Abstract
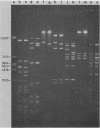
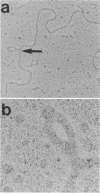
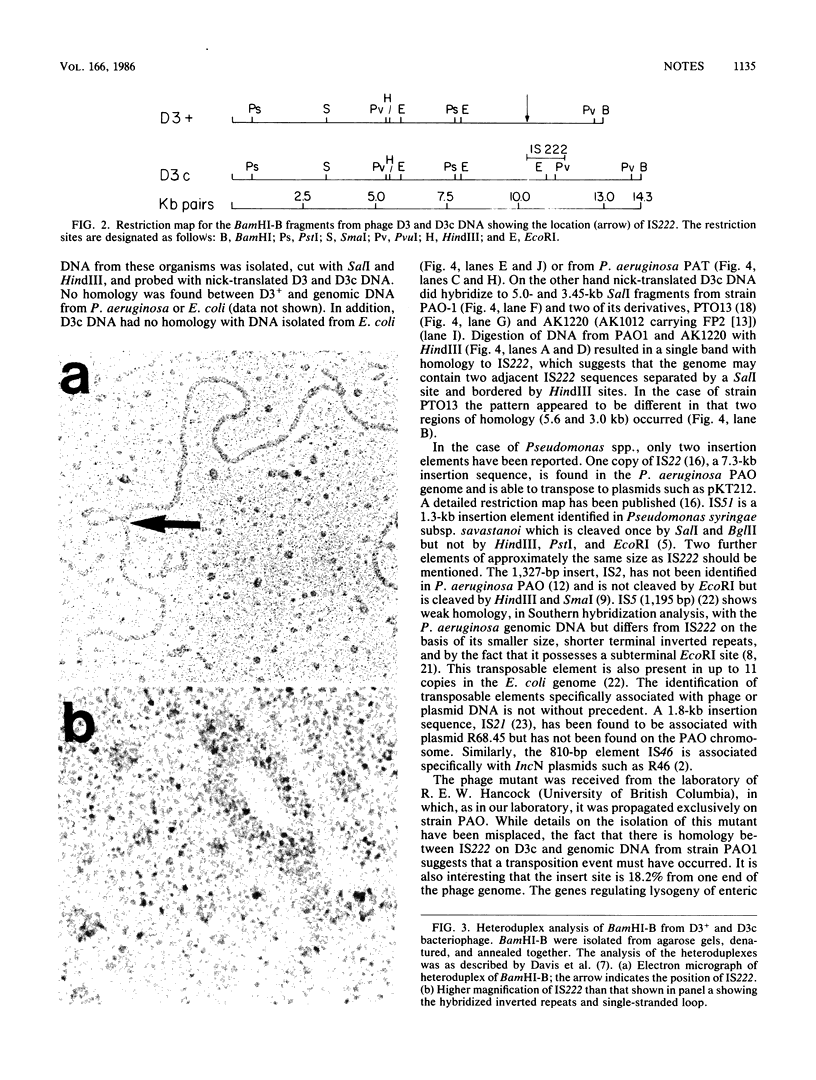
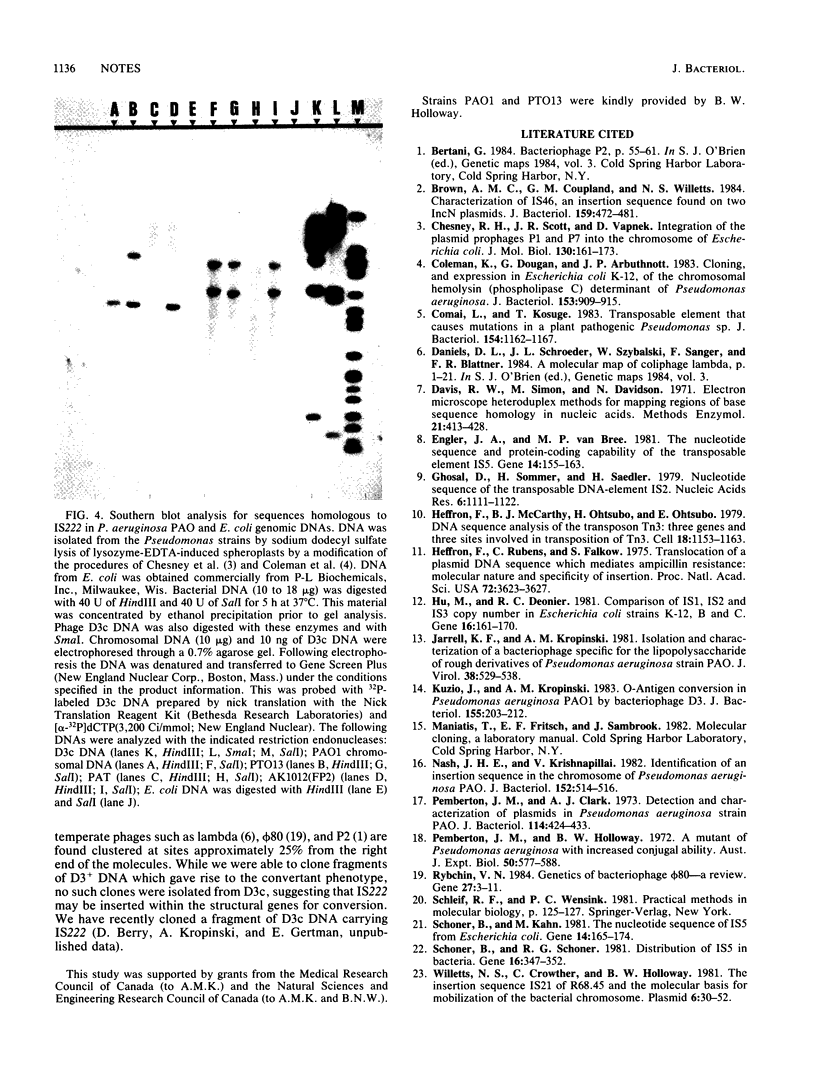
A new insertion element, IS222, was identified to be associated with the DNA of a mutant strain of the converting Pseudomonas aeruginosa bacteriophage D3. The insertion sequence was 1,350 base pairs in size and possessed terminal inverted repeats. The nucleotide sequence contained single cleavage sites for EcoRI and PvuI but none for BamHI, PstI, HindIII, SmaI, or SalI. By Southern hybridization analysis, no homology was found with genomic DNA from P. aeruginosa PAT or Escherichia coli. Genomic DNA from the phage host, P. aeruginosa PAO, contained two sequences homologous to IS222.
Full text
PDF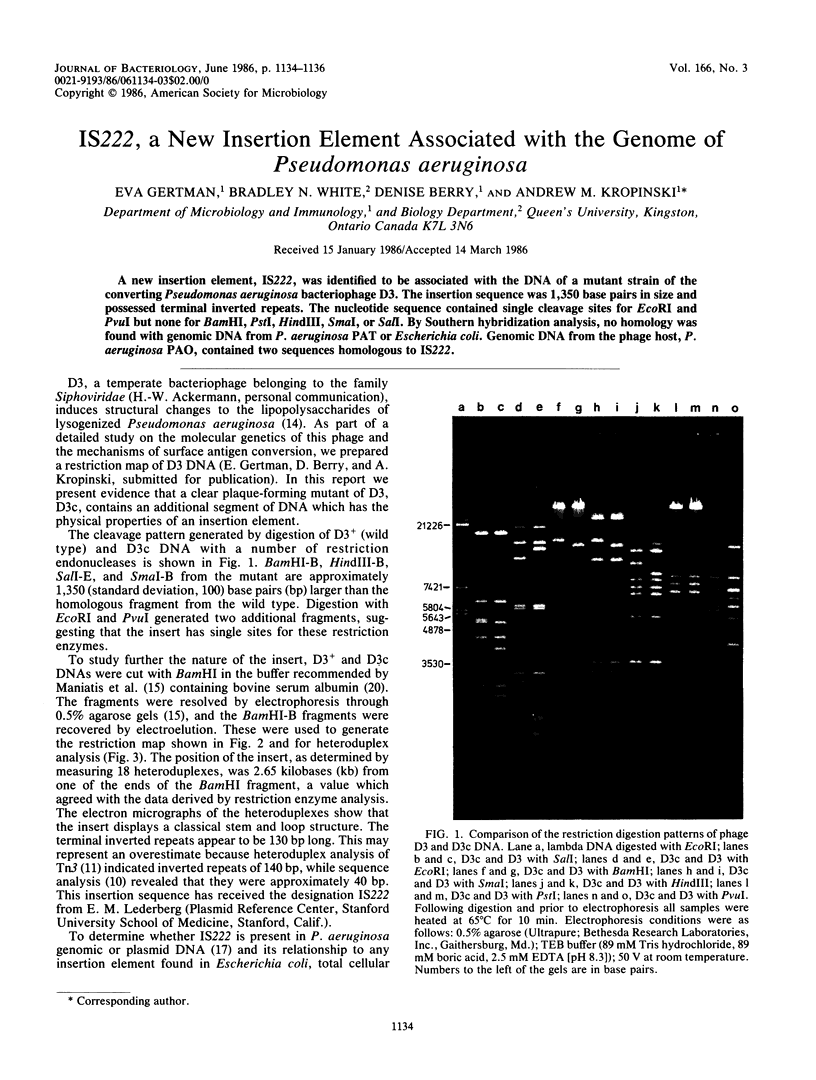
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown A. M., Coupland G. M., Willetts N. S. Characterization of IS46, an insertion sequence found on two IncN plasmids. J Bacteriol. 1984 Aug;159(2):472–481. doi: 10.1128/jb.159.2.472-481.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman K., Dougan G., Arbuthnott J. P. Cloning, and expression in Escherichia coli K-12, of the chromosomal hemolysin (phospholipase C) determinant of Pseudomonas aeruginosa. J Bacteriol. 1983 Feb;153(2):909–915. doi: 10.1128/jb.153.2.909-915.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comai L., Kosuge T. Transposable element that causes mutations in a plant pathogenic Pseudomonas sp. J Bacteriol. 1983 Jun;154(3):1162–1167. doi: 10.1128/jb.154.3.1162-1167.