Abstract
As the oxidation of low density lipoprotein (LDL) lipids may be a key event in atherogenesis, there is interest in antioxidants as potential anti-atherogenic compounds. Here we report that α-tocopheryl hydroquinone (α-TQH2) strongly inhibited or completely prevented the (per)oxidation of ubiquinol-10 (CoQ10H2), α-tocopherol (α-TOH), and both surface and core lipids in LDL exposed to either aqueous or lipophilic peroxyl radicals, Cu2+, soybean lipoxygenase, or the transition metal-containing Ham’s F-10 medium in the absence or presence of human monocyte-derived macrophages. The antioxidant activity of α-TQH2 was superior to that of several other lipophilic hydroquinones, including endogenous CoQ10H2, which is regarded as LDL’s first line of antioxidant defence. At least three independent activities contributed to the antioxidant action of α-TQH2. First, α-TQH2 readily associated with LDL and instantaneously reduced the lipoprotein’s ubiquinone-10 to CoQ10H2, thereby maintaining this antioxidant in its active form. Second, α-TQH2 directly intercepted aqueous peroxyl radicals, as indicated by the increased rate of its consumption with increasing rates of radical production, independent of LDL’s content of CoQ10H2 and α-TOH. Third, α-TQH2 rapidly quenched α-tocopheroxyl radical in oxidizing LDL, as demonstrated directly by electron paramagnetic resonance spectroscopy. Similar antioxidant activities were also seen when α-TQH2 was added to high-density lipoprotein or the protein-free Intralipid, indicating that the potent antioxidant activity of α-TQH2 was neither lipoprotein specific nor dependent on proteins. These results suggest that α-TQH2 is a candidate for a therapeutic lipid-soluble antioxidant. As α-tocopherylquinone is formed in vivo at sites of oxidative stress, including human atherosclerotic plaque, and biological systems exist that reduce the quinone to the hydroquinone, our results also suggest that α-TQH2 could be a previously unrecognized natural antioxidant.
Keywords: vitamin E, tocopherol-mediated peroxidation, co-antioxidation, atherogenesis
The oxidation of low density lipoprotein (LDL) is regarded as one of the early and key events in atherogenesis (1). As a result of the breakdown of oxidized lipids, LDL’s apolipoprotein B-100 (apoB) may become modified, and this can result in the uncontrolled cellular uptake of the lipoprotein, leading to the formation of lipid-laden “foam” cells (1, 2). Oxidized lipid component(s) may also be responsible for recognition of modified LDL, e.g., by the thrombospondin CD 36 receptor (3). In addition, oxidized LDL has many additional pro-atherogenic activities, so that inhibition of LDL lipid (per)oxidation might be beneficial and retard atherogenesis (4).
Recently, we proposed a novel molecular mechanism of radical-initiated lipid peroxidation in isolated LDL (5–7) and extended it to other isolated lipoproteins and to lipoproteins in human plasma (8–10). This model, referred to as tocopherol-mediated peroxidation (TMP), predicts that α-tocopherol (α-TOH, biologically the most active form of vitamin E) present in lipoproteins will aid the “entry” of radical oxidants into the particle by acting as a phase-transfer agent. Once inside, the radical will be present predominantly as α-tocopheroxyl radical (α-TO⋅) that, under relatively mild oxidizing conditions, will initiate and propagate the formation of lipid hydroperoxides by acting as the lipid peroxidation chain-carrying species. This chain transfer activity of α-TOH is inhibited by either high rates of radical entry into the lipoprotein particle (resulting in radical-radical termination reactions) (6, 10) or the presence of suitable reductants capable of “exporting” the radical from the lipoprotein back into the aqueous compartment (11). Human blood plasma (12) and interstitial fluids (13) contain several such reductants, referred to as co-antioxidants (11). Of these, ubiquinol-10 (CoQ10H2) (14) and ascorbate (15) form the first line of non-proteinaceous antioxidant defence; in their presence, α-TOH efficiently protects the lipids in isolated LDL and plasma against in vitro oxidation (15–17).
It is not known how and where LDL becomes oxidized during atherogenesis. However, oxidation most likely takes place in the subendothelial space where, at least at the late stages of the disease, the levels of oxidized lipids are approximately 105-fold higher (17) than in plasma of severely diseased subjects (18). Despite such high levels of oxidized lipids, human atherosclerotic plaque contains large amounts of ascorbate and α-TOH when expressed per protein and oxidizable lipid, respectively (17). This could suggest that lipid peroxidation in the intima proceeds via TMP, perhaps within micro-environments from which aqueous co-antioxidants such as ascorbate are excluded. In such a case, lipid-soluble co-antioxidants that associate with LDL could conceivably be of greater importance than aqueous co-antioxidants in the inhibition of TMP, and possibly atherogenesis.
Previous in vitro screening of a large number of natural and synthetic compounds for co-antioxidant activity (19) indicated high efficacy for hydroquinones. We now report on a group of lipophilic hydroquinones as powerful inhibitors of LDL lipid peroxidation. Among them, α-tocopheryl hydroquinone (α-TQH2) was found to be most potent, capable of efficiently reducing α-TO⋅ as well as directly scavenging aqueous radicals and reducing ubiquinone-10 (CoQ10) to CoQ10H2 in LDL, thereby also maintaining this co-antioxidant in the active form.
