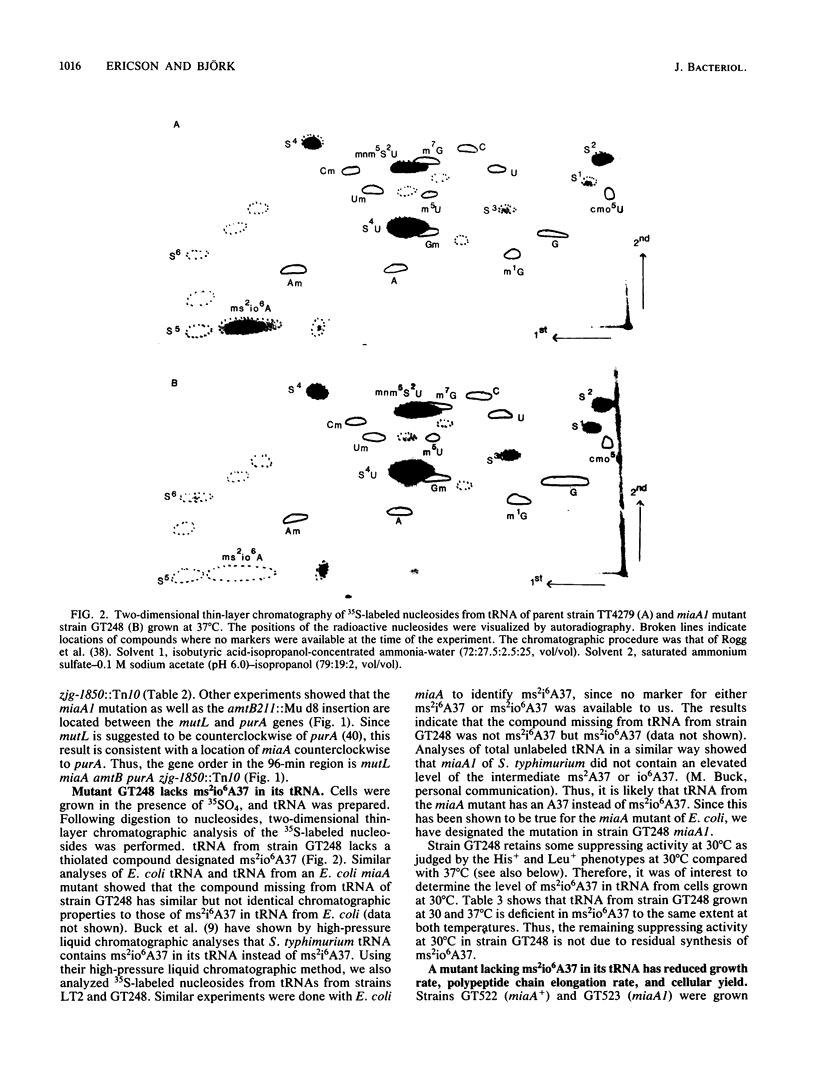
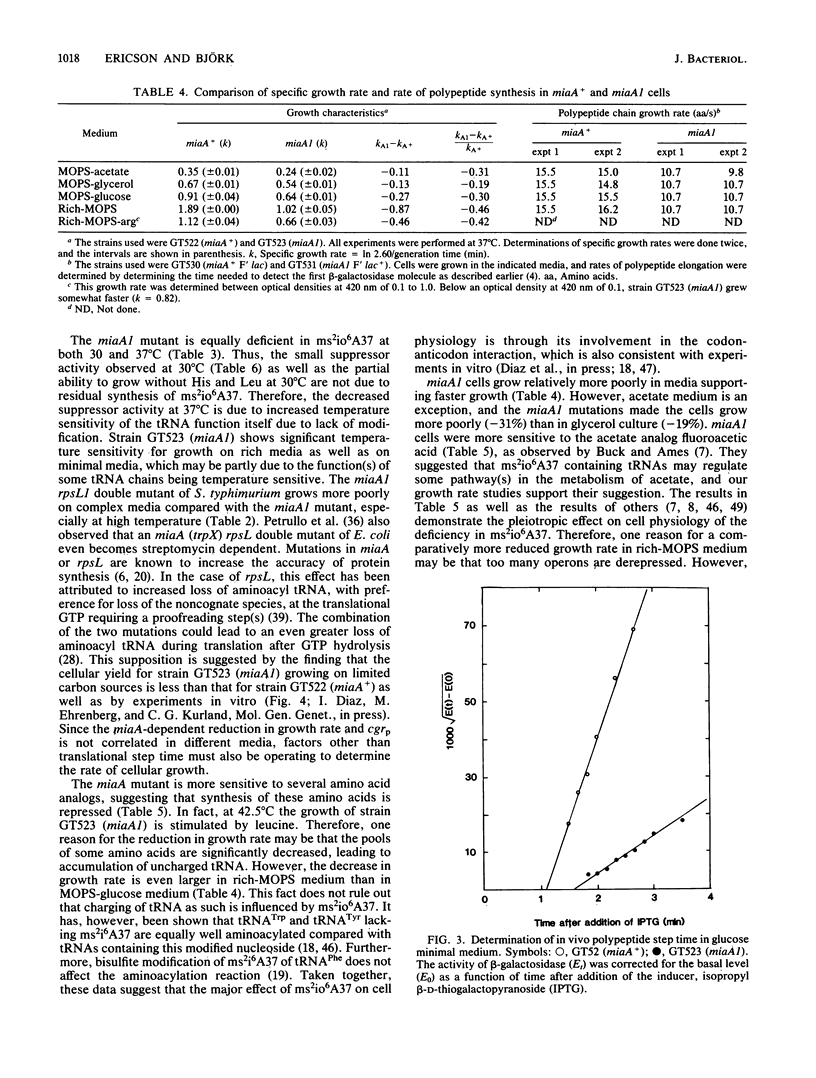
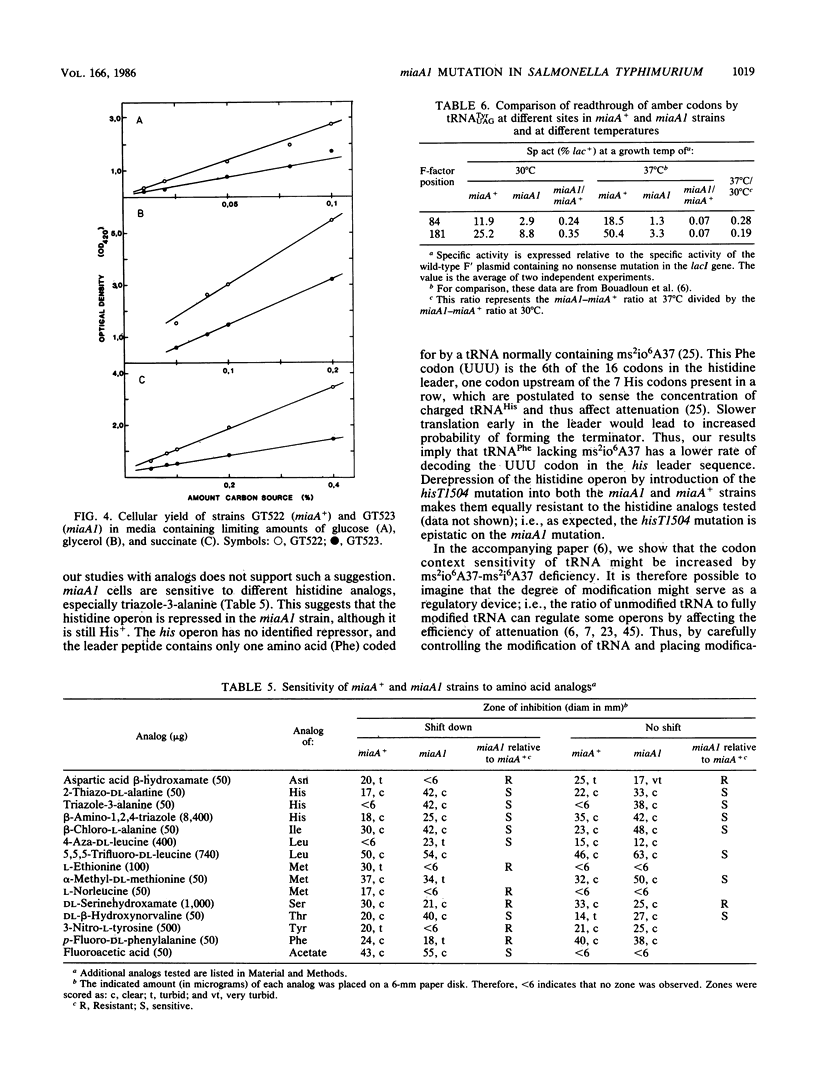
Abstract
A strain of Salmonella typhimurium LT2 was isolated, which harbors a mutation acting as an antisuppressor toward an amber suppressor derivative, supF30, of tRNATyr1. The mutant is deficient in cis-2-methylthioribosylzeatin[N6-(4-hydroxyisopentenyl)-2-me thylthioadenosine, ms2io6A], which is a modification normally present next to the anticodon (position 37) in tRNA reading codons starting with uridine. The gene miaA, defective in the mutant, is located close to and counterclockwise of the purA gene at 96 min on the chromosomal map of S. typhimurium with the gene order mutL miaA purA. Growth rate of the mutant was reduced 20 to 50%, and the effect was more pronounced in media supporting fast growth. Translational chain elongation rate at 37 degrees C was reduced from 16 amino acids per s in the wild-type cell to 11 amino acids per s in the miaA1 mutant in the four different growth media tested. The cellular yield in limiting glucose, glycerol, or succinate medium was reduced for the miaAI mutant compared with wild-type cells, with 49, 41, and 57% reductions, respectively. The miaAI mutation renders the cell more sensitive or resistant toward several amino acid analogs, suggesting that the deficiency in ms2io6A influences the regulation of several amino acid biosynthetic operons. We suggest that tRNAPhe, lacking ms2io6A, translates a UUU codon in the early histidine leader sequence with lowered efficiency, leading to repression of the his operon.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agris P. F., Armstrong D. J., Schäfer K. P., Söll D. Maturation of a hypermodified nucleoside in transfer RNA. Nucleic Acids Res. 1975 May;2(5):691–698. doi: 10.1093/nar/2.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björk G. R. A novel link between the biosynthesis of aromatic amino acids and transfer RNA modification in Escherichia coli. J Mol Biol. 1980 Jul 5;140(3):391–410. doi: 10.1016/0022-2836(80)90391-5. [DOI] [PubMed] [Google Scholar]
- Björk G. R., Neidhardt F. C. Physiological and biochemical studies on the function of 5-methyluridine in the transfer ribonucleic acid of Escherichia coli. J Bacteriol. 1975 Oct;124(1):99–111. doi: 10.1128/jb.124.1.99-111.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossi L., Ruth J. R. The influence of codon context on genetic code translation. Nature. 1980 Jul 10;286(5769):123–127. doi: 10.1038/286123a0. [DOI] [PubMed] [Google Scholar]
