Abstract
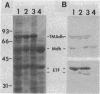
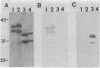
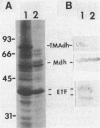
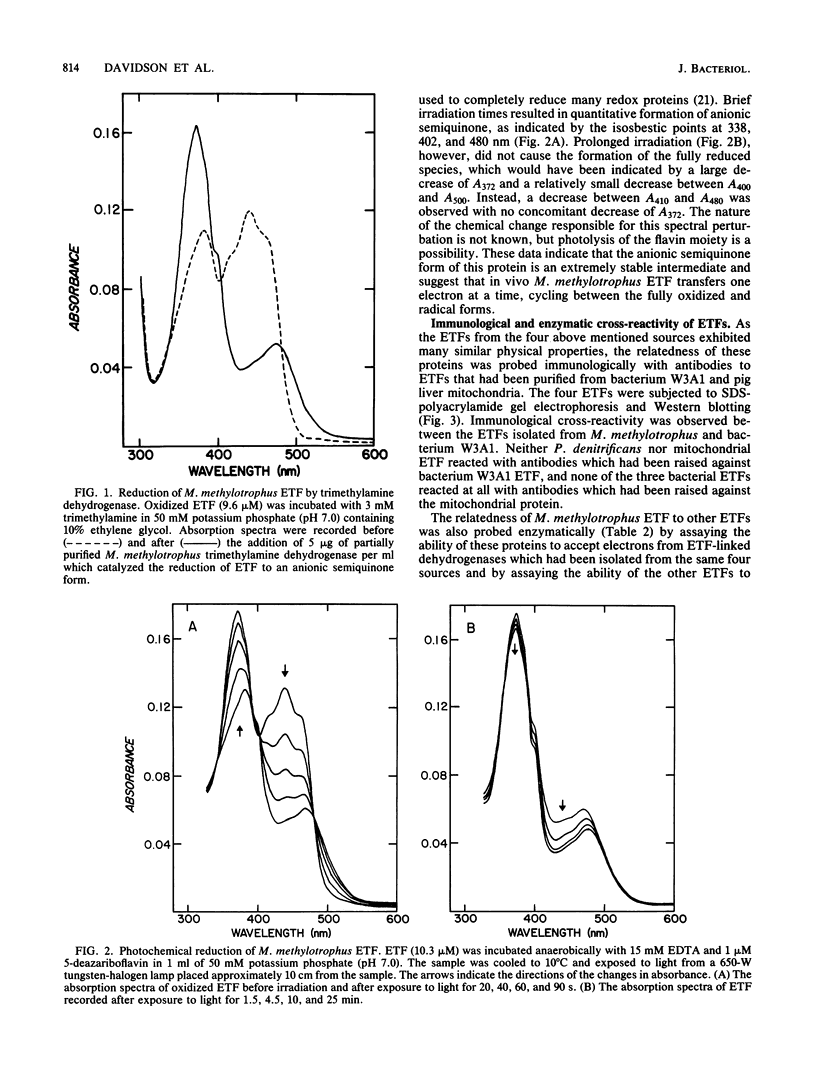
When grown on methylated amines as a carbon source, Methylophilus methylotrophus synthesizes an electron transfer flavoprotein (ETF) which is the natural electron acceptor of trimethylamine dehydrogenase. It is composed of two dissimilar subunits of 38,000 and 42,000 daltons and 1 mol of flavin adenine dinucleotide. It was reduced by trimethylamine dehydrogenase to a stable anionic semiquinone form, which could not be converted, either enzymatically or chemically, to the fully reduced dihydroquinone. This ETF exhibited spectral properties which were nearly identical to ETFs from bacterium W3A1, Paracoccus denitrificans, and pig liver mitochondria. M. methylotrophus ETF cross-reacted immunologically and enzymatically with the ETF of bacterium W3A1 but not with the other two ETFs. In M. methylotrophus and bacterium W3A1, ETF and trimethylamine dehydrogenase were each expressed during growth on trimethylamine and were each absent during growth on methanol.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blankenhorn G. Nicotinamide-dependent one-electron and two-electron (flavin) oxidoreduction: thermodynamics, kinetics, and mechanism. Eur J Biochem. 1976 Aug 1;67(1):67–80. doi: 10.1111/j.1432-1033.1976.tb10634.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Choong Y. S., Massey V. Stabilization of lactate oxidase flavin anion radical by complex formation. J Biol Chem. 1980 Sep 25;255(18):8672–8677. [PubMed] [Google Scholar]
- Colby J., Zatman L. J. Tricarboxylic acid-cycle and related enzymes in restricted facultative methylotrophs. Biochem J. 1975 Jun;148(3):505–511. doi: 10.1042/bj1480505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby J., Zatman L. J. Trimethylamine metabolism in obligate and facultative methylotrophs. Biochem J. 1973 Jan;132(1):101–112. doi: 10.1042/bj1320101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. B., Quayle J. R. The autotrophic growth of Micrococcus denitrificans on Methanol. Biochem J. 1975 Sep;150(3):569–571. doi: 10.1042/bj1500569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson V. L. Regulation by carbon source of enzyme expression and slime production in bacterium W3A1. J Bacteriol. 1985 Nov;164(2):941–943. doi: 10.1128/jb.164.2.941-943.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRISELL W. R., MACKENZIE C. G. Separation and purification of sarcosine dehydrogenase and dimethylglycine dehydrogenase. J Biol Chem. 1962 Jan;237:94–98. [PubMed] [Google Scholar]
- Husain M., Davidson V. L. An inducible periplasmic blue copper protein from Paracoccus denitrificans. Purification, properties, and physiological role. J Biol Chem. 1985 Nov 25;260(27):14626–14629. [PubMed] [Google Scholar]
- Husain M., Stankovich M. T., Fox B. G. Measurement of the oxidation-reduction potentials for one-electron and two-electron reduction of electron-transfer flavoprotein from pig liver. Biochem J. 1984 May 1;219(3):1043–1047. doi: 10.1042/bj2191043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain M., Steenkamp D. J. Electron transfer flavoprotein from pig liver mitochondria. A simple purification and re-evaluation of some of the molecular properties. Biochem J. 1983 Feb 1;209(2):541–545. doi: 10.1042/bj2090541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain M., Steenkamp D. J. Partial purification and characterization of glutaryl-coenzyme A dehydrogenase, electron transfer flavoprotein, and electron transfer flavoprotein-Q oxidoreductase from Paracoccus denitrificans. J Bacteriol. 