Abstract
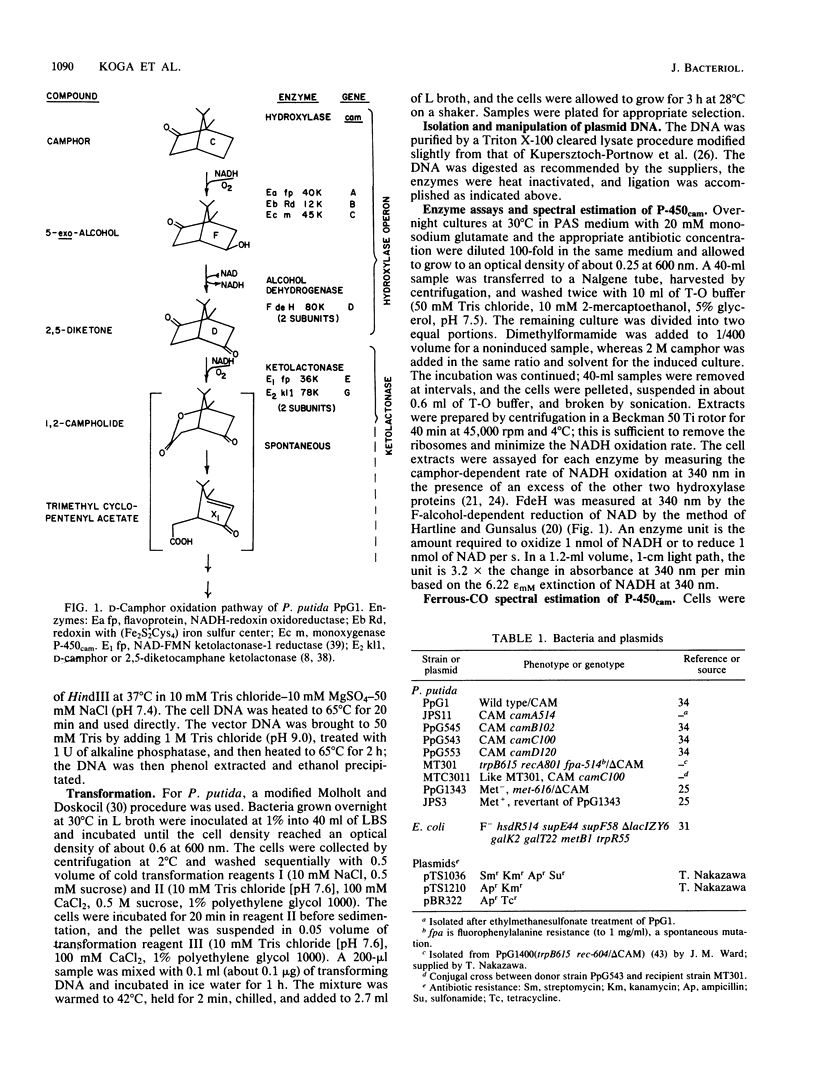
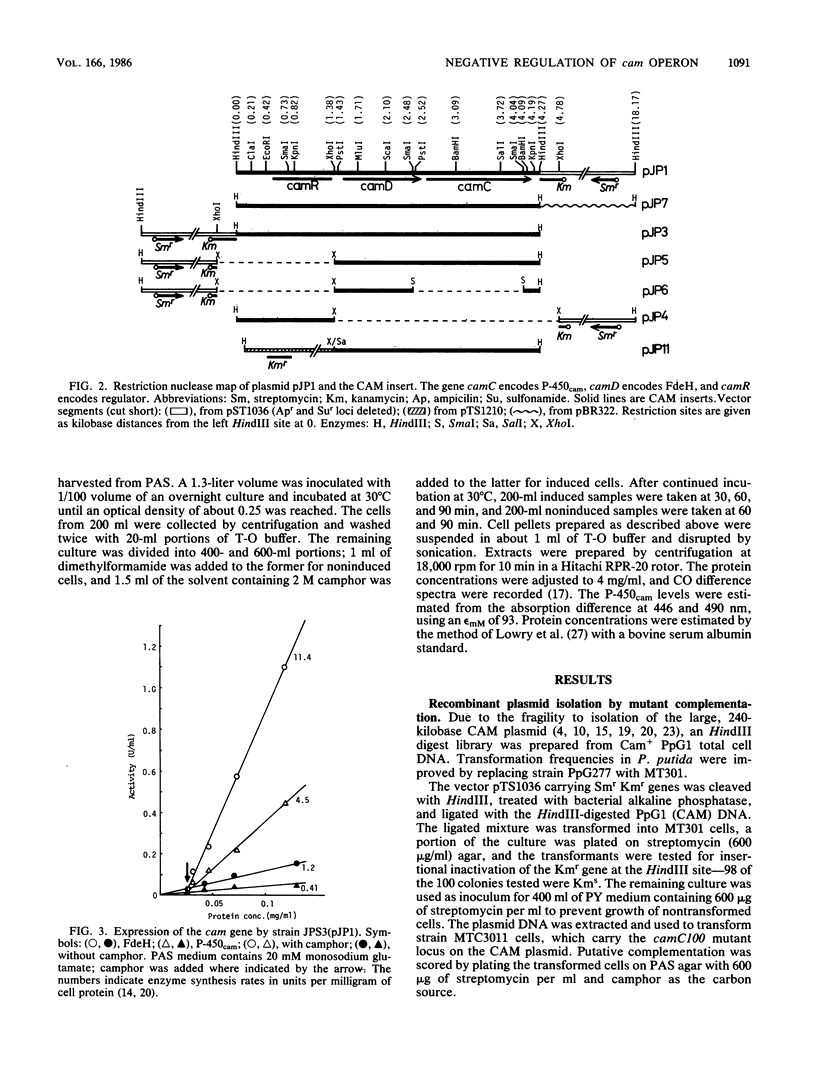
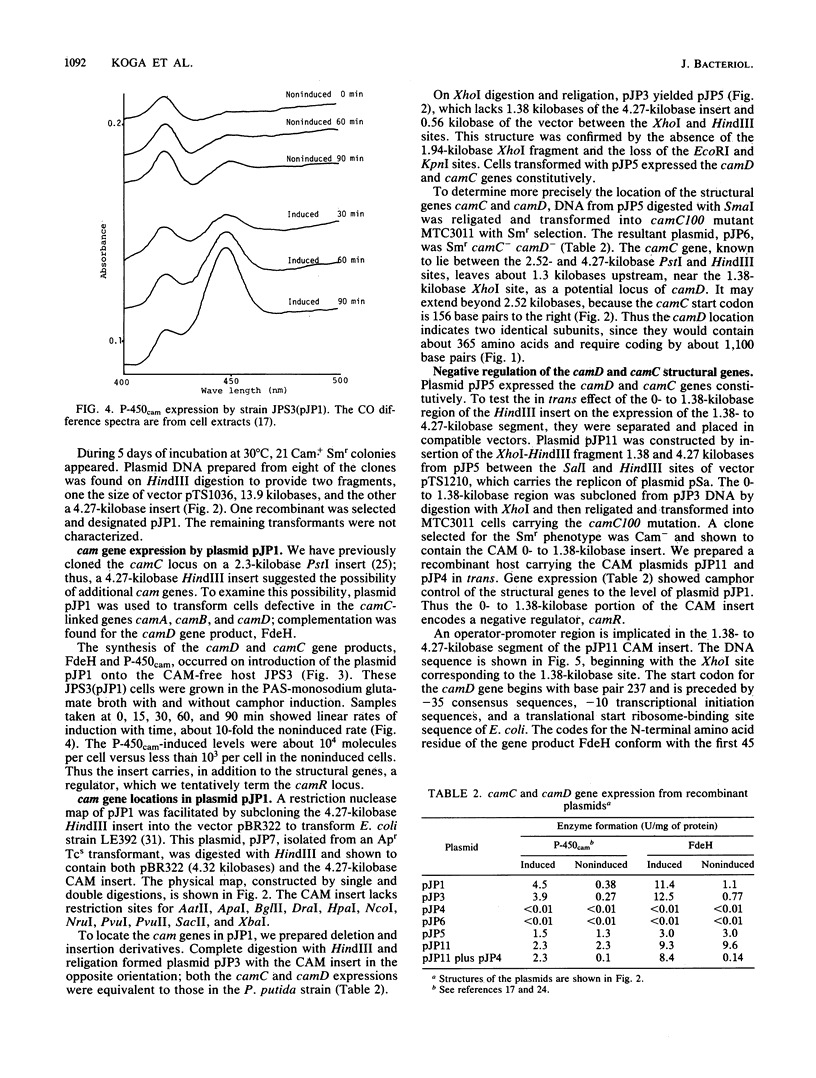
A 4.27-kilobase insert from a HindIII DNA library of Pseudomonas putida carrying the CAM plasmid allowed coordinate expression of genes camD and camC under control of camR, an upstream regulator. The camC gene specifies cytochrome P-450cam, and camD specifies the 5-exo-alcohol dehydrogenase. A 1.38-kilobase deletion from the insert results in the constitutive expression of genes camC and camD; transformation in trans restores the substrate control, indicating that camR is a negative regulator.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoyama Y., Yoshida Y. The 14alpha-demethylation of lanosterol by a reconstituted cytochrome P-450 system from yeast microsomes. Biochem Biophys Res Commun. 1978 Nov 14;85(1):28–34. doi: 10.1016/s0006-291x(78)80006-0. [DOI] [PubMed] [Google Scholar]
- CONRAD H. E., DUBUS R., GUNSALUS I. C. An enzyme system for cyclic ketone lactonization. Biochem Biophys Res Commun. 1961 Nov 29;6:293–297. doi: 10.1016/0006-291x(61)90382-5. [DOI] [PubMed] [Google Scholar]
- CONRAD H. E., HEDEGAARD J., GUNSALUS I. C. LASTONE INTERMEDIATES IN THE MICROBIAL OXIDATION OF (+)-CAMPHOR. Tetrahedron Lett. 1965 Mar 10;10:561–565. doi: 10.1016/s0040-4039(00)89996-7. [DOI] [PubMed] [Google Scholar]
- Cardini G., Jurtshuk P. The enzymatic hydroxylation of n-octane by Corynebacterium sp. strain 7E1C. J Biol Chem. 1970 Jun 10;245(11):2789–2796. [PubMed] [Google Scholar]
- Chakrabarty A. M. Genetic basis of the biodegradation of salicylate in Pseudomonas. J Bacteriol. 1972 Nov;112(2):815–823. doi: 10.1128/jb.112.2.815-823.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty A. M., Gunsalus I. C. Chromosomal mobilization from a recA mutant of Pseudomonas putida. Mol Gen Genet. 1979 Oct 2;176(1):151–154. doi: 10.1007/BF00334307. [DOI] [PubMed] [Google Scholar]
- Currier T. C., Nester E. W. Isolation of covalently closed circular DNA of high molecular weight from bacteria. Anal Biochem. 1976 Dec;76(2):431–441. doi: 10.1016/0003-2697(76)90338-9. [DOI] [PubMed] [Google Scholar]