- Bouadloun F., Srichaiyo T., Isaksson L. A., Björk G. R. Influence of modification next to the anticodon in tRNA on codon context sensitivity of translational suppression and accuracy. J Bacteriol. 1986 Jun;166(3):1022–1027. doi: 10.1128/jb.166.3.1022-1027.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck M., Ames B. N. A modified nucleotide in tRNA as a possible regulator of aerobiosis: synthesis of cis-2-methyl-thioribosylzeatin in the tRNA of Salmonella. Cell. 1984 Feb;36(2):523–531. doi: 10.1016/0092-8674(84)90245-9. [DOI] [PubMed] [Google Scholar]
- Buck M., Griffiths E. Iron mediated methylthiolation of tRNA as a regulator of operon expression in Escherichia coli. Nucleic Acids Res. 1982 Apr 24;10(8):2609–2624. doi: 10.1093/nar/10.8.2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck M., McCloskey J. A., Basile B., Ames B. N. cis 2-Methylthio-ribosylzeatin (ms2io6A) is present in the transfer RNA of Salmonella typhimurium, but not Escherichia coli. Nucleic Acids Res. 1982 Sep 25;10(18):5649–5662. doi: 10.1093/nar/10.18.5649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byström A. S., Hjalmarsson K. J., Wikström P. M., Björk G. R. The nucleotide sequence of an Escherichia coli operon containing genes for the tRNA(m1G)methyltransferase, the ribosomal proteins S16 and L19 and a 21-K polypeptide. EMBO J. 1983;2(6):899–905. doi: 10.1002/j.1460-2075.1983.tb01519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan R. K., Botstein D., Watanabe T., Ogata Y. Specialized transduction of tetracycline resistance by phage P22 in Salmonella typhimurium. II. Properties of a high-frequency-transducing lysate. Virology. 1972 Dec;50(3):883–898. doi: 10.1016/0042-6822(72)90442-4. [DOI] [PubMed] [Google Scholar]
- Chumley F. G., Menzel R., Roth J. R. Hfr formation directed by tn10. Genetics. 1979 Apr;91(4):639–655. doi: 10.1093/genetics/91.4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby D. S., Schedl P., Guthrie C. A functional requirement for modification of the wobble nucleotide in tha anticodon of a T4 suppressor tRNA. Cell. 1976 Nov;9(3):449–463. doi: 10.1016/0092-8674(76)90090-8. [DOI] [PubMed] [Google Scholar]
