TY, et al. pygopus Encodes a nuclear protein essential for wingless/Wnt signaling. Development. 2002;129:4089–101. doi: 10.1242/dev.129.17.4089. [DOI] [PubMed] [Google Scholar]
- Belenkaya TY, et al. Drosophila Dpp morphogen movement is independent of dynamin-mediated endocytosis but regulated by the glypican members of heparan sulfate proteoglycans. Cell. 2004;119:231–44. doi: 10.1016/j.cell.2004.09.031. [DOI] [PubMed] [Google Scholar]
- Bernfield M, et al. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem. 1999;68:729–77. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- Bornemann DJ, et al. Abrogation of heparan sulfate synthesis in Drosophila disrupts the Wingless, Hedgehog and Decapentaplegic signaling pathways. Development. 2004;131:1927–38. doi: 10.1242/dev.01061. [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–15. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Cabernard C, Affolter M. Distinct roles for two receptor tyrosine kinases in epithelial branching morphogenesis in Drosophila. Dev Cell. 2005;9:831–42. doi: 10.1016/j.devcel.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Cabernard C, et al. Cellular and molecular mechanisms involved in branching morphogenesis of the Drosophila tracheal system. J Appl Physiol. 2004;97:2347–53. doi: 10.1152/japplphysiol.00435.2004. [DOI] [PubMed] [Google Scholar]
- Chou TB, Perrimon N. The autosomal FLP-DFS technique for generating germline mosaics in Drosophila melanogaster. Genetics. 1996;144:1673–9. doi: 10.1093/genetics/144.4.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coumoul X, Deng CX. Roles of FGF receptors in mammalian development and congenital diseases. Birth Defects Res C Embryo Today. 2003;69:286–304. doi: 10.1002/bdrc.10025. [DOI] [PubMed] [Google Scholar]
- Dammai V, et al. Drosophila awd, the homolog of human nm23, regulates FGF receptor levels and functions synergistically with shi/dynamin during tracheal development. Genes Dev. 2003;17:2812–24. doi: 10.1101/gad.1096903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, Kankel DR. l(1)trol and l(1)devl, loci affecting the development of the adult central nervous system in Drosophila melanogaster. Genetics. 1992;130:523–37. doi: 10.1093/genetics/130.3.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbordes SC, Sanson B. The glypican Dally-like is required for Hedgehog signalling in the embryonic epidermis of Drosophila. Development. 2003;130:6245–55. doi: 10.1242/dev.00874. [DOI] [PubMed] [Google Scholar]
- Dubrulle J, Pourquie O. fgf8 mRNA decay establishes a gradient that couples axial elongation to patterning in the vertebrate embryo. Nature. 2004;427:419–22. doi: 10.1038/nature02216. [DOI] [PubMed] [Google Scholar]
- Esko JD, Selleck SB. Order out of chaos: assembly of ligand binding sites in heparan sulfate. Annu Rev Biochem. 2002;71:435–71. doi: 10.1146/annurev.biochem.71.110601.135458. [DOI] [PubMed] [Google Scholar]
- Franch-Marro X, et al. Glypicans shunt the Wingless signal between local signalling and further transport. Development. 2005;132:659–66. doi: 10.1242/dev.01639. [DOI] [PubMed] [Google Scholar]
- Gabay L, et al. In situ activation pattern of Drosophila EGF receptor pathway during development. Science. 1997a;277:1103–6. doi: 10.1126/science.277.5329.1103. [DOI] [PubMed] [Google Scholar]
- Gabay L, et al. MAP kinase in situ activation atlas during Drosophila embryogenesis. Development. 1997b;124:3535–41. doi: 10.1242/dev.124.18.3535. [DOI] [PubMed] [Google Scholar]
- Ghabrial AS, Krasnow MA. Social interactions among epithelial cells during tracheal branching morphogenesis. Nature. 2006;441:746–9. doi: 10.1038/nature04829. [DOI] [PubMed] [Google Scholar]
- Gisselbrecht S, et al. heartless encodes a fibroblast growth factor receptor (DFR1/DFGF-R2) involved in the directional migration of early mesodermal cells in the Drosophila embryo. Genes Dev. 1996;10:3003–17. doi: 10.1101/gad.10.23.3003. [DOI] [PubMed] [Google Scholar]
- Golembo M, et al. The Drosophila embryonic midline is the site of Spitz processing, and induces activation of the EGF receptor in the ventral ectoderm. Development. 1996;122:3363–70. doi: 10.1242/dev.122.11.3363. [DOI] [PubMed] [Google Scholar]
- Golic KG. Site-specific recombination between homologous chromosomes in Drosophila. Science. 1991;252:958–61. doi: 10.1126/science.2035025. [DOI] [PubMed] [Google Scholar]
