Abstract
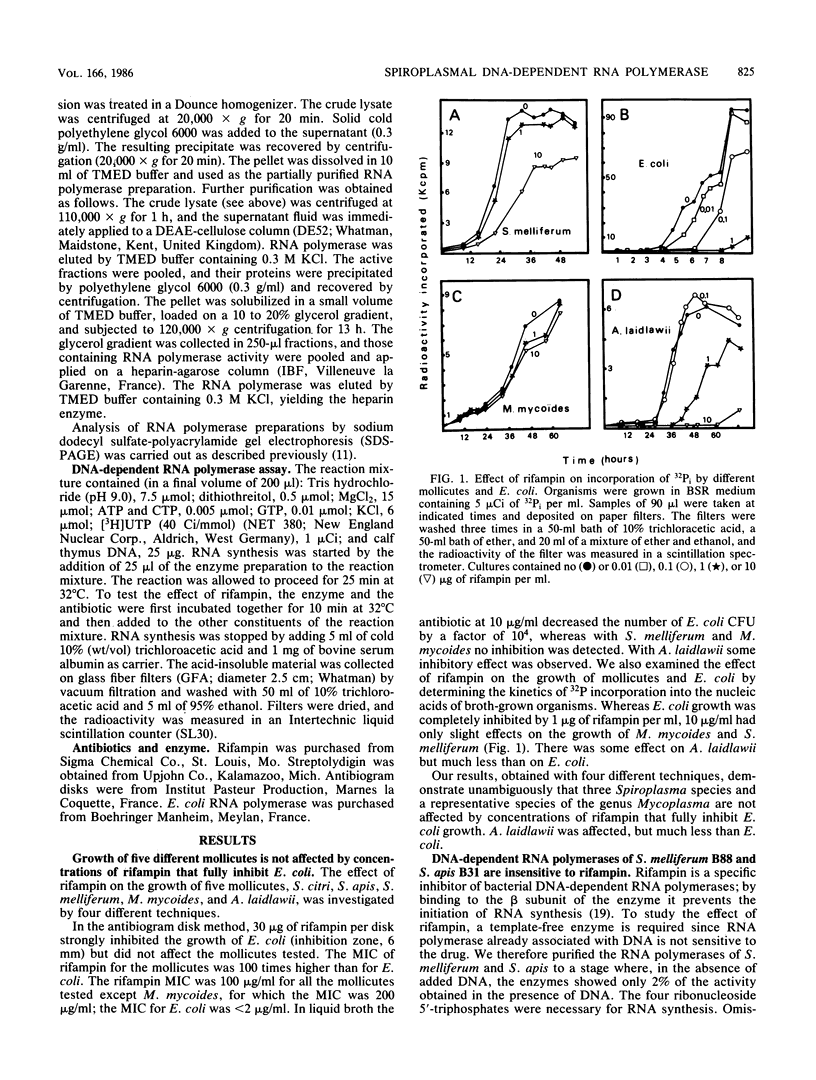
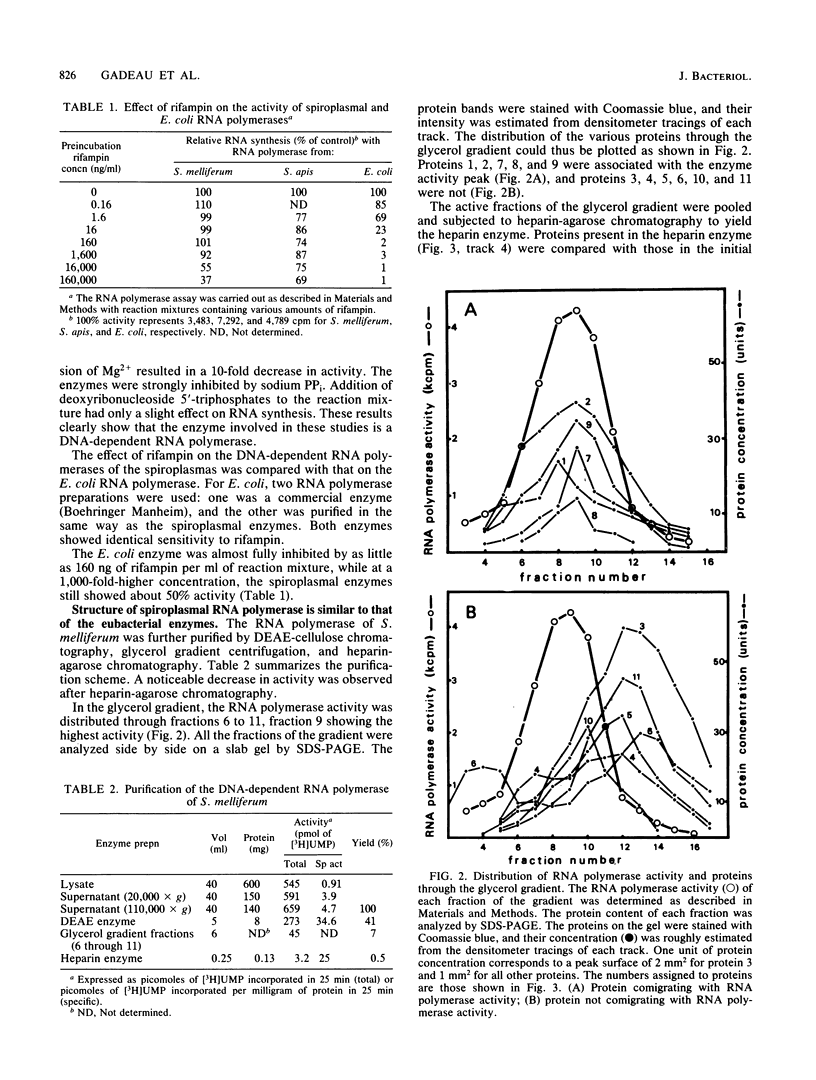
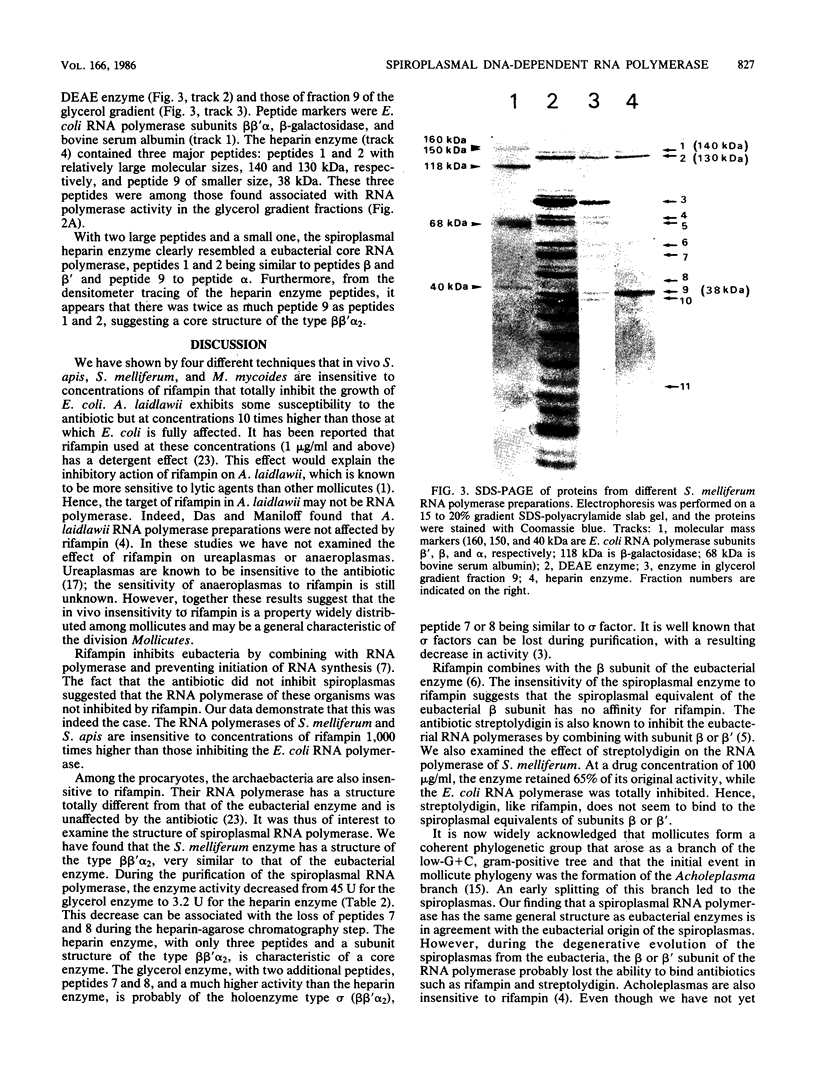
The effect of rifampin on five mollicutes (Spiroplasma citri, Spiroplasma melliferum, Spiroplasma apis, Acholeplasma laidlawii, and Mycoplasma mycoides) was compared with that on Escherichia coli. We found that, in contrast to wild-type E. coli, mollicutes were insensitive to rifampin. DNA-dependent RNA polymerases from S. melliferum and S. apis were purified to the stage where the enzymes were dependent on the addition of exogenous templates for activity. The enzymes were then tested for their sensitivity to rifampin. Spiroplasmal enzymes were at least 1,000 times less sensitive to rifampin than the corresponding E. coli enzyme. This result provides a molecular basis for the resistance of mollicutes to rifampin. The RNA polymerase of S. melliferum was further purified and its subunit composition was investigated. The RNA polymerase