- Cortese R., Landsberg R., Haar R. A., Umbarger H. E., Ames B. N. Pleiotropy of hisT mutants blocked in pseudouridine synthesis in tRNA: leucine and isoleucine-valine operons. Proc Natl Acad Sci U S A. 1974 May;71(5):1857–1861. doi: 10.1073/pnas.71.5.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg S. P., Yarus M., Soll L. The effect of an Escherichia coli regulatory mutation on transfer RNA structure. J Mol Biol. 1979 Nov 25;135(1):111–126. doi: 10.1016/0022-2836(79)90343-7. [DOI] [PubMed] [Google Scholar]
- Gallagher P. J., Schwartz I., Elseviers D. Genetic mapping of pheU, an Escherichia coli gene for phenylalanine tRNA. J Bacteriol. 1984 May;158(2):762–763. doi: 10.1128/jb.158.2.762-763.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gefter M. L., Russell R. L. Role modifications in tyrosine transfer RNA: a modified base affecting ribosome binding. J Mol Biol. 1969 Jan 14;39(1):145–157. doi: 10.1016/0022-2836(69)90339-8. [DOI] [PubMed] [Google Scholar]
- Goddard J. P., Lowdon M. The effect upon aminoacylation of bisulphite addition to 2-methylthio-N6-isopentenyl adenosine of Escherichia coli phenylalanine tRNA. FEBS Lett. 1981 Aug 3;130(2):221–222. doi: 10.1016/0014-5793(81)81124-6. [DOI] [PubMed] [Google Scholar]
- Gowrishankar J., Pittard J. Regulation of phenylalanine biosynthesis in Escherichia coli K-12: control of transcription of the pheA operon. J Bacteriol. 1982 Jun;150(3):1130–1137. doi: 10.1128/jb.150.3.1130-1137.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosjean H., Nicoghosian K., Haumont E., Söll D., Cedergren R. Nucleotide sequences of two serine tRNAs with a GGA anticodon: the structure-function relationships in the serine family of E. coli tRNAs. Nucleic Acids Res. 1985 Aug 12;13(15):5697–5706. doi: 10.1093/nar/13.15.5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janner F., Vögeli G., Fluri R. The antisuppressor strain sin1 of Schizosaccharomyces pombe lacks the modification isopentenyladenosine in transfer RNA. J Mol Biol. 1980 May 15;139(2):207–219. doi: 10.1016/0022-2836(80)90305-8. [DOI] [PubMed] [Google Scholar]
