Abstract
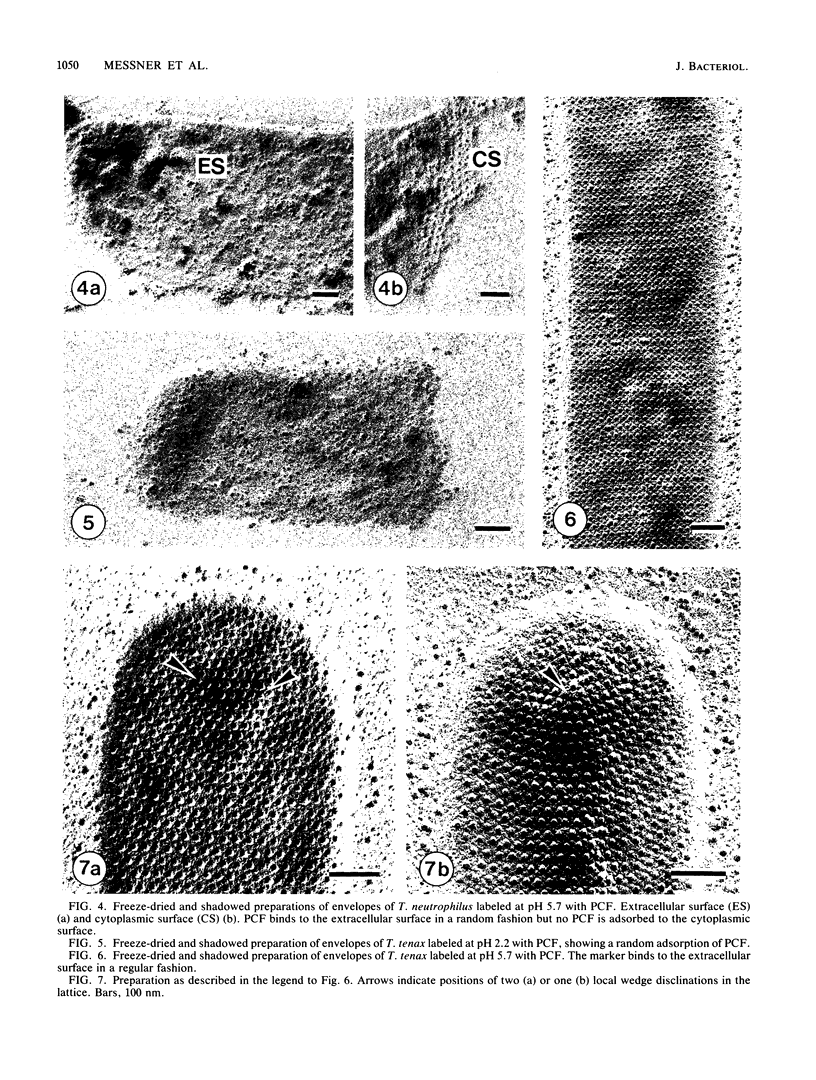
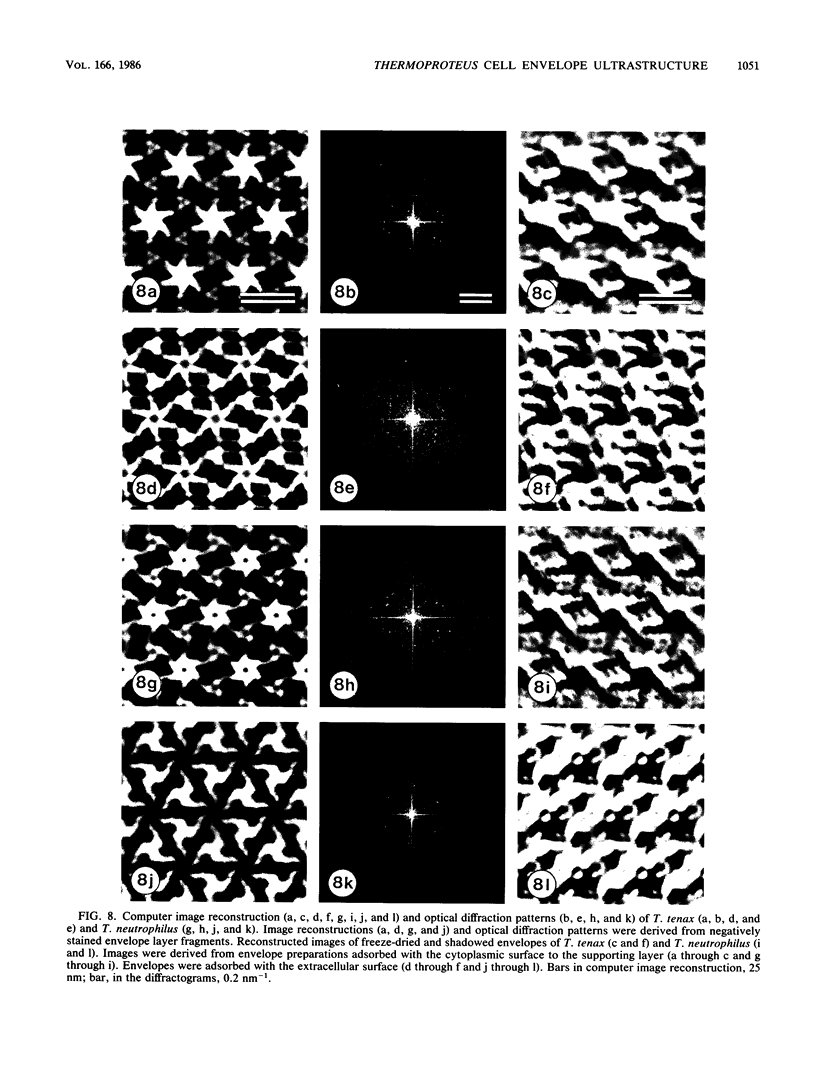
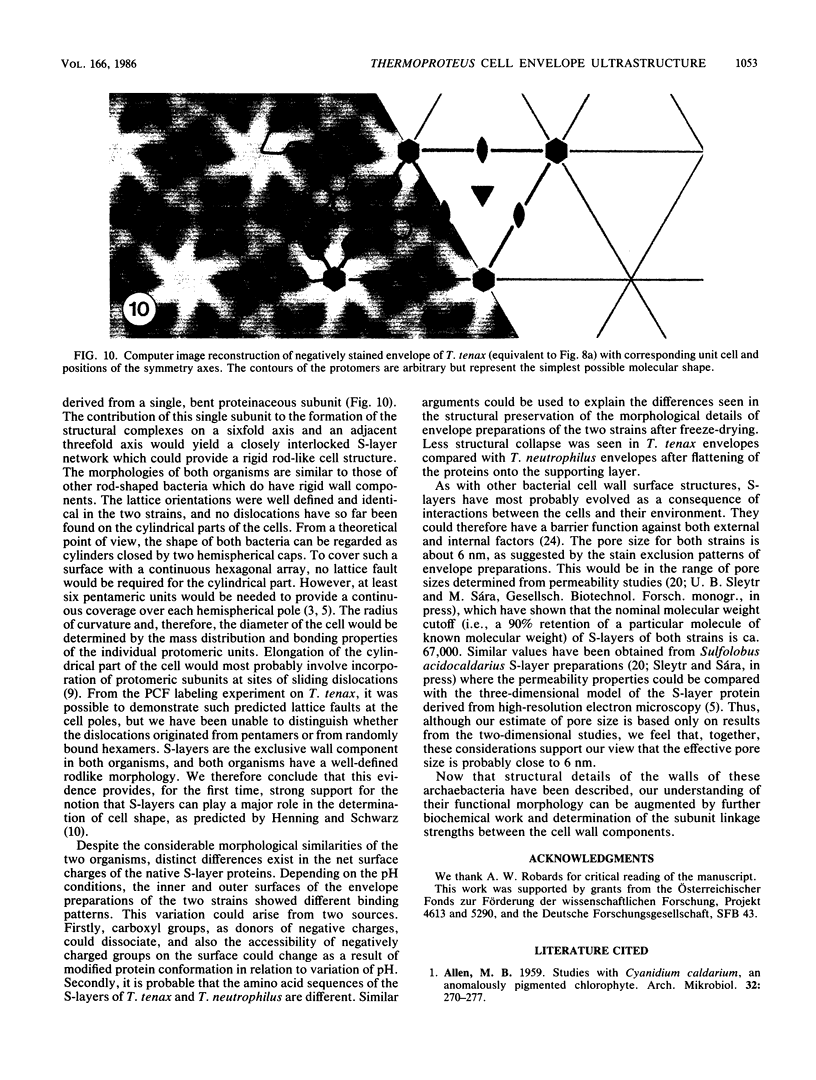
The ultrastructures of the regular surface layers (S-layers) of the extremely thermophilic archaebacteria Thermoproteus tenax and Thermoproteus neutrophilus were examined by freeze-etching, freeze-drying, and negative staining methods combined with optical and digital image enhancement. In both strains, a monolayer of macromolecules arranged in hexagonal arrays with center-to-center spacings of approximately 30 nm was the only component of the cell wall. The gross morphologies of the S-layer lattices of the two organisms were similar and showed the same handedness in the arrangement of the protomers of the morphological units. Striking differences were found in the anionic charge distributions on the surfaces of the two S-layer proteins as determined by labeling with polycationic ferritin. Analysis of the lattice orientation, together with the number and distribution of lattice faults on intact cells, provided a strong indication that the S-layers of both organisms have a shape-determining function.