MATERIALS AND METHODS
Native LDL and high density lipoprotein (HDL) were isolated from fresh plasma by 2-h density ultracentrifugation (20). Where indicated, LDL was enriched with (21) or depleted of (10) α-TOH in vitro, or by isolation from plasma obtained from a vitamin E-deficient (FIVE) patient (22, 23) either denied vitamin E supplements for 5 consecutive days (α-TOH-depleted) or after 2 or 5 days of vitamin E supplementation [partially or fully α-TOH-replenished samples, respectively (10)]. Before use, the lipoproteins were passed over two consecutive PD-10 columns (Pharmacia) or, for those prepared from the FIVE patient’s plasma, dialyzed against 50 mM of phosphate-buffered saline (PBS; pH 7.4). For peroxynitrite (ONOO−) oxidations, LDL was prepared in 200 mM of phosphate buffer (pH 7.2). All buffers used were treated with Chelex-100 (Bio-Rad) to remove contaminating transition metals.
LDL and HDL at the concentration indicated were oxidized under an atmosphere of air using 2,2′-azobis(2-amidinopropane) dihydrochloride (AAPH), 2,2′-azobis(2,4-dimethylvaleronitrile) (AMVN) (Polysciences), soybean 15-lipoxygenase (SLO; type V, Sigma), CuSO4, ONOO− (prepared according to ref. 24), or hypochlorite (−OCl, added as NaOCl) at the concentration indicated or by incubation in Ham’s F-10 medium in the presence and absence of human monocyte-derived macrophages (MDM), prepared as described (25). Hydroquinones were prepared freshly in ethanol and added at 10 μM final concentration [ethanol <1% (vol/vol)], with the appropriate volume of ethanol added to the control samples. The hydroquinones used were tert-butylhydroquinone, 2,5-di-tert-butylhydroquinone (2,5-DTBHQ), and 3,5-di-tert-butylhydroquinone (3,5-DTBHQ) (Aldrich), α-TQH2 and 1,4-dihydroxy-2-(3-hydroxy-3-methylbutyl)-3,5,6-trimethyl-1,4-benzene (α-PQH2). α-TQH2 and α-PQH2 were prepared immediately before use by sodium dithionite reduction of α-tocopheryl quinone (RRR-α-TQ) (Kodak or Acros) and 2-(3-hydroxy-3-methylbutyl)-3,5,6-trimethyl-1,4-benzoquinone (provided by C. Suarna, Heart Research Institute, Sydney, Australia), respectively (ɛ285 nm = 4075 M−1⋅cm−1).
For the stability assay, α-TQH2 (10 μM final concentration) was added to native LDL, α-TOH-depleted LDL, fresh HDL, or Intralipid [20% (wt/vol); Pharmacia] diluted 10 times in PBS. The samples were then incubated at 37°C and aliquots were withdrawn at various time points. The extracts were then analyzed for α-TQH2 and α-TQ and, where applicable, for CoQ10H2 and CoQ10 as described below. A total LDL lipid extract was prepared by extracting the lipoprotein into chloroform (26).
EPR experiments were performed using a Bruker (Billerica, MA) ESP 300 spectrometer fitted with an X-band cavity and, at 20 mW, frequency 9.4 GHz and averaging the output from five successive accumulations with sweep time of 20.5 s. For generation of α-TO⋅ (27), LDL (7.2 μM in apoB) was incubated with SLO (1 mg/ml) at 37°C for 5 min before EPR spectra were recorded in the presence of ethanol (control) or 10 μM of α-TQH2.
Aliquots (50–200 μl) of the lipoprotein samples were extracted with methanol/hexane and analyzed for α-TOH, CoQ10, and CoQ10H2, unoxidized lipids (cholesterol and cholesteryl esters), hydroperoxides of cholesteryl esters, and phosphatidyl choline (PC-OOH) by reversed-phase HPLC with electrochemical, ultraviolet, and chemiluminescence detection, respectively, as described (20), except that UV234 nm absorbance rather than chemiluminescence detection was used for cholesteryl ester hydroperoxides. The UV absorbance-based method detects both the cholesteryl ester hydroperoxides and hydroxides [referred to as CE-O(O)H]. Standards of cholesteryllinoleate hydroperoxides [used for CE-O(O)H] and PC-OOH were prepared as described in (20). The concentration of the various analytes was determined by area comparison with authentic standards, using free cholesterol as internal standard for CE-O(O)H, α-TOH, CoQ10, and CoQ10H2, and α-TOH as internal standard for α-TQH2 and α-TQ, measured as described in ref. 28. The quinones and hydroquinones were measured immediately upon generation of appropriate samples to avoid adventitious autoxidation upon storage. Determination of triglyceride hydroperoxides in oxidizing Intralipid was performed as described previously (8). Protein was determined by the bicinchoninic acid assay using bovine serum albumin as standard. The concentrations of LDL and HDL were calculated from the cholesterol determination, assuming a protein molecular mass of 500 and 35 kDa or 550 and 35 molecules of free cholesterol per LDL and HDL particle, respectively.