has one small and two large subunits. The structure of S. melliferum RNA polymerase therefore resembles that of the eubacterial enzymes in spite of its insensitivity to rifampin.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archer D. B. The structure and functions of the mycoplasma membrane. Int Rev Cytol. 1981;69:1–44. doi: 10.1016/s0074-7696(08)62319-0. [DOI] [PubMed] [Google Scholar]
- Das J., Maniloff J. Replication of mycoplasmavirus MVL51. IV. Inhibition of viral synthesis by rifampin. J Virol. 1976 Jun;18(3):969–976. doi: 10.1128/jvi.18.3.969-976.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halling S. M., Burtis K. C., Doi R. H. beta' subunit of bacterial RNA polymerase is responsible for streptolydigin resistance in Bacillus subtilis. Nature. 1978 Apr 27;272(5656):837–839. doi: 10.1038/272837a0. [DOI] [PubMed] [Google Scholar]
- Kumar S. A. The structure and mechanism of action of bacterial DNA-dependent RNA polymerase. Prog Biophys Mol Biol. 1981;38(3):165–210. doi: 10.1016/0079-6107(81)90013-4. [DOI] [PubMed] [Google Scholar]
- Mouches C., Bové J. M., Tully J. G., Rose D. L., McCoy R. E., Carle-Junca P., Garnier M., Saillard C. Spiroplasma apis, a new species from the honey-bee Apis mellifera. Ann Microbiol (Paris) 1983 May-Jun;134A(3):383–397. [PubMed] [Google Scholar]
- Rabussay D., Zillig W. A rifampicin resistent rna-polymerase from E. coli altered in the beta-subunit. FEBS Lett. 1969 Oct 21;5(2):104–106. doi: 10.1016/0014-5793(69)80305-4. [DOI] [PubMed] [Google Scholar]
- Sippel A., Hartmann G. Mode of action of rafamycin on the RNA polymerase reaction. Biochim Biophys Acta. 1968 Mar 18;157(1):218–219. doi: 10.1016/0005-2787(68)90286-4. [DOI] [PubMed] [Google Scholar]
- Woese C. R., Maniloff J., Zablen L. B. Phylogenetic analysis of the mycoplasmas. Proc Natl Acad Sci U S A. 1980 Jan;77(1):494–498. doi: 10.1073/pnas.77.1.494. [DOI] [PMC free article] [PubMed] [Google Scholar]