Full text
PDF
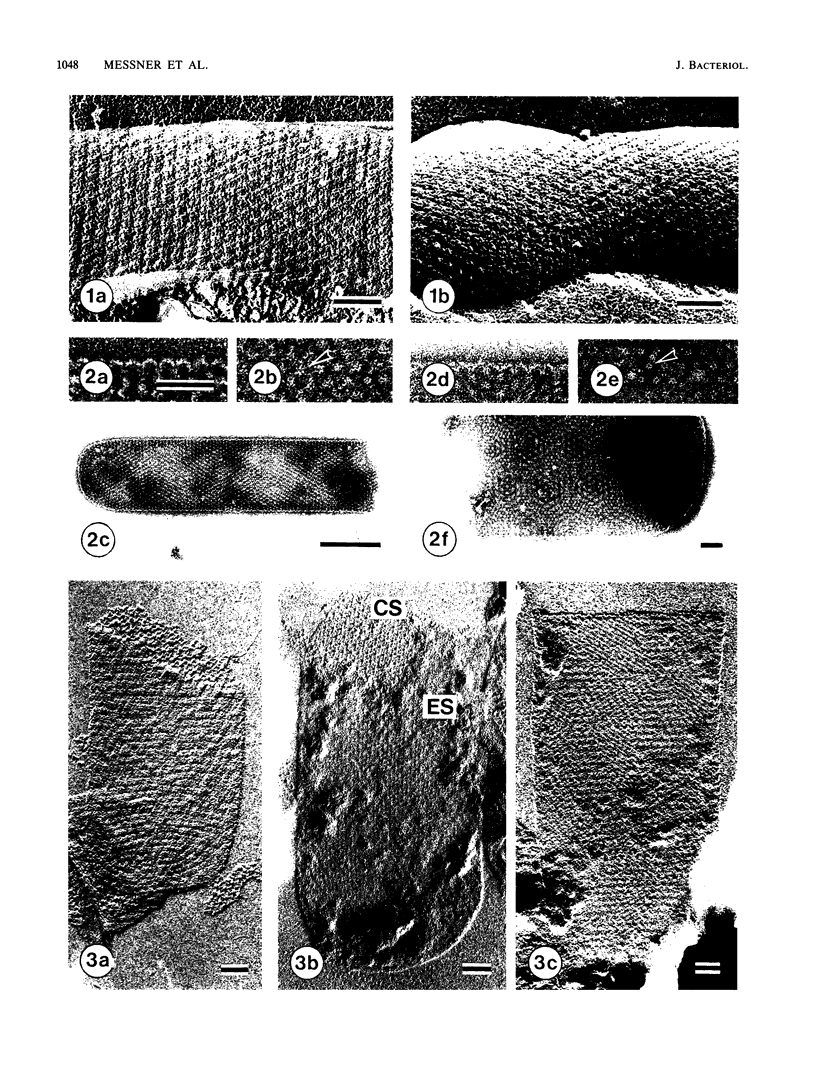



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN M. B. Studies with Cyanidium caldarium, an anomalously pigmented chlorophyte. Arch Mikrobiol. 1959;32(3):270–277. doi: 10.1007/BF00409348. [DOI] [PubMed] [Google Scholar]
- Baumeister W., Kübler O., Zingsheim H. P. The structure of the cell envelope of Micrococcus radiodurans as revealed by metal shadowing and decoration. J Ultrastruct Res. 1981 Apr;75(1):60–71. doi: 10.1016/s0022-5320(81)80100-1. [DOI] [PubMed] [Google Scholar]
- CASPAR D. L., KLUG A. Physical principles in the construction of regular viruses. Cold Spring Harb Symp Quant Biol. 1962;27:1–24. doi: 10.1101/sqb.1962.027.001.005. [DOI] [PubMed] [Google Scholar]
- Chang J. J., Leonard K., Arad T., Pitt T., Zhang Y. X., Zhang L. H. Structural studies of the outer envelope of Chlamydia trachomatis by electron microscopy. J Mol Biol. 1982 Nov 15;161(4):579–590. doi: 10.1016/0022-2836(82)90409-0. [DOI] [PubMed] [Google Scholar]
- Deatherage J. F., Taylor K. A., Amos L. A. Three-dimensional arrangement of the cell wall protein of Sulfolobus acidocaldarius. J Mol Biol. 1983 Jul 15;167(4):823–848. doi: 10.1016/s0022-2836(83)80113-2. [DOI] [PubMed] [Google Scholar]
- Fischer F., Zillig W., Stetter K. O., Schreiber G. Chemolithoautotrophic metabolism of anaerobic extremely thermophilic archaebacteria. Nature. 1983 Feb 10;301(5900):511–513. doi: 10.1038/301511a0. [DOI] [PubMed] [Google Scholar]
- Glaubert A. M., Sleytr U. B. Analysis of regular arrays of subunits on bacterial surfaces: evidence for a dynamic process of assembly. J Ultrastruct Res. 1975 Jan;50(1):103–116. doi: 10.1016/s0022-5320(75)90012-x. [DOI] [PubMed] [Google Scholar]
- Harris W. F., Scriven L. E. Function of dislocations in cell walls and membranes. Nature. 1970 Nov 28;228(5274):827–829. doi: 10.1038/228827a0. [DOI] [PubMed] [Google Scholar]
