Abstract
Membrane vesicles and the F1-ATPase from Clostridium thermoaceticum were examined by electron microscopy. F1-ATPase particles projecting from the vesicles have a diameter of 10 to 12 nm. The F1-ATPase has an alpha 3 beta 3 gamma delta structure. The alpha and beta subunits are most likely arranged in an alternating sequence around a central protein mass consisting of the gamma and delta subunits.
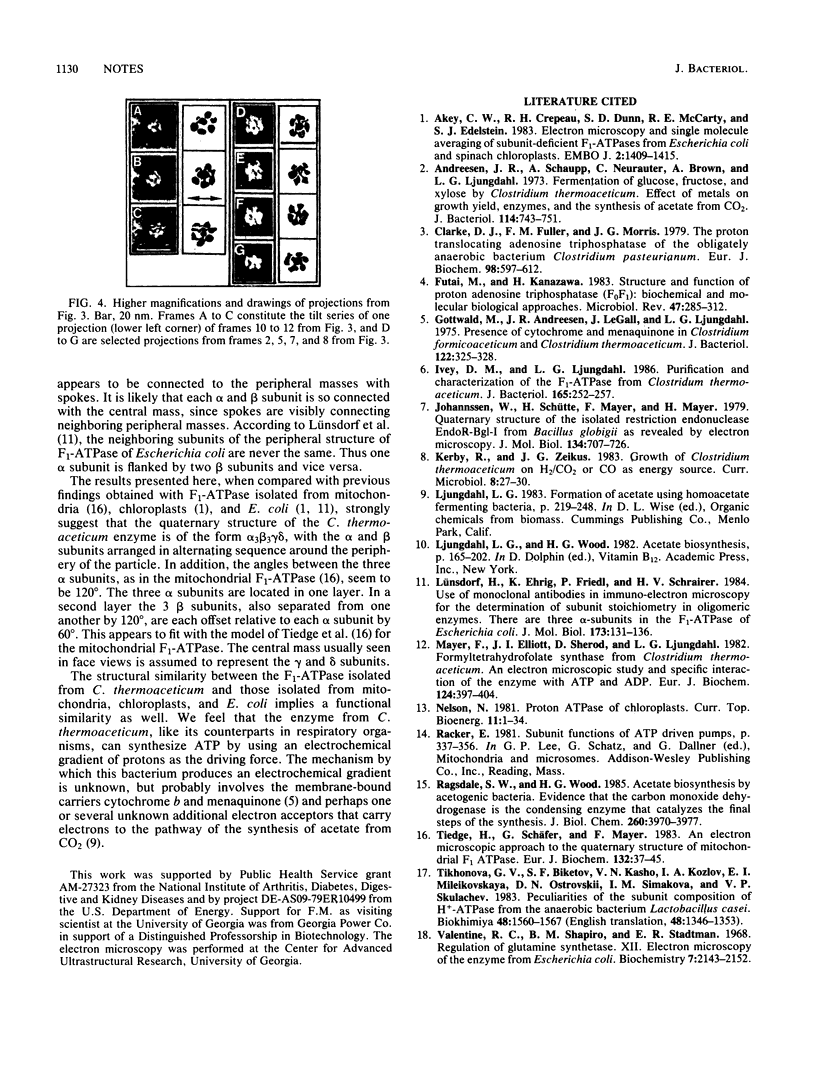
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akey C. W., Crepeau R. H., Dunn S. D., McCarty R. E., Edelstein S. J. Electron microscopy and single molecule averaging of subunit-deficient F1-ATPases from Escherichia coli and spinach chloroplasts. EMBO J. 1983;2(8):1409–1415. doi: 10.1002/j.1460-2075.1983.tb01599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreesen J. R., Schaupp A., Neurauter C., Brown A., Ljungdahl L. G. Fermentation of glucose, fructose, and xylose by Clostridium thermoaceticum: effect of metals on growth yield, enzymes, and the synthesis of acetate from CO 2 . J Bacteriol. 1973 May;114(2):743–751. doi: 10.1128/jb.114.2.743-751.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke D. J., Fuller F. M., Morris J. G. The proton-translocating adenosine triphosphatase of the obligately anaerobic bacterium Clostridium pasteurianum. 1. ATP phosphohydrolase activity. Eur J Biochem. 1979 Aug 1;98(2):597–612. doi: 10.1111/j.1432-1033.1979.tb13222.x. [DOI] [PubMed] [Google Scholar]
- Futai M., Kanazawa H. Structure and function of proton-translocating adenosine triphosphatase (F0F1): biochemical and molecular biological approaches. Microbiol Rev. 1983 Sep;47(3):285–312. doi: 10.1128/mr.47.3.285-312.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottwald M., Andreesen J. R., LeGall J., Ljungdahl L. G. Presence of cytochrome and menaquinone in Clostridium formicoaceticum and Clostridium thermoaceticum. J Bacteriol. 1975 Apr;122(1):325–328. doi: 10.1128/jb.122.1.325-328.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivey D. M., Ljungdahl L. G. Purification and characterization of the F1-ATPase from Clostridium thermoaceticum. J Bacteriol. 1986 Jan;165(1):252–257. doi: 10.1128/jb.165.1.252-257.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannssen W., Schütte H., Mayer F., Mayer H. Quaternary structure of the isolated restriction endonuclease EndoR.Bgl I from Bacillus globigii as revealed by electron microscopy. J Mol Biol. 1979 Nov 15;134(4):707–726. doi: 10.1016/0022-2836(79)90481-9. [DOI] [PubMed] [Google Scholar]
- Lünsdorf H., Ehrig K., Friedl P., Schairer H. U. Use of monoclonal antibodies in immuno-electron microscopy for the determination of subunit stoichiometry in oligomeric enzymes. There are three alpha-subunits in the F1-ATPase of Escherichia coli. J Mol Biol. 1984 Feb 15;173(1):131–136. doi: 10.1016/0022-2836(84)90408-x. [DOI] [PubMed] [Google Scholar]
- Mayer F., Elliott J. I., Sherod D., Ljungdahl L. G. Formyltetrahydrofolate synthase from Clostridium thermoaceticum. An electron microscopic study and specific interaction of the enzyme with ATP and ADP. Eur J Biochem. 1982 May 17;124(2):397–404. [PubMed] [Google Scholar]
- Ragsdale S. W., Wood H. G. Acetate biosynthesis by acetogenic bacteria. Evidence that carbon monoxide dehydrogenase is the condensing enzyme that catalyzes the final steps of the synthesis. J Biol Chem. 1985 Apr 10;260(7):3970–3977. [PubMed] [Google Scholar]
- Tiedge H., Schäfer G., Mayer F. An electron microscopic approach to the quaternary structure of mitochondrial F1-ATPase. Eur J Biochem. 1983 Apr 15;132(1):37–45. doi: 10.1111/j.1432-1033.1983.tb07322.x. [DOI] [PubMed] [Google Scholar]
- Valentine R. C., Shapiro B. M., Stadtman E. R. Regulation of glutamine synthetase. XII. Electron microscopy of the enzyme from Escherichia coli. Biochemistry. 1968 Jun;7(6):2143–2152. doi: 10.1021/bi00846a017. [DOI] [PubMed] [Google Scholar]