RESULTS AND DISCUSSION
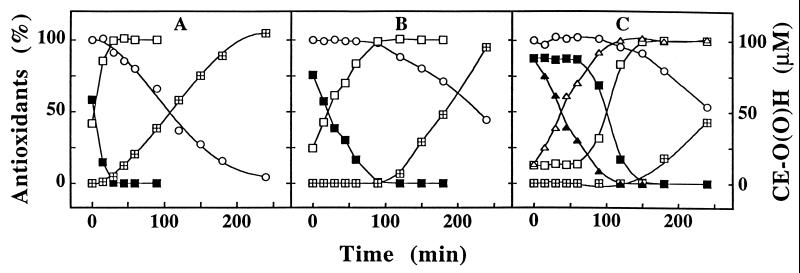
Previous studies have shown that in freshly isolated LDL 60–80% of endogenous coenzyme Q is present as CoQ10H2 (29) and that upon exposure to aqueous or lipophilic radical oxidants, CoQ10H2 is converted to CoQ10 before the concomitant consumption of α-TOH and accumulation of CE-O(O)H and PC-OOH (see refs. 30 and 31). Fig. 1 confirms these results for a constant flux of aqueous peroxyl radicals (ROO⋅) produced from AAPH and, for the first time, shows that the initial levels of CoQ10H2 increased upon addition of either 2,5-DTBHQ (Fig. 1B) or α-TQH2 (Fig. 1C) to native LDL before oxidation. Addition of α-PQH2 or 3,5-DTBHQ, but not tert-butylhydroquinone, butylated hydroxytoluene (10 μM in ethanol), superoxide dismutase (1000 units/ml), or catalase (1000 units/ml), also increased the initial level of LDL’s CoQ10H2 (data not shown). The presence of 2,5-DTBHQ (Fig. 1B) or 3,5-DTBHQ (data not shown) reduced the rate of ROO⋅-induced CoQ10H2 oxidation, whereas α-TQH2 (Fig. 1C) and α-PQH2 (data not shown) completely prevented the onset of CoQ10H2 oxidation for up to 1 h, during which time α-TQH2 was oxidized stoichiometrically to α-TQ. Importantly, the presence of any of the five hydroquinones delayed the consumption of α-TOH and accumulation of CE-O(O)H, with the order of efficacy being α-TQH2 > α-PQH2 > 2,5-DTBHQ = 3,5-DTBHQ > tert-butylhydroquinone (partially shown in Fig. 1).
Figure 1.
Hydroquinones protect LDL’s cholesteryl esters from ROO⋅-induced peroxidation. An LDL solution (1.2 μM in apoB) was incubated at 37°C with 2 mM of AAPH in the absence (A) or presence (B) of 10 μM of 2,5-DTBHQ or α-TQH2 (C). At the time points indicated, aliquots were taken and analyzed for CoQ10H2 (▪), CoQ10 (□), α-TOH (○), and CE-O(O)H (⊞) and, where applicable, for α-TQH2 (▴) and α-TQ (▵). The data shown represent mean values of three separate experiments with a variation of <15%. The initial concentrations of CoQ10H2, CoQ10, and α-TOH in the LDL solution were 0.73 ± 0.15, 0.43 ± 0.09, and 9.5 ± 0.9 μM, respectively. The initial levels of α-TQH2 and α-TQ measured after addition of the freshly prepared hydroquinone to LDL varied between 8.9 to 9.1 and 1.1 to 1.6 μM, respectively. Note that the sum of CoQ10H2 plus CoQ10 and that of α-TQH2 plus α-TQ represent 100%.
To assess the ability of α-TQH2 to “incorporate” into LDL, the hydroquinone (final concentration 10 μM in ethanol) was added to the lipoprotein emulsion (1.3 μM in apoB) and placed on ice for ≈0.5–1 min; the LDL was subsequently gel filtered (using cold PBS) through two sequential PD-10 columns, after which 96% and 86% of the α-TQH2 added was recovered with LDL. In addition, in a separate experiment, 10 μM of α-TQH2 was added to different LDL samples (1 ml each) prepared from three different subjects (concentration of LDL 1.2, 0.8, and 1.5 μM apoB, respectively), after which the individual LDL samples were re-isolated by 2-h density gradient ultracentrifugation (20). Recovery of total tocopherylquinone expressed as the sum of α-TQ and α-TQH2 in such re-isolated LDL was 84 ± 4.1% (mean ± SD, n = 3) of the total tocopherylquinone in LDL prior to centrifugation. Together, these results indicated that the majority of the added α-TQH2 associated strongly with LDL.
Because substantial amounts of α-TQ are present in extracts of human atherosclerotic plaque (17), and cells can reduce α-TQ to α-TQH2 (28, 32), we tested the ability of the hydroquinone to inhibit LDL lipid oxidation initiated by different oxidants. As can be seen from Table 1, α-TQH2 was highly efficient in protecting LDL lipids against either AAPH, AMVN, SLO, Cu2+, or Ham’s F-10 medium in the presence and absence of MDM. Examination of the kinetics of lipid oxidation revealed that for each oxidant used, α-TQH2 was consumed before CoQ10H2 (as shown in Fig. 1 for AAPH), indicating that α-TQH2 not only effectively suppressed lipid peroxidation but did so in preference to CoQ10H2, itself regarded as a first line of LDL’s antioxidant defence (14, 16).
Table 1.
α-TQH2 effectively inhibits LDL lipid peroxidation induced by different oxidants
| Oxidant* | Onset of consumption,† min
|
Accumulation of LOOH,‡ μM
|
||
|---|---|---|---|---|
| CoQ10H2 | α-TOH | CE-O(O)H | PC-OOH | |
| AAPH | ||||
| − | 0 | 30 ± 10 | 20.4 ± 3.9 | 7.8 ± 3.1 |
| + | 20 ± 10 | 150 ± 25 | 0 | 0 |
| AMVN | ||||
| − | 0 | 30 ± 15 | 36.6 ± 11.5 | 10.5 ± 2.5 |
| + | 120 ± 2 | 270 ± 28 | 0 | 0 |
| SLO | ||||
| − | 0 | 33 ± 13 | 24.8 ± 8 | 9.3 ± 2.2 |
| + | 140 ± 20 | >450 | 0.6 ± 0.35 | 0 |
| Cu2+ (1)§ | ||||
| − | 0 | 0 | 27 ± 3.6 | ND |
| + | <5¶ | 35 ± 5 | 4 ± 1.2 | ND |
| Cu2+ (2) | ||||
| − | 0 | 30 ± 15 | 16.2 ± 3.3 | 4.3 ± 0.3 |
| + | 90 ± 11 | >360 | 0 | 0 |
| F-10 | ||||
| − | 0 | 145 ± 45 | 9.5 ± 4 | 1.8 ± 0.3 |
| + | >720 | >720 | 0 | 0 |
| MDM | ||||
| − | 0 | 95 ± 40 | 16.8 ± 11 | 3.3 ± 1.2 |
| + | 300 | >720 | 0 | 0 |
The results shown represent means ± SD from three separate experiments from two to three different donors. ND, not determined.