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler J. A., van Bree M. P. The nucleotide sequence and protein-coding capability of the transposable element IS5. Gene. 1981 Aug;14(3):155–163. doi: 10.1016/0378-1119(81)90111-6. [DOI] [PubMed] [Google Scholar]
- Ghosal D., Sommer H., Saedler H. Nucleotide sequence of the transposable DNA-element IS2. Nucleic Acids Res. 1979 Mar;6(3):1111–1122. doi: 10.1093/nar/6.3.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffron F., McCarthy B. J., Ohtsubo H., Ohtsubo E. DNA sequence analysis of the transposon Tn3: three genes and three sites involved in transposition of Tn3. Cell. 1979 Dec;18(4):1153–1163. doi: 10.1016/0092-8674(79)90228-9. [DOI] [PubMed] [Google Scholar]
- Heffron F., Rubens C., Falkow S. Translocation of a plasmid DNA sequence which mediates ampicillin resistance: molecular nature and specificity of insertion. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3623–3627. doi: 10.1073/pnas.72.9.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M., Deonier R. C. Comparison of IS1, IS2 and IS3 copy number in Escherichia coli strains K-12, B and C. Gene. 1981 Dec;16(1-3):161–170. doi: 10.1016/0378-1119(81)90072-x. [DOI] [PubMed] [Google Scholar]
- Jarrell K. F., Kropinski A. M. Isolation and characterization of a bacteriophage specific for the lipopolysaccharide of rough derivatives of Pseudomonas aeruginosa strain PAO. J Virol. 1981 May;38(2):529–538. doi: 10.1128/jvi.38.2.529-538.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzio J., Kropinski A. M. O-antigen conversion in Pseudomonas aeruginosa PAO1 by bacteriophage D3. J Bacteriol. 