- Feyereisen R., Pratt G. E., Hamnett A. F. Enzymic synthesis of juvenile hormone in locust corpora allata: evidence for a microsomal cytochrome P-450 linked methyl farnesoate epoxidase. Eur J Biochem. 1981 Aug;118(2):231–238. doi: 10.1111/j.1432-1033.1981.tb06391.x. [DOI] [PubMed] [Google Scholar]
- Franklin F. C., Bagdasarian M., Bagdasarian M. M., Timmis K. N. Molecular and functional analysis of the TOL plasmid pWWO from Pseudomonas putida and cloning of genes for the entire regulated aromatic ring meta cleavage pathway. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7458–7462. doi: 10.1073/pnas.78.12.7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin F. C., Williams P. A. Construction of a partial diploid for the degradative pathway encoded by the TOL plasmid (pWWO) from Pseudomonas putida mt-2: evidence for the positive nature of the regulation by the xyIR gene. Mol Gen Genet. 1980 Jan;177(2):321–328. doi: 10.1007/BF00267445. [DOI] [PubMed] [Google Scholar]
- Fulco A. J., Kim B. H., Matson R. S., Narhi L. O., Ruettinger R. T. Nonsubstrate induction of a soluble bacterial cytochrome P-450 monooxygenase by phenobarbital and its analogs. Mol Cell Biochem. 1983;53-54(1-2):155–161. doi: 10.1007/BF00225251. [DOI] [PubMed] [Google Scholar]
- Grund A. D., Gunsalus I. C. Cloning of genes for naphthalene metabolism in Pseudomonas putida. J Bacteriol. 1983 Oct;156(1):89–94. doi: 10.1128/jb.156.1.89-94.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunsalus C., Gunsalus C. F., Chakrabarty A. M., Sikes S., Crawford I. P. Fine structure mapping of the tryptophan genes in Pseudomonas putida. Genetics. 1968 Nov;60(3):419–435. doi: 10.1093/genetics/60.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunsalus I. C., Wagner G. C. Bacterial P-450cam methylene monooxygenase components: cytochrome m, putidaredoxin, and putidaredoxin reductase. Methods Enzymol. 1978;52:166–188. doi: 10.1016/s0076-6879(78)52019-3. [DOI] [PubMed] [Google Scholar]
- Haniu M., Armes L. G., Yasunobu K. T., Shastry B. A., Gunsalus I. C. Amino acid sequence of the Pseudomonas putida cytochrome P-450. II. Cyanogen bromide peptides, acid cleavage peptides, and the complete sequence. J Biol Chem. 1982 Nov 10;257(21):12664–12671. [PubMed] [Google Scholar]