LDL (0.8–1.2 μM apoB) was supplemented with 10 μM α-TQH2 (+) or the appropriate volume of ethanol (−) immediately before oxidation. AAPH was used at 2 mM, AMVN at 1 mM, and SLO at 4 μg/ml. Cu2+ was used at either 16.7 (1) or 1.5 (2) molecules of Cu2+ per LDL particle. For cell-enhanced oxidation, LDL was diluted 5 times with sterile Ham’s F-10 medium and incubated in the absence (F-10) or presence (MDM) of ≈106 MDM per well.
Onset of oxidation refers to the first signs of significant decrease of CoQ10H2 or α-TOH.
The extent of lipid hydroperoxide accumulation was determined at a time where 20% of the initial α-TOH was depleted in the control, α-TQH2-free LDL samples. The times required for this varied for the different oxidants and were approximately: AAPH, 140 min; AMVN, 315 min; SLO, 315 min; Cu2+ (2), 180 min; F-10, 300 min; and MDM, 210 min.
For Cu2+ under condition (1), 25 μM of α-TQH2 was added and the level of CE-O(O)H accumulation was compared after complete α-TOH consumption and at a time of maximum peroxidation in the control LDL (≈120 min), as under these very strong oxidizing conditions only little lipid hydroperoxides accumulate as long as the vitamin is present (for example, see ref. 10).
Five minutes represents the earliest time point measured, where there was already detectable CoQ10H2 consumption.
All of the above agents have been demonstrated to be able to oxidize LDL’s lipids via TMP (10). We next tested the antioxidant efficacy of α-TQH2 for LDL exposed to ONOO− (≤500 mol per mol apoB) and −OCl (≤1100 mol per mol apoB), as these oxidants can react via nucleophilic attack. α-TQH2 also protected LDL lipids from such oxidants, as judged by the decrease in both the accumulation of CE-O(O)H and the consumption of CoQ10H2 and α-TOH. These results indicate that α-TQH2 is an outstanding antioxidant for LDL’s lipids. Preliminary experiments indicate, however, that α-TQH2 is less able to inhibit the oxidation of apoB induced by −OCl and ONOO−, as assessed by its inability to inhibit the loss of the tryptophan fluorescence by these oxidants (data not shown). This may suggest that α-TQH2 is not an efficient antioxidant for LDL’s protein, although this requires further examination. It is noteworthy that apoB is the major initial target for −OCl (33), whereas for the group of oxidants that peroxidize LDL via TMP, the lipoprotein lipid components are initially the major targets.
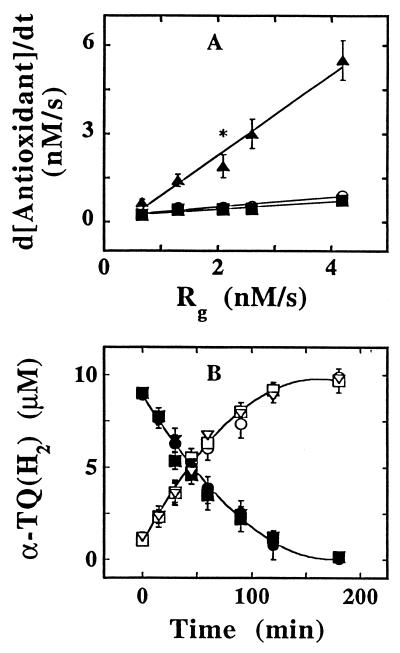
We next investigated the mechanism(s) by which α-TQH2 exhibited such strong antioxidant activity for LDL’s lipids. Fig. 2A shows the rates of oxidation of α-TOH, CoQ10H2, and α-TQH2 in LDL exposed to increasing rates (Rg) of generation of ROO⋅. As predicted from the model of TMP and shown previously (6), the rate of α-TOH consumption was largely unaffected by changes in Rg. Similarly unaffected was the rate of CoQ10H2 consumption (Fig. 2A), indicating that this antioxidant acted primarily as a co-antioxidant, i.e., reacting with α-TO⋅ rather than ROO⋅. In sharp contrast with α-TOH and CoQ10H2, the rate of α-TQH2 consumption closely matched and increased with increasing Rg, i.e., d[α-TQH2]/dt ≈ Rg (Fig. 2A). Also, the rates of α-TQH2 consumption remained largely unaltered when LDL (0.75 μM apoB) containing different concentrations of α-TOH was oxidized by 0.5 mM of AAPH. Thus, 0.81, 0.65, and 0.85 nmol α-TQH2 liter−1⋅s−1 were oxidized in AAPH-exposed, in vitro α-TOH-depleted, native and in vitro α-TOH-enriched LDL containing 0, 8.2, and 101.6 mol of α-TOH per mol apoB, respectively. In the same experiment, the corresponding rates of α-TOH oxidation were 0.26 and 1.2 nmol liter−1⋅s−1 for the native and α-TOH-supplemented LDL, respectively. Thus, α-TQH2 appeared to directly intercept at least some of the lipid peroxidation-inducing ROO⋅.
Figure 2.
