Abstract
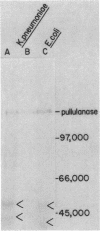
Pullulanase is a starch-debranching enzyme produced by the gram-negative bacterium Klebsiella pneumoniae. In this organism, the enzyme is first exported to the outer membrane and is subsequently released into the growth medium. Evidence reported here indicates that pullulanase is a lipoprotein. It is apparently synthesized as a precursor with a 19-residue-long signal sequence and modified by the covalent attachment of palmitate to the cysteine residue which becomes the amino terminus after cleavage of the signal sequence. In this respect, pullulanase is similar to some penicillinases produced by gram-positive bacteria which are initially exported to the cell surface and subsequently released into the medium. However, pullulanase and the penicillinases differ in one important aspect, namely, that the extracellular pullulanase still carries the covalently attached fatty acyls, whereas extracellular penicillinases lack the modified amino-terminal cysteine together with a limited number of other residues from the amino terminus.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chapon C., Raibaud O. Structure of two divergent promoters located in front of the gene encoding pullulanase in Klebsiella pneumoniae and positively regulated by the malT product. J Bacteriol. 1985 Nov;164(2):639–645. doi: 10.1128/jb.164.2.639-645.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisele B., Rasched I. R., Wallenfels K. Molecular characterization of pullulanase from Aerobacter aerogenes. Eur J Biochem. 1972 Mar 15;26(1):62–67. doi: 10.1111/j.1432-1033.1972.tb01739.x. [DOI] [PubMed] [Google Scholar]
- Felmlee T., Pellett S., Lee E. Y., Welch R. A. Escherichia coli hemolysin is released extracellularly without cleavage of a signal peptide. J Bacteriol. 1985 Jul;163(1):88–93. doi: 10.1128/jb.163.1.88-93.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S., Wu H. C. Accumulation of prolipoprotein in Escherichia coli mutants defective in protein secretion. J Bacteriol. 1985 Mar;161(3):949–954. doi: 10.1128/jb.161.3.949-954.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope G. C., Dean A. C. Pullulanase synthesis in klebsiella (aerobacter) aerogenes strains growing in continuous culture. Biochem J. 1974 Nov;144(2):403–411. doi: 10.1042/bj1440403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard S. P., Buckley J. T. Protein export by a gram-negative bacterium: production of aerolysin by Aeromonas hydrophila. J Bacteriol. 1985 Mar;161(3):1118–1124. doi: 10.1128/jb.161.3.1118-1124.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y. X., Ching G., Inouye M. Comparison of the lipoprotein gene among the enterobacteriaceae. DNA sequence of Morganella morganii lipoprotein gene and its expression in Escherichia coli. J Biol Chem. 1983 Jul 10;258(13):8139–8145. [PubMed] [Google Scholar]
- Inouye S., Wang S., Sekizawa J., Halegoua S., Inouye M. Amino acid sequence for the peptide extension on the prolipoprotein of the Escherichia coli outer membrane. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1004–1008. doi: 10.1073/pnas.74.3.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Bassford P. J., Jr, Beckwith J. Protein localization in E. coli: is there a common step in the secretion of periplasmic and outer-membrane proteins? Cell. 1981 Jun;24(3):707–717. doi: 10.1016/0092-8674(81)90097-0. [DOI] [PubMed] [Google Scholar]
- Lory S., Tai P. C., Davis B. D. Mechanism of protein excretion by gram-negative bacteria: Pseudomonas aeruginosa exotoxin A. J Bacteriol. 1983 Nov;156(2):695–702. doi: 10.1128/jb.156.2.695-702.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luedtke R., Owen C. S., Karush F. Proximity of antibody binding sites studied by fluorescence energy transfer. Biochemistry. 1980 Mar 18;19(6):1182–1192. doi: 10.1021/bi00547a023. [DOI] [PubMed] [Google Scholar]
- Michaelis S., Chapon C., D'Enfert C., Pugsley A. P., Schwartz M. Characterization and expression of the structural gene for pullulanase, a maltose-inducible secreted protein of Klebsiella pneumoniae. J Bacteriol. 1985 Nov;164(2):633–638. doi: 10.1128/jb.164.2.633-638.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima S. Post-translational modification and processing of outer membrane prolipoproteins in Escherichia coli. Mol Cell Biochem. 1984;60(1):5–15. doi: 10.1007/BF00226297. [DOI] [PubMed] [Google Scholar]
- Neugebauer K., Sprengel R., Schaller H. Penicillinase from Bacillus licheniformis: nucleotide sequence of the gene and implications for the biosynthesis of a secretory protein in a Gram-positive bacterium. Nucleic Acids Res. 1981 Jun 11;9(11):2577–2588. doi: 10.1093/nar/9.11.2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J. B., Caulfield M. P., Lampen J. O. Lipoprotein nature of Bacillus licheniformis membrane penicillinase. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3511–3515. doi: 10.1073/pnas.78.6.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmura K., Nakamura K., Yamazaki H., Shiroza T., Yamane K., Jigami Y., Tanaka H., Yoda K., Yamasaki M., Tamura G. Length and structural effect of signal peptides derived from Bacillus subtilis alpha-amylase on secretion of Escherichia coli beta-lactamase in B. subtilis cells. Nucleic Acids Res. 1984 Jul 11;12(13):5307–5319. doi: 10.1093/nar/12.13.5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perumal N. B., Minkley E. G., Jr The product of the F sex factor traT surface exclusion gene is a lipoprotein. J Biol Chem. 1984 May 10;259(9):5357–5360. [PubMed] [Google Scholar]
- Priest F. G. Extracellular enzyme synthesis in the genus Bacillus. Bacteriol Rev. 1977 Sep;41(3):711–753. doi: 10.1128/br.41.3.711-753.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley A. P., Schnaitman C. A. Factors affecting the electrophoretic mobility of the major outer membrane proteins of Escherichia coli in polyacrylamide gels. Biochim Biophys Acta. 1979 Nov 23;581(1):163–178. doi: 10.1016/0005-2795(79)90233-2. [DOI] [PubMed] [Google Scholar]
- Pugsley A. P., Schwartz M. Colicin E2 release: lysis, leakage or secretion? Possible role of a phospholipase. EMBO J. 1984 Oct;3(10):2393–2397. doi: 10.1002/j.1460-2075.1984.tb02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz G., Braun V. Cell-bound and secreted proteases of Serratia marcescens. J Bacteriol. 1985 Mar;161(3):1002–1009. doi: 10.1128/jb.161.3.1002-1009.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K., Sarvas M., Garoff H., Helenius A. Membrane-bound and secreted forms of penicillinase from Bacillus licheniformis. J Mol Biol. 1978 Dec 25;126(4):673–690. doi: 10.1016/0022-2836(78)90015-3. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E., Mocca L. F. Five structural classes of major outer membrane proteins in Neisseria meningitidis. J Bacteriol. 1981 Apr;146(1):69–78. doi: 10.1128/jb.146.1.69-78.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wöhner G., Wöber G. Pullulanase, an enzyme of starch catabolism, is associated with the outer membrane of Klebsiella. Arch Microbiol. 1978 Mar;116(3):303–310. doi: 10.1007/BF00417856. [DOI] [PubMed] [Google Scholar]