1985 Aug;163(2):709–715. doi: 10.1128/jb.163.2.709-715.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y., Dabrowski C., Tanaka K. Separation and properties of five distinct acyl-CoA dehydrogenases from rat liver mitochondria. Identification of a new 2-methyl branched chain acyl-CoA dehydrogenase. J Biol Chem. 1983 Jan 25;258(2):1066–1076. [PubMed] [Google Scholar]
- Kasprzak A. A., Steenkamp D. J. Localization of the major dehydrogenases in two methylotrophs by radiochemical labeling. J Bacteriol. 1983 Oct;156(1):348–353. doi: 10.1128/jb.156.1.348-353.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Massey V., Hemmerich P. Photoreduction of flavoproteins and other biological compounds catalyzed by deazaflavins. Biochemistry. 1978 Jan 10;17(1):9–16. doi: 10.1021/bi00594a002. [DOI] [PubMed] [Google Scholar]
- Mayhew S. G., Massey V. Purification and characterization of flavodoxin from Peptostreptococcus elsdenii. J Biol Chem. 1969 Feb 10;244(3):794–802. [PubMed] [Google Scholar]
- McKean M. C., Frerman F. E., Mielke D. M. General acyl-CoA dehydrogenase from pig liver. Kinetic and binding studies. J Biol Chem. 1979 Apr 25;254(8):2730–2735. [PubMed] [Google Scholar]
- McNerney T., O'connor M. L. Regulation of enzymes associated with C-1 metabolism in three facultative methylotrophs. Appl Environ Microbiol. 1980 Aug;40(2):370–375. doi: 10.1128/aem.40.2.370-375.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens J. D., Keddie R. M. The nitrogen nutrition of soil and herbage coryneform bacteria. J Appl Bacteriol. 1969 Sep;32(3):338–347. doi: 10.1111/j.1365-2672.1969.tb00981.x. [DOI] [PubMed] [Google Scholar]
- Schnaitman C., Greenawalt J. W. Enzymatic properties of the inner and outer membranes of rat liver mitochondria. J Cell Biol. 1968 Jul;38(1):158–175. doi: 10.1083/jcb.38.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankovich M. T., Schopfer L. M., Massey V. Determination of glucose oxidase oxidation-reduction potentials and the oxygen reactivity of fully reduced and semiquinoid forms. J Biol Chem. 1978 Jul 25;253(14):4971–4979. [PubMed] [Google Scholar]
- Steenkamp D. J., Gallup M. The natural flavorprotein electron acceptor of trimethylamine dehydrogenase. J Biol Chem. 1978 Jun 25;253(12):4086–4089. [PubMed] [Google Scholar]
- Steenkamp D. J., Husain M. The effect of tetrahydrofolate on the reduction of electron transfer flavoprotein by sarcosine and dimethylglycine dehydrogenases. Biochem J. 1982 Jun 1;203(3):707–715. doi: 10.1042/bj2030707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenkamp D. J., Mallinson J. Trimethylamine dehydrogenase from a methylotrophic bacterium. I. Isolation and steady-state kinetics. Biochim Biophys Acta. 1976 May 13;429(3):705–719. doi: 10.1016/0005-2744(76)90319-3. [DOI] [PubMed] [Google Scholar]
- Thorpe C., Matthews R. G., Williams C. H., Jr Acyl-coenzyme A dehydrogenase from pig kidney. Purification and properties. Biochemistry. 1979 Jan 23;18(2):331–337. doi: 10.1021/bi00569a016. [DOI] [PubMed] [Google Scholar]
- Weaver C. A., Lidstrom M. E. Methanol dissimilation in Xanthobacter H4-14: activities, induction and comparison to Pseudomonas AM1 and Paracoccus denitrificans. J Gen Microbiol. 1985 Sep;131(9):2183–2197. doi: 10.1099/00221287-131-9-2183. [DOI] [PubMed] [Google Scholar]