The consumption of α-TQH2 during LDL oxidation is dependent on the rate of ROO⋅ production but independent of the α-TOH content of the lipoprotein. (A) LDL (1 μM in apoB), control or supplemented with 10 μM α-TQH2, was oxidized with increasing concentrations of AAPH, and the rates of consumption of CoQ10H2 (▪) and α-TOH (○) in the control and α-TQH2 (▴) in the supplemented LDL estimated from the linear portion of their respective depletion curves. Rg was calculated from Rg = 1.3 × 10−3 [AAPH] nM/s (34). The data shown are average values obtained from two or three (indicated by asterisk) independent experiments, with the range indicated by the vertical lines. (B) LDL (1.5 μM in apoB) from a FIVE patient depleted (circles), partially (squares), or fully re-supplemented with α-TOH (triangles) was incubated with 1 mM AAPH in the presence of 10 μM of α-TQH2. At the time points indicated, an aliquot was taken and analyzed for α-TQH2 (solid symbols) and α-TQ (open symbols). The data shown are mean values ± SD of three independent experiments performed with LDL prepared from plasma of one FIVE patient.
To rule out the possibility of an artefact with the above LDL samples whose α-TOH content was manipulated in vitro, we performed similar AAPH-induced oxidation experiments with lipoproteins containing different endogenous levels of α-TOH isolated from the plasma of a FIVE patient (see Materials and Methods and ref. 10). As was the case with the in vitro manipulated samples, the rates of oxidation of α-TQH2 to α-TQ were the same despite the up to 10-fold different initial concentrations of α-TOH in the LDL samples from the FIVE patient (Fig. 2B). Similar rates of α-TQH2 oxidation were also observed with the FIVE patient’s HDL samples that contained different amount of α-TOH (data not shown), demonstrating that α-TQH2 can react with ROO⋅ independent of the levels of α-TOH and the apolipoprotein present. Under all conditions tested, α-TQ was formed stoichiometrically from α-TQH2. Also, the presence of α-TQH2 completely prevented the ROO⋅-induced accumulation of CE-O(O)H and PC-OOH in the FIVE patient’s LDL and HDL for at least the first 3 hr of oxidation, whereas in the absence of α-TQH2, the hydroperoxides accumulated at rates directly proportional to the levels of endogenous α-TOH (data not shown), in agreement with our recent observation (10). Furthermore, α-TQH2 completely prevented the peroxidation of Intralipid (diluted 1:10 in PBS) incubated at 37°C with 2 mM of AAPH (data not shown). These results confirm that under these mild oxidizing conditions, α-TOH acts as a prooxidant for lipid emulsions (6, 7), and demonstrate that α-TQH2 effectively prevents this prooxidant activity, independent of the presence of protein(s).
We next examined a possible role of coenzyme Q in the antioxidant activity of α-TQH2. For this, LDL enriched some 3-fold with CoQ10H2 by dietary supplementation with CoQ10 (29) was exposed to AAPH in the presence and absence of α-TQH2. Under all conditions α-TQH2 was consumed before and completely prevented the oxidation of CoQ10H2 in agreement with the results shown in Fig. 1. More importantly, the rates of α-TQH2 oxidation were independent of the initial level of LDL’s CoQ10H2 (data not shown), indicating that α-TQH2 acted independently of CoQ10H2.
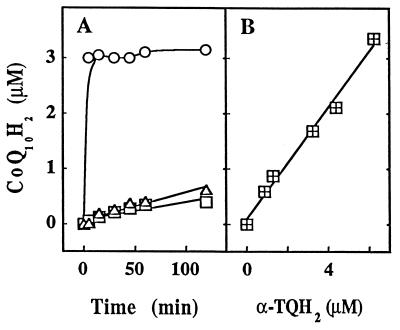
A striking feature of the treatment of LDL with α-TQH2 was the ability of the hydroquinone to rapidly reduce CoQ10 (see above). Fig. 3A shows the results of an experiment where in vivo CoQ10H2-enriched LDL (29) was first allowed to autoxidize until all coenzyme Q was present as CoQ10, before the lipoprotein was placed on ice, 10 μM of α-TQH2 was added, and the lipoprotein was then incubated at 37°C. Addition of α-TQH2 to such LDL resulted in the instantaneous formation of CoQ10H2, whereas a slower reduction of CoQ10 was observed when either an organic extract of such LDL (redissolved in ethanol) or an ethanolic solution of authentic CoQ10 was used (Fig. 3A), similar to the situation when LDL-associated CoQ10 was exposed to HepG2 and red blood cells (35). Whether the very rapid reduction of CoQ10 by α-TQH2 plays a physiological role in the maintenance of extracellular coenzyme Q in its reduced, antioxidant active form appears unlikely because healthy human blood does not contain measurable α-TQH2. However, the fact that several of the hydroquinones tested here are able to perform this reduction may suggest a role for a physiological hydroquinone, such as the reduced form of vitamin K, in the maintenance of circulating CoQ10H2, though this requires testing. What is clear is that the rapid reduction of CoQ10 by α-TQH2 was protein independent, as it was also observed when α-TQH2 was added to either (in vivo CoQ10-enriched) HDL or commercial Intralipid (data not shown).
Figure 3.