- Gryzik T, Muller HA. FGF8-like1 and FGF8-like2 encode putative ligands of the FGF receptor Htl and are required for mesoderm migration in the Drosophila gastrula. Curr Biol. 2004;14:659–67. doi: 10.1016/j.cub.2004.03.058. [DOI] [PubMed] [Google Scholar]
- Hacker U, et al. The Drosophila sugarless gene modulates Wingless signaling and encodes an enzyme involved in polysaccharide biosynthesis. Development. 1997;124:3565–73. doi: 10.1242/dev.124.18.3565. [DOI] [PubMed] [Google Scholar]
- Hacker U, et al. Heparan sulphate proteoglycans: the sweet side of development. Nat Rev Mol Cell Biol. 2005;6:530–41. doi: 10.1038/nrm1681. [DOI] [PubMed] [Google Scholar]
- Han C, et al. Distinct and collaborative roles of Drosophila EXT family proteins in morphogen signalling and gradient formation. Development. 2004a;131:1563–75. doi: 10.1242/dev.01051. [DOI] [PubMed] [Google Scholar]
- Han C, et al. Drosophila glypicans control the cell-to-cell movement of Hedgehog by a dynamin-independent process. Development. 2004b;131:601–11. doi: 10.1242/dev.00958. [DOI] [PubMed] [Google Scholar]
- Han C, et al. Drosophila glypicans Dally and Dally-like shape the extracellular Wingless morphogen gradient in the wing disc. Development. 2005;132:667–79. doi: 10.1242/dev.01636. [DOI] [PubMed] [Google Scholar]
- Itoh N, Ornitz DM. Evolution of the Fgf and Fgfr gene families. Trends Genet. 2004;20:563–9. doi: 10.1016/j.tig.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Johnson KG, et al. Axonal heparan sulfate proteoglycans regulate the distribution and efficiency of the repellent slit during midline axon guidance. Curr Biol. 2004;14:499–504. doi: 10.1016/j.cub.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Kamimura K, et al. Drosophila heparan sulfate 6-O-sulfotransferase (dHS6ST) gene. Structure, expression, and function in the formation of the tracheal system. J Biol Chem. 2001;276:17014–21. doi: 10.1074/jbc.M011354200. [DOI] [PubMed] [Google Scholar]
- Kamimura K, et al. Specific and flexible roles of heparan sulfate modifications in Drosophila FGF signaling. J Cell Biol. 2006;174:773–8. doi: 10.1083/jcb.200603129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khare N, Baumgartner S. Dally-like protein, a new Drosophila glypican with expression overlapping with wingless. Mech Dev. 2000;99:199–202. doi: 10.1016/s0925-4773(00)00502-5. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick CA, et al. Spatial regulation of Wingless morphogen distribution and signaling by Dally-like protein. Dev Cell. 2004;7:513–23. doi: 10.1016/j.devcel.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Klambt C, et al. breathless, a Drosophila FGF receptor homolog, is essential for migration of tracheal and specific midline glial cells. Genes Dev. 1992;6:1668–78. doi: 10.1101/gad.6.9.1668. [DOI] [PubMed] [Google Scholar]
- Kreuger J, et al. Opposing activities of Dally-like glypican at high and low levels of Wingless morphogen activity. Dev Cell. 2004;7:503–12. doi: 10.1016/j.devcel.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Kuhnlein RP, Schuh R. Dual function of the region-specific homeotic gene spalt during Drosophila tracheal system development. Development. 1996;122:2215–23. doi: 10.1242/dev.122.7.2215. [DOI] [PubMed] [Google Scholar]
- Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–61. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- Lin X. Functions of heparan sulfate proteoglycans in cell signaling during development. Development. 2004;131:6009–21. doi: 10.1242/dev.01522. [DOI] [PubMed] [Google Scholar]
- Lin X, et al. Heparan sulfate proteoglycans are essential for FGF receptor signaling during Drosophila embryonic development. Development. 1999;126:3715–23. doi: 10.1242/dev.126.17.3715. [DOI] [PubMed] [Google Scholar]
- Lin X, Perrimon N. Dally cooperates with Drosophila Frizzled 2 to transduce Wingless signalling. Nature. 1999;400:281–4. doi: 10.1038/22343. [DOI] [PubMed] [Google Scholar]
- Llimargas M. Wingless and its signalling pathway have common and separable functions during tracheal development. Development. 2000;127:4407–17. doi: 10.1242/dev.127.20.4407. [DOI] [PubMed] [Google Scholar]
- Lum L, et al. Identification of Hedgehog pathway components by RNAi in Drosophila cultured cells. Science. 2003;299:2039–45. doi: 10.1126/science.1081403. [DOI] [PubMed] [Google Scholar]