- Hansen J. B., Olsen R. H. Isolation of large bacterial plasmids and characterization of the P2 incompatibility group plasmids pMG1 and pMG5. J Bacteriol. 1978 Jul;135(1):227–238. doi: 10.1128/jb.135.1.227-238.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartline R. A., Gunsalus I. C. Induction specificity and catabolite repression of the early enzymes in camphor degradation by Pseudomonas putida. J Bacteriol. 1971 May;106(2):468–478. doi: 10.1128/jb.106.2.468-478.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedegaard J., Gunsalus I. C. Mixed function oxidation. IV. An induced methylene hydroxylase in camphor oxidation. J Biol Chem. 1965 Oct;240(10):4038–4043. [PubMed] [Google Scholar]
- Inouye S., Nakazawa A., Nakazawa T. Molecular cloning of gene xylS of the TOL plasmid: evidence for positive regulation of the xylDEGF operon by xylS. J Bacteriol. 1981 Nov;148(2):413–418. doi: 10.1128/jb.148.2.413-418.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri M., Ganguli B. N., Gunsalus I. C. A soluble cytochrome P-450 functional in methylene hydroxylation. J Biol Chem. 1968 Jun 25;243(12):3543–3546. [PubMed] [Google Scholar]
- Koga H., Rauchfuss B., Gunsalus I. C. P450cam gene cloning and expression in Pseudomonas putida and Escherichia coli. Biochem Biophys Res Commun. 1985 Jul 16;130(1):412–417. doi: 10.1016/0006-291x(85)90432-2. [DOI] [PubMed] [Google Scholar]
- Kupersztoch-Portnoy Y. M., Lovett M. A., Helinski D. R. Strand and site specificity of the relaxation event for the relaxation complex of the antibiotic resistance plasmid R6K. Biochemistry. 1974 Dec 31;13(27):5484–5490. doi: 10.1021/bi00724a005. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Madyastha K. M., Meehan T. D., Coscia C. J. Characterization of a cytochrome P-450 dependent monoterpene hydroxylase from the higher plant Vinca rosea. Biochemistry. 1976 Mar 9;15(5):1097–1102. doi: 10.1021/bi00650a023. [DOI] [PubMed] [Google Scholar]
- Molholt B., Doskocil J. Increased transformation frequency in E. coli. Biochem Biophys Res Commun. 1978 May 30;82(2):477–483. doi: 10.1016/0006-291x(78)90899-9. [DOI] [PubMed] [Google Scholar]