α-TQH2 efficiently and stoichiometrically reduces CoQ10 present in intact LDL but not LDL lipid extracts or organic solution. (A) In vivo CoQ10H2-enriched (30) LDL (1.1 μM in apoB) was allowed to autoxidize until all of the coenzyme Q was present as CoQ10. The intact lipoprotein (○), its total lipid extract (▵), or an ethanolic solution of CoQ10 (□) were then placed on ice before 10 μM of α-TQH2 was added. The samples were placed at 37°C and aliquots were removed at the time points indicated and analyzed for CoQ10H2. The initial CoQ10 concentration was 3.1 ± 0.25, 2.62 ± 0.20, and 10 ± 0 μM (mean ± SD; n = 3) for intact LDL, LDL lipid extract, and organic CoQ10 solution, respectively. The data shown are average values derived from three independent experiments with <3% variation for all conditions. (B) In vivo CoQ10H2-enriched (29) LDL (0.93 μM in apoB) was preincubated at 37°C for ≈8 hr and then left at room temperature overnight for most of the CoQ10H2 to autoxidize. Such LDL preparation contained 0.3 and 3 μM of CoQ10H2 and CoQ10, respectively, and was hydroperoxide-free as determined by HPLC (see text). Increasing amounts of α-TQH2 were then added and the formation of CoQ10H2 monitored at 37°C. The data shown are mean values derived from a single experiment performed in triplicate, with variation <5%.
The conversion of LDL’s CoQ10 to CoQ10H2 was quantitative and required two molecules of α-TQH2 for the two electron reduction of each molecule of CoQ10 (Fig. 3B). This indicates that the reaction may be complex, perhaps involving semiquinone radicals. As superoxide dismutase did not affect CoQ10 reduction by α-TQH2 (data not shown), it appears that superoxide anion does not play a role here.
Similar to CoQ10H2 (d[CoQ10H2]/dt = 97 ± 11 pmol liter−1⋅s−1), α-TQH2 dissolved in ethanol at 10 μM and incubated at 37°C autoxidized linearly at a rate of 127 ± 5.6 pmol liter−1⋅s−1. By contrast when added to LDL, HDL, or Intralipid incubated at 37°C, the levels of α-TQH2 remained unchanged for at least 5 hr, independent of the coenzyme Q and α-TOH concentrations present in the emulsions, suggesting that for presently unknown reason(s), lipid emulsions stabilize α-TQH2.
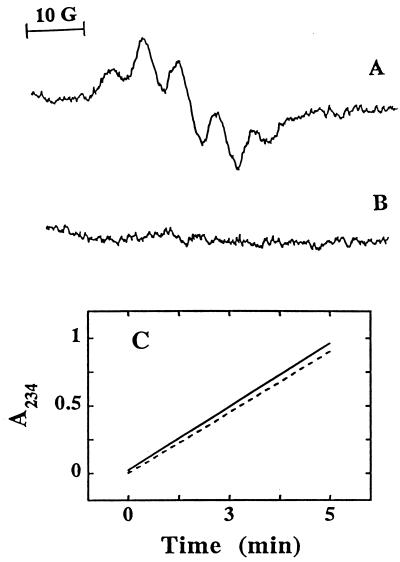
Previous results suggested that α-TQH2 is also capable of directly reducing α-TO⋅ in alcohol/water mixtures (36) or micelles (19). We therefore tested whether α-TQH2 was also able to react with α-TO⋅ in LDL. For this, we incubated LDL with SLO that resulted in detectable α-TO⋅ (27) (Fig. 4A). Addition of α-TQH2 to such oxidizing LDL resulted in immediate disappearance of the phenoxyl radical (Fig. 4B). Importantly, α-TQH2 had no inhibitory effect on the activity of SLO (Fig. 4C), thereby ruling out the possibility that the elimination of the radical signal was due to the prevention of its formation. These results demonstrate that α-TQH2 is able to directly react with α-TO⋅ in oxidizing LDL.
Figure 4.
Direct interaction of α-TQH2 with α-TO⋅ in LDL. α-TO⋅ was generated in LDL (7.2 μM in apoB) by incubation with SLO (1 mg/ml) at 37°C, and recorded by EPR spectroscopy before (A) and after (B) addition of 10 μM of α-TQH2 at the same region of field as detailed in the text. (C) The changes in UV234 nm absorbance associated with the conversion of linoleic acid (0.1 mM in PBS) to linoleic acid hydroperoxide by SLO (1 mg/ml) in the absence (dashed line) and presence (solid line) of 10 μM α-TQH2. The data shown are from a single experiment, representative of three separate experiments.
In summary, the results presented show evidence for three distinct antioxidant activities of α-TQH2, i.e., direct radical scavenging and reduction of both α-TO⋅ and CoQ10. Various compounds have been shown to both directly scavenge radicals and reduce α-TO⋅ (see ref. 19). What makes α-TQH2 exceptional is the apparent preference with which it becomes oxidized before CoQ10H2 (which thus far has been regarded the first line of lipophilic antioxidant defence), and the efficacy with which it prevents, rather than attenuates, lipid peroxidation in LDL and HDL exposed to different types of radical oxidants. Consistent with this, the one-electron reduction potential of α-TQH2 is expected to be lower than that of CoQ10H2 (and ascorbate) (for example, see ref. 37), and the rate constant for the reaction of α-TQH2 with α-TO⋅ is greater than that for CoQ10H2 though slightly lower than that for ascorbate (36).
In humans, who do not synthesize α-TQ, the quinone is most likely formed by oxidation of α-TOH. Under normal conditions, significant concentrations of α-TQ or α-TQH2 are not present in human tissues and fluids. By contrast, α-TQ has been detected in biological systems exposed to oxidative stress, such as plasma obtained during ischemia induced by crossclamping (38) or in advanced atherosclerotic plaques, where ≈9% of intimal α-TOH is present as α-TQ (17). This raises the possibility that α-TQH2 could be formed in vivo under conditions where α-TOH oxidation occurs and a suitable reducing system for α-TQ exists (28). If so, α-TQH2 may represent a previously unrecognized, highly effective natural antioxidant that could contribute to the protection of intimal lipids from oxidation. Also, in this case a potential contribution of α-TQH2 formation may need to be considered when evaluating the in vivo antioxidant activities of vitamin E, particularly under conditions of severe oxidative stress.