1983 Jul;155(1):203–212. doi: 10.1128/jb.155.1.203-212.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash J. H., Krishnapillai V. Identification of an insertion sequence in the chromosome of Pseudomonas aeruginosa PAO. J Bacteriol. 1982 Oct;152(1):514–516. doi: 10.1128/jb.152.1.514-516.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pemberton J. M., Clark A. J. Detection and characterization of plasmids in Pseudomonas aeruginosa strain PAO. J Bacteriol. 1973 Apr;114(1):424–433. doi: 10.1128/jb.114.1.424-433.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pemberton J. M., Holloway B. W. A mutant of Pseudomonas aeruginosa with increased conjugational ability. Aust J Exp Biol Med Sci. 1972 Oct;50(5):577–588. doi: 10.1038/icb.1972.51. [DOI] [PubMed] [Google Scholar]
- Rybchin V. N. Genetics of bacteriophage phi 80--a review. Gene. 1984 Jan;27(1):3–11. doi: 10.1016/0378-1119(84)90233-6. [DOI] [PubMed] [Google Scholar]
- Schoner B., Kahn M. The nucleotide sequence of IS5 from Escherichia coli. Gene. 1981 Aug;14(3):165–174. doi: 10.1016/0378-1119(81)90112-8. [DOI] [PubMed] [Google Scholar]
- Schoner B., Schoner R. G. Distribution of IS5 in bacteria. Gene. 1981 Dec;16(1-3):347–352. doi: 10.1016/0378-1119(81)90093-7. [DOI] [PubMed] [Google Scholar]
- Willetts N. S., Crowther C., Holloway B. W. The insertion sequence IS21 of R68.45 and the molecular basis for mobilization of the bacterial chromosome. Plasmid. 1981 Jul;6(1):30–52. doi: 10.1016/0147-619x(81)90052-4. [DOI] [PubMed] [Google Scholar]