- Murray N. E., Brammar W. J., Murray K. Lambdoid phages that simplify the recovery of in vitro recombinants. Mol Gen Genet. 1977 Jan 7;150(1):53–61. doi: 10.1007/BF02425325. [DOI] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- Palchaudhuri S. Molecular characterization of hydrocarbon degradative plasmids in Pseudomonas putida. Biochem Biophys Res Commun. 1977 Jul 25;77(2):518–525. doi: 10.1016/s0006-291x(77)80010-7. [DOI] [PubMed] [Google Scholar]
- Rheinwald J. G., Chakrabarty A. M., Gunsalus I. C. A transmissible plasmid controlling camphor oxidation in Pseudomonas putida. Proc Natl Acad Sci U S A. 1973 Mar;70(3):885–889. doi: 10.1073/pnas.70.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait R. C., Close T. J., Rodriguez R. L., Kado C. I. Isolation of the origin of replication of the IncW-group plasmid pSa. Gene. 1982 Nov;20(1):39–49. doi: 10.1016/0378-1119(82)90085-3. [DOI] [PubMed] [Google Scholar]
- Taylor D. G., Trudgill P. W. Camphor revisited: studies of 2,5-diketocamphane 1,2-monooxygenase from Pseudomonas putida ATCC 17453. J Bacteriol. 1986 Feb;165(2):489–497. doi: 10.1128/jb.165.2.489-497.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudgill P. W., DuBus R., Gunsalus I. C. Mixed function oxidation. V. Flavin interaction with a reduced diphosphopyridine nucleotide dehydrogenase, one of the enzymes participating in camphor lactonization. J Biol Chem. 1966 Mar 10;241(5):1194–1205. [PubMed] [Google Scholar]
- Unger B. P., Gunsalus I. C., Sligar S. G. Nucleotide sequence of the Pseudomonas putida cytochrome P-450cam gene and its expression in Escherichia coli. J Biol Chem. 1986 Jan 25;261(3):1158–1163. [PubMed] [Google Scholar]
- White R. E., Coon M. J. Oxygen activation by cytochrome P-450. Annu Rev Biochem. 1980;49:315–356. doi: 10.1146/annurev.bi.49.070180.001531. [DOI] [PubMed] [Google Scholar]
- White R. E., McCarthy M. B., Egeberg K. D., Sligar S. G. Regioselectivity in the cytochromes P-450: control by protein constraints and by chemical reactivities. Arch Biochem Biophys. 1984 Feb 1;228(2):493–502. doi: 10.1016/0003-9861(84)90015-8. [DOI] [PubMed] [Google Scholar]
- Yen K. M., Gunsalus I. C. Regulation of naphthalene catabolic genes of plasmid NAH7. J Bacteriol. 1985 Jun;162(3):1008–1013. doi: 10.1128/jb.162.3.1008-1013.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]