Because of its outstanding antioxidant activity in the in vitro systems used here, a potential protective function of α-TQH2 as a therapeutic antioxidant deserves consideration. Relevant to this, hydroquinones are potential carcinogens and cytotoxic agents, although these deleterious activities, at least in the case of tocopherol hydroquinones, are lowest for the α-form, probably due to its inability to give rise to Michael adducts (39). Also, tocopheryl quinones inhibit vitamin K-dependent carboxylase activity (40), and this could result in an anti-clotting action, although to our knowledge this action has not been demonstrated in vivo. On the other hand, there is early literature demonstrating a beneficial effect of administration of relatively large doses of α-TQ or α-TQH2 in the prevention of muscular dystrophy in rabbits and rats without apparent side effects (41, 42). α-TQ and α-TQH2 possess vitamin E activity (for example, see ref. 42), and this may be due to their conversion to α-TOH (43). Oral supplementation of humans with α-TQ results in low micromolar plasma levels of both α-TQ and α-TQH2 (28, 43). Together, these results suggest that α-TQ and α-TQH2 are non-toxic and that humans not only take up the quinone but also reduce it to the hydroquinone, and therefore warrant testing of α-TQ/α-TQH2 as a potential therapeutic antioxidant.
Acknowledgments
We thank Drs. A. Kohlschütter and A. Kontush (University of Hamburg) for the plasma samples from a vitamin-E deficient patient, Drs. A. Baoutina for preparing MDM, C. Suarna for synthesizing 2-(3-hydroxy-3-methylbutyl)-3,5,6-trimethyl-1,4-benzoquinone, J. Upston for measuring SLO activity, W. Schwartz for preparing ONOO−, and Ms. J. Letters for technical assistance. Dr. K. Ingold is acknowledged for helpful discussions and communicating results before publication. This work was supported by ASTRA Hässle, Sweden, and the Australian National Health & Medical Research Council Grant 940915 to R.S.
ABBREVIATIONS
- AAPH
2,2′-azobis(2-amidinopropane) dihydrochloride
- AMVN
2,2′-azobis(2, 4-dimethylvaleronitrile)
- apoB
apolipoprotein B-100
- CE-O(O)H
cholesteryl ester hydroperoxides plus cholesteryl ester hydroxides
- CoQ10
ubiquinone-10
- CoQ10H2
ubiquinol-10
- 2
5-DTBHQ, 2,5-di-tert-butylhydroquinone
- 3
5-DTBHQ, 2,6-di-tert-butylhydroquinone
- EPR
electron paramagnetic resonance
- FIVE
vitamin E-deficient patient
- HDL
high density lipoproteins
- LDL
low density lipoprotein
- MDM
human monocyte-derived macrophage
- −OCl
hypochlorite
- ONOO−
peroxynitrite
- PC-OOH
phosphatidyl choline hydroperoxides
- α-PQH2
1,4-di-hydroxy-2-(3-hydroxy-3-methylbutyl)-3,5,6-trimethylbenzene
- ROO⋅
peroxyl radical
- SLO
soybean 15-lipoxygenase
- TMP
tocopherol-mediated peroxidation
- α-TOH
α-tocopherol
- α-TO⋅
α-tocopheroxyl radical
- α-TQH2
α-tocopheryl hydroquinone
- α-TQ
α-tocopheryl quinone
References
- 1.Steinberg D, Parthasarathy S, Carew T E, Khoo J C, Witztum J L. N Engl J Med. 1989;320:915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein J L, Ho Y K, Basu S K, Brown M S. Proc Natl Acad Sci USA. 1979;76:333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nicholson A C, Frieda S, Pearce A, Silverstein R L. Arterioscler Thromb Vasc Biol. 1995;15:269–275. doi: 10.1161/01.atv.15.2.269. [DOI] [PubMed] [Google Scholar]
- 4.Berliner J A, Heinecke J W. Free Radical Biol Med. 1996;20:707–727. doi: 10.1016/0891-5849(95)02173-6. [DOI] [PubMed] [Google Scholar]
- 5.Bowry V W, Ingold K U, Stocker R. Biochem J. 1992;288:341–344. doi: 10.1042/bj2880341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowry V W, Stocker R. J Am Chem Soc. 1993;115:6029–6044. [Google Scholar]
- 7.Ingold K U, Bowry V W, Stocker R, Walling C. Proc Natl Acad Sci USA. 1993;90:45–49. doi: 10.1073/pnas.90.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohr D, Stocker R. Arterioscl Thromb. 1994;14:1186–1192. doi: 10.1161/01.atv.14.7.1186. [DOI] [PubMed] [Google Scholar]
- 9.Neuzil J, Stocker R. J Biol Chem. 1994;269:16712–16719. [PubMed] [Google Scholar]
- 10.Neuzil J, Thomas S R, Stocker R. Free Radical Biol Med. 1997;22:57–71. doi: 10.1016/s0891-5849(96)00224-9. [DOI] [PubMed] [Google Scholar]
- 11.Bowry V W, Mohr D, Cleary J, Stocker R. J Biol Chem. 1995;270:5756–5763. doi: 10.1074/jbc.270.11.5756. [DOI] [PubMed] [Google Scholar]