- Kandler O., König H. Chemical composition of the peptidoglycan-free cell walls of methanogenic bacteria. Arch Microbiol. 1978 Aug 1;118(2):141–152. doi: 10.1007/BF00415722. [DOI] [PubMed] [Google Scholar]
- Kistler J., Aebi U., Kellenberger E. Freeze drying and shadowing a two-dimensional periodic specimen. J Ultrastruct Res. 1977 Apr;59(1):76–86. doi: 10.1016/s0022-5320(77)80030-0. [DOI] [PubMed] [Google Scholar]
- Roberts K., Shaw P. J., Hills G. J. High-resolution electron microscopy of glycoproteins: the crystalline cell wall of Lobomonas. J Cell Sci. 1981 Oct;51:295–313. doi: 10.1242/jcs.51.1.295. [DOI] [PubMed] [Google Scholar]
- Salmon E. D., DeRosier D. A surveying optical diffractometer. J Microsc. 1981 Sep;123(Pt 3):239–247. doi: 10.1111/j.1365-2818.1981.tb02468.x. [DOI] [PubMed] [Google Scholar]
- Saxton W. O., Baumeister W. The correlation averaging of a regularly arranged bacterial cell envelope protein. J Microsc. 1982 Aug;127(Pt 2):127–138. doi: 10.1111/j.1365-2818.1982.tb00405.x. [DOI] [PubMed] [Google Scholar]
- Sleytr U. B., Messner P. Crystalline surface layers on bacteria. Annu Rev Microbiol. 1983;37:311–339. doi: 10.1146/annurev.mi.37.100183.001523. [DOI] [PubMed] [Google Scholar]
- Sleytr U. B. Regular arrays of macromolecules on bacterial cell walls: structure, chemistry, assembly, and function. Int Rev Cytol. 1978;53:1–62. doi: 10.1016/s0074-7696(08)62240-8. [DOI] [PubMed] [Google Scholar]
- Stewart M., Beveridge T. J. Structure of the regular surface layer of Sporosarcina ureae. J Bacteriol. 1980 Apr;142(1):302–309. doi: 10.1128/jb.142.1.302-309.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrigley N. G. The lattice spacing of crystalline catalase as an internal standard of length in electron microscopy. J Ultrastruct Res. 1968 Sep;24(5):454–464. doi: 10.1016/s0022-5320(68)80048-6. [DOI] [PubMed] [Google Scholar]