- Johnston H. M., Barnes W. M., Chumley F. G., Bossi L., Roth J. R. Model for regulation of the histidine operon of Salmonella. Proc Natl Acad Sci U S A. 1980 Jan;77(1):508–512. doi: 10.1073/pnas.77.1.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalnins A., Otto K., Rüther U., Müller-Hill B. Sequence of the lacZ gene of Escherichia coli. EMBO J. 1983;2(4):593–597. doi: 10.1002/j.1460-2075.1983.tb01468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersten H. On the biological significance of modified nucleosides in tRNA. Prog Nucleic Acid Res Mol Biol. 1984;31:59–114. doi: 10.1016/s0079-6603(08)60375-x. [DOI] [PubMed] [Google Scholar]
- Kurland C. G., Ehrenberg M. Optimization of translation accuracy. Prog Nucleic Acid Res Mol Biol. 1984;31:191–219. doi: 10.1016/s0079-6603(08)60378-5. [DOI] [PubMed] [Google Scholar]
- Laten H., Gorman J., Bock R. M. Isopentenyladenosine deficient tRNA from an antisuppressor mutant of Saccharomyces cerevisiae. Nucleic Acids Res. 1978 Nov;5(11):4329–4342. doi: 10.1093/nar/5.11.4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. H., Albertini A. M. Effects of surrounding sequence on the suppression of nonsense codons. J Mol Biol. 1983 Feb 15;164(1):59–71. doi: 10.1016/0022-2836(83)90087-6. [DOI] [PubMed] [Google Scholar]
- Murray M. L., Hartman P. E. Overproduction of hisH and hisF gene products leads to inhibition of cell cell division in Salmonella. Can J Microbiol. 1972 May;18(5):671–681. doi: 10.1139/m72-105. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ny T., Thomale J., Hjalmarsson K., Nass G., Björk G. R. Non-coordinate regulation of enzymes involved in transfer RNA metabolism in Escherichia coli. Biochim Biophys Acta. 1980 Apr 30;607(2):277–284. doi: 10.1016/0005-2787(80)90080-5. [DOI] [PubMed] [Google Scholar]
- Palmer D. T., Blum P. H., Artz S. W. Effects of the hisT mutation of Salmonella typhimurium on translation elongation rate. J Bacteriol. 1983 Jan;153(1):357–363. doi: 10.1128/jb.153.1.357-363.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam S. L., Koch A. L. Complications in the simplest cellular enzyme assay: lysis of Escherichia coli for the assay of beta-galactosidase. Anal Biochem. 1975 Feb;63(2):350–360. doi: 10.1016/0003-2697(75)90357-7. [DOI] [PubMed] [Google Scholar]
- Rogg H., Brambilla R., Keith G., Staehelin M. An improved method for the separation and quantitation of the modified nucleosides of transfer RNA. Nucleic Acids Res. 1976 Jan;3(1):285–295. doi: 10.1093/nar/3.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruusala T., Kurland C. G. Streptomycin preferentially perturbs ribosomal proofreading. Mol Gen Genet. 1984;198(2):100–104. doi: 10.1007/BF00328707. [DOI] [PubMed] [Google Scholar]