- 12.Frei B, Stocker R, Ames B N. Proc Natl Acad Sci USA. 1988;85:9748–9752. doi: 10.1073/pnas.85.24.9748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dabbagh A J, Frei B. J Clin Invest. 1995;96:1958–1966. doi: 10.1172/JCI118242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stocker R, Bowry V W, Frei B. Proc Natl Acad Sci USA. 1991;88:1646–1650. doi: 10.1073/pnas.88.5.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frei B, England L, Ames B N. Proc Natl Acad Sci USA. 1989;86:6377–6381. doi: 10.1073/pnas.86.16.6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stocker R, Frei B. In: Oxidative Stress: Oxidants and Antioxidants. Sies H, editor. London: Academic; 1991. pp. 213–243. [Google Scholar]
- 17.Suarna C, Dean R T, May J, Stocker R. Arterioscler Thromb Vasc Biol. 1995;15:1616–1624. doi: 10.1161/01.atv.15.10.1616. [DOI] [PubMed] [Google Scholar]
- 18.Cleary J, Mohr D, Adams M R, Celermajer D S, Stocker R. Free Radical Res. 1997;26:175–182. doi: 10.3109/10715769709097796. [DOI] [PubMed] [Google Scholar]
- 19.Witting P K, Westerlund C, Stocker R. J Lipid Res. 1996;37:853–867. [PubMed] [Google Scholar]
- 20.Sattler W, Mohr D, Stocker R. Methods Enzymol. 1994;233:469–489. doi: 10.1016/s0076-6879(94)33053-0. [DOI] [PubMed] [Google Scholar]
- 21.Esterbauer H, Dieber-Rotheneder M, Striegl G, Waeg G. Am J Clin Nutr. 1991;53:314S–321S. doi: 10.1093/ajcn/53.1.314S. [DOI] [PubMed] [Google Scholar]
- 22.Traber M G, Sokol R J, Burton G W, Ingold K U, Papas A M, Huffaker J E, Kayden H J. J Clin Invest. 1990;85:397–407. doi: 10.1172/JCI114452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Traber M G, Sokol R J, Kohlschütter A, Yokota T, Muller D P, Dufour R, Kayden H J. J Lipid Res. 1993;34:201–210. [PubMed] [Google Scholar]
- 24.Pryor W A, Cueto R, Jin X, Koppenol W H, Ngu-Schwemlein M, Squadrito G L, Uppu P L, Uppu R M. Free Radical Biol Med. 1995;18:75–83. doi: 10.1016/0891-5849(94)00105-s. [DOI] [PubMed] [Google Scholar]
- 25.Christen S, Thomas S R, Garner B, Stocker R. J Clin Invest. 1994;93:2149–2158. doi: 10.1172/JCI117211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bligh E G, Dyer W J. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 27.Kalyanaraman B, Antholine W E, Parthasarathy S. Biochim Biophys Acta. 1990;1035:286–292. doi: 10.1016/0304-4165(90)90090-j. [DOI] [PubMed] [Google Scholar]
- 28.Kohar I, Baca M, Suarna C, Stocker R, Southwell-Keely P. Free Radical Biol Med. 1995;19:197–207. doi: 10.1016/0891-5849(95)00010-u. [DOI] [PubMed] [Google Scholar]
- 29.Mohr D, Bowry V W, Stocker R. Biochim Biophys Acta. 1992;1126:247–254. doi: 10.1016/0005-2760(92)90237-p. [DOI] [PubMed] [Google Scholar]
- 30.Thomas S R, Witting P K, Stocker R. J Biol Chem. 1996;271:32714–32721. doi: 10.1074/jbc.271.51.32714. [DOI] [PubMed] [Google Scholar]
- 31.Thomas S R, Neuzil J, Stocker R. Arterioscl Thromb Vasc Biol. 1996;16:687–696. doi: 10.1161/01.atv.16.5.687. [DOI] [PubMed] [Google Scholar]
- 32.Bindoli A, Valente M, Cavallini L. Biochem Intern. 1985;10:753–761. [PubMed] [Google Scholar]
- 33.Hazell L J, Stocker R. Biochem J. 1993;290:165–172. doi: 10.1042/bj2900165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niki E, Saito M, Yoshikawa Y, Yamamoto Y, Kamiya Y. Bull Chem Soc Jpn. 1986;59:471–477. [Google Scholar]
- 35.Stocker R, Suarna C. Biochim Biophys Acta. 1993;1158:15–22. doi: 10.1016/0304-4165(93)90090-u. [DOI] [PubMed] [Google Scholar]
- 36.Mukai K, Itoh S, Morimoto H. J Biol Chem. 1992;267:22277–22281. [PubMed] [Google Scholar]
- 37.Steenken S, Neta P. J Phys Chem. 1982;86:3661–3667. [Google Scholar]
- 38.Murphy M E, Kolvenbach R, Aleksis M, Hansen R, Sies H. Free Radical Biol Med. 1992;13:95–100. doi: 10.1016/0891-5849(92)90069-s. [DOI] [PubMed] [Google Scholar]
- 39.Thornton D E, Jones K H, Jiang Z, Zhang H, Liu G, Cornwell D G. Free Radical Biol Med. 1995;18:963–976. doi: 10.1016/0891-5849(94)00210-b. [DOI] [PubMed] [Google Scholar]
- 40.Dowd P, Zheng Z B. Proc Natl Acad Sci USA. 1995;92:8171–8175. doi: 10.1073/pnas.92.18.8171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mackenzie J B, Rosenkrantz H, Ulick S, Milhorat A T. J Biol Chem. 1950;183:655–662. [Google Scholar]
- 42.Mackenzie J B, Mackenzie C G. J Nutr. 1959;67:223–235. doi: 10.1093/jn/67.2.223. [DOI] [PubMed] [Google Scholar]
- 43.Moore A N J, Ingold K U. Free Radical Biol Med. 1997;22:931–934. doi: 10.1016/s0891-5849(96)00276-6. [DOI] [PubMed] [Google Scholar]