- Michelson AM, et al. Dual functions of the heartless fibroblast growth factor receptor in development of the Drosophila embryonic mesoderm. Dev Genet. 1998;22:212–29. doi: 10.1002/(SICI)1520-6408(1998)22:3<212::AID-DVG4>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Nakato H, et al. The division abnormally delayed (dally) gene: a putative integral membrane proteoglycan required for cell division patterning during postembryonic development of the nervous system in Drosophila. Development. 1995;121:3687–702. doi: 10.1242/dev.121.11.3687. [DOI] [PubMed] [Google Scholar]
- Ornitz DM. FGFs, heparan sulfate and FGFRs: complex interactions essential for development. Bioessays. 2000;22:108–12. doi: 10.1002/(SICI)1521-1878(200002)22:2<108::AID-BIES2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Ornitz DM, Itoh N. Fibroblast growth factors. Genome Biol. 2001;2:REVIEWS3005. doi: 10.1186/gb-2001-2-3-reviews3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrimon N, et al. Generating lineage-specific markers to study Drosophila development. Dev Genet. 1991;12:238–52. doi: 10.1002/dvg.1020120309. [DOI] [PubMed] [Google Scholar]
- Sato M, Kornberg TB. FGF is an essential mitogen and chemoattractant for the air sacs of the drosophila tracheal system. Dev Cell. 2002;3:195–207. doi: 10.1016/s1534-5807(02)00202-2. [DOI] [PubMed] [Google Scholar]
- Shiga Y, Tanaka-Matakatsu M, Hayashi S. A nuclear GFP beta-galactosidase fusion protein as a marker for morphogenesis in living Drosophila. Dev Growth Differ. 1996;38:99–106. [Google Scholar]
- Shishido E, et al. Two FGF-receptor homologues of Drosophila: one is expressed in mesodermal primordium in early embryos. Development. 1993;117:751–61. doi: 10.1242/dev.117.2.751. [DOI] [PubMed] [Google Scholar]
- Shishido E, et al. Requirements of DFR1/Heartless, a mesoderm-specific Drosophila FGF-receptor, for the formation of heart, visceral and somatic muscles, and ensheathing of longitudinal axon tracts in CNS. Development. 1997;124:2119–28. doi: 10.1242/dev.124.11.2119. [DOI] [PubMed] [Google Scholar]
- Spring J, et al. Drosophila syndecan: conservation of a cell-surface heparan sulfate proteoglycan. Proc Natl Acad Sci U S A. 1994;91:3334–8. doi: 10.1073/pnas.91.8.3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stathopoulos A, et al. pyramus and thisbe: FGF genes that pattern the mesoderm of Drosophila embryos. Genes Dev. 2004;18:687–99. doi: 10.1101/gad.1166404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland D, et al. branchless encodes a Drosophila FGF homolog that controls tracheal cell migration and the pattern of branching. Cell. 1996;87:1091–101. doi: 10.1016/s0092-8674(00)81803-6. [DOI] [PubMed] [Google Scholar]
- Takei Y, et al. Three Drosophila EXT genes shape morphogen gradients through synthesis of heparan sulfate proteoglycans. Development. 2004;131:73–82. doi: 10.1242/dev.00913. [DOI] [PubMed] [Google Scholar]
- The I, et al. Hedgehog movement is regulated through tout velu-dependent synthesis of a heparan sulfate proteoglycan. Mol Cell. 1999;4:633–9. doi: 10.1016/s1097-2765(00)80214-2. [DOI] [PubMed] [Google Scholar]
- Thisse B, Thisse C. Functions and regulations of fibroblast growth factor signaling during embryonic development. Dev Biol. 2005;287:390–402. doi: 10.1016/j.ydbio.2005.09.011. [DOI] [PubMed] [Google Scholar]
- van den Heuvel M, et al. Mutations in the segment polarity genes wingless and porcupine impair secretion of the wingless protein. Embo J. 1993;12:5293–302. doi: 10.1002/j.1460-2075.1993.tb06225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt A, et al. Perlecan participates in proliferation activation of quiescent Drosophila neuroblasts. Dev Dyn. 2002;224:403–12. doi: 10.1002/dvdy.10120. [DOI] [PubMed] [Google Scholar]
- Xu T, Rubin GM. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development. 1993;117:1223–37. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]
- Yoffe KB, et al. Evidence for engrailed-independent wingless autoregulation in Drosophila. Dev Biol. 1995;170:636–50. doi: 10.1006/dbio.1995.1243. [DOI] [PubMed] [Google Scholar]
- Zhang X, et al. Reciprocal epithelial-mesenchymal FGF signaling is required for cecal development. Development. 2006;133:173–80. doi: 10.1242/dev.02175. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