- Sanderson K. E., Roth J. R. Linkage map of Salmonella typhimurium, Edition VI. Microbiol Rev. 1983 Sep;47(3):410–453. doi: 10.1128/mr.47.3.410-453.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleif R., Hess W., Finkelstein S., Ellis D. Induction kinetics of the L-arabinose operon of Escherichia coli. J Bacteriol. 1973 Jul;115(1):9–14. doi: 10.1128/jb.115.1.9-14.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmieger H. Phage P22-mutants with increased or decreased transduction abilities. Mol Gen Genet. 1972;119(1):75–88. doi: 10.1007/BF00270447. [DOI] [PubMed] [Google Scholar]
- Scott J. F., Roth J. R., Artz S. W. Regulation of histidine operon does not require hisG enzyme. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5021–5025. doi: 10.1073/pnas.72.12.5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan M. A., Cannon J. F., Webb F. H., Bock R. M. Antisuppressor mutation in Escherichia coli defective in biosynthesis of 5-methylaminomethyl-2-thiouridine. J Bacteriol. 1985 Jan;161(1):368–376. doi: 10.1128/jb.161.1.368-376.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbough C. L., Jr, Neill R. J., Landsberg R., Ames B. N. Pseudouridylation of tRNAs and its role in regulation in Salmonella typhimurium. J Biol Chem. 1979 Jun 25;254(12):5111–5119. [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Vacher J., Grosjean H., Houssier C., Buckingham R. H. The effect of point mutations affecting Escherichia coli tryptophan tRNA on anticodon-anticodon interactions and on UGA suppression. J Mol Biol. 1984 Aug 5;177(2):329–342. doi: 10.1016/0022-2836(84)90460-1. [DOI] [PubMed] [Google Scholar]
- Yanofsky C. Mutations affecting tRNATrp and its charging and their effect on regulation of transcription termination at the attenuator of the tryptophan operon. J Mol Biol. 1977 Jul 15;113(4):663–677. doi: 10.1016/0022-2836(77)90229-7. [DOI] [PubMed] [Google Scholar]
- Zurawski G., Brown K., Killingly D., Yanofsky C. Nucleotide sequence of the leader region of the phenylalanine operon of Escherichia coli. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4271–4275. doi: 10.1073/pnas.75.9.4271. [DOI] [PMC free article] [PubMed] [